Abstract
BACKGROUND
Esophageal bolus clearance requires a preferential esophagogastric pressure gradient sustained for a sufficient period. We aimed to validate a high-resolution manometry (HRM) paradigm for predicting bolus clearance.
METHODS
Twenty volunteers and 30 patients were studied with HRM during barium swallows with concurrent fluoroscopy. Simultaneous bolus domain pressure and esophagogastric junction (EGJ) obstruction pressure were plotted and flow permissive time was tallied during which the bolus domain pressure exceeded the EGJ obstruction pressure. Distal peristaltic integrity was assessed at incrementally increasing pressure isobaric contour thresholds from 15–40 mmHg. ROC analysis was performed to assess the sensitivity and specificity of cutoff values for flow permissive time and peristaltic amplitude for predicting incomplete clearance as verified fluoroscopically.
RESULTS
Flow permissive time ≤2.5 s had a sensitivity of 86% and specificity of 92% for predicting incomplete clearance. In contrast, a 30-mmHg peristaltic amplitude had a sensitivity of only 48% and specificity of 88%. Incomplete clearance was variably attributable to functional EGJ obstruction, hiatus hernia, or impaired peristalsis.
CONCLUSIONS
A detailed analysis of intraluminal pressure gradients in the distal esophagus and across the EGJ in the postdeglutitive period predicts esophageal bolus clearance with far greater accuracy than any threshold value of peristaltic amplitude.
INTRODUCTION
Although manometry accurately defines esophageal motor patterns associated with poor bolus transit, it is limited in its ability to predict bolus clearance. One problem with conventional manometry is the lack of a sufficient spatial resolution to determine subtle defects in the peristaltic contractile wavefront that might lead to impaired clearance. This limitation was highlighted in a recent investigation demonstrating that, in experienced hands, high-resolution manometry (HRM) is superior to conventional manometry in predicting bolus transit (1). In that investigation, successful bolus transit was predicted based on the presence of a compatible high-resolution pressure topography motor pattern, capitalizing on the tools of HRM.
Although peristaltic function is undoubtedly an important determinant of esophageal clearance, the obstructing pressure at the esophagogastric junction (EGJ) must also be considered. Bolus clearance across the EGJ is, in fact, dependent on two conditions: (a) a flow permissive pressure gradient between the intrabolus pressure and the maximal EGJ obstructing pressure and (b) adequate persistence of that gradient to facilitate emptying of a specified volume. We recently utilized the tools of HRM to validate a novel analysis paradigm that accurately predicted bolus clearance across the EGJ in 20 normal subjects (2). By plotting the pressure within the bolus domain simultaneously against the maximal pressure along the EGJ during the postdeglutitive period, we were able to quantify periods during which a flow permissive pressure gradient was present. With concomitant fluoroscopy, we demonstrated that trans-EGJ flow only occurred during periods characterized by a positive bolus driving pressure (BDP). In addition, we were also able to establish normative ranges that may be useful in predicting abnormal bolus transit. Thus, the goal of the present study was to validate BDP plots against concomitant fluoroscopy in predicting bolus clearance in asymptomatic controls and patients presenting with reflux or dysphagia. In addition, we sought to compare the utility of BDP plots to measures of peristaltic integrity in predicting bolus clearance.
METHODS
Patients
Concurrent HRM and fluoroscopy studies were done on 20 normal controls (7 men, ages 20–45) and 30 patients (11 men, ages 32–64). The control group consisted of volunteers without gastrointestinal symptoms or history of upper gastrointestinal tract surgery and all were without any significant medical condition. The patients were recruited from the GI clinic at the Northwestern Medical Faculty Foundation. Fourteen patients had reflux complaints and were being evaluated for antireflux surgery while another 12 patients had a chief complaint of dysphagia. Four of the reflux patients and five of the dysphagia patients also had concomitant chest pain. In addition, four patients with postfundoplication dysphagia symptoms were studied. The study protocol was approved by the Northwestern University Institutional Review Board and informed consent was obtained from each subject prior to beginning the study.
