Abstract
The endoplasmic reticulum (ER) is the major site in the cell for protein folding and trafficking and is central to many cellular functions. Failure of the ER's adaptive capacity results in activation of the unfolded protein response (UPR), which intersects with many different inflammatory and stress signaling pathways. These pathways are also critical in chronic metabolic diseases such as obesity, insulin resistance, and type 2 diabetes. The ER and related signaling networks are emerging as a potential site for the intersection of inflammation and metabolic disease.
Introduction
Energy and nutrient management in all organisms requires the highly regulated and coordinated operation of many homeostatic systems. Much of the development and evolution of these systems has taken place in the absence of pressures that we now experience as modern humans, including excess nutrients, new dietary components, lack of physical activity, and an increased life span. In fact, the requirements for the duration as well as the magnitude of adaptive responses have dramatically increased due to both increases in life expectancy and a chronic lifetime exposure to the stress signals associated with caloric excess. Consequently, biological responses are unable to cope with these challenges and homeostatic systems gradually deteriorate giving rise to an array of pathologies.
In the past century, the incidence of chronic metabolic diseases, particularly obesity and type 2 diabetes, has increased dramatically in the developed and developing worlds (Hossain et al., 2007) and in children (Ogden et al., 2010). Increased adiposity and abnormal insulin action are associated with an array of health problems including a markedly increased risk for type 2 diabetes, fatty liver, hepato-biliary and gallbladder diseases, cardiovascular pathologies, neurodegenerative disorders, asthma, and a variety of cancers (Hotamisligil, 2006). This disease cluster resembles longevity-related systemic deterioration. Addressing the central mechanisms underlying these pathologies will have implications for aging and should lead to new therapeutic approaches for treating these conditions.
One potential emerging mechanism involves the endoplasmic reticulum (ER), the organelle responsible for protein folding, maturation, quality control, and trafficking. When the ER becomes stressed due to the accumulation of newly synthesized unfolded proteins, the unfolded protein response (UPR) is activated. The three branches of the canonical UPR intersect with a variety of inflammatory and stress signaling systems including the NF-κB-IκB kinase (IKK) and JNK-AP1 pathways, as well as networks activated by oxidative stress, all of which can influence metabolism (Figure 1). Inflammation and ER stress are linked at many levels: both are short-term adaptive systems necessary for the function and survival of the organism, and both are detrimental when chronically engaged. In this Review, I discuss ER stress responses and their intersection with inflammatory pathways in the context of metabolic homeostasis and disease (Figure 2).
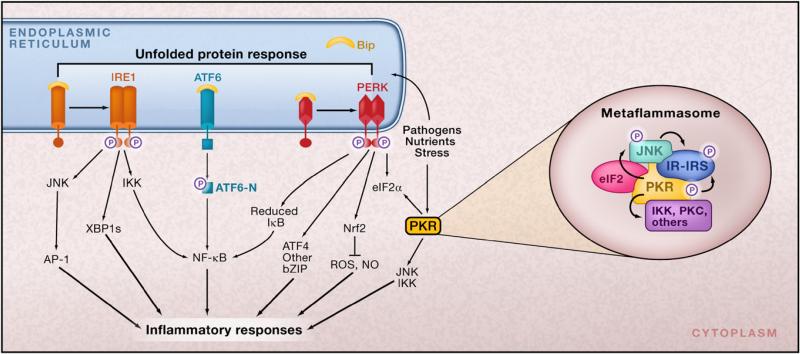
Figure 1. ER Stress and Inflammation.
There are several potential avenues through which ER function is linked to inflammatory signaling. In response to ER stress, the three branches of the unfolded protein response (UPR) are activated. In the first branch, PERK-mediated attenuation of translation results in release of NF-κB from its inhibitor IκB. NF-κB moves to the nucleus and switches on expression of a variety of different genes involved in inflammatory pathways, such as those encoding the cytokines IL-1 and TNF-α. In the second branch of the UPR, activated IRE1α recruits tumor necrosis factor-α (TNF-α)-receptor-associated factor 2 (TRAF2), and this complex interacts with JNK and IκB kinase (IKK) and activates them. These inflammatory kinases then phosphorylate and activate downstream mediators of inflammation. The third branch of the UPR, the ATF6 pathway, also activates NF-κB. There is also crosstalk between the three branches. For example, spliced X-box binding protein 1 (XBP1s), as well as ATF4, induce production of the inflammatory cytokines IL-8, IL-6, and monocyte chemoattractant protein 1 (MCP1) by human endothelial cells. XBP1s has also been implicated in production of IFN-β when ER stress is combined with activation of Toll-like receptor (TLR) signaling and in IFN-α production by dendritic cells. The ability of XBP1s to expand the capacity of the ER for protein folding (and ER calcium stores) enables it to mediate calcium-dependent inflammatory responses in human bronchial epithelial cells, which produce IL-8. (Inset) Assembly of a putative metabolic inflammasome or metaflammasome. This protein complex integrates pathogen and nutrient sensing with ER stress, inflammatory kinases, insulin action, and metabolic homeostasis. The eIF2α kinase PKR (double-stranded RNA-activated protein kinase) is a core component of the metaflammasome and interacts directly with several inflammatory kinases such as IKK and JNK, insulin receptor signaling components such as IRS1, and the translational machinery via eIF2α. Nutrients, inflammatory mediators, and ER stress regulate PKR activity.
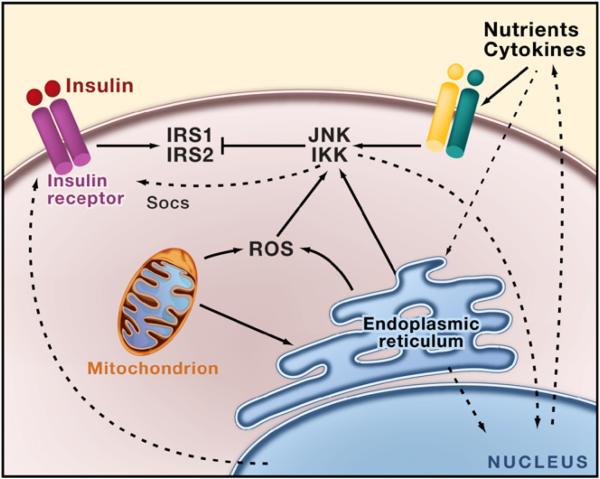
Figure 2. Insulin Signaling, Inflammation, and Stress Signals.
In obesity, inflammatory mediators and lipids activate a signaling cascade that triggers inflammatory kinases such as JNK and IKK as well as protein kinase C, S6K, mTOR, and ERK. The activation of JNK and IKK results in the inhibition of insulin action in part through serine phosphorylation of insulin receptor substrates (IRS) 1 and 2. Energy or nutrient excess can trigger ER stress, which is directly linked to activation of inflammatory signaling pathways that then block insulin action and transcriptionally regulate production of inflammatory cytokines. Reactive oxygen species (ROS) that are produced during organelle stress and mitochondrial dysfunction also contribute to this cycle. The consequences are increased ER stress, increased inflammation, inhibition of insulin action, and possibly leptin action, culminating in systemic metabolic dysfunction.
ER and the Canonical Unfolded Protein Response
The ER is a vast membranous network responsible for the synthesis, maturation, and trafficking of a wide range of proteins. It is also a critical site for Ca2+ homeostasis. As a central regulator of protein folding, quality control, trafficking, and targeting, the ability of the ER to adapt its capacity to manage synthetic, metabolic, and other adverse conditions is of paramount importance for the cell. Under conditions that challenge ER function, particularly an increase in newly synthesized, unfolded proteins in the ER lumen, this organelle elicits an elaborate adaptive response known as the UPR (Ron and Walter, 2007).
In eukaryotic cells, monitoring of the ER lumen and signaling through the canonical branches of the UPR are mediated by three ER membrane-associated proteins, PERK (PKR-like eukaryotic initiation factor 2α kinase), IRE1 (inositol requiring enzyme 1), and ATF6 (activating transcription factor-6). In a well-functioning and “stress-free” ER, these three transmembrane proteins are bound by a chaperone, BiP/GRP78, in their intralumenal domains (amino-terminal of IRE1 and PERK and carboxy-terminal of ATF6) and rendered inactive (Bertolotti et al., 2000; Shen et al., 2002). There may also be additional mechanisms controlling the activity of each UPR sensor and simple disruption of the interaction with BIP may not always result in constitutive activation (Oikawa et al., 2007; Zhou et al., 2006). Accumulation of improperly folded proteins and increased protein cargo in the ER results in the recruitment of BiP away from these UPR sensors. This and potentially other yet to be discovered lumenal events result in oligomerization and activation of the two kinases, PERK and IRE1, and engage a complex downstream signaling pathway (Ron and Walter, 2007). Activation of the third branch of the UPR requires translocation of ATF6 to the Golgi apparatus where it is processed by the serine protease site-1 protease (S1P) and the metalloprotease site-2 protease (S2P) to produce an active transcription factor (Chen et al., 2002). ATF6 is reduced in response to ER stress, and only the reduced monomeric ATF6 can reach the Golgi apparatus, indicating that redox status is also a potential determinant of ATF6 activation (Nadanaka et al., 2007). Together these three arms mitigate ER stress by reducing protein synthesis, facilitating protein degradation, and increasing production of chaperones that help proteins in the ER lumen to fold (Figure 1). The result is that the ER stress resolves, and if it does not then the cell is functionally compromised and may undergo apoptosis.
The oldest branch of the UPR, in an evolutionary sense, is mediated through the stress-regulated kinase and ribonuclease IRE1, which is conserved from yeast to humans (Calfon et al., 2002; Patil and Walter, 2001). The endoribonuclease activity of IRE1α cleaves a 26 base-pair segment from the mRNA of the X-box binding protein-1 (XBP1), creating an alternative message that is translated into the active (or spliced) form of the transcription factor (XBP1s) (Sidrauski and Walter, 1997). XBP1s, alone or in conjunction with ATF6α, launches a transcriptional program to produce chaperones (such as Grp78) and proteins involved in ER biogenesis, phospholipid synthesis, ER-associated protein degradation (ERAD), and secretion (for example, EDEM, ERdj4, PDI). Thus, XBP1s activates one of the major pathways for enhancing the folding capacity of the ER and for dealing with ER stress (Lee et al., 2003). XBP1 was the only known substrate of IRE1, but recently this endoribonuclease was reported to target other mRNAs during ER stress, although through a different mechanism. These mRNAs are degraded by IRE1, thereby preventing their translation, an additional measure to relieve ER stress (Hollien et al., 2009; Hollien and Weissman, 2006). This is an intriguing new aspect of signaling through the regulated IRE1-dependent decay (RIDD) pathway that is likely to produce additional insights into the biological outcomes of ER stress beyond the simple degradation of unwanted mRNAs.
