Abstract
The discovery of avian cochlear hair cell regeneration in the late 1980’s and the concurrent development of new techniques in molecular and developmental biology generated a renewed interest in understanding the genetic mechanisms that regulate hair cell development in the embryonic avian and mammalian cochlea and regeneration in the mature avian cochlea. Research from many labs has demonstrated that the development of the inner ear utilizes a complex series of genetic signals and pathways to generate the endorgans, specify cell identities, and establish innervation patterns found in the inner ear. Recent studies have shown that the Notch signaling pathway, the Atoh1/Hes signaling cascade, the stem cell marker Sox2, and some of the unconventional myosin motor proteins are utilized to regulate distinct steps in inner ear development. While many of the individual genes involved in these pathways have been identified from studies of mutant and knockout mouse cochleae, the interplay of all these signals into a single systemic program that directs this process needs to be explored. We need to know not only what genes are involved, but understand how their gene products interact with one another in a structural and temporal framework to guide hair cell and supporting cell differentiation and maturation.
Keywords: Cochlea, Hair Cell, Supporting Cell, Genetic Regulation, Notch Pathway, Development, Regeneration
Hearing impairment affects almost 49 million people in the US and over 249 million worldwide. Approximately 17% of children under age 18 have a hearing loss and the incidence increases with age: roughly 30% of people over 65 and over 90% of people over 80 have a substantial hearing loss. Although rarely life-threatening, hearing loss affects more people than epilepsy, multiple sclerosis, spinal injury, stroke, Huntington’s and Parkinson’s diseases combined (Hudspeth, 1997) and has a huge financial impact on our economy and lifestyle. Hearing impairment is mainly caused by damage to the hair cells, the sensory cells in the cochlea (Figure 1). In mammals, the absence of these cells results in permanent hearing loss because they are generated only during embryonic development (Ruben, 1967) and must last throughout a lifetime.
Figure 1.
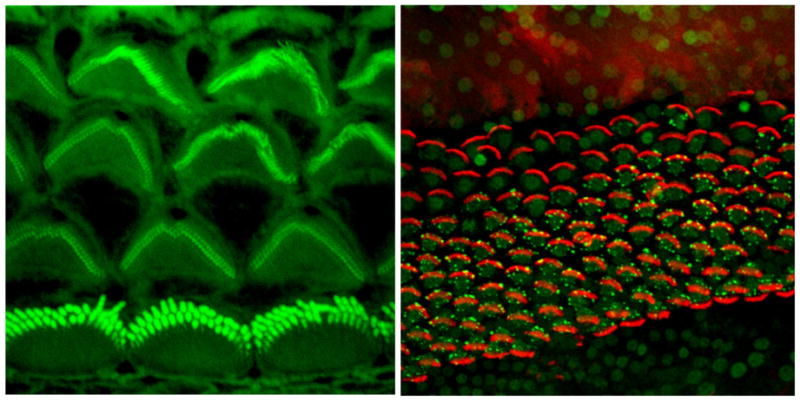
Confocal micrographs of the luminal surface of the postnatal day 3 mouse organ of Cortiwhere actin is labeled with phalloidin (green) and of the regenerating mature chick basilar papilla where actin is labeled with phalloidin (red) and the early apoptosis marker TIAR is labeled with a specific antibody (green).
In birds, however, this is not an issue, as they have the ability to regenerate cochlear hair cells throughout their lifetime (Cotanche, 1987; Cruz et al., 1987; Corwin & Cotanche, 1988; Ryals & Rubel, 1988; Lippe et al., 1991). This is not merely a delayed developmental response, as six-year-old quails (which is three years beyond their average lifespan) can regenerate hair cells as readily as newborn chicks (Ryals & Rubel, 1988). If this regeneration could be induced in the mammalian cochlea, it would offer a potential therapeutic treatment for sensorineural hearing loss in humans. Based on the recent clinical successes of the electronic cochlear implant, the goal of current researchin this area is to utilize regeneration to develop a “biological cochlear implant” to restore hearing function in the human cochlea. Thus, understanding the mechanisms that regulate avian hair cell regeneration and how this correlates with mammalian hair cell development, will be important for pursuing strategies to induce mammalian hair cell regeneration. These strategies can be approached either through the manipulation of existing, intrinsic cochlear cells to become replacement hair cells or through the engineering of stem cells for transplantation and differentiation within a damaged mammalian cochlea to make new hair cells.
There is significant evidence from the developmental literature that the Notch signaling pathway plays two key roles in cochlear development. The first of these occurs early in otocyst development and involves Notch1 and Jagged1 in defining the presumptive sensory epithelium through a lateral induction process (Daudet & Lewis, 2005; Kiernan et al., 2006; Daudet et. al., 2007; Hayashi et al., 2008). The second mechanism occurs later in cochlear development and is involved in determining hair cell and supporting cell fates through a lateral inhibition receptor/ligand interaction (Adam et al., 1998; Lanford et al., 1999; Morrison et al., 1999; Kiernan et al., 2005b). It has been proposed by several groups that the Notch lateral inhibition pathway initiates hair cell differentiation. However, detailed analyses of the published data indicate that the initial differentiation of hair cells is independent of the Notch lateral inhibition pathway. However, once the initial four rows of cochlear hair cells differentiate, the Notch pathway is apparently utilized to keep other progenitor cells from differentiating as supernumerary hair cells. Subsequently, if some of those initial four rows of hair cells are damaged or lost during the late embryonic or early post-natal periods, the dynamics of the Notch pathway are altered so that the progenitor cells formerly prohibited from differentiating into hair cells can now do so. Thus, the Notch pathway does appear to play a critical role in limiting the hair cell population during development. During hair cell regeneration in the bird cochlea, the Notch pathway is recruited once again to limit hair cell fates in the re-establishment of the hair cell and supporting cell mosaic in the sensory epithelium (Stone & Rubel, 1999; Daudet et al.,2009). Moreover, if regeneration can be manipulated to help generate new hair cells in the mature mammalian cochlea, then these cells will also need to express the Notch pathway genes at the appropriate time and place.