High-Resolution Manometry
A solid-state manometric assembly with 36 circumferential sensors spaced at 1-cm intervals (outer diameter 4.2 mm) was used to study intraluminal pressure from the pharynx to the stomach (Sierra Scientific Instruments Inc., Los Angeles, CA). Prior to recording, the transducers were calibrated at 0–300 mmHg using an externally applied pressure in a calibration chamber. The response characteristics of each sensing element were such that they could record pressure transients in excess of 6,000 mmHg/s and were accurate to within 1 mmHg of atmospheric pressure for measurements obtained for at least the final 5 min of the study, immediately prior to thermal recalibration (3).
Subjects underwent transnasal placement of the HRM assembly. Studies were done in a supine position after at least a 6-h fast and the manometric assembly was positioned to record from the hypopharynx to the stomach with about three intragastric sensors. The catheter was fixed in place by taping the catheter to the nose. The manometric protocol included a 5-min basal period to assess sphincter pressure followed by ten 5 mL and one each of 1 mL, 10 mL, and 20 mL water swallows. After completion of the manometric protocol, the subjects were placed in the supine position under a C-arm fluoroscope (Easy Diagnostics, Phillips Medical Systems, Shelton, CT) and shielded below the umbilicus with a lead apron, along with a lead collar for thyroid protection. Fluoroscopic images were recorded using a DVD recorder (LG RC797T) and were synchronized with manometric data from the high-resolution solid-state catheter using a video timer (model VC 436, Thalner Electronics Laboratories, Ann Arbor, MI) that encodes time in hundredths of a second on each video frame. Two 5-mL swallows were recorded with the potential for a repeat swallow if the first was technically inadequate.
HRM data were subsequently analyzed using custom programs written in Matlab (The MathWorks Inc., Natick, MA, version 7.5.9.342 R2007b). Characterization of the pressure morphology across the esophagus and EGJ was performed with computer programs customized for displaying HRM data as color pressure topography plots and spatial pressure variation plots as described previously (4, 5).
BDP Plots
Utilizing concepts originally described by Ren et al. that focused on the relationship between peristalsis and intrabolus pressure (6), we developed an analysis paradigm that tracked and plotted instantaneous intrabolus pressure and maximal EGJ pressure (Fig. 1). A surrogate measurement for intrabolus pressure was created called the bolus domain pressure. The bolus domain pressure was defined as the pressure midway between the ramp-up or inflexion point of the peristaltic wavefront and the proximal margin of the crural component of the EGJ after the swallow (Fig. 1B). In instances of either weak or failed peristalsis, a default bolus domain pressure measurement was performed 2 cm above the proximal margin of the crural component of the EGJ. The bolus domain pressure was quantified during the distal esophageal contraction spanning from the transition zone to the termination of peristalsis. In instances of failed peristalsis, a time period of 8 s beginning at the transition zone was assessed. Obstructing pressure within the EGJ was measured as the maximum pressure within the EGJ high-pressure zone extending through a 4-cm span starting at the proximal margin of the crural diaphragmatic component. This anatomic zone was chosen as it represents the point of maximal resistance for esophageal emptying (7).
Figure 1.