When activated, ATF6 moves to the nucleus to stimulate the expression of genes containing ER stress elements (ERSE-I, -II), UPR elements (UPRE), and cAMP response elements (CRE) in their promoters. ERAD as well as production of the ER degradation-enhancing α-mannosidase-like protein (EDEM) are boosted by these events, facilitating clearance and degradation of misfolded proteins from the ER lumen (Kokame et al., 2001; Yoshida et al., 1998). Currently, ATF6 and XBP1—a target of ATF6, which is further processed and activated by the ribonuclease activity of the IRE1 branch—are viewed as the predominant regulators of the transcriptional response programs triggered during the UPR (Yoshida et al., 2000). In addition to regulating XBP1 mRNA expression, ATF6 can also interact directly with the XBP1 protein to target UPR quality-control genes. Interestingly, however, mice lacking ATF6α or ATF6β do not have a major phenotype, whereas the dual deficiency is lethal (Wu et al., 2007; Yamamoto et al., 2007). This suggests either highly specialized adaptive functions carried out through this branch or the existence of further redundancies in the transcriptional responses of the UPR.
The recent identification of a number of ATF6α-related proteins suggests that the traditional model of a three-armed UPR may not be complete. To date, five proteins—Luman (CREB3), Oasis (CREB3L1), BBF2H7 (CREB3L2), CREBH (CREB3L3), and Tisp40 (CREB4, CREB3L4)—share a region of high sequence similarity with ATF6α: a transmembrane domain adjacent to a conserved bZIP region (Bailey and O'Hare, 2007). Like ATF6α, these proteins are anchored to the ER and in response to activation by specific stimuli undergo regulated intramembrane proteolysis in the Golgi and subsequent translocation to the nucleus. These factors have all been implicated in the ER stress response due to their ability to respond to traditional ER stressors, activate known UPR targets, or show activity at UPR response elements (Bailey and O'Hare, 2007). Despite the similarities among these transmembrane bZIPs and ATF6α, differences in activating stimuli, tissue distribution, and response element binding indicate unique roles for each of these factors in regulating the UPR. For example, CREBH may respond to inflammatory stimuli induced by lipopolysaccharide and cytokines in the liver and may help to integrate the UPR with the acute phase response by stimulating the production of serum amyloid, serum amyloid P component, and C-reactive protein (Zhang et al., 2006a), whereas Oasis is highly expressed in the skeletal system with a potential role in bone formation and osteoblast activity (Murakami et al., 2009). The existence of a family of ATF6-like proteins with distinct tissue distributions and activation profiles suggests the evolution of a more fine-tuned ER stress response in mammals that allows for a distinct response depending on the cell or tissue involved, the specific stressor encountered, and the duration of the stress. Of course, the transcriptional program of the UPR may have even greater complexity beyond this transcription factor family and may involve other as yet unknown molecules that carry out specialized functions, including those related to metabolism.
Activation of the third arm of the UPR through PERK results in phosphorylation of eIF2α (eukaryotic translational initiation factor 2α) at serine 51, which converts eIF2α to a competitor of eIF2B and reduces the rate of formation of the ternary complex, resulting in reduced global protein synthesis and a subsequent reduction in the workload of the ER (Harding et al., 1999; Shi et al., 1998). PERK is one of four protein kinases that can mediate eIF2α phosphorylation; the other three kinases are PKR (double-stranded RNA-activated protein kinase), GCN2 (general control non-derepressible kinase 2), and HRI (heme-regulated inhibitor kinase). The role of eIF2α kinases other than PERK in ER stress remains unclear, although recent studies show that PKR is activated during ER stress and influences the UPR and related inflammatory signaling events (Nakamura et al., 2010) (Figure 1). In addition to an overall reduction in protein synthesis, this branch of the UPR is also linked to broad transcriptional regulation through several distinct mechanisms, including the transcriptional regulation of ribosomal RNA (DuRose et al., 2009). This results in activation of ATF4 (activating transcription factor-4), Nrf2 (nuclear erythroid 2 p45-related factor 2), and NF-κB (nuclear factor kappa β), a master transcription factor with numerous functions including regulation of the inflammatory response. ATF4 is produced through alternative translation and induces expression of genes involved in apoptosis (CHOP, C/EBP homologous protein), ER redox control (ERO1, endoplasmic reticulum oxidoreductin), the negative feedback release of eIF2α inhibition (Gadd34, growth arrest, and DNA damage-inducible protein), and glucose metabolism (fructose 1,6-bisphosphate; glucokinase, and phosphoenolpyruvate carboxykinase) (Harding et al., 2000b; Ma et al., 2002). PERK-dependent phosphorylation triggers dissociation of Nrf2/Keap1 complexes and allows subsequent Nrf2 nuclear import (Cullinan et al., 2003). Recent studies have shown that NF-κB can be activated through this pathway via translational suppression of inhibitory kappa B (IκB), resulting in the regulation of mediators of inflammation (Deng et al., 2004; Jiang et al., 2003) such as IL-6 and TNF-α.
The selective increase in production or activity of a subset of proteins by PERK also aids in recovery from stress. For example, one consequence of ER stress is the accumulation of reactive oxygen species (ROS) that promotes a state of oxidative stress (Cullinan and Diehl, 2006). In anticipation of generating these potentially hazardous by-products, the UPR has incorporated an antioxidant defense system as well. PERK signaling, via activation of the Nrf2 and ATF4 transcription factors, engages survival responses, coordinates the convergence of ER stress with oxidative stress signaling, and orchestrates the execution of the antioxidant response element-dependent gene transcription program. This includes expression of genes encoding heme oxygenase-1 (HO-1), thioredoxin reductase 1 (TXNRD1), and the glutathione S-transferases GSTP1, GSTM1, and GSTm2 (Cullinan and Diehl, 2006).
It is clear that the UPR does not always result in successful alleviation of ER stress and establishment of a functional equilibrium in the ER. Often driven by severe or prolonged stress signals, the UPR can induce cell death via apoptosis (Rao et al., 2004). Like most other ER stress responses, the signaling pathways involved in this response are tightly regulated. CHOP is induced through the PERK pathway, caspase-12 and JNK signaling are activated, and proapoptotic Bcl-2 proteins BAX and BAK are switched on by the IRE1α pathway (Hetz et al., 2006; Rao et al., 2004). In addition, ER stress-induced IRE1α phosphorylation leads to the recruitment of TRAF2 (tumor necrosis factor receptor-associated factor 2) and ASK1 (apoptosis signal-regulating kinase 1) to the cytosolic leaflet of the ER membrane (Kaneko et al., 2003). Activation of both the PERK and IRE1 pathways also leads to regulation of the NF-κB-IKK signaling pathway during ER stress through activation of IKK or degradation of the p65 subunit (Deng et al., 2004; Hu et al., 2006). The ATF6 branch can also regulate NF-κB activity (Yamazaki et al., 2009). All of these signals contribute to the triggering of apoptotic responses when ER stress is excessive, prolonged, or insufficiently neutralized.
The mechanisms resulting in a commitment to cell death in response to ER stress remain a challenging aspect of ER biology that has yet to be decoded. Differential activation of the three UPR pathways may be a critical determinant of apoptosis (Ron and Walter, 2007). For example, attenuation of IRE1 and ATF6 activities by persistent ER stress and prolonged signaling through PERK can create an appropriate condition for apoptosis (Lin et al., 2007). Similarly, disproportionate engagement of the ribonuclease activity of IRE1 (which produces XBP1s as a protective measure) versus its ability to trigger JNK activity (or release proapoptotic mediators) (Hetz et al., 2006) and divergent effects of IRE1-mediated XBP1 splicing and IRE1α-triggered degradation of mRNAs localized in the ER may be critical determinants of the life and death outcomes resulting from ER stress (Han et al., 2009). Hence, it is likely that IRE1 is a critical lever in the UPR that controls commitment to cell death or promotes survival (Ron and Walter, 2007). But there remain important gaps in our understanding of the ability of individual UPR initiators to recognize or respond to various forms of ER stress. Moreover, additional work is required to determine how and whether different branches of the UPR are specialized to respond to particular conditions and different cellular environments by engaging distinct survival responses. Although disruption of distal death mediators has yielded important insights, the proximal engagement of these pathways is also vital in linking ER stress to physiological functions and disease pathogenesis.
Although the function of the ER is predominantly viewed from a protein processing perspective, many conditions other than (or in addition to) increased protein synthesis and the presence of mutant or misfolded proteins can also trigger UPR activation. These conditions include an imbalance in ER calcium levels, glucose and energy deprivation, hypoxia, pathogens or pathogen-associated components, certain lipids, and toxins. In particular, studies to explore ER function under metabolic and inflammatory challenge may prove fruitful and are discussed in the following sections.
ER and Metabolism
The ER is a principal site of protein synthesis and maturation and, together with the Golgi apparatus, the transportation and release of correctly folded proteins. As a result, much of the systemic physiology related to its dysfunction has been viewed in the context of its lumenal adaptation to protein processing and folding. I would like to emphasize three critical emerging aspects of ER biology relevant to energy and substrate metabolism. First, the physiological consequences of ER dysfunction cannot simply be explained by cell-autonomous responses to ensure proper protein folding or the differential survival of cells suffering from ER stress. Second, the ER can be viewed as a “nutrient-sensing” apparatus, establishing specific functional links with metabolic responses including links with endocrine networks that have systemic actions (Figure 3). Third, cells burdened with a high secretory or metabolic capacity do not all face similar challenges and hence may not resort to the same strategies to maintain their ER, regulate the UPR, or commit to apoptosis.