Hair Cell Regeneration in the Avian Cochlea
Hair cell regeneration in the mature avian cochlea is a response to damage of the existing sensory cells caused by sound exposure, aminoglycoside treatment, laser ablation, or genetic mutation (Cotanche, 1987; Cruz et al., 1987; Corwin & Cotanche, 1988; Ryals & Rubel, 1988; Lippe et al., 1991; Warchol & Corwin, 1996; Gleich et al., 1997; reviewed in Stone & Cotanche, 2007; Cotanche, 2008). The loss of hair cells induces non-sensory supporting cells to undergo either direct transdifferentiation, where a supporting cell changes its gene expression to become a hair cell without dividing, or mitotic proliferation, where a supporting cell divides to produce two progenitor cells, in order to generate replacement hair cells. This results in a nearly complete structural and functional recovery of the cochlea. Yet, the regenerative response is limited in both time and quantity, so that the number of new cells produced is just enough to replace the number of hair cells lost, as well as replacing the supporting cells utilized in direct transdifferentiation and mitotic proliferation (Stone & Cotanche, 1994; Bhave et al., 1995). Hair cell differentiation through supporting cell transdifferentiation and proliferation occurs either concurrent with or directly after hair cell death and ejection from the sensory epithelium (Corwin et al., 1991; Stone & Cotanche, 1994; Bhave et al., 1995; Stone et al., 1999; Mangiardi et al., 2004; Duncan et al., 2006). Our recent work has focused on demonstrating that the dying hair cells in the gentamicin-treated chick cochlea regulate the onset and progression of supporting cells through both direct transdifferentiation and mitotic proliferation. Evidence for this is seen in the regenerating chick utricle, where blocking hair cell death with apoptotic inhibitors rescues these cells, but also reduces the level of ongoing supporting cell mitoses needed to generate replacement cells (Matsui et al., 2002). Moreover, the number of dying hair cells will specifically regulate the choice of direct transdifferentiation or mitotic proliferation as a means for producing new hair cells (Stone & Cotanche, 1994; Cotanche, 2008). Identifying the genes and proteins involved in the regulation of regeneration will enable us to experimentally induce new hair cell production and potentially harness this process for therapeutic replacement of hair cells in mammals and ultimately in humans suffering from sensorineural hearing loss.
Dual mechanisms for regenerating hair cells
While supporting cell mitotic proliferation is the major source of regenerated hair cells, there is also clear evidence that up to a third of the new hair cells arise through direct transdifferentiation, where supporting cells change their gene expression and differentiate into hair cells without going through mitosis (Roberson et al., 1996; Adler et al., 1997; Baird et al., 2000; Roberson et al., 2004; Morest & Cotanche, 2004; Duncan et al., 2006;Cafaro et al., 2007). While direct transdifferentiation appears to be a rapid and early source of new hair cells (Roberson et al., 2004; Duncan et al., 2006), its overall effectiveness is limited because there is a loss of one supporting cell for each new hair cell made. Thus, there must be a point where supporting cell mitotic proliferation is activated not only to make new hair cells, but also to replace the supporting cells lost to direct transdifferentiation. Ultimately, the regenerating sensory epithelium must produce enough new hair cells and supporting cells to structurally and functionally repopulate the damaged region. Estimates from previous studies (Cotanche et al., 1987) indicate that there are approximately 2000 hair cells in the proximal 30% of the chick BP, the area damaged by a single gentamicin injection (Roberson et al., 2000). At a ratio of 3 to 4 supporting cells per hair cell in the proximal basilar papilla (Goodyear & Richardson, 1997), there should be 6000 to 8000 supporting cells in this region. All 2000 of the hair cells in the proximal 30% of the basilar papilla are lost during gentamicin damage in our model. About one-third (667) of these hair cells are replaced by direct transdifferentiation (Roberson et al., 1996;Roberson et al., 2004). The other two-thirds (1334) are replaced by mitosis. Thus, 667 supporting cells (~8% of the total) should be lost due to direct transdifferentiation, while 1334 supporting cells(~16%) should be involved in undergoing mitosis to produce one new hair cell and one new supporting cell. That means that 4000–6000 of the supporting cells (76%)within the region of hair cell loss should not be directly involved in regeneration. Yet, according to data from Bhave et al. (1995) all the supporting cells in the damaged region (proximal 30%) and beyond down-regulate statin and up-regulate PCNA in response togentamicin treatment. Moreover, our in situ hybridization data for α-tectorin indicate that all of the supporting cells in the basal 20–30% down-regulate expression of α-tectorin by 24h after gentamicin with a recovery seen by 48–72h (Figure 2). So even though only a third of the supporting cells are actively involved in regeneration, all of them within the region of hair cell loss seem to respond, at least initially, to the loss of the hair cells.
Figure 2.
