Abstract
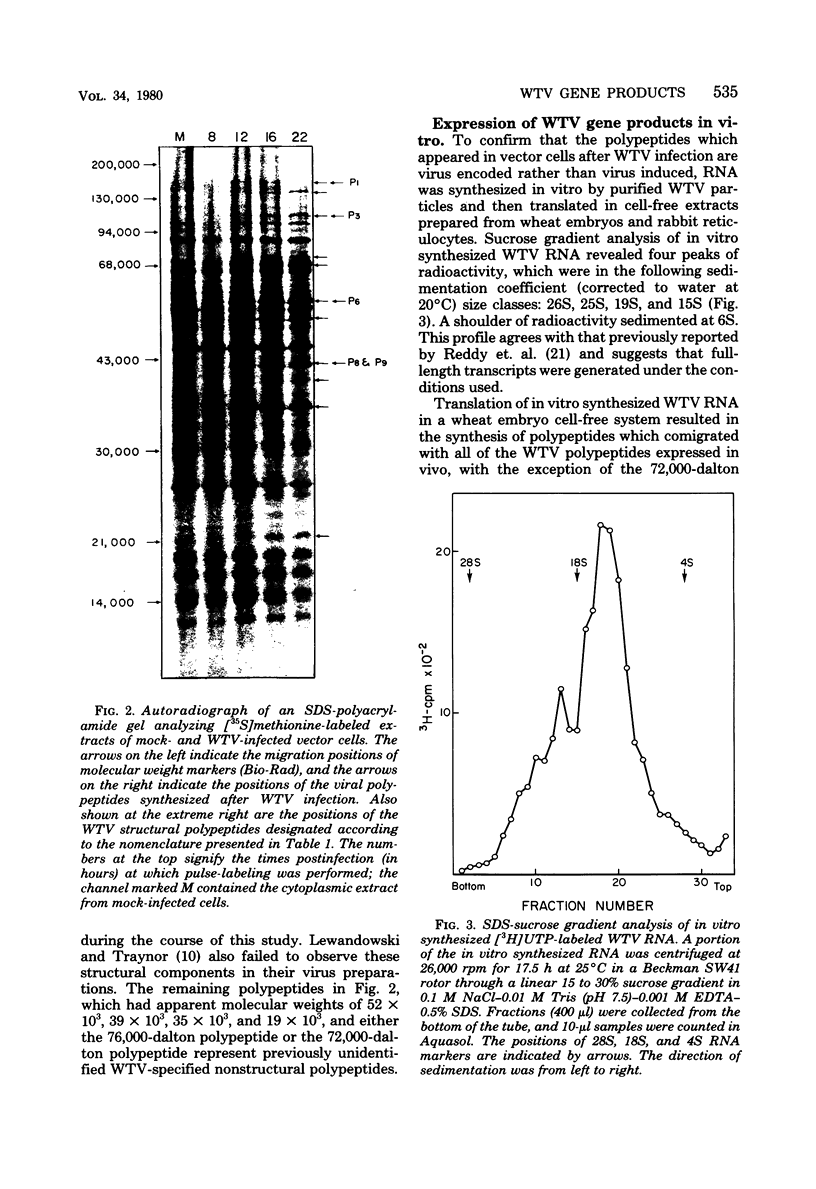
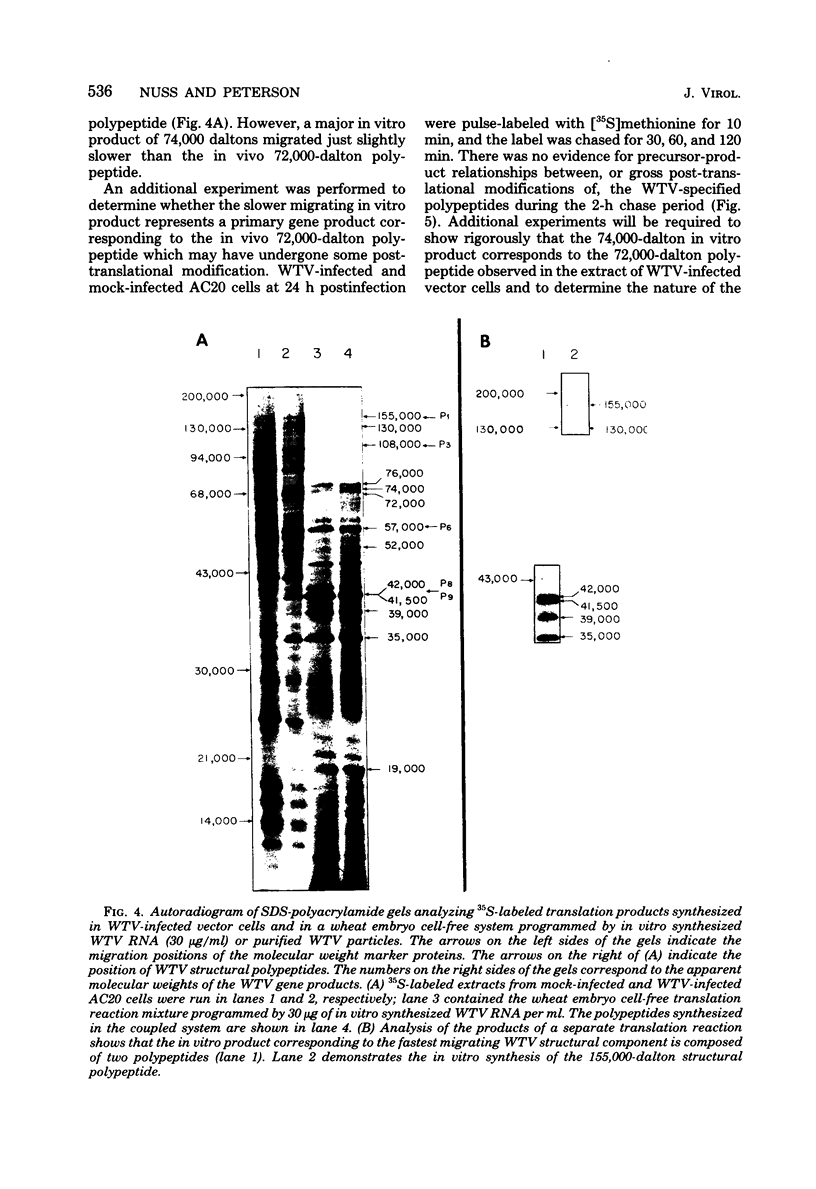
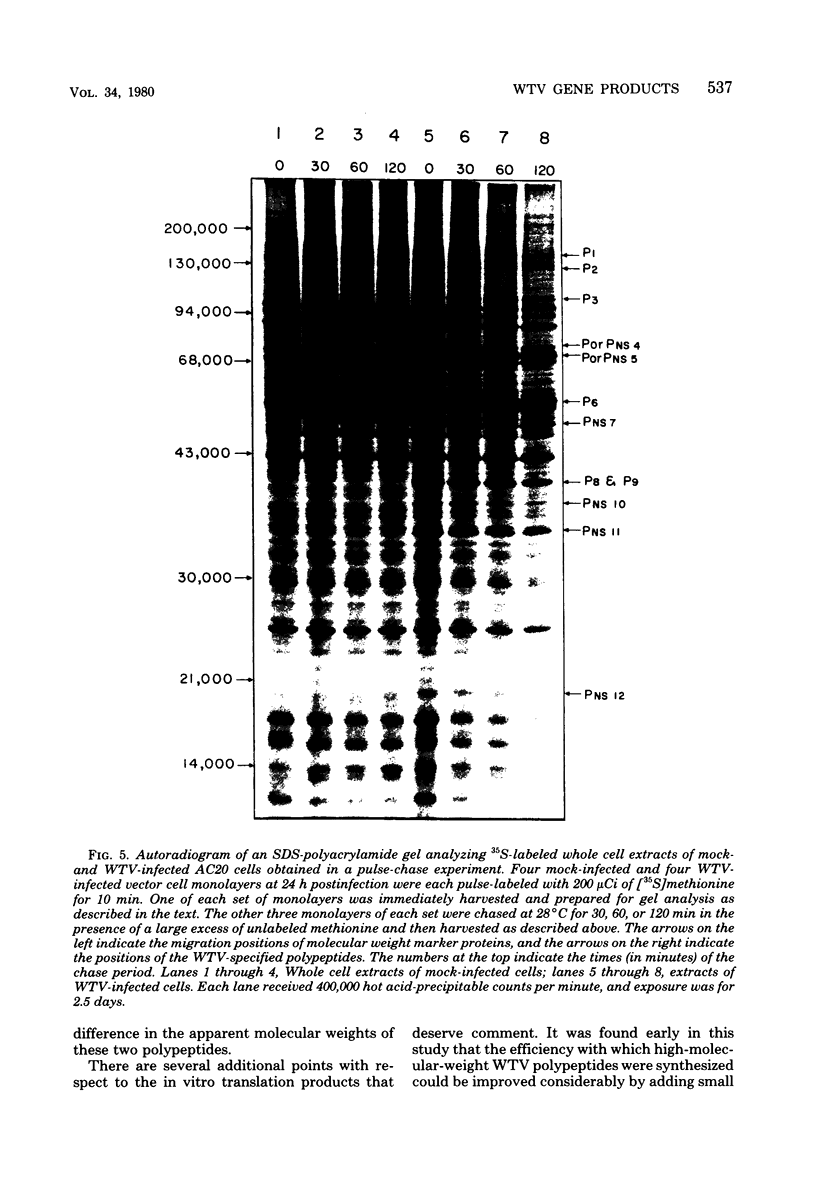
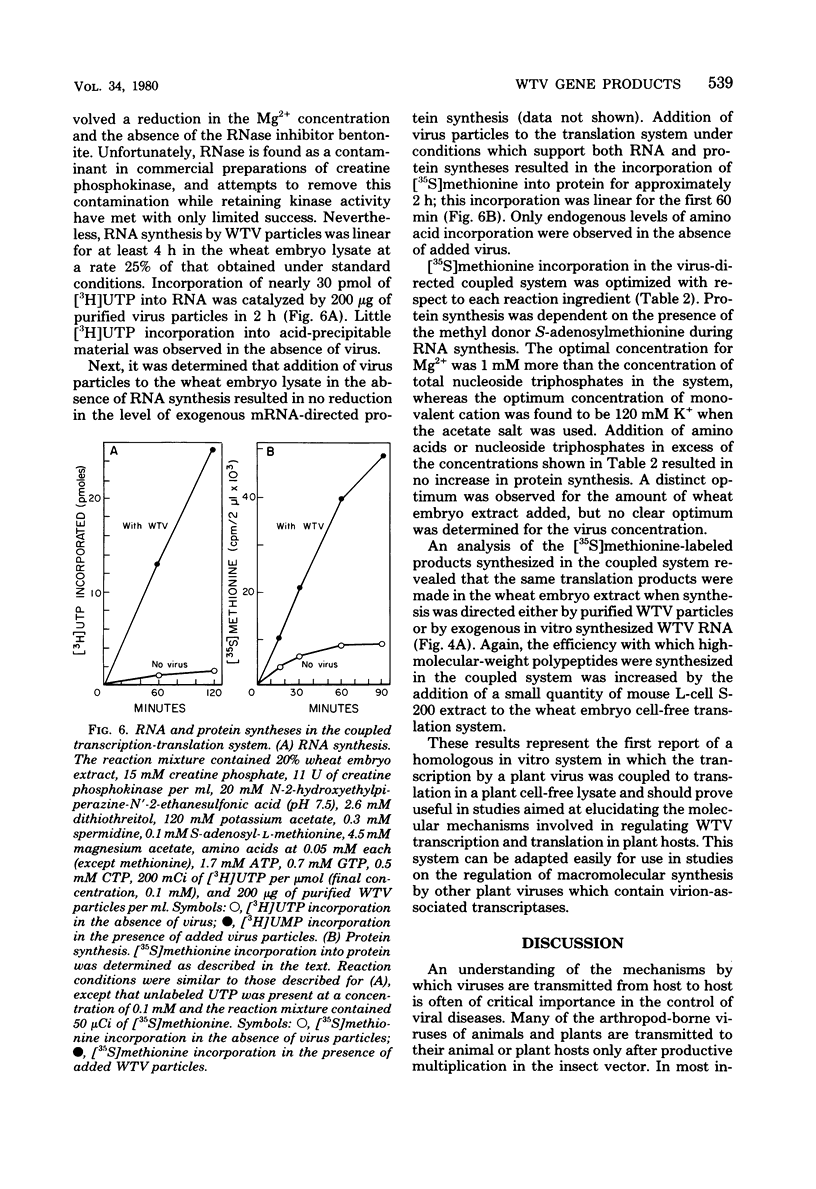
Infection of Agallia constricta vector cell monolayers with wound tumor virus results in the synthesis of 12 virus-specific polypeptides. Confirmation that these polypeptides are virus encoded rather than virus induced was obtained by cell-free translation of in vitro synthesized viral mRNA. In addition, transcription by purified wound tumor virus particles was coupled with translation of the resulting transcripts in a wheat embryo cell-free extract. Six previously described structural polypeptides, one presumptive structural polypeptide, and five previously unidentified nonstructural polypeptides were synthesized in infected vector cell monolayers, in cell-free extracts directed by in vitro synthesized viral mRNA, and in the homologous plant cell-free system, in which viral transcription was coupled with translation. Pulse-chase experiments revealed no evidence of precursor-product relationships for the wound tumor virus-specific polypeptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black D. R., Knight C. A. Ribonucleic acid transcriptase acitvity in purified wound tumor virus. J Virol. 1970 Aug;6(2):194–198. doi: 10.1128/jvi.6.