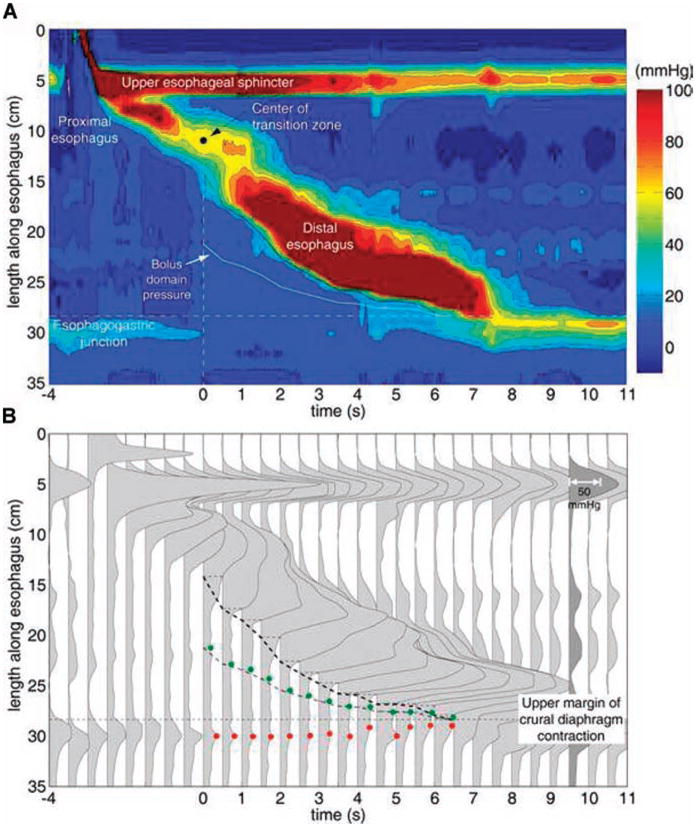
Pressure topography (A) and spatial pressure variation (B) representations of a normal swallow. The proximal esophagus, the distal esophagus, and the transition zone that separates them are clearly shown in the pressure topography plot. Time 0 corresponds to center of the manometrically defined transition zone. The heavy black isobaric contour line represents the 30-mmHg threshold and the trajectory of the bolus domain pressure is illustrated midway between the 30-mmHg isobaric contour and the proximal margin of the EGJ. Panel B illustrates a series of spatial pressure variation plots at 0.5-s intervals of the same swallow. The darkened plot (9.5 s) shows the pressure scaling. This method provides a convenient means to visualize intraluminal pressure gradients responsible for bolus transit. The trajectory of the 30-mmHg isobaric contour (dashed line), the maximal EGJ obstruction pressure (red circle), and the bolus domain pressure (green circle) are also shown.
Simultaneous bolus domain pressure and EGJ relaxation pressure were overlayed to assess the relationship between these two parameters during swallows (Fig. 2). Given that the error of pressure measurement was about 1 mmHg, we established a cutoff value of 0 mmHg as the minimal gradient at which flow could potentially occur. For each swallow, the time that the bolus domain pressure was greater than or equal to the EGJ relaxation pressure was defined as the flow permissive time. All pressures were referenced to atmospheric pressure.
Figure 2.
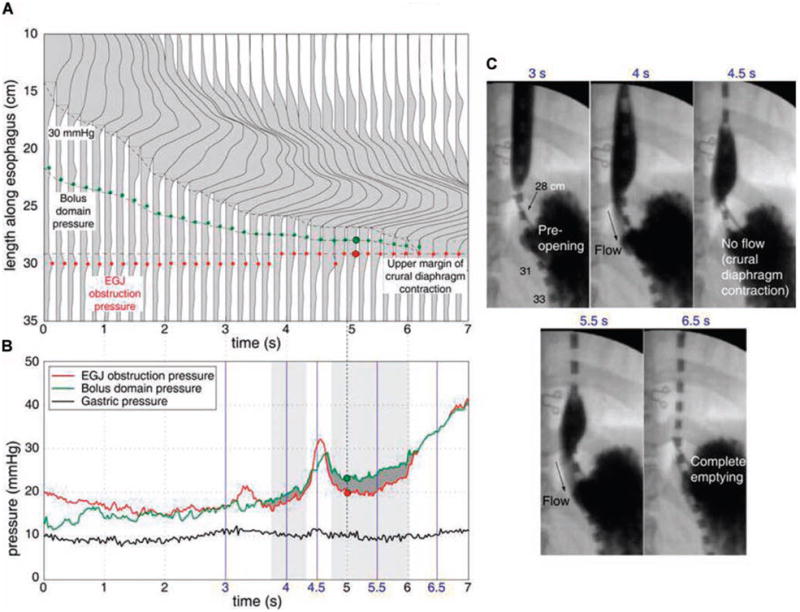
Bolus driving pressure (BDP) analysis for a normal swallow. Panel A illustrates a detailed spatial pressure variation plot of distal esophageal peristalsis of the swallow shown in Figure 1 with increased time resolution to 0.2 s. Panel B shows the BDP analysis in which the instantaneous bolus domain pressure, EGJ obstruction pressure, and gastric pressure are plotted together. Periods during which the bolus domain pressure exceeds EGJ obstruction pressure are deemed flow permissive and highlighted by the dark gray fill. Fluoroscopically determined periods of flow are shown by the gray rectangular boxes. Panel C illustrates X-ray images of esophageal bolus emptying obtained from concurrent fluoroscopy at the times indicated in blue font. Note the hiatal closure at 4.5 s resulting from a crural diaphragm contraction.