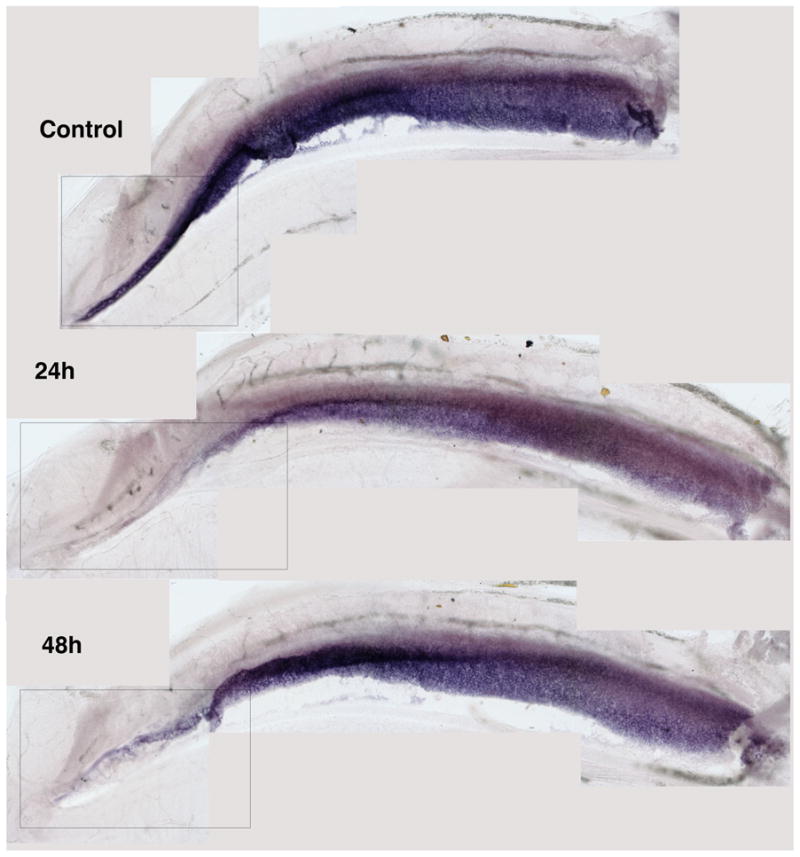
Whole mount in situ hybridization of the chick cochlea forα-Tectorin, a gene expressed in supporting cells for synthesizing proteins needed to maintain the extracellular matrix of the tectorial membrane. This gene is down-regulated in all supporting cells within 24h of a single gentamicin injection, but begins to be re-expressed in some supporting cells by 48h after the gentamicin.
The signals that induce direct transdifferentiation in a subset of the supporting cells as an initial response to hair cell loss remain undefined, as are those that lead to the switch from direct transdifferentiation to mitotic proliferation. However, studies of both the developing mouse cochlea and the regenerating chick cochlea suggest that cell fate pathways, such as the Notch signaling pathway and the Atoh1 signaling pathway, regulate the precursor cells in the sensory epithelium to select either a hair cell or supporting cell fate (Fekete et al., 1998; Bermingham et al, 1999; Lanford et al., 1999; Stone & Rubel, 1999; Kelley, 2002; Woods et al., 2004; Kelley, 2007; Cafaro et al., 2007; Daudet et al., 2009). We hypothesize that very early on in the dying process, hair cells send signals to adjacent supporting cells to activate Atoh1 and initiate hair cell differentiation through direct transdifferentiation. Subsequently, there must be a threshold reached in the number of supporting cells available to commit to direct transdifferentiation that stops this pathway and leads to the activation of the mitotic proliferation pathway instead.
Regeneration as a recapitulation of development
One of the initial hypotheses proposed in our early regeneration studies was that the new hair cells were arising by processes similar to those which occurred during embryonic development in the cochlea (Cotanche, 1987; Corwin & Cotanche, 1988). Indeed, morphological and ultrastructural studies of regeneration and chick cochlear development showed many common events between the two. Moreover, a number of structural similarities were seen between development and regeneration in the chick cochlea and embryonic development in the mouse organ of Corti. These include an initial period of cell proliferation followed by a differentiation of the post-mitotic progenitor cells into hair cells and supporting cells, culminating in a maturation of the stereociliary bundles and establishment of neural connectivity. As tools developed in mouse genomics and data from the human genome project became available during the 1990’s, researchers were able to begin assessing the genetic mechanisms that regulate the development of the mouse cochlear sensory epithelium and the cell fate choices made during the differentiation of hair cells and supporting cells within the organ of Corti (Barald & Kelley, 2004; Kelley, 2007). Again, as with structural development, many common genetic pathways were found between the developing mouse cochlea and the developing and regenerating chick cochlea (Lewis, 1991; Lee & Cotanche, 1995, 1996; Adam et al, 1998; Fekete & Wu, 2002; Daudet & Lewis, 2005, Stone & Rubel, 1999).
However, one major difference identified between mammals and birds was the expression of proliferation inhibitors during organ of Corti development (Chen & Segil, 1999; Lowenheim et al., 1999; Chen et al, 2003; Mantela et al., 2005; Sage et al., 2005, 2006), and this was thought to be responsible for the mature mammalian cochlea’s inability to regenerate. While p27 is expressed in hair cells and supporting cells of the mature avian basilar papilla (Torchinsky et al., 1999), its presence does not seem to be altered in supporting cells during regeneration. Furthermore, the continued presence of p27 does not appear to inhibit the initiation of regeneration. The presence of other proliferation inhibitors in the chick basilar papilla, such as Rb and p19, has not been defined. Genetic manipulation of the mammalian cochlea has shown that when these proliferation inhibitors are knocked down the supporting cells can continue to proliferate beyond their normal embryonic time window, which can lead to the production of excess supporting cells and supernumerary hair cells (Chen & Segil, 1999; Lowenheim et al., 1999; Chen et al., 2003; Mantela et al., 2005; Sage et al., 2005, 2006; Weber et al., 2008). However, this abnormal proliferation of the supporting cells induces a massive wave of apoptosis in both hair cells and supporting cells that leads to a complete disruption of the sensory epithelium and a subsequent profound hearing loss. Thus, future attempts to induce mammalian cochlear regeneration will need to find a way to temporarily turn off the cell proliferation inhibitors and block the signals that result in widespread cell death in the sensory epithelium. Moreover, once supporting cell proliferation is reinitiated, it will be important to induce the new cells to assume a cochlear progenitor fate and differentiate into new hair cells and supporting cells. Evidence from in vitro studies of the embryonic mouse cochlear cells expressing Atoh1 suggest that these cells can also regulate the differentiation of their neighboring cells to produce both new hair cells and supporting cells in a pattern reminiscent of the normal organ of Corti (Woods et al., 2004). Whether this can occur in a mature, damaged sensory epithelium is not yet clear.