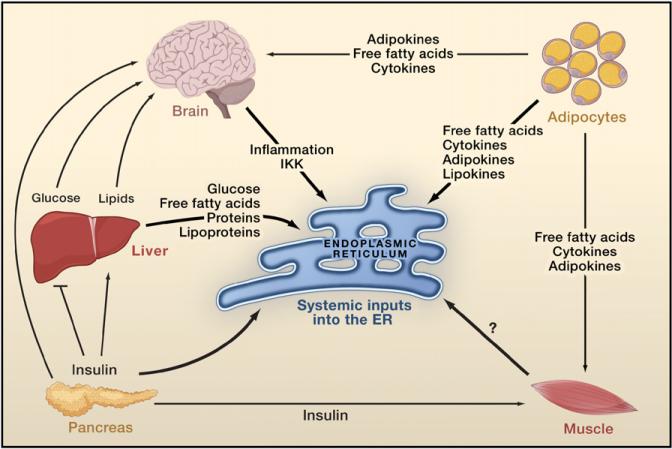
Figure 3. Crosstalk among Organs in Metabolic Regulation.
Glucose derived from the diet or endogenous sources stimulates insulin secretion by the β cells of the pancreas. Insulin decreases glucose production by the liver, inhibits fat lipolysis, and increases glucose uptake by fat and muscle. Fat cells (adipocytes) release free fatty acids that increase gluconeogenesis and lipogenesis by the liver and reduce glucose uptake by muscle and insulin secretion by the pancreas. In addition, adipocytes secrete adipokines such as the proinflammatory cytokine TNF-α, leptin, and adiponectin that regulate food intake, energy expenditure, and insulin sensitivity either directly or via the brain. Adipocytes also secrete lipokines that modulate lipid metabolism in the liver and glucose metabolism in muscle. The brain monitors metabolite signals (such as serum glucose, free fatty acid levels) as well as hormones (insulin, leptin) from peripheral tissues resulting in the regulation of whole-body metabolism. The ER functions as a nutrient sensor. Obesity is associated with increased adiposity, chronic inflammation, and insulin resistance. Within the adipocyte, elevated lipid storage, lipogenesis, and adipokine synthesis may act as stress signals for the ER. In the liver, increased protein synthesis, lipogenesis, lipid transport, and gluconeogenesis influence ER function. An increased demand for insulin synthesis in pancreatic β cells may lead to disturbed ER homeostasis. An increase in circulating free fatty acids and inflammatory cytokines could also trigger ER stress in the hypothalamus through activation of IkB kinase (IKK). It is not clear how obesity-induced metabolic stress influences ER function in muscle.
For example, in an antibody-secreting plasma cell or an insulin-producing pancreatic β cell, the main task may be to adapt their ER capacity (that is, protein-folding capacity) to meet the fluctuations in demand for protein synthesis and secretion (Ron and Walter, 2007). Accordingly, survival signals and apoptotic triggers engaged by the UPR may fit better with the involvement of UPR in pathologies associated with these cell types. However, other metabolically active cells such as hepatocytes and adipocytes face additional challenges with respect to ER homeostasis. For example, liver cells secrete not only large quantities of proteins (millions of molecules per minute) but also complex lipoproteins with unique processing demands coupled to lipid status. Liver cells are also the major providers of glucose and lipid to the entire body. Similarly, adipocytes store enormous quantities of lipids yet produce and secrete abundant proteins, lipids, and lipid mediators under unique structural and architectural constraints (Rosen and Spiegelman, 2006; Scherer, 2006; reviewed in Gregor and Hotamisligil, 2007). In addition to these structural and metabolic aspects, the UPR also may play a role in the differentiation of adipocytes. However, our current understanding of how UPR signaling affects adipocytes is limited to in vitro studies. A key unanswered question is how adipogenesis and adipocyte function are regulated in whole animals by ER stress and related signaling networks, especially through the principal transcriptional branches of the UPR pathway.
UPR in Glucose and Lipid Metabolism
Metabolic programs linked to the ER should not be considered simply as pathways that are activated during ER stress. Instead, I suggest that the ER should be viewed as an essential apparatus in the coordination of metabolic responses through its ability to control the synthetic and catabolic pathways of various nutrients (Figure 3). In the case of glucose, all three canonical UPR branches are engaged with glucose metabolism. One of the first phenotypes detected in genetic models of UPR deficiency was defective gluconeogenesis in the livers of PERK-deficient mice or in animals harboring a homozygous mutation that eliminates eIF2α phosphorylation on serine 51 (Harding et al., 2000a; Scheuner et al., 2001). As PERK was subsequently implicated in the survival and function of pancreatic islet cells, the disturbed glucose metabolism seen in PERK-deficient mice and in eIF2α mutant mice has been linked with islet cell dysfunction or death triggered by ER failure (Back et al., 2009). A recent study further addressed the regulation of eIF2α phosphorylation in liver and uncovered complex mechanisms that might be related to liver glucose metabolism during chronic ER stress (Oyadomari et al., 2008). Mice expressing a Gadd34 C-terminal fragment in the liver exhibited marked problems in defending blood glucose during fasting due to diminished hepatic glycogen reserves as well as defective gluconeogenesis (Oyadomari et al., 2008). In the context of excess energy, however, these animals retain a healthy profile, potentially due to diminished hepatic lipogenesis. Future work should determine whether ER stress also affects glucose metabolism in other tissues through this or related mechanisms and the potential role of other eIF2α kinases in these phenotypes (Figure 4).
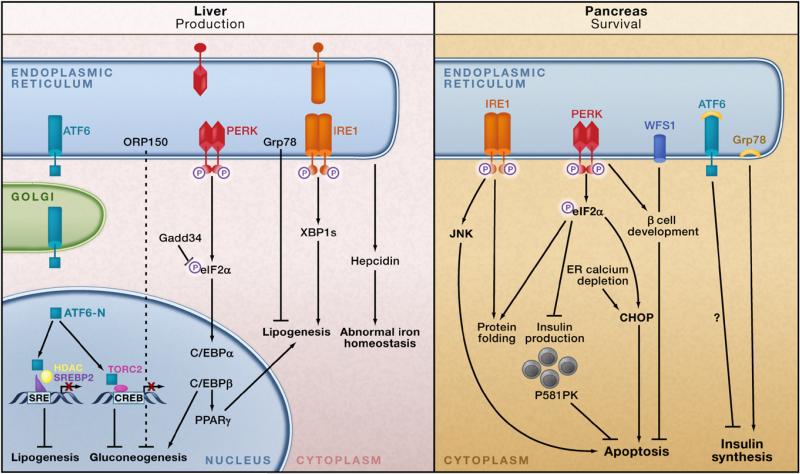
Figure 4. ER Stress, the UPR, and Metabolic Homeostasis.
ER stress interferes with lipid metabolism, glucose homeostasis, and iron homeostasis in the liver (left panel). In response to ER stress, the cleaved form of ATF6 (ATF6-N) translocates to the nucleus and binds to SRE-bound SREBP2, which recruits the histone deacetylase HDAC1 that blocks SRE-mediated transcriptional activation. In obesity, ATF6-N can also bind to TORC2, decreasing the interaction of TORC2 with CREB and downregulating hepatic gluconeogenesis. Activated PERK phosphorylates eIF2α, which in turn increases production of C/EBPα and C/EBPβ proteins in the liver. These transcription factors induce expression of genes involved in lipid synthesis and gluconeogenesis. The spliced form of X-box binding protein 1 (XBP1s) can activate a subset of genes involved in lipogenesis. Overexpression of the chaperone Grp78 inhibits the ER stress-induced activation of SREBP1 and 2 and reduces hepatic steatosis in obese mice. ORP150 overexpression in the liver also reduces gluconeogenesis. The liver is also important for regulating iron homeostasis. ER stress may induce production of the iron-regulating peptide hormone hepcidin in hepatocytes, leading to abnormal iron homeostasis. The pancreatic β cell must maintain the proper balance between insulin synthesis, folding, and secretion, and the ER is intricately involved in this process (right panel). In early development, PERK is required for β cell formation and survival. IRE1 is required for maintenance of insulin production; eIF2α phosphorylation is necessary to halt insulin synthesis until folding demands have been met. The UPR-induced chaperone p58IPK is also necessary for β cell survival. Loss of the WFS1 gene, which encodes the ER transmembrane protein wolframin, results in increased ER stress and cell death. Unresolved ER stress in β cells may lead to apoptosis through IRE1-activated JNK phosphorylation and also through the downstream UPR mediator CHOP. CHOP downregulation during high insulin demand results in the survival and expansion of β cells and improved glucose homeostasis.
Interestingly, transcriptional networks activated during ER stress directly target the expression of genes important in glucose metabolism (Wang et al., 2009). Signaling networks that control the metabolic destiny of nutrients, such as amino acids and glucose, are also integrated with ER function. The ER is exquisitely sensitive to glucose availability and energy fluctuations, and glucose synthesis or breakdown pathways are transcriptionally regulated during the UPR. For example, genes involved in glucose output and glycogen synthesis such as glycogen synthase 1 are targets of XBP1 (Acosta-Alvear et al., 2007; Lee et al., 2008). Recently, ATF6 has also been linked to regulation of liver glucose production through regulated interaction with CRTC2 (CREB-regulated transcription coactivator 2, also called TORC2) (Wang et al., 2009). In this model, acute ER stress triggers the dephosphorylation and nuclear entry of CRTC2 in cultured cells, which in turn promotes the expression of ER quality-control genes through an association with ATF6. In response to ER stress, CRTC2 binds to ATF6 and shuttles to the nucleus where it occupies the XBP1 promoter. Interestingly, ATF6 activation may reduce hepatic glucose output by disrupting the CREB–CRTC2 interaction, thereby inhibiting CRTC2 occupancy at the promoters of genes involved in gluconeogenesis. This interaction between ATF6 and CRTC2 may be an important component of the disruption of hepatic gluconeogenesis in obesity and type 2 diabetes as overexpression of ATF6 in the livers of obese animals appears to be beneficial in reversing the effects of CRTC2 on the gluconeogenic program (Figure 4).
The three major UPR pathways have also been implicated in the regulation of cellular lipogenesis, and the ER plays an important role in both fatty acid synthesis and cholesterol metabolism. The SREBP family of transcription factors are resident in the ER. In response to low sterol levels or insulin, SREBP proteins trans-locate to the Golgi and are processed into active transcription factors (Brown et al., 2000). Activation of SREBP proteins also occurs during ER stress and is critical for the regulation of target genes involved in cholesterol metabolism (SREBP1a, SREBP2) or lipid (SREBP1c) synthesis (Gregor and Hotamisligil, 2007). In fact, SREBP transcription factors may be critical mediators in the integration of lipid metabolism with the UPR. In addition, the ER is the site of triglyceride formation, particularly in adipocytes and possibly in liver cells as well (Wolins et al., 2006). The potential role of the ER in the regulation of lipid droplet number, composition, and size and in lipogenesis and lipolysis remains an important but unexplored area (Gregor and Hotamisligil, 2007).