The integrity of distal esophageal peristalsis was assessed using the isobaric contour tool in ManoView analysis software. The isobaric contour tool allows delineation of the anatomical/temporal boundaries of a pressure domain of user-designated magnitude (30 mmHg in Fig. 1). Threshold pressures for the isobaric contour measurement beginning at 15 mmHg and progressing in 5 mmHg increments to 40 mmHg were analyzed and peristaltic function was characterized as normal (intact isobaric contour), or failed if a defect ≥2 cm was noted in the isobaric contour. All pressures were referenced to intragastric pressure.
Agreement Between Fluoroscopy and HRM in Assessing Bolus Clearance
An independent and blinded assessment of trans-hiatal emptying was made from fluoroscopy (NL) and compared with an independent assessment of flow permissive time from the BDP analysis (SKG). Previous work reported that the normative range for flow permissive time was 2.5–5.5 s and that bolus clearance across the EGJ typically required greater than 2.0 s (8). Thus, we tested the agreement between bolus clearance on fluoroscopy and flow permissive time using cutoff values beginning at 1.5 s and progressing in 0.5 s increments. A receiver operator characteristic (ROC) analysis was performed to predict the best cutoff value for predicting bolus clearance based on flow permissive time and on peristaltic performance.
Statistical Analysis
Agreement between fluoroscopically ascertained bolus clearance and the manometric prediction of bolus clearance was performed by testing the agreement between two dichotomous variables. Fluoroscopically ascertained clearance was defined as complete or incomplete while the manometric prediction of clearance was based on the flow permissive time period. One hundred swallows were available for the agreement analysis. ROC analysis was performed to assess the sensitivity and specificity of cutoff values for flow permissive time and peristaltic front pressure in predicting incomplete clearance.
RESULTS
Nineteen of the 20 normal subjects had complete bolus clearance on fluoroscopy for both 5 mL swallows. One normal subject had aperistalsis and incomplete emptying. Of the 30 patients, 17 had complete clearance, 7 had one swallow with incomplete clearance (postfundoplication, 1; gastroesophageal reflux disease (GERD), 4; dysphagia, 2), and 6 had both swallows with impaired clearance (postfundoplication, 3; GERD, 2; dysphagia, 1).
Agreement Between Fluoroscopy and HRM in Predicting Clearance
The agreement between fluoroscopy and the BDP analysis was assessed using the 100 swallows obtained in the 20 asymptomatic controls and the 30 patients. Thus, the data set was comprised of 21 swallows with incomplete clearance and 79 swallows with complete clearance. ROC analysis determined that a flow permissive time cutoff value of 2.5 s had the best performance in determining complete clearance (Fig. 3) with a sensitivity and specificity of 85.7% and 92.4%, respectively. This was associated with a positive predictive value of 75% and a negative predictive value of 96% for incomplete clearance. It was extremely rare to have a flow permissive time greater than 2.5 s and not completely clear the bolus during a 5-mL supine swallow (3 out of 100 swallows). Furthermore, it was noted that the 3 swallows with incomplete clearance occurred in 2 subjects with a >2-cm hiatal hernia with the impairment in esophageal emptying occurring by the mechanism of retrograde escape of the bolus from the hernia sac into the esophagus after the termination of peristalsis.
Figure 3.