Genetic specification of the cochlear sensory epithelium
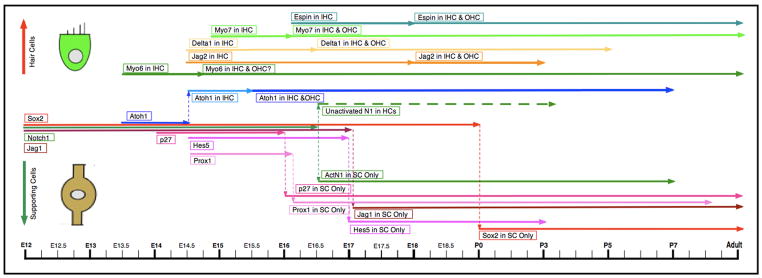
The Notch signaling pathway comprises a complex of genes that have been found to control cell fate determination in developing tissues of all metazoans (Doe & Goodman, 1985; Lewis, 1991; Artavanis-Tsakonas et al., 1999; Louvi & Artavanis-Tsakonas, 2006). Early in otocyst development, the Notch pathway is thought to define the presumptive sensory epithelium through a lateral induction process (Daudet & Lewis, 2005; Kiernan et al., 2006; Daudet et. al., 2007; Hayashi et al., 2008). Once the sensory epithelium is established, the cells undergo a series of Notch-directed lateral inhibition choices to determine which cells will differentiate into hair cells and which will become supporting cells (Figure 3). These decisions are made by the segregating of Delta1, Jagged2/Serrate2, and Atoh1 into presumptive hair cells, while Notch1, Jagged1/Serrate1, and the Hes family of genes are restricted to supporting cells (Kelley, 2006, 2007). At embryonic day 12.5 (E12.5) in the mouse, Notch1, Jagged1, and Sox2 are expressed in all cells within the presumptive cochlear sensory epithelium (Lanford et al., 1999; Woods et al., 2004; Kiernan et al., 2005b; Dabdoub et al., 2008). Most of these signals are initially found in the base of the cochlea and spread to the apex with time. However, p27, an inhibitor of cell proliferation, is expressed in the opposite pattern. It initially appears in the cochlear apex beginning at E12.5 and reaches the base by E14 (Chen & Segil, 1999; Lee et al., 2006). This pattern matches the wave of terminal mitoses in the mouse cochlea originally described by Ruben (1967) and defines a region in the presumptive sensory epithelium termed the Zone of Nonproliferating Cells, or ZNPC (Chen & Segil, 1999). The ZNPC is thought to represent the boundaries of the region specified to become the developing organ of Corti.
Figure 3.
Timeline of gene expression in mammalian cochlear hair cell/supporting cell differentiation. In the timeline, expression of the genes/proteins in all progenitor cells is represented by the lines in the center on the left side of the timeline. The line shifts up when one of these components becomes restricted to hair cells; while for those that become restricted to supporting cells, the line shifts down. The left end of each line represents the time when these genes/proteins are first expressed, while the right end of the line represents the time they stop being expressed. The lines that begin within the hair cell field (top) are for genes/proteins that are only ever expressed in hair cells, and not in progenitor cells. Lines that reach the left and right edges of the timeline represent genes/proteins that have begun their expression before E12, or continue to be expressed in the adult cochlea, respectively.
Genetic expression patterns during mammalian embryonic hair cell differentiation
Atoh1 (the gene formerly known as Math1) has been identified as the earliest hair-cell-specific gene required for definitive hair cell development, because the loss of Atoh1 results in the failure of any hair cell differentiation in the mouse cochlea (Bermingham et al., 1999). Although the deletion of Sox2 or Eya1 also leads to a lack of hair cell differentiation, this is thought to involve a loss of the progenitor cell population in general and not a specific block in hair cell differentiation (Kiernan et al., 2005a; Kalatzis et al., 1998; Zou et al., 2008). At E13.5, Atoh1 mRNA is broadly expressed in cells throughout the presumptive sensory epithelium, as determined by in situ hybridization (Lanford et al., 2000; Woods et al., 2004; Matei et al., 2005). These Atoh1-positive cells are the progenitor cells that will subsequently differentiate further into presumptive hair cells and supporting cells. Interestingly, a single row of cells within the Atoh1-positive progenitor cells labels with antibodies to myosin VI at this time (Montcouquiol & Kelley, 2003). These myosin VI-positive cells will give rise to the single row of inner hair cells in the organ of Corti. One day later, at E14.5, the presumptive inner hair cells begin to express higher levels of Atoh1 than their neighbors, as determined by Atoh1-GFP reporter expression(Bermingham-McDonogh et al., 2006). In addition, these same cells begin to expressDelta1 and Jagged2 mRNA (Morrison et al., 1999; Lanford et al., 1999; Hartman et al., 2007). AtE15.5the three rows of OHC can be identified with the Atoh1-GFP reporter mouse(Bermingham-McDonogh et al., 2006)and by E17 only the four rows of hair cells express Atoh1 mRNA (Lanford et al., 2000). AtE16.5-E18Delta1 and Jagged2 mRNA can be localized withinthe three rows of OHC, as well (Morrison et al., 1999; Lanford et al., 1999; Murata et al., 2006; Kelley, 2007).
There appears to be a temporal discepancy in the localization of Atoh1 mRNA between the in situ hybridization experiments and those using the Atoh1-GFP reporter mouse. It has been suggested that the promoter used in the Atoh1-GFP mouse enables the Atoh1 protein to act on its own promoter, therefore enhancing the GFP signals in cells where it is most active (Kelley, personal communication). Therefore, the signal would be stronger in the presumptive hair cells at a time when both the presumptive hair cells and presumptive supporting cells still express the Atoh1 mRNA. This is suggested in the results presented above, but a careful comparative study of mRNA expression and Atoh1-GFP expression during the key E13.5 to E17 time window is lacking.
Currently, it is not known if myosin VI labels the differentiating outer hair cells before they can be identified by Atoh1-GFP atE15.5. A second hair cell-specific protein, myosin VIIa, appears in the presumptive inner hair cells of the cochlea by E15.5, coincident with the localization of Delta1 mRNA to these cells (Chen et al., 2002). Myosin VIIa protein is localized to the three rows of outer hair cells by E16, 12 hours after Atoh1-GFP and 12 hours before Delta1 mRNA are localized to these same cells (Chen et al., 2002;Bermingham-McDonogh et al., 2006; Morrison et al., 1999).