In liver cells, XBP1s, downstream of the UPR sensor IRE1α, regulates the transcription of many genes involved in fatty acid synthesis, including Scd-1 (stearoyl-CoA desaturase-1), Acc2 (acetyl-CoA carboxylases 2), and Dgat2 (diacyl glycerol acyl transferase 2). Consequently, mice with a liver-specific XBP1 deficiency exhibited reductions in serum triglycerides and cholesterol and did not develop hepatic steatosis (fatty liver) when placed on a high carbohydrate diet to stimulate hepatic lipogenesis (Lee et al., 2008). It will be interesting to explore the role of XBP1 in hepatic lipid production as well as secretion in other contexts and also the physiological window in which XBP1 activity regulates lipid metabolism. In a related vein, deletion of another UPR sensor, PERK, from mammary epithelial cells in mice compromises the free fatty acid content of milk due to the lack of expression of genes involved in fatty acid synthesis, such as Fas (fatty acid synthase), Acl (ATP citrate lyase), and Scd-1 (Bobrovnikova-Marjon et al., 2008). In this case, a defect in SREBP1 activation, a major transcriptional regulator of lipogenic gene expression, appears to result in this pattern. Finally, investigation of liver lipogenesis under chemical ER stress using loss-of-function mouse models for multiple UPR proteins indicated an impact of the UPR on lipid metabolism (Rutkowski et al., 2008). When exposed to tunicamycin (which disrupts glycosylation of newly synthesized proteins resulting in ER stress), liver tissue exhibited downregulation in lipid metabolic pathways of many genes, such as Fas, Srebp1, PGC-1α (peroxisome proliferator-activated receptor-coactivator), CEBPα (CCAAT enhancer binding protein α), PPARα (peroxisome proliferator-activated receptor α). Livers from mice lacking ATF6, IRE1, or p58IK or from an eIF2α mutant strain all showed even greater loss of metabolic gene expression during tunicamycin treatment compared to livers from wild-type mice. Notably, the extreme level of ER stress induced by tunicamycin is not physiological and is beyond what the liver experiences during metabolic fluctuations or even in most disease states. Nevertheless, these data are consistent with links between ER stress and liver lipogenesis. In contrast, livers from mice lacking Chop can sustain metabolic gene expression under tunicamycin challenge, pointing to CHOP as a possible mediator of decreased gene expression under severe ER stress and a cause of hepatocyte death. The principal function of CHOP may be related to cell death induced by severe ER stress. In a more physiological setting, overexpression of Gadd34, the phosphatase of UPR-activated, phosphorylated eIF2α (which would be expected to decrease CHOP expression) resulted in decreased hepatic steatosis in mice on a high-fat diet (Oyadomari et al., 2008). Interestingly, loss of ATF6α or p58IPK also resulted in hepatic steatosis (Rutkowski et al., 2008). This seemingly paradoxical result (hepatic steatosis during defective liver lipogenesis) is likely to be due to a loss of liver lipoprotein production (Rutkowski et al., 2008) (Figure 4).
This somewhat puzzling picture of the regulation of lipogenesis by the UPR demands further experimentation in physiologically relevant settings and with new animal models. We may be able to better understand the metabolic impact of ER stress by distinguishing between a physiological setting for the UPR (high glucose, dietary exposure, or lactation) and an extreme, death-inducing UPR (for example, liver cells treated with tunicamycin). It may be that under severe and perhaps nonphysiological stress, UPR-mediated CHOP activity regulates a decrease in metabolic gene expression as the cells spiral toward death. However, in a physiological setting, the UPR may lead to enhanced lipogenesis, as seen with XBP1s and PERK in liver tissue and mammary epithelial cells, respectively. Indeed, the working UPR could be seen as an important adaptive response in cells poised to handle high metabolic loads (lipid synthesis in the liver; milk production in mammary epithelia). Some of the alterations in lipid metabolism may also be defensive (or adaptive), for example, synthesizing lipids to protect the composition of lipid membranes, to prevent toxicity, and to regulate deposition into droplets. Such preventive alterations may be active during the early adaptive stages of the UPR but then may be shut down upon commitment to death. This latter response is central to the function and survival of macrophages under conditions of lipotoxic stress (Maxfield and Tabas, 2005). We recently demonstrated that lipid chaperones such as aP2 (Furuhashi and Hotamisligil, 2008) are crucial for linking toxic lipids such as saturated fatty acids or oxidized lipoproteins to ER stress and survival pathways (Erbay et al., 2009). In such a setting, the ability to engage de novo lipogenesis pathways appears to be disrupted by the lipid chaperones. When these chaperones are blocked or lacking, macrophages can mount “defensive lipogenesis,” which involves transcribing lipogenic genes as well as alterations in cellular lipid composition. The ability of lipids to “defend” cells under stress conditions is vital for survival responses, as well as for eliciting inflammatory signals such as MCP-1 and TNF-α emanating from metabolically challenged immune cells. Accordingly, aP2-deficient macrophages exhibit marked resistance to ER stress (Erbay et al., 2009). As the lipotoxicity of immune cells such as macrophages is a common feature of obesity and atherosclerosis, the action of lipid chaperones in controlling lipid-induced ER stress has critical implications for these diseases and their complications (Erbay et al., 2009; Makowski et al., 2001). How ER stress in immune cells relates to chronic metabolic disease remains to be studied.
If the ER functions as a nutrient-sensing organelle as suggested by the findings described above, other nutrient-sensing pathways would be predicted to be closely linked to ER responses. Activation of the nutrient-responsive mTOR signaling pathway stimulates protein synthesis and folding in the ER (Polak and Hall, 2009). Hence, it is possible that in the presence of excess nutrients, this pathway is stimulated adding to the translational demand and contributing to ER stress and metabolic dysfunction. Here, we are unable to cover this important pathway in detail and so only mention a few intriguing findings directly related to ER function. Tuberous sclerosis is a condition characterized by constitutive activation of the mTOR pathway due to mutations in the TSC-1 and TSC-2 genes (Harrington et al., 2004; Kwiatkowski and Manning, 2005; Zhang et al., 2009b). Interestingly, tuberous sclerosis is associated with severe cellular insulin resistance. In recent studies, we have demonstrated that this hyperactivity of the mTOR pathway promotes ER stress and contributes to inhibition of insulin receptor signaling, at least in part, through JNK-mediated serine phosphorylation of IRS1 (insulin receptor substrate 1) (Ozcan et al., 2008) (Figure 2). Interestingly, mTOR activity is increased in obesity (Khamzina et al., 2005), and this augmented signaling may play a role in abnormal insulin action associated with the obese state. Previously, mTOR itself and mTOR-mediated activation of S6K-1 have been reported as potential mechanisms for inhibiting insulin action through direct phosphorylation of IRS1 (Tzatsos and Kandror, 2006; Um et al., 2004). Our recent work also places ER stress and JNK activation in the feedback regulation of insulin action through the mTOR pathway. It is possible that the obesity-related increase in mTOR activity itself may contribute to the ER stress response seen in this condition, although it is unlikely that this activity alone is a causal event. Moreover, mTOR signaling has a role in the induction of SREBP1 activity, potentially through direct or indirect regulation of SREBP1 trafficking and/or processing within the ER/Golgi. This also demonstrates that the ER has an important role in integrating protein synthesis and glucose-induced lipogenesis (Porstmann et al., 2008; Powers, 2008).
A study of tumor angiogenesis revealed that the TSC-1 protein is a direct target of IKK-β kinase, leading to its degradation and consequent activation of the mTOR pathway (Lee et al., 2007a). This finding raises the possibility that activation of inflammatory kinases may be linked to the mTOR and ER stress pathways through this route. In fact, disruption of the mTOR pathway in various tissues results in an array of metabolic abnormalities, although how these disorders are linked to ER homeostasis or alterations in inflammatory responses still requires further investigation (Polak and Hall, 2009). Overall, there are important gaps in our understanding of the interactions and balance between nutrient-sensing mechanisms and the different arms of the UPR as they relate to metabolism (whether it is fostered or shut down) (Figure 4). Thus, nutrient (and energy) deprivation, as well as nutrient surplus, may be perceived by the ER in many different ways leading to the mounting of different adaptive responses. Such adaptive responses also engage additional stress signals and inflammatory pathways, to which we now turn.
The UPR and Inflammation
A close examination of ER stress and UPR pathways has demonstrated many links to major inflammatory and stress signaling networks, including the activation of the JNK-AP1 and NF-κB-IKK pathways (Deng et al., 2004; Hu et al., 2006), as well as production of ROS and nitric oxide (Cullinan and Diehl, 2006; Gotoh and Mori, 2006). Notably, these are also the pathways and mechanisms that play a central role in obesity-induced inflammation and metabolic abnormalities, particularly abnormal insulin action (Hotamisligil, 2006) (Figure 1). For example, during ER stress, IRE1α triggers a key inflammatory signaling pathway through activation of JNK, which can transcriptionally regulate many inflammatory genes (Urano et al., 2000). The obesity-induced expression of proinflammatory cytokines (including TNF-α, IL-6, and MCP-1) is also suppressed in mice lacking JNK and contributes to protection from insulin resistance and type 2 diabetes (Hotamisligil et al., 1993; Tuncman et al., 2006; Vallerie et al., 2008). However, it remains to be determined to what extent obesity-induced JNK activation and the subsequent alterations in inflammatory responses can be explained by ER stress, or to what extent JNK activation or inflammation could account for the metabolic effects of ER stress. Interestingly, through multiple and distinct mechanisms, both the IRE1α and PERK branches of the UPR can lead to activation of the NF-κB-IKK pathway. This pathway also plays a critical role in the induction of multiple inflammatory mediators and has been implicated in insulin resistance (Deng et al., 2004; Hu et al., 2006; Shoelson et al., 2006). Recently, the ATF6 branch of the UPR has also been linked to NF-κB-IKK signaling, suggesting that specific inflammatory triggers may signal through different branches of the UPR (Yamazaki et al., 2009). In cellular systems, experimental induction of the UPR has been reported to cause increased expression of proinflammatory molecules such as IL-8, IL-6, MCP-1, and TNF-α (Li et al., 2005). The ER-resident transcription factor, CREBH, also may be a regulator of acute phase response genes in the liver (Zhang et al., 2006a). Both CREBH and CHOP may play a role in the regulation of the peptide hormone hepcidin, which may link iron metabolism to inflammatory output via the ER (Vecchi et al., 2009).