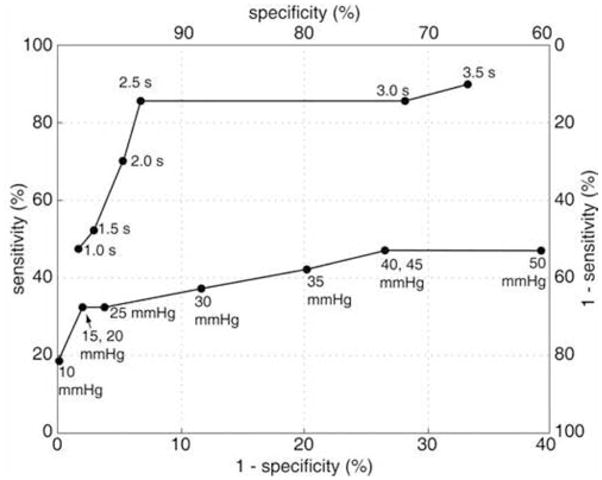
Receiver operating characteristic curves of BDP analysis predicted incomplete clearance and peristaltic amplitude predicted incomplete clearance versus fluoroscopically confirmed incomplete clearance. The optimal BDP threshold value is 2.5 s with both sensitivity and specificity in the range of 90%. Peristaltic amplitude was a far less perfect predictor of impaired clearance with maximal sensitivity of less than 50% and an optimal threshold less well defined, residing in the 15–30 mmHg range.
In contrast to the BDP analysis, agreement between fluoroscopically ascertained clearance and clearance predicted by peristaltic performance was poor. ROC analysis revealed that a contiguous wavefront between 15 and 30 mmHg in the distal esophagus was associated with the best predictive value for determining incomplete bolus transit (Fig. 3). However, this was only associated with a sensitivity of 47.6% and a specificity of 88% and a distinct pressure threshold based on the ROC analysis could not be established. A peristaltic wavefront with an intact isobaric contour set at 20 mmHg had a positive predictive value for predicting incomplete clearance of 88%. However, there were 11 patients that had incomplete bolus transit despite having a peristaltic wavefront with an intact isobaric contour of 50 mmHg. These subjects were noted to have either an impaired EGJ relaxation pressure due to a functional obstruction (fundoplication) or a hiatal hernia.
Functional Parameters Responsible for Abnormal Bolus Transit
In addition to providing the flow permissive time, the spatial pressure variation plots and the bolus domain pressure plots also provide mechanistic information regarding incomplete clearance. The mechanistic etiology of impaired transit in the 21 swallows with incomplete clearance were 3 with dropped peristalsis, 3 with hypotensive peristalsis, 7 with a functional obstruction at the EGJ with intact distal peristalsis, 4 with hiatal hernia resulting in retrograde escape, 2 with focal defects >2 cm in the distal esophagus, 1 with weak spastic contractions, and 1 with a transition zone defect. As evidenced above, incomplete clearance can occur in the context of normal peristalsis if EGJ anatomy or function is altered. Figure 4 illustrates an example of incomplete clearance in a subject with a hiatal hernia. The manometric evidence of a hiatal hernia is noted from the double pressure peaks corresponding to the lower esophageal sphincter and the crural diaphragm (at time 12 s in Fig. 4A). In this example, the bolus domain pressure measurement is positioned within the hiatal sac during the final stages of the swallow and the flow permissive time is approximately 1.5 s. Although flow through the hiatal canal occurs at 9 s, the flow permissive period is too brief and the bolus is retained in the hiatal sac until retrograde escape occurs at 13 s, after the LES after-contraction subsides. Note the drop in the bolus domain pressure as the EGJ obstruction pressure increases with crural contraction and the hiatal sac decompresses and pressurizes the distal esophageal body at 13–14 s.
Figure 4.