Thus, myosin VI appears to be the earliest overt marker to appear in the subset of cells that will become hair cells within the broaderband of Atoh1 mRNA-positive cells of the developing organ of Corti. Delta1 and Jagged2 mRNA, and myosin VIIa protein exhibit a similarlyrestricted expression in hair cellstwo to three days later. Interestingly, while both myosin VI and myosin VIIa are expressed very early in hair cell specification, neither are required for hair cell differentiation, as mutant mice lacking either of the two genes develop normally throughout embryogenesis and the hair cells only begin to fall apart during postnatal maturation (Self et al., 1998, 1999). However, a double knockout for both myosin VI and VIIa may show that hair cell differentiation cannot occur when both are missing. Thus, even though myosin VI and myosin VIIa expression do not appear to be critical to early hair cell differentiation, they do specifically identify those cells that have already committed to a hair cell fate even earlier than can be detected with Atoh1 mRNA or Atoh1-GFP expression. Either we have not yet been able to detect the changes in Atoh1 mRNA or protein levels that signal the discrete beginning of hair cell differentiation in these cells, or there is someas-yet-unidentified gene that isactivated in the presumptive hair cells before Atoh1 levels are increasedwhich also induces myosin VI expression. This protein could then play a role in localizing and reinforcing the expression of Atoh1 within these presumptive hair cells. This would then be coordinated with a turning off of Atoh1 expression in adjacent progenitor cells and an activation of signals specific for determining supporting cell fate. There is some evidence that Eya1 may play such a role (Zou et al., 2008).
At E16-E17, activated Notch1, Jagged1, p27, Prox1, and Hes5 expression are localized to supporting cells, and Sox2 expression is much stronger in supporting cells than in hair cells (Lanford et al., 2000; Kiernan et al., 2005a; Murata et al., 2006; Bermingham-McDonogh et al., 2006; Kelley, 2007; Dabdoub et al., 2008). Sox2 disappears completely from hair cells by P0, but is retained in supporting cells throughout maturity (Oesterle et al., 2008; Dabdoub et al., 2008). The Notch pathway genes are thought to function through lateral inhibition to prevent adjacent progenitor cells from differentiating as hair cells during cochlear development. These Notch interactions do not appear to have any role in stimulating the differentiation of hair cells, because selective knockouts of Delta1 and Jagged2 result in excessive hair cell differentiation at the expense of supporting cells, rather than eliminating the differentiation of hair cells (Kiernan et al., 2005b; Brooker et al., 2006). This second wave of Notch pathway genes appears to be involved in preventing neighboring cells from acquiring a hair cell fate once the original four rows of hair cells have differentiated. Moreover, altering the Notch pathway does not seem to be able to induce hair cell specification in undamaged mature avian basilar papillae, or in damaged mammalian organs of Corti (Daudet et al., 2009; Batts et al., 2009).
Between postnatal day 0 (P0) and P3 the hair cell fate genes Atoh1, Delta1, and Jagged2 are selectively down-regulated in the developing hair cells and by P7 are no longer detected in the organ of Corti (Lanford et al., 2000; Murata et al., 2006). Meanwhile, activated Notch1 and Hes5 are down-regulated in the differentiated supporting cells, while expression of Sox2, p27, and Jagged1 are retained through maturity (Chen & Segil, 1999; Lanford et al., 2000; Oesterle et al., 2008). Once the key Notch pathway genes have performed their developmental roles in establishing the sensory epithelium and defining the correct mosaic of hair cells and supporting cells, these genes are no longer required to maintain cell identity in the mature organ of Corti and are down-regulated. Therefore, these developmentalcell fate genes are only needed during the initial stages of development in the cochlear sensory epithelium and appear to function as genetic “training wheels”. Once the cells have established their identities as hair cells or supporting cells, they can maintain these cell fates throughout the remainder of their lives without the active functioning of the Notch lateral inhibition pathway genes or the Atoh1/Hes family of genes.
The genes that continue to be expressed in mature supporting cells only, i.e., Sox2, p27, Notch1, and Jagged1, are initially found in all cells of the prosensory epithelium and become restricted to supporting cells only late in development, well after the hair cells have differentiated. This corroborates the hypothesis that supporting cells are the direct descendants of the multipotent progenitor cells in the sensory epithelium and, therefore, they may be good targets for initiating a regenerative capacity in the mature mammalian organ of Corti. In addition, since Notch1, Sox2, p27, and Jagged1 are retained in the presumptive hair cells as they undergo differentiation and are only lost from the sensory cells later in development, it suggests that continued expression of these particulargenes at the levels present in hair cells from E13.5 through P0 does not actively interfere with hair cell differentiation during development. It is possible that the activity of the proteins for these genes is diminished or inactivated during this period, even though their mRNAs are still present. However, overexpression of these genes in experimental situations may lead to levels of their respective proteins high enough to disrupt hair cell differentiation (Dabdoub et al., 2008).