The relationship between ER stress and inflammation is not likely to be one-sided. Inflammatory mediators and activation of cellular stress pathways, such as the JNK and IKK pathways, may have a negative impact on ER function. However, the interplay between inflammation and ER stress may depend on the cell type, and a great deal of work is still needed to understand the major parameters that determine a cell's susceptibility to inflammatory signals and subsequent effects on ER homeostasis. Indeed, much of the work done to date is based on in vitro experiments. However, recent studies in the brain provide in vivo evidence supporting the model that both ER stress and inflammation are able to activate each other and to inhibit normal cellular metabolism (Zhang et al., 2008). In this setting, activation of IKK-β can lead to ER stress, and activation of ER stress can stimulate IKK-β. One can speculate that the cell may have a central node through which different stress responses send their signals. This central node may then, in turn, “decide” which responses to elicit and may activate specific pathways related to the intrinsic status of the organelle. For example, the inflammatory response may be activated and may signal to this node, which then activates the UPR. Depending upon the cell type, these responses and the components of such a node may differ. In the adipocyte, for instance, inflammatory signals may not cause UPR activation (or may do so only when the cell is under metabolic stress), but activation of the UPR may elicit an inflammatory response.
It will be fascinating to unravel this integrated network of responses to cellular stress and how it relates to the folding environment of the ER. A simple place to start could be to investigate the differences between cells where the UPR is activated by inflammation and cells where it is not. Studies such as this would begin to shed light on the complex regulation of stress responses at the cost or conservation of metabolic function (Figures 1 and 2). Recently, we have discovered a unique role for the eIF2α kinase PKR in linking nutrients and ER stress to inflammation and metabolic regulation (Nakamura et al., 2010). PKR is markedly activated by lipids and during obesity plays a critical role in the activation of JNK and the inflammatory response. Interestingly, PKR also directly interferes with insulin action by interacting with IRS1. Thus, PKR could assemble a putative metabolically activated inflammatory complex—called the metabolic inflammasome or metaflammasome—that then integrates insulin action, pathogen responses, and translational control with nutrient sensing and ER stress. Formation of a metaflammasome and its activation by nutrients and ER stress may explain the functional overlap between multiple signaling pathways, such as JNK, IKK, and others, in modulating metabolism (Figure 1).
Chronic ER stress and activation of the UPR may also result in oxidative stress, causing a toxic accumulation of ROS within the cell (Cullinan and Diehl, 2006). This occurs due to the UPR-stimulated upregulation of protein chaperones involved in disulfide bond formation in the ER lumen. The enzymes responsible for forming disulfide bonds (Ero1p and Erv2p) use oxidation/reduction reactions, with molecular oxygen as the final electron recipient. This reduced molecular oxygen accumulates during increased protein folding due to UPR activation and is toxic to cells (Haynes et al., 2004). The UPR has evolved to anticipate this increase in ROS especially during ER stress via PERK-mediated activation of an antioxidant program through the transcription factor Nrf2 to neutralize toxic species. Despite these protective measures, prolonged UPR activation still results in increased ROS levels that may elicit inflammatory responses, thereby providing yet another potential link between ER stress and inflammation. Finally, recent studies suggest that nitric oxide (NO) generation could also be related to ER stress (Uehara et al., 2006). NO-induced S-nitrosylation of protein disulfide isomerase inhibits its enzymatic activity, leading to the accumulation of polyubiquitinated proteins, activation of the UPR, and death of neurons. Also, the relationship between ER stress and oxidative stress is not one-sided as ROS generated through inflammation or damage to organelles (such as mitochondria) could accelerate ER dysfunction.
The close link between ER stress and inflammation is a likely contributor to the integration of ER function and metabolic homeostasis, given the critical role of inflammation in obesity, insulin resistance, type 2 diabetes, and many other clustering metabolic disorders (Hotamisligil, 2006). In particular, chronic inflammation of adipose tissue (and recruitment of professional immune cells to this site) (Alexaki et al., 2009; Feuerer et al., 2009; Liu et al., 2009; Nishimura et al., 2009; Winer et al., 2009), whether triggered metabolically or through injury or death of adipocytes, can be linked to a diverse set of stress responses emerging from the ER. In addition to macrophages, the potential importance of immune cells in adipose tissue was shown in studies on T cell subpopulations and mast cells, which are present in adipose tissue and are regulated by obesity (Feuerer et al., 2009; Nishimura et al., 2009). Similar events may occur in the liver and pancreas as well, suggesting that integration of inflammatory and metabolic pathways could influence all major metabolic tissues. How the ER and its integrated stress signaling systems relate to the function of these immune cells in metabolic tissues remains unclear. In the context of chronic inflammation, current evidence is pointing to metabolic cells (such as adipocytes and hepatocytes) as the drivers of metabolic homeostasis, which provide signals that disrupt immune effectors and propagate inflammatory alterations. However, additional studies are needed to elucidate the metabolic importance of the link between ER stress and inflammatory pathways, as well as the full metabolic impact of alterations in the number or function of immune cell populations in metabolic tissues in vivo.
ER Stress, Autophagy, and Inflammation
Autophagy is a highly regulated process involved in the turnover of long-lived proteins and damaged organelles (Yorimitsu and Klionsky, 2005). Autophagy is linked to the ER at many levels and is likely to be a critical component of normal ER function. For example, ER stress can induce autophagy in mammalian cells (Ogata et al., 2006), with several canonical UPR pathways implicated in this interaction (Kouroku et al., 2007; Ogata et al., 2006). Why does ER stress induce autophagy? One possible answer is “because it needs it,” especially during recovery from stress. The ubiquitin-proteasome and autophagy-lysosome systems are the two major degradation routes in eukaryotic cells, and the ER is connected to the proteosome and to autophagy during the degradation of misfolded proteins. Hence, ER stress-induced autophagy may have evolved as an alternate mechanism to dispose of misfolded proteins in the ER lumen that cannot be removed through ERAD. A second use for autophagy during ER stress may be the degradation of the damaged ER itself. Given the dynamic nature of the ER, UPR-induced auto-phagic degradation of the ER could be an integral player in ER plasticity, replenishment, and homeostasis (Figure 5). Although mTOR is a potential upstream signal that may regulate autophagy in obesity (Xie and Klionsky, 2007), neither the regulation of autophagy nor its impact on insulin action under conditions of nutrient excess is well understood. Furthermore, recent studies have identified an unconventional, mTOR-independent form of autophagy that further complicates the link between these pathways (Nishida et al., 2009).
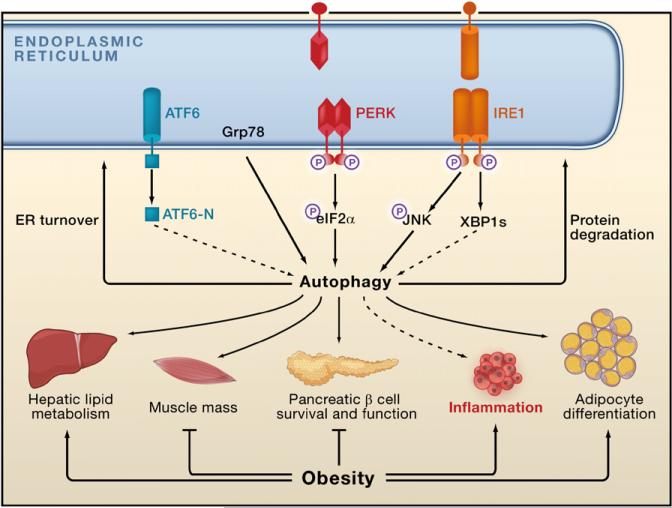
Figure 5. ER Stress, Autophagy, Inflammatory Signals, and Metabolism.
The unfolded protein response (UPR) has been implicated in ER stress-induced autophagy, thus implicating autophagy in ER homeostasis. Potential actions of autophagy in stress recovery could include degradation of misfolded proteins and the promotion of ER turnover. Autophagy may also be involved in lipid droplet formation in the liver, b cell survival and function, adipocyte differentiation, muscle mass regulation, and inflammatory responses, all of which are disturbed in obesity. A role for autophagy in insulin action is not known.
Growing evidence indicates that aberrant regulation of autophagy may be detrimental to metabolism. Loss in mice of p62, a protein important for the efficiency of autophagy, results in mature-onset obesity and leptin resistance, as well as metabolic abnormalities (Rodriguez et al., 2006). Two studies demonstrated that autophagy is essential for pancreatic islet function and survival and that a lack of autophagy leads to islet degeneration and reduced insulin secretion (Ebato et al., 2008; Jung et al., 2008). There is also evidence that autophagy is selectively involved in the delivery of lipid droplets for lysosomal degradation (Singh et al., 2009a). Loss of the autophagy protein Atg7 in mouse adipose tissue results in developmental and metabolic abnormalities (Singh et al., 2009b), and Atg7 deficiency in muscle results in muscle atrophy (Masiero et al., 2009).
Autophagy also functions in diverse aspects of immunity. It is now clear that the autophagy pathway plays an important role in eliminating intracellular pathogens (Levine and Deretic, 2007). Autophagy also contributes to MHC class II-restricted endogenous antigen presentation and to production of type I interferons in response to Toll-like receptor (TLR) signaling (Lee et al., 2007b; Levine and Deretic, 2007). Lastly, autophagy directly affects B and T cell homeostasis (Levine and Deretic, 2007) and is an effector of Th1/Th2 T cell responses (Harris et al., 2007). Genome-wide association studies (GWAS) have identified two genes encoding proteins within the autophagy pathway, Atg16L and IRGM, that are associated with inflammatory bowel disease (Hampe et al., 2007; Parkes et al., 2007). Interestingly, in response to lipopolysaccharide, macrophages lacking Atg16L or Atg7 show enhanced production of inflammatory cytokines such as IL-1 and IL-6 (Saitoh et al., 2008). There is also evidence for the regulation of autophagy by inflammatory factors such as TNF-α that are linked to obesity and type 2 diabetes. Some studies suggest that JNK activation is required for the induction of ER stress-induced autophagy (Ogata et al., 2006). Also, there may be crosstalk between the NF-κB signaling pathway and autophagy during tumor progression (Djavaheri-Mergny et al., 2006). Thus, inflammatory pathways that are linked to the UPR and metabolic disease are also linked to autophagy. However, the physiological and pathophysiological consequences of the connection between autophagy and ER stress in metabolic homeostasis and its implications for insulin action are still unanswered questions and require studies with new animal models, especially those with enhanced autophagic responses in target tissues (Figure 5).