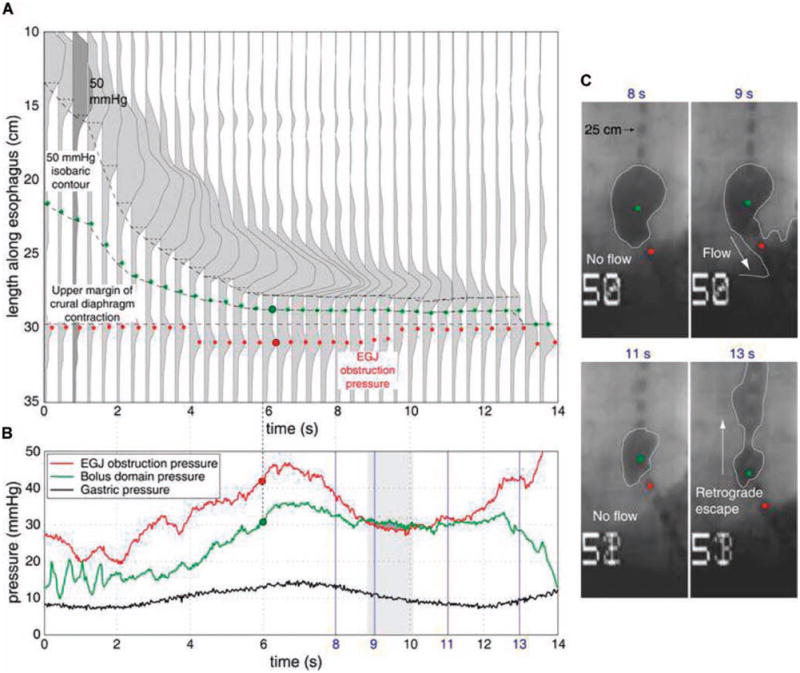
BDP analysis for a hiatal hernia patient with impaired emptying illustrated as in Figure 2. A 50-mmHg isobaric contour was chosen as the point of inflexion since the bolus domain pressure exceeded 30 mmHg in the hernia sac. The trajectory of the 50 mmHg isobaric contour, the bolus domain pressure measurement location (green dots), and the location of maximum EGJ obstruction pressure (red dots) are shown. Panel B shows the BDP analysis. The period during which the intrabolus pressure exceeds maximum EGJ obstruction pressure was deemed flow permissive and indicated by the heavily grayed area. The period of fluoroscopically determined flow is shown by the gray rectangular box. Panel C shows the corresponding X-ray images obtained at the times indicated in blue font. Note that the hiatal closure at 11 s is followed by a retrograde escape of the bolus at 13 s with corresponding depressurization of the hernia sac as seen by the drop in the bolus domain pressure from 12 to 14 s in panel B.
In the example illustrated in Figure 5, incomplete clearance occurs in the context of a functional obstruction despite a strong peristaltic wavefront in a postfundoplication patient. Despite the normal peristaltic function, the time limited nature of the peristaltic contraction coupled with the abnormally high EGJ outflow pressure results in their being bolus retained in the esophagus after the termination of peristalsis.
Figure 5.
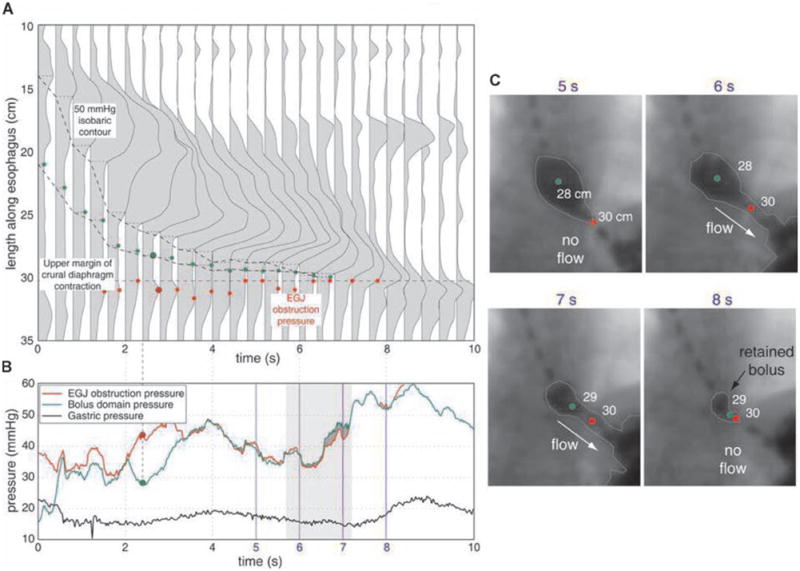
BDP analysis for a postfundoplication patient with dysphagia illustrated as in Figures 2 and 4. Note the relatively high magnitude of the EGJ obstruction pressure compared to the normal swallow shown in Figure 2. Also note the retained bolus in the distal esophagus at the conclusion of esophageal peristalsis in panel C, 8s.