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart W., Nuss D. L., Paoletti E. Effect of UV irradiation on the expression of vaccinia virus gene products synthesized in a cell-free system coupling transcription and translation. J Virol. 1978 Jun;26(3):673–680. doi: 10.1128/jvi.26.3.673-680.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart W., Paoletti E., Nuss D. L. Cell-free translation of purified virion-associated high-molecular-weight RNA synthesized in vitro by vaccinia virus. J Virol. 1978 Dec;28(3):905–916. doi: 10.1128/jvi.28.3.905-916.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Chiu R. J., Black L. M. Assay of wound tumor virus by the fluorescent cell counting technique. Virology. 1969 Apr;37(4):667–677. doi: 10.1016/0042-6822(69)90285-2. [DOI] [PubMed] [Google Scholar]
- Chiu R. J., Black L. M. Monolayer cultures of insect cell lines and their inoculation with a plant virus. Nature. 1967 Sep 2;215(5105):1076–1078. doi: 10.1038/2151076a0. [DOI] [PubMed] [Google Scholar]
- Hsu H. T., McBeath J. H., Black L. M. The comparative susceptibilities of cultured vector and nonvector leafhopper cells to three plant viruses. Virology. 1977 Sep;81(2):257–262. doi: 10.1016/0042-6822(77)90142-8. [DOI] [PubMed] [Google Scholar]