ER Stress in Obesity and Diabetes
Given that the UPR is closely integrated with stress signaling, inflammation, and JNK activation and the fact that obesity stresses the ER (due to an increase in synthetic demand, alterations in energy availability, and activation of inflammatory pathways), we have postulated that obesity may lead to ER stress in metabolically active tissues. Indeed, in both the adipose and liver of mice chronically fed a high-fat diet, PERK and IRE1α phosphorylation and JNK activity are significantly increased compared to lean animals (Ozcan et al., 2004). Similarly, in the severe ob/ob mouse model of genetic obesity resulting from leptin deficiency, both adipose and liver exhibit characteristics of ER stress, including increased levels of phosphorylated PERK and IRE1α as well as increased JNK activity.
The causality between susceptibility to ER stress and insulin resistance was supported by genetic manipulation of XBP1 in cultured cells and whole animals (Ozcan et al., 2004). XBP1-deficient cells exhibit markedly increased sensitivity to ER stress and succumb to insulin resistance, at least in part, through IRE1α-dependent activation of JNK and serine phosphorylation or degradation of IRS1. In contrast, cells experimentally equipped with higher levels of activated XBP1s become refractory to ER stress and exhibit protection against insulin resistance. XBP1 haploinsufficiency in mice replicates the findings in cultured cells, as these animals succumb to ER stress and develop hyperinsulinemia, hyperglycemia, and impaired glucose and insulin tolerance compared to wild-type animals. XBP1 haploinsufficiency results in a small but significant increase in body weight, and in the liver and adipose tissues of these mice there is increased phosphorylation of PERK and IRE1α and also increased JNK activity coupled with a loss of insulin sensitivity (Figure 4).
Several recent studies provide additional support for the involvement of ER stress in obesity, insulin sensitivity, and type 2 diabetes, verifying a role for the UPR in this process. For example, the ER chaperone protein ORP150 (oxygen-regulated protein 150), which is activated during the UPR as a protective response, plays a role in mouse metabolic homeostasis (Nakatani et al., 2005; Ozawa et al., 2005). ORP150 deficiency, either in the whole organism or specifically in the liver, results in impaired glucose tolerance and decreased insulin receptor signaling through phosphorylation of IRS1 (Nakatani et al., 2005). Conversely, overexpression of ORP150 in a mouse model of obesity and diabetes leads to significantly improved glucose tolerance and enhanced insulin receptor signaling. In a similar vein, overexpression of the ER chaperone Grp78 in the liver of obese mice has beneficial metabolic effects (Kammoun et al., 2009). Lipogenic gene expression and hepatic steatosis decreased and insulin sensitivity increased in obese mice overexpressing Grp78. Interestingly, a phenotype reminiscent of this was also reported for animals missing one allele of Grp78. In this model, Grp78 heterozygosity during development resulted in a compensatory increase in a large array of other ER chaperones, which enhanced overall ER folding capacity and consequently metabolic homeostasis. In another genetic model, the UPR was compromised by a mutation in eIF2α such that inhibitory phosphorylation of the protein could not occur (Scheuner et al., 2001). In heterozygous mice carrying this mutant form of eIF2α, the ER is dysfunctional, and when these animals are placed on a high-fat diet they develop obesity and a phenotype reminiscent of type 2 diabetes. Although ER dysfunction in the pancreas may play a primary role in this phenotype, it is interesting to note that eIF2α mutants also exhibit an expanded adipose tissue phenotype, although this might be secondary to systemic alterations. Taken together, these animal models provide ample evidence to link ER function with various metabolic pathways related to obesity and diabetes.
ER stress and the UPR also play an important role in the pancreas by acting on islet survival and function (Figure 4). Indeed, this mechanism may be a potential cause of rare forms of juvenile diabetes (Zhang et al., 2002). For example, PERK-deficient pancreatic β cells are more susceptible to ER stress-induced apoptosis. PERK-deficient mice develop severe hyperglycemia soon after birth due to defects in islet proliferation and increased apoptosis (Harding et al., 2001). Interestingly, different models of conditional PERK deletion in islets suggest that this role of PERK may be more important during β cell development than in the adult (Zhang et al., 2006b). Preventing eIF2α phosphorylation in pancreatic β cells also results in development of diabetes, potentially due to oxidative damage (Back et al., 2009). The absence of p58 (IPK), an ER chaperone, also promotes β cell failure (Ladiges et al., 2005). The PERK-eIF2α pathway is also critical for islet survival and function in humans. A loss-of-function mutation in PERK causes a heritable form of juvenile diabetes called Wallcot-Rallison syndrome, characterized by severe defects in pancreatic β cells (Zhang et al., 2002). Additionally, mutations in the WFS1 gene in humans, which encodes the ER transmembrane protein wolframin, have been linked to an increased incidence of diabetes in patients with Wolfram syndrome (Fonseca et al., 2005).
It is generally accepted that ER stress plays a critical role in islet biology and type 2 diabetes. Chronic insulin resistance dramatically increases the demand on the islets for insulin production. As pro-insulin is processed in the ER into mature insulin, the increase in insulin production places a strain on ER function in the pancreatic islets resulting in activation of the UPR. In addition, many factors associated with obesity and type 2 diabetes, including free fatty acids and inflammatory cytokines, can also trigger or exacerbate ER stress (Borradaile et al., 2006; Kharroubi et al., 2004); and islets from mice and humans with type 2 diabetes exhibit signs of ER stress (Huang et al., 2007). In fact, CHOP is important for islet cell death and loss of Chop protects islets from apoptosis in the Akita mouse model, which produces a mutant form of insulin (Oyadomari et al., 2002). When Chop-deficient animals are crossed with db/db mice (an extreme obesity and diabetes model characterized by the rapid demise of β cells), the death of β cells is prevented (Song et al., 2008). Global profiling of genes in islet cells in response to ER stress revealed the early degradation of Ins1 and Ins2 mRNAs (Pirot et al., 2007). Hence, the two major pathological arms of type 2 diabetes—peripheral insulin resistance and defective insulin secretion—are both related to defects in ER function. Finally, it is likely that ER stress and related inflammatory and stress signaling pathways are involved in the survival and function of islets in type 1 diabetes (Figure 4). Additional and ongoing studies should address this important question, which may carry significant clinical ramifications.
The identification of the critical role of inflammatory signals and ER stress in obesity and diabetes has stimulated studies of these inflammatory pathways in the central nervous system (see Review by C.K. Glass et al. on page 918 of this issue). Early suggestions for a potential link between inflammation and metabolic regulation in the brain came from studies showing obesity-induced inflammatory alterations in the hypothalamus (De Souza et al., 2005) and reduced weight gain in JNK-deficient mice fed a high-fat diet (Hirosumi et al., 2002). Activation of IKK-β in the brain causes ER stress and interferes with leptin signaling (Zhang et al., 2008). Alternatively, IKK-β deletion in the brain reduces ER stress in mice fed a high-fat diet, suggesting that overactivation of this inflammatory pathway causes ER stress, leptin resistance, and increased food intake and body weight gain. However, a simple linear line between these factors cannot be drawn at this point. Although IKK-β activity does cause ER stress, ER stress can also activate NF-κB. NF-κB activation can be reversed by reducing ER stress using tauro-ursodeoxycholic acid, a conjugated bile acid with chemical chaperone activity (Zhang et al., 2008). Accordingly, in this model of brain inflammation mediated by the NF-κB pathway, ER stress and the inflammatory response seem to be in a reciprocal relationship, with both influencing leptin sensitivity. However, further studies will be necessary to support the potential role of ER stress in leptin sensitivity.
Other observations support a role for ER stress in defects in metabolic regulation by the central nervous system. Animals with XBP1 haploinsufficiency, which experience higher levels of ER stress, also show increased body weight (Ozcan et al., 2004). In contrast, treatment with tauro-ursodeoxycholic acid or another chemical chaperone, phenyl butyric acid, can reduce weight gain (Basseri et al., 2009; Ozcan et al., 2006), although the underlying mechanisms are not understood. In cellular systems, it has been suggested that ER stress may be associated with leptin action and that a chemical chaperone can protect leptin action by alleviating ER stress (Hosoi et al., 2008). Leptin resistance can be induced by administration of ER stress-inducing chemicals into the brain (Zhang et al., 2008), although the physiological implications of such a treatment remain unclear. However, a similar pattern is also reported in a brain-specific XBP1 deficiency mouse model, which revealed increased levels of phosphorylated PERK and decreased leptin sensitivity associated with a higher food intake and increased body weight compared to control animals (Ozcan et al., 2009). In addition, other nutrient-sensing networks such as mTOR or the eIF2α kinase GCN2 likely are involved in metabolic signaling in the central nervous system through ER stress and inflammation or alternative mechanisms (Hao et al., 2005; Maurin et al., 2005). All of these pathways also carry the potential to regulate insulin sensitivity in the brain. Much more work is needed to establish a causal link between leptin signaling and ER stress or related inflammatory networks in the central nervous system, and to dissect these from the regulation of insulin sensitivity. In particular, as these pathways are intimately linked to survival, it is essential to separate the impact on the survival of specific brain cell populations and functional alterations, especially in loss-of-function models.