Gene expression patterns during avian embryonic hair cell differentiation
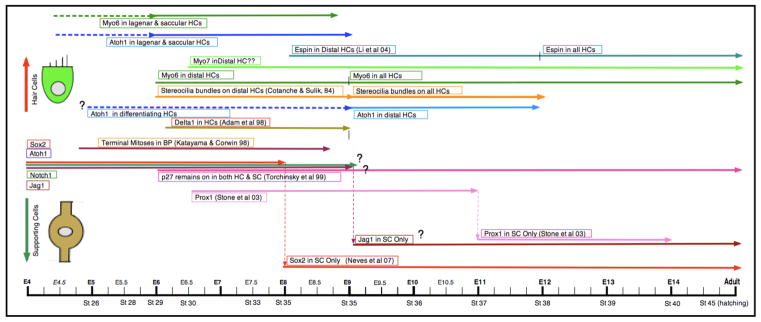
While most of the research on the Notch signaling pathway has been carried out in the embryonic mouse organ of Corti, a few parallel studies have been performed in the developing and regenerating avian cochlea (Figure 4). The benefit of the avian system in these experiments is that we can explore how closely the developmental pathways match those in the mouse and, in addition, how they are recapitulated during regeneration in the mature cochlea. In development, Notch1 is expressed as early as E1.5-E2 in the otic placode and is present in all otocyst cells by E5 (Adam et al., 1998). Jagged1/Serrate1 is localized to the ventral surface of the otocyst by E3.5 and in the prosensory epithelium by E5. Notch1 and Jagged1/Serrate1 expression at these early times is thought to function in the lateral induction of the sensory epithelia (Daudet & Lewis, 2005; Daudet et al., 2007). Sox2 is localized to the prosensory epithelium by E3-E4 and is found in all cells of the developing basilar papilla by E5, but by E8 it is already restricted to the supporting cells (Neves et al., 2007). Atoh1 mRNA appears in the anterior and posterior semicircular canals by E3 and is strongly expressed in a subset of these cells by E4 (Pujades et al., 2006). In our preliminary studies, we see strong expression of Atoh1 protein in the lagena and saccule as early as E6 and faint label in the cells of the basilar papilla by E9. Since hair cells begin differentiating in the distal basilar papilla by E6 and reach the proximal region by E9, Atoh1 expression in the presumprive hair cells would be expected to precede this time window. Currently, we are working on refining the expression patterns of Atoh1 in the developing chick basilar papilla between E5 and E9. Delta1 appears in the prosensory epithelium by E5, is seen throughout the differentiating hair cells in the basilar papilla by E6-E8, but is only transiently expressed and is gone from the developing sensory epithelia by E9 (Adam et al., 1998). In our preliminary studies, we see clear labeling for myosin VI protein in the distal basilar papilla at E6 and throughout the entire sensory epithelium by E9. This early evidence parallels our previous SEM studies which show that the first hair cells are identifiable by E6 in the distal end and throughout the basilar papilla by E9 (Cotanche & Sulik, 1984). Espin, a critical component of the developing stereociliary bundle, is seen in the distal hair cells by E8 and throughout all the hair cells by E12 (Li et al., 2004; Sekerková et al., 2006). Unfortunately, there is not yet enough data for the embryonic chick basilar papilla on the expression of many of the genes or proteins that have been defined in detail for the embryonic mouse cochlea. In general, the data that have been collected so far suggest that the genetic program forgenerating hair cells and supporting cells in the avian inner ear are similar to those utilized in the developing mouse cochlea. However, further detailed studies are needed to make more accurate comparisons between the two systems.
Figure 4.
Timeline of gene expression in avian cochlear hair cell/supporting cell development. In the timeline, expression of the genes/proteins in differentiating hair cells is represented by the lines beginning on the left side of the timeline. Data based on published results include the reference in the line descriptor. Data without references are based on preliminary studies in our lab.
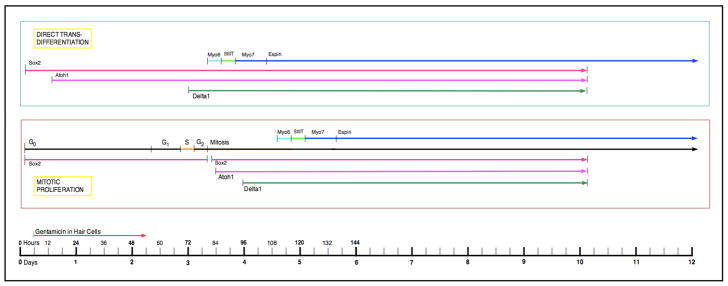
Genetic regulation of avian cochlear regeneration
The mature chick cochlea exhibits continued expression of Notch1 and Jagged1/Serrate1 in the supporting cells of the basilar papilla (Stone & Rubel, 1999; Daudet et al., 2009). During regeneration induced by gentamicin, Atoh1 protein appears in the supporting cells very early, within 9–15h after the gentamicin injection (Cafaro et al., 2007; Chapman et al., 2008). By 3–4 days, Delta1 mRNA is upregulated in the region of damage (Stone & Rubel, 1999; Daudet et al., 2009). Myosin VI first appears in newhair cells generated by direct transdifferentiation at 78h and myosin VIIa appears at 90h (Duncan et al., 2006). By 10D, both Atoh1 and Delta1 expression has been down-regulated in the new hair cells (Stone & Rubel, 1999; Chapman et al., 2009). Thus, at least at the level of gene expression, the signaling pathways utilized in embryonic development are re-employed during the differentiation of hair cells and supporting cells during regeneration.
Summary and Conclusions
Studies of the gene pathways and expression patterns in the development and regeneration of hair cells and supporting cells in the mammalian and avian cochlea have demonstrated a number of proteins involved in the Notch lateral inhibition pathway and the Atoh1/Hes family of genes. It will be important to understand the normal cascade of these signals in the developing cochlea that give rise to cochlear progenitor cells and then guide their subsequent decisions to become either hair cells or supporting cells. While many of the individual genes involved in these decisions have been identified from studies of mutant and knockout mouse cochleae, the interplay of all these signals into a single systemic program that directs this process needs to be explored more fully. We need to know not only what genes are involved, but understand how their gene products interact with one another in a structural and temporal framework to guide hair cell and supporting cell differentiation and maturation.
Currently, the major obstacle to inducing regeneration in the mammalian cochlea seems to be the expression of several proliferation inhibitors during the later stages of cochlear development. Initial genetic experiments leading to the elimination or conditional knockout of these inhibitors have demonstrated renewed or continued proliferation within the sensory epithelium. However, this has not yet led to the production of new hair cells and often causes rampant apoptosis throughout the sensory epitheilum and the death of all the hair cells and supporting cells. A greater understanding of the genetic components in progenitor cell proliferation and hair cell and supporting cell differentiation will be critical to our ability to regulate the regeneration induced by controlled down-regulation of specific proliferation inhibition signals in the mammalian cochlea.
Figure 5.