- Kimura I., Black L. M. Some factors affecting infectivity assays of wound-tumor virus on cell monolayers from an insect vector. Virology. 1971 Nov;46(2):266–276. doi: 10.1016/0042-6822(71)90029-8. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Traynor B. L. Comparison of the structure and polypeptide composition of three double-stranded ribonucleic acid-containing viruses (diplornaviruses): cytoplasmic polyhedrosis virus, wound tumor virus, and reovirus. J Virol. 1972 Nov;10(5):1053–1070. doi: 10.1128/jvi.10.5.1053-1070.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Herbst E. J. Stimulation of in vitro transcription of T4 DNA by the polyamine spermidine. Arch Biochem Biophys. 1975 Aug;169(2):513–521. doi: 10.1016/0003-9861(75)90194-0. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Deletion mutations of the genome segments of wound tumor virus. Virology. 1974 Oct;61(2):458–473. doi: 10.1016/0042-6822(74)90282-7. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Electrophoretic separation of all components of the double-stranded RNA of wound tumor virus. Virology. 1973 Aug;54(2):557–562. doi: 10.1016/0042-6822(73)90168-2. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Estimate of absolute specific infectivity of wound tumor virus purified with polyethylene glycol. Virology. 1973 Jul;54(1):150–159. doi: 10.1016/0042-6822(73)90124-4. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Increase of wound tumor virus in leafhoppers as assayed on vector cell monolayers. Virology. 