Emergence of ER Stress in Metabolic Disease
Obesity is a condition where the organism needs to adapt to and function under chronic exposure to high energy and nutrient intake. This adaptation alone increases the demand on the synthetic and storage machinery at several sites, including liver, adipose tissue, and pancreas, all of which are central players in metabolic homeostasis. In equilibrium, hepatocytes maintain one of the highest protein synthesis rates in the body and can produce millions of proteins per minute. The overwhelming majority of these peptides are processed in the ER (Ricca et al., 1978). Similarly, pancreatic exocrine cells and adipocytes also are burdened with very high rates of protein synthesis, which further increases in the presence of obesity and insulin resistance. Adipocytes are perhaps one of the most unusual cell types as they store molar quantities of lipids but also actively secrete peptides and lipids. Given this configuration, adipocytes operate under challenging conditions even under normal physiological circumstances, with only a small percentage of the total volume of the cell allocated to accommodate organelles and cytoplasm (Gregor and Hotamisligil, 2007). During the nutrient and energy surplus associated with obesity, adipocytes enlarge further, alter their endocrine capacity, store (or try to store) more lipids, undergo severe architectural changes, and may suffer from hypoxia. It is possible then that these cells may have developed additional strategies to maintain ER integrity. Similarly, liver cells start to accumulate lipids during obesity, which rarely occurs under normal physiological conditions, and this could compromise the functional integrity of the ER in hepatocytes. Hence, the major cell types controlling systemic metabolic homeostasis may become highly sensitive to ER stress under conditions of obesity. This is due to increased demands on the synthetic machinery as a result of nutrient excess, saturation of storage capacity, and many other factors, which together create a challenging milieu in which the ER must carry out its regular functions and sustain its protein-folding capacity. But there is as yet no formal evidence supporting the contribution of increased protein synthesis to the emergence of ER stress in obesity and type 2 diabetes. So, this “synthesis-driven” view may not be accurate or may not apply equally to all sites of obesity-induced ER stress. For example, it is possible that obesity features a defect in ERAD that limits the disposal capacity of the ER. Alternatively, the main problem compromising ER function could be insufficient chaperone capacity. Finally, misfolding or trafficking of a limited number of specific proteins with critical functions could disrupt metabolism even at milder levels of ER stress. In other words, one could speculate that obesity may represent an isolated protein-folding disease. For example, the folding status of apolipoprotein B100, which is a challenging protein for the ER to process and secrete, could influence the performance of the ER (Ota et al., 2008). These questions are of critical importance and warrant additional studies to dissect the etiology of ER dysfunction in obesity and diabetes.
One can envision that excess nutrients themselves may serve as signals that induce or worsen ER stress. For example, the metabolism and trafficking of free fatty acids are abnormal in obesity, and excess free fatty acids induce ER stress in hepatocytes, cardiomyoblasts, pancreatic β cells, and macrophages (Borradaile et al., 2006; Kharroubi et al., 2004; Wei et al., 2006). Certain free fatty acids can induce JNK activation directly or indirectly resulting in insulin resistance in adipocytes, hepatocytes, and pancreatic islets, whereas others mediate beneficial hormonal and metabolic effects (Cao et al., 2008). The mechanisms underlying the effects of certain lipids on ER function remain unclear. In macrophages, lipid chaperone proteins have a remarkable role in mediating lipid-induced ER stress responses (Erbay et al., 2009). Whether or not similar mechanisms have implications for insulin resistance or diabetes remains to be determined. Nevertheless, insulin resistance, whether triggered by lipids or inflammatory pathways, results in alterations in glucose availability and flux, a well-established condition that contributes to ER stress in many cell types. Furthermore, in the microenvironment of adipose tissue, the individual cells that constitute the tissue may still be glucose, nutrient, or energy deprived relative to their needs or availability in their subcellular compartments. For example, studies in rats have shown potentially defective vascularization of adipose tissue in obesity (West et al., 1987). Interestingly, the adipose tissue of obese mice may also be hypoxic, another condition that can induce ER stress (Hosogai et al., 2007). A more intriguing possibility is that nutrients directly engage inflammatory signaling nodes, which are linked to ER function. A compelling example for such a mechanism is the pathogen sensor PKR, which can respond to nutrients and ER stress and is linked to multiple inflammatory pathways and insulin action (Nakamura et al., 2010).
ER stress and the UPR are connected to inflammatory pathways and may contribute to the production of inflammatory mediators. Conversely, inflammation can also induce or propagate the UPR. For example, exposure to TNF-α can activate the UPR in mouse fibrosarcoma cells, potentially through generation of ROS, which themselves can cause ER stress (Xue et al., 2005). Whether such a mechanism is broadly applicable to other cell types remains to be determined. However, adipose tissue from obese or insulin-resistant mice and humans do exhibit signs of oxidative stress, which can lead to further insulin resistance (Houstis et al., 2006). As stated earlier, inflammatory signals can trigger mTOR activity through TSC proteins, contributing to the emergence or maintenance of ER stress and associated responses. In summary, there are several vicious cycles that integrate ER dysfunction with stress and inflammatory signaling pathways. Perhaps a working model could be that ER stress in obesity is triggered by energy and nutrient metabolism and propagated by the engagement of a multitude of stress signals—such as inflammatory mediators, reactive oxygen species, and nitric oxide—which themselves could emerge due to ER stress. Specific evidence pinpointing these pathways as contributory mechanisms in metabolic dysfunction due to ER stress remains to be established. Nevertheless, these stress signals and byproducts will certainly worsen the cellular milieu in which the ER has to operate. Similar to many other biological phenomena related to metabolic homeostasis, the “chicken or egg” question is a challenge here as well, especially considering the unique needs and demands of different metabolic cells such as pancreatic β cells and adipocytes.
Therapeutic Targeting of ER Dysfunction
An important question when considering therapeutic opportunities is the relevance of observations made in cells or animals to human disease. One critical proof of principle implicating ER function in human metabolic disease comes from genetic studies of human diseases such as Wallcot-Rallison syndrome (Zhang et al., 2002) or Wolfram syndrome (Fonseca et al., 2005). Recent studies provide strong evidence in support of a role for ER stress in human metabolic disease. Three independent groups studied human subjects in the context of metabolic disease and found a strong relationship between obesity and ER stress. Sharma et al. (2008) demonstrated a significant positive correlation between increasing body mass index and expression of various ER stress markers in human subcutaneous adipose tissue. Boden et al. (2008) performed proteomic analyses comparing adipose tissue from lean and obese individuals and found significant upregulation of several proteins involved in the ER stress response. Finally, we showed that when obese patients undergo marked weight loss due to gastric by-pass surgery, there is a decrease in ER stress markers in liver and adipose tissue (Gregor et al., 2009). ER stress indicators have been observed in human atherosclerotic lesions, in a manner similar to the vascular lesions of mouse (Tabas et al., 2009). These studies are important as they reveal that the causal disease mechanisms discovered in mice are also present in human disease and exhibit similar regulation. Although correlative, this is nonetheless important confirmation and motivation for continuing work on the role of ER stress in obesity and type 2 diabetes. Currently, much of the data on the regulation of ER stress in human metabolic disease has focused on the involvement of the IRE1 and PERK branches of the UPR, whereas the ATF6 branch has not been as well studied due to lack of effective reagents for studying this pathway in human cells. In light of this, it is important to note that genetic variation at the ATF6 and ORP150 loci has been linked to insulin resistance, further supporting a role for ER stress in human metabolic disease (Kovacs et al., 2002; Thameem et al., 2006).
The ER is an attractive potential therapeutic target in part because ER adaptive responses have not had time to evolve to deal with the chronic stresses encountered due to recent changes in lifestyle, such as excess nutrient availability and obesity. Thus, maintenance or enhancement of proper ER function may be able to prevent chronic metabolic disease. Such “organelle therapy” may also be needed to disengage stress pathways from insulin signaling or other metabolic responses. Our lab demonstrated that two chemical chaperones, phenyl butyric acid and tauro-ursodeoxycholic acid, that relieve ER stress could protect liver cells from chemically induced ER stress and whole animals from obesity-induced ER stress. These chaperones increased systemic insulin sensitivity, established normoglycemia, and reduced fatty liver disease in obese mice (Ozcan et al., 2006). These treatments suppressed ER stress and inflammatory kinase signaling and enhanced insulin receptor signaling in adipose and liver tissues. At least in experimental models of obesity and diabetes, these agents exhibit therapeutic efficacy; however, additional work is needed to definitively link their activity to the ER. Additionally, studies in lipid-induced models of ER stress have shown that apoB100 secretion is inhibited in the liver by ER stress, an important contributor to hepatic steatosis (Ota et al., 2008). Interestingly, this inhibition of apoB100 secretion in liver insulin resistance can also be prevented by chemical chaperones in vitro and in vivo (Ota et al., 2008). Whether these approaches can be translated into treatments for human disease remains unknown, but there are certainly limitations with these chemicals due to the high doses required to produce the desired effect and relatively undesirable pharmacokinetics. More studies are needed to elucidate how enhancing the activity of endogenous chaperones and boosting protein folding in metabolically active tissues affects the adaptive capacity of the UPR under metabolic stress and to develop specific strategies to do so. This concept is even applicable to plants, which benefit from increased chaperone expression or capacity under stress (Alvim et al., 2001). However, the nature and magnitude of the response to elevated chaperone levels under metabolic stresses such as obesity remain to be determined. It will be exciting to investigate individual molecular chaperones and the transcription factors that control them, such as ATF6 and XBP1, in the liver, adipose tissue, muscle, and pancreas. Concerted upregulation of protein-folding chaperones or the programs leading to their coordinated regulation may prove beneficial to a metabolically overloaded cell and may be a powerful approach for treating chronic metabolic diseases.
We have shown that ER modification by chemical chaperones or targeting lipid chaperones in macrophages and adipocytes have therapeutic efficacy against atherosclerosis in mouse models (Erbay et al., 2009). Both murine and human atherosclerotic vascular lesions exhibit signs of ER stress, and the cholesterol-induced apoptosis of macrophages involves ER stress (Devries-Seimon et al., 2005; Tabas et al., 2009). Although the mechanisms and targets of ER stress and the atheroprotective effect of chemical chaperones are still unclear, these findings suggest that multiple metabolic diseases could be tackled through functional management of the ER. Taken together, these experiments provide proof of principle in a preclinical setting that the ER can be chemically targeted to enhance its functional capacity and that such strategies may provide potential new therapeutic avenues. It will be fascinating to test the efficacy of some of these chemical modalities in humans with metabolic disease as some of the chemical chaperones are already approved for testing in humans.