Timeline of gene expression in avian cochlear hair cell/supporting cell regeneration. In the timeline, expression of the genes/proteins in regenerating hair cells produced by both direct transdifferentiation and mitotic proliferation are represented by the lines beginning on the left side of the timeline.
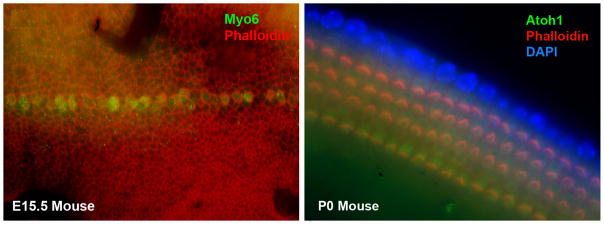
Figure 6.
Expression of myosin VI protein in early differentiation of inner hair cells in the mouse embryonic cochlea at E15 and of Atoh1 expression in the differentiating hair cells in the mammal cochlea at postnatal day 0.
Acknowledgments
Many thanks to the members of the Cotanche laboratory who have carried out much of the original work described in this paper. Our appreciation also to all our colleagues who have made numerous outstanding scientific advances in this area. Research from our laboratory has been graciously supported by NIH/NIDCD, The National Organization for Hearing Research Foundation, The Deafness Research Foundation, The American Hearing Research Foundation, The Kramer/Dosberg Foundation, The Sarah Fuller Fund, The Patterson Trust, and The Caroline Bass Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ developement. Dev. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Adler H, Komeda M, Raphael Y. Further evidence for supporting cell conversion in the damaged avian basilar papilla. Int J Dev Neurosci. 1997;15:375–385. doi: 10.1016/s0736-5748(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;2284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Baird R, Burton M, Fashena D, Naeger R. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci USA. 2000;97:11722–11729. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Dev. 2004;131:4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Batts SA, Shoemaker CR, Raphael Y. Notch signaling and Hes labeling in the normal and drug-damaged organ of Corti. Hear Res. 2009;249:15–22. doi: 10.1016/j.heares.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. J Comp Neurol. 2006;496:172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave SA, Stone JS, Rubel EW, Coltrera MD. Cell cycle progression in gentamicin-damaged avian cochleas. J Neurosci. 1995;15:4618–4628. doi: 10.1523/JNEUROSCI.15-06-04618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Dev. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors as well as differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Chapman BJ, Kaiser CL, Cotanche DA. Characterization of Math1 positive cells in the regenerating avian cochlea: Creating a timeline for Math1 upregulation after gentamicin treatment in vivo. Assoc Res Otolaryngol Abstr. 2008;31:189. [Google Scholar]
- Chapman BJ, Cotanche DA, Kaiser CL. Expression of Math1 positive cells during mitotic proliferation in the regenerating chick cochlea. Assoc Res Otolaryngol Abstr. 2009;32:728. [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Dev. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Dev. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel M, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Jones JE, Katayama A, Kelley MW, Warchol ME. Hair cell regeneration: The identities of progenitor cells, potential triggers and instructive cues. Regeneration of Vertebrate Sensory Cells. In: Bock GR, Whelan J, editors. Ciba Foundation Symposium. Vol. 160. John Wiley & Sons; New York: 1991. pp. 103–130. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Saunders JC, Tilney LG. Hair cell damage produced by acoustic trauma in the chick cochlea. Hearing Res. 1987;25:267–286. doi: 10.1016/0378-5955(87)90098-0. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30:181–196. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Genetic and pharmacological intervention for treatment/prevention of hearing loss. J Commun Disord. 2008;41:421–43. doi: 10.1016/j.jcomdis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA, Sulik KK. The development of stereociliary bundles in the cochlear duct of chick embryos. Dev Brain Res. 1984;16:181–193. doi: 10.1016/0165-3806(84)90024-5. [DOI] [PubMed] [Google Scholar]
- Cruz RM, Lambert PM, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones J, Fritsch B, Cheah K, Pevny L, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Dev. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Ariza-McNaughton L, Lewis J. Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Dev. 2007;134:2369–2378. doi: 10.1242/dev.001842. [DOI] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ, Goodman CS. Early events in insect neurogenesis. II. The role of cell interactions and cell lineage in the determination of neuronal precursor cells. Dev Biol. 1985;111:206–219. doi: 10.1016/0012-1606(85)90446-4. [DOI] [PubMed] [Google Scholar]
- Duncan LJ, Anderson JK, Williamson KE, Mangiardi DA, Matsui JI, Cotanche DA. Pattern of myosin expression during hair cell death and regeneration in the chick cochlea. J Comp Neurol. 2006;499:691–701. doi: 10.1002/cne.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete D, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Gleich O, Dooling RJ, Presson JC. Evidence for supporting cell proliferation and hair cell differentiation in the basilar papilla of adult Belgian Waterslager canaries Serinus canarius. J Comp Neurol. 1997;377:5–14. doi: 10.1002/(sici)1096-9861(19970106)377:1<5::aid-cne2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Goodyear R, Richardson G. Pattern formation in the basilar papilla: evidence for cell rearrangement. J Neurosci. 1997;17:6289–6301. doi: 10.1523/JNEUROSCI.17-16-06289.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B, Hayashi T, Nelson B, Bermingham-McDonogh O, Reh T. Dll3 is expressed in developing hair cells in the mammalian cochlea. Developmental Dynamics. 2007;236:2875–2883. doi: 10.1002/dvdy.21307. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kokubo H, Hartman B, Ray C, Reh T, Bermingham-McDonogh O. Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Developmental Biology. 2008;316:87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. How hearing happens. Neuron. 1997;19:947–950. doi: 10.1016/s0896-6273(00)80385-2. [DOI] [PubMed] [Google Scholar]
- Kalatzis V, Sahly I, El-Amraoui A, Petit C. Eya1 expression in the developing ear and kidney: Towards the understanding of the pathogenesis of branchio-oto-renal (BOR) syndrome. Developmental Dynamics. 1998;213:486–499. doi: 10.1002/(SICI)1097-0177(199812)213:4<486::AID-AJA13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Determination and commitment of mechanosensory hair cells. ScientificWorldJournal. 2002;2:1079–1094. doi: 10.1100/tsw.2002.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Hair cell development: commitment through differentiation. Brain Res. 2006;109:172–185. doi: 10.1016/j.brainres.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Kelley MW. Cellular commitment and differentiation in the organ of Corti. Int J Dev Neurosci. 2007;51:571–583. doi: 10.1387/ijdb.072388mk. [DOI] [PubMed] [Google Scholar]