DISCUSSION
The primary determinants of normal bolus transit during swallowing are a preferential pressure gradient and a sufficient time interval during which flow can occur. Given that peristalsis is a time-limited event, there is only a finite time window during which a positive esophago-gastric pressure gradient can be generated to promote esophageal emptying. We recently described and validated an HRM paradigm that quantified the presence and persistence of this flow permissive time by plotting the relationship between bolus domain pressure and EGJ obstruction pressure in the postdeglutitive period (2). In the current study, we applied this paradigm to patient data to test its performance as a predictor of fluoroscopically ascertained impaired esophageal clearance. The major finding was that a flow permissive time of 2.5 s had a sensitivity of 86% and specificity of 92% for determining incomplete clearance and that the BDP analysis was greatly superior to peristaltic amplitude in predicting impaired clearance. In addition, the BDP analysis provided mechanistic information regarding the etiology of impaired clearance.
Although we are not the first investigators to suggest that intrabolus pressure is important in understanding bolus transit mechanics, the current work represents the first attempt to reduce bolus transit from the distal esophagus to a mechanical relationship between driving pressure and outflow resistance at the EGJ. Our methodology built upon previous work utilizing combined HRM and fluoroscopy at the upper esophageal sphincter also suggesting transphincteric intrabolus pressure gradients to have important pathological implications (9). Also fundamental to the current study, in the body of the esophagus, Ren et al. used concurrent fluoroscopy and conventional manometry to demonstrate the relationship between effective bolus transport and the difference between closure pressure of the peristaltic contraction and intrabolus pressure (6). The goal of the present study was to utilize the advantages of HRM to determine if manometry alone could be leveraged to accurately predict bolus transit across the EGJ in patients with a spectrum of pathological conditions. Using the normative data for esophago-gastric flow permissive time in 20 controls and mathematical modeling data of antegrade esophageal emptying (8), we performed an ROC analysis for the predictive value of flow permissive time that is optimal to predict complete clearance. Our findings suggest that a cutoff value of ≤2.5 s had the best predictive value for incomplete clearance with a sensitivity of 86% and specificity of 92%.
Our findings using the BDP analysis are consistent with other recent work applying high-resolution manometry to predict bolus transit. Fox et al. recently compared high-resolution manometry to conventional manometry in predicting bolus transit and provided strong evidence that, at least in experienced hands, HRM was superior to conventional manometry in predicting bolus transit (1). Using a more liberal definition of abnormal bolus transit (bolus escape with delayed clearance), these investigators reported a sensitivity of 90% and a specificity of 100% for predicting abnormal bolus transit. We utilized the more stringent definition of no retained bolus and were still able to maintain a high level of sensitivity and specificity. In addition, the BDP analysis has the added benefit of reducing bolus transit prediction to a defined algorithm that derives a single number, hopefully making it less subjective and more reproducible.
Although the agreement between flow permissive time <2.5 s and fluoroscopically confirmed incomplete clearance of 86% approximates the reported accuracy of multichannel intraluminal impedance as a predictor of complete bolus transit (97%), it does not provide the same detail as intraluminal impedance in terms of bolus position and overall transit time. Residual intraesophageal bolus does not create a distinct pressure signal as is the case with impedance studies. In this context, high-resolution manometry should be viewed as complementary rather than competitive with impedance measurement and the most robust esophageal function test would integrate both technologies. In that setting, the BDP analysis would assess the etiology of impaired transit while the impedance data would characterize the occurrence and distribution of impaired bolus clearance, analogous to fluoroscopy. This would enable a distinction between a transition zone defect versus a peristaltic defect of the distal esophagus as the primary contributor of impaired transit.
