Abstract
Rationale
3α-OH-5α[β]-pregnan-20-one (THP) is a positive modulator of the GABAA receptor (GABAR), which underlies its reported anxiolytic effect. However, there