Alternative therapies to reduce ER stress or to modify organelle function may also involve directly targeting molecules that regulate the UPR. Salubrinal is a small molecule that prevents dephosphorylation of eIF2α (Boyce et al., 2005). Treatment of cells with salubrinal leads to protection against ER stress-induced cell death in vitro and in vivo (Sokka et al., 2007). However, it is unclear whether this chemical strategy could be beneficial for treating metabolic disease as different cell types respond differently to salubrinal (Cnop et al., 2007). It is also possible that activation of the IRE1 pathway in a manner that does not lead to JNK activation could be beneficial, as recently suggested in islet cells (Lipson et al., 2006). Intriguingly, some chemicals, such as PPAR agonists or salicylates, that are in clinical trials or currently used for treating type 2 diabetes affect the activity of critical ER molecules (Palakurthi et al., 2001; Silva et al., 2007). Similar arguments, although speculative, could be made for metformin, rapamycin, or AMP-K activators, which modulate nutrient-sensing and related pathways (Tsang et al., 2007; Zhang et al., 2009a). One wonders whether at least some of the metabolically beneficial effects of these agents are due to their ability to modulate ER function or the UPR.
Finally, another way of relieving ER stress may be through the action of metabolic hormones. Recent data have shown that activation of the receptor for glucagon-like peptide-1 (GLP-1) reduces ER stress in pancreatic β cells (Yusta et al., 2006). A reciprocal relationship may exist wherein some hormones, which depend upon the ER for their translation and secretion, may act on target cells in ways that protect the biological function of that cell thus ensuring continued hormone action, a self-preserving activity. Given the example of GLP-1 receptor activation in relieving ER stress-induced apoptosis, it will be interesting to investigate other hormones for such potential protective effects. Regardless, many questions need to be addressed and many new strategies developed before the true potential of “ER therapy” can be realized.
I would like to end by stating an apparent paradox in thinking about therapeutic strategies for modulating insulin sensitivity and the pathways that block it through targeting ER stress and JNK activation. If ER stress is causal to metabolic deterioration and is induced by energy or nutrient surplus, then it follows that the UPR is linked to insulin resistance. Insulin is a major anabolic hormone and so insulin resistance may help the ER to cope by transiently shutting off synthetic pathways. In fact, perhaps one can even consider that insulin resistance is a bona fide component of the UPR. If true, then a critical consideration is that therapeutic strategies that achieve insulin sensitivity without addressing an underlying ER dysfunction, or JNK activation, or other inflammatory stresses may not be sustainable or even beneficial in the long term because they would increase the burden on the ER. Perhaps there should be a revision of the classic models on which therapeutic strategies for treating obesity, type 2 diabetes, and atherosclerosis are predicated.
I propose that the ER is the cell's ultimate exposure-monitoring device, which is sensitive to a variety of environmental signals, from nutrients and toxins to pathogens. Hence, the ER represents a rich platform for understanding interactions between environmental signals and the biological infrastructure. Its inherent variations and adaptability provide new therapeutic possibilities for treating a number of serious metabolic diseases.
ACKNOWLEDGMENTS
G.S.H. is supported by the NIH, American Diabetes Association, and the Juvenile Diabetes Research Foundation. I am grateful to the students and fellows who contributed to the studies in my group. I thank Margaret Gregor Gustafson and Ling Yang for helpful discussions and thoughtful comments. G.S.H. is on the Scientific Advisory Board, is a shareholder, and receives research support from Syndexa Pharmaceuticals.
REFERENCES
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, Stathopoulos EN, Tsapis A, Castanas E. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J. Immunol. 2009;183:5948–5956. doi: 10.4049/jimmunol.0901186. [DOI] [PubMed] [Google Scholar]
- Alvim FC, Carolino SM, Cascardo JC, Nunes CC, Martinez CA, Otoni WC, Fontes EP. Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol. 2001;126:1042–1054. doi: 10.1104/pp.126.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey D, O'Hare P. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid. Redox Signal. 2007;9:2305–2321. doi: 10.1089/ars.2007.1796. [DOI] [PubMed] [Google Scholar]
- Basseri S, Lhotak S, Sharma AM, Austin RC. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J. Lipid Res. 2009;50:2486–2501. doi: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, Diehl JA. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc. Natl. Acad. Sci. USA. 2008;105:16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 2002;277:13045–13052. doi: 10.1074/jbc.M110636200. [DOI] [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J. Biol. Chem. 2007;282:3989–3997. doi: 10.1074/jbc.M607627200. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavaheri-Mergny M, Amelotti M, Mathieu J, Besancon F, Bauvy C, Souquere S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J. Biol. Chem. 2006;281:30373–30382. doi: 10.1074/jbc.M602097200. [DOI] [PubMed] [Google Scholar]
- DuRose JB, Scheuner D, Kaufman RJ, Rothblum LI, Niwa M. Phosphorylation of eukaryotic translation initiation factor 2alpha coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol. Cell. Biol. 2009;29:4295–4307. doi: 10.1128/MCB.00260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J. Biol. Chem. 2005;280:39609–39615. doi: 10.1074/jbc.M507426200. [DOI] [PubMed] [Google Scholar]
- Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MG, Hotamisligil GS. Adipocyte stress: The endoplasmic reticulum and metabolic disease. J. Lipid Res. 2007;48:1905–1914. doi: 10.1194/jlr.R700007-JLR200. [DOI] [PubMed] [Google Scholar]
- Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC, Cavener DR, McGrath BC, Rudell JB, Koehnle TJ, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000a;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000b;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk–/– mice reveals a role for translational control in secretory cell survival. Mol. Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J. Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Sasaki M, Miyahara T, Hashimoto C, Matsuo S, Yoshii M, Ozawa K. Endoplasmic reticulum stress induces leptin resistance. Mol. Pharmacol. 2008;74:1610–1619. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N. Engl. J. Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, Haataja L, Gurlo T, Butler AE, Wu X, Soeller WC, Butler PC. Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1656–E1662. doi: 10.1152/ajpendo.00318.2007. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappa B in response to diverse cellular stresses. Mol. Cell. Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Chung KW, Won Kim J, Kim J, Komatsu M, Tanaka K, Nguyen YH, Kang TM, Yoon KH, Kim JW, et al. Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- Kokame K, Kato H, Miyata T. Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J. Biol. Chem. 2001;276:9199–9205. doi: 10.1074/jbc.M010486200. [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Yang X, Permana PA, Bogardus C, Baier LJ. Polymorphisms in the oxygen-regulated protein 150 gene (ORP150) are associated with insulin resistance in Pima Indians. Diabetes. 2002;51:1618–1621. doi: 10.2337/diabetes.51.5.1618. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum. Mol. Genet. 2005;14:R251–R258. doi: 10.1093/hmg/ddi260. Spec No. 2. [DOI] [PubMed] [Google Scholar]
- Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, MacAuley A, Goodman AG, LeBoeuf RC, Katze MG. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54:1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, et al. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007a;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007b;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat. Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab. 2009;10:507–515. doi: 10.1016/j.cmet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Maurin AC, Jousse C, Averous J, Parry L, Bruhat A, Cherasse Y, Zeng H, Zhang Y, Harding HP, Ron D, et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- Nadanaka S, Okada T, Yoshida H, Mori K. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol. Cell. Biol. 2007;27:1027–1043. doi: 10.1128/MCB.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. 2005;280:847–851. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature. 2009;461:654–658. doi: 10.1038/nature08455. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevelance of high body mass index in US children and adolescents. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Oikawa D, Kimata Y, Kohno K. Self-association and BiP dissociation are not sufficient for activation of the ER stress sensor Ire1. J. Cell Sci. 2007;120:1681–1688. doi: 10.1242/jcs.002808. [DOI] [PubMed] [Google Scholar]
- Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J. Clin. Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, Tamatani T, Yamagata K, Miyagawa J, Kitao Y, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr., Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor gamma and mediated by inhibition of translation initiation. Cancer Res. 2001;61:6213–6218. [PubMed] [Google Scholar]
- Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, et al. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn's disease susceptibility. Nat. Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 2001;13:349–355. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- Pirot P, Naamane N, Libert F, Magnusson NE, Orntoft TF, Cardozo AK, Eizirik DL. Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia. 2007;50:1006–1014. doi: 10.1007/s00125-007-0609-0. [DOI] [PubMed] [Google Scholar]
- Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T. Cell growth control: mTOR takes on fat. Mol. Cell. 2008;31:775–776. doi: 10.1016/j.molcel.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Ricca GA, Liu DS, Coniglio JJ, Richardson A. Rates of protein synthesis by hepatocytes isolated from rats of various ages. J. Cell. Physiol. 1978;97:137–146. doi: 10.1002/jcp.1040970202. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, Serrano M, Auwerx J, Diaz-Meco MT, Moscat J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab. 2006;3:211–222. doi: 10.1016/j.cmet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev. Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J. Clin. Endocrinol. Metab. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Silva AM, Wang D, Komar AA, Castilho BA, Williams BR. Salicylates trigger protein synthesis inhibition in a protein kinase R-like endoplasmic reticulum kinase-dependent manner. J. Biol. Chem. 2007;282:10164–10171. doi: 10.1074/jbc.M609996200. [DOI] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009a;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 2009b;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J. Neurosci. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Seimon T, Timmins J, Li G, Lim W. Macrophage apoptosis in advanced atherosclerosis. Ann. N Y Acad. Sci. 2009;1173(Suppl 1):E40–E45. doi: 10.1111/j.1749-6632.2009.04957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thameem F, Farook VS, Bogardus C, Prochazka M. Association of amino acid variants in the activating transcription factor 6 gene (ATF6) on 1q21-q23 with type 2 diabetes in Pima Indians. Diabetes. 2006;55:839–842. doi: 10.2337/diabetes.55.03.06.db05-1002. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov. Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol. Cell. Biol. 2006;26:63–76. doi: 10.1128/MCB.26.1.63-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isom-erase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS ONE. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- West DB, Prinz WA, Francendese AA, Greenwood MR. Adipocyte blood flow is decreased in obese Zucker rats. Am. J. Physiol. 1987;253:R228–R233. doi: 10.1152/ajpregu.1987.253.2.R228. [DOI] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat. Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Xue X, Piao JH, Nakajima A, Sakon-Komazawa S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H, Nakano H. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, et al. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Zhang BB, Zhou G, Li C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009a;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Huang J, Duvel K, Boback B, Wu S, Squillace RM, Wu CL, Manning BD. Insulin stimulates adipogenesis through the Akt-TSC2-mTORC1 pathway. PLoS ONE. 2009b;4:e6189. doi: 10.1371/journal.pone.0006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006a;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006b;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc. Natl. Acad. Sci. USA. 2006;103:14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]