1972 Nov;50(2):412–421. doi: 10.1016/0042-6822(72)90393-5. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Isolation and replication of mutant populations of wound tumor virions lacking certain genome segments. Virology. 1977 Jul 15;80(2):336–346. doi: 10.1016/s0042-6822(77)80009-3. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., MacLeod R. Polypeptide components of wound tumor virus. Virology. 1976 Apr;70(2):274–282. doi: 10.1016/0042-6822(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Rhodes D. P., Lesnaw J. A., Macleod R., Banerjee A. K., Black L. M. In vitro transcription of wound tumor virus RNA by virion-associated RNA transcriptase. Virology. 1977 Jul 15;80(2):356–361. doi: 10.1016/s0042-6822(77)80011-1. [DOI] [PubMed] [Google Scholar]
- Rhodes D. P., Reddy D. V., MacLeod R., Black L. M., Banerjee A. K. In vitro synthesis of RNA containing 5'-terminal structure 7nG(5')ppp(5')Apm... by purified wound tumor virus. Virology. 1977 Feb;76(2):554–559. doi: 10.1016/0042-6822(77)90237-9. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M., Sperling R. The cell-free synthesis and assembly of viral specific polypeptides into TMV particles. Virology. 1974 May;59(1):307–313. doi: 10.1016/0042-6822(74)90227-x. [DOI] [PubMed] [Google Scholar]