- Kiernan A, Pelling A, Leung K, Tang A, Bell D, Tease C, Lovell-Badge R, Steel K, Cheah K. Sox2 is required for sensory organ development in the mammalan inner ear. Nature. 2005a;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan A, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005b;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:27–38. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford P, Shailam R, Norton C, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J Assoc Res Otolaryngol. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Cotanche DA. Detection of β-actin mRNA by RT-PCR in normal and regenerating chicken cochleae. Hear Res. 1995;87:9–15. doi: 10.1016/0378-5955(95)00072-c. [DOI] [PubMed] [Google Scholar]
- Lee KH, Cotanche DA. Potential role of bFGF and retinoic acid in the regeneration of chicken cochlear hair cells. Hear Res. 1996;94:1–13. doi: 10.1016/0378-5955(95)00220-0. [DOI] [PubMed] [Google Scholar]
- Lee Y-S, Liu F, Segil N. A morphogenetic wave of p27kip1 transcription directs cell cycle exit during organ of Corti development. Dev. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Lewis J. Rules for the production of sensory cells. Ciba Foundation Symposia. 1991;160:25–39. [PubMed] [Google Scholar]
- Li H, Liu H, Balt S, Mann S, Corrales CE, Heller S. Correlation of expression of the actin filament-bundling protein espin with stereociliary bundle formation in the developing inner ear. J Comp Neurol. 2004;468:125–134. doi: 10.1002/cne.10944. [DOI] [PubMed] [Google Scholar]
- Lippe WR, Westbrook EW, Ryals BM. Hair cell regeneration in the chicken cochlea following aminoglycoside toxicity. Hear Res. 1991;56:203–210. doi: 10.1016/0378-5955(91)90171-5. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signallling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackeny CM, Zenner HP. Gene disruption of p27 (Kip1) allows cell proliferation in the postnatal and adult organ of cort. Proc Natl Acad Sci USA. 1999;30:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiardi DA, Williamson KM, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004;475:1–18. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- Mantela J, Jiang Z, Ylikoski J, Fritzsch B, Zacksenhaus E, Pirvola U. The retinoblastoma gene pathway regulates the postmitotic state of hair cells of the mouse inner ear. Development. 2005;132:2377–2388. doi: 10.1242/dev.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Developmental Dynamics. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui J, Ogilvie J, Warchol M. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M, Kelley MW. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J Neurosci. 2003;23:9469–9478. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morest DK, Cotanche DA. Regeneration of the inner ear as a model of neural plasticity. J Neurosci Res. 2004;78:455–460. doi: 10.1002/jnr.20283. [DOI] [PubMed] [Google Scholar]
- Morrison A, Hodgetts C, Gossler A, Hrabe de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Murata J, Tokunaga A, Okano H, Kubo T. Mapping of Notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. J Comp Neurol. 2006;497:502–518. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neural and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor R, Forge A, Hume C. Sox2 and Jagged1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol. 2008;9:65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujades C, Kamaid A, Alsina B, Giraldez F. BMP-signaling regulates the generation of hair cells. Dev Biol. 2006;292:55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdiffer-entiation gives rise to new hair cells in regenerating avian auditory epithelium. Aud Neurosci. 1996;2:195–205. [Google Scholar]
- Roberson DW, Alosi JA, Messana EP, Nedder AP, Cotanche DA. Effect of violation of the labyrinth on the sensory epithelium in the chick cochlea. Hear Res. 2000;141:155–164. doi: 10.1016/s0378-5955(99)00218-x. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse. A radioautographic study of terminal mitosis. Acta Otolaryngol Suppl. 1967;220:1–44. [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang D-S, Garcia-Añoveros J, Hinds PW, Corwin JT, Corey DP, Chen Z-Y. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Vollrath MA, Brown MC, Hinds PW, Corey DP, Vetter DE, Chen ZY. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. PNAS. 2006;103:7345–7350. doi: 10.1073/pnas.0510631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerková G, Zheng L, Mugnaini E, Bartles J. Differential expression of espin isoforms during epithelial morphogenesis, stereociliogenesis and postnatal maturation in the developing inner ear. Dev Biol. 2006;291:83–95. doi: 10.1016/j.ydbio.2005.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self T, Mahony M, Fleming J, Walsh J, Brown SDM, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Dev. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- Self T, Sobe T, Copeland NG, Jenkins NA, Avraham KB, Steel KP. Role of myosin VI in the differentiation of cochlear hair cells. Dev Biol. 1999;214:331–341. doi: 10.1006/dbio.1999.9424. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J Comp Neurol. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Neurosci. 2007;51:633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Dev. 1999;126:961–973. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Stone JS, Choi Y-S, Woolley SMN, Yamashita H, Rubel EW. Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. J Neurocytol. 1999;28:863–876. doi: 10.1023/a:1007022205821. [DOI] [PubMed] [Google Scholar]
- Torchinsky C, Messana EP, Arsura M, Cotanche DA. Regulation of p27Kip1 during gentamicin mediated hair cell death. J Neurocytol. 1999;28:913–924. doi: 10.1023/a:1007082424477. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996;16:5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Corbett MK, Chow LM, Valentine MB, Baker SJ, Zuo J. Rapid cell-cyclereentry and cell death after acute inactivation of the retinoblastoma gene product inpostnatal cochlear hair cells. PNAS. 2008;105:781–785. doi: 10.1073/pnas.0708061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim E-H, Jin D, Fritzsch B, Xu P-X. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–3356. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]