In conclusion, we have developed and validated a novel HRM paradigm for assessing the pressure dynamics responsible for bolus clearance following a swallow. The esophagogastric flow permissive period can be readily delineated from BDP plot analyses, and a flow permissive time of <2.5 s was found to be the most sensitive and specific threshold for predicting incomplete esophageal clearance of a 5-mL bolus. Furthermore, graphical manipulation of the HRM data into spatial pressure variation plots and the BDP plots helped define the primary determinant of impaired clearance by differentiating instances of impaired peristalsis, exaggerated outflow obstruction, and anatomic derangement, particularly hiatus hernia. Clearly each of these conditions has unique management implications, and these findings illustrate the potential for enhancing the clinical utility of HRM studies by designing secondary analysis based on physiological and mechanical principles.
STUDY HIGHLIGHTS.
What Is Current Knowledge
Measurement of peristaltic amplitude with conventional manometry poorly predicts esophageal clearance.
Esophageal clearance is dependent upon the balance between peristaltic integrity and outflow obstruction at the esophagogastric junction (EGJ).
What Is New Here
High-resolution manometry can simultaneously track peristaltic clearing wave pressure, intrabolus pressure, and residual EGJ pressure during the postdeglutitive period, thereby quantifying the time during which the intraluminal pressure gradient favors outflow (flow permissive time).
Flow permissive time >2.5 s is strongly correlated with complete esophageal clearance as verified with concurrent fluoroscopy.
Flow permissive time is a much better predictor of esophageal clearance than is peristaltic amplitude because it accounts for both peristaltic and EGJ abnormalities.
Acknowledgments
Financial support: This work was supported by R01 DC00646 (PJK) from the Public Health Service and the AGA June and Donald O. Castell Esophageal Clinical Research Award (JEP).
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: John Pandolfino, M.D.
Specific author contributions: John Pandolfino: hypothesis, study design, data analysis, primary author; Sudip K. Ghosh: hypothesis, study design, computer programming, analysis; Nilesh Lodhia: data analysis; Peter J. Kahrilas: study design, co-author.
Potential competing interests: None.
References
- 1.Fox M, Hebbard G, Janiak P, et al. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–42. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh SK, Kahrilas PJ, Lodhia N, et al. Utilizing intraluminal pressure differences to predict esophageal bolus flow dynamics. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1023–8. doi: 10.1152/ajpgi.00384.2007. [DOI] [PubMed] [Google Scholar]
- 3.Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: A challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–49. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677–84. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh SK, Pandolfino JE, Zhang Q, et al. Quantifying esophageal peristalsis with high-resolution manometry: A study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G988–97. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ren J, Massey BT, Dodds WJ, et al. Determinants of intrabolus pressure during esophageal peristaltic bolus transport. Am J Physiol. 1993;264:G407–13. doi: 10.1152/ajpgi.1993.264.3.G407. [DOI] [PubMed] [Google Scholar]
- 7.Pandolfino JE, Shi G, Curry J, et al. Esophagogastric junction distensibility: A factor contributing to sphincter incompetence. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1052–8. doi: 10.1152/ajpgi.00279.2001. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh SK, Kahrilas PJ, Zaki T, et al. The mechanical basis of impaired esophageal emptying post fundoplication. Am J Physiol Gastrointest Liver Physiol. 2005;289:G21–35. doi: 10.1152/ajpgi.00235.2004. [DOI] [PubMed] [Google Scholar]
- 9.Pal A, Williams RB, Cook IJ, et al. Intrabolus pressure gradient identifies pathological constriction in the upper esophageal sphincter during flow. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1037–48. doi: 10.1152/ajpgi.00030.2003. [DOI] [PubMed] [Google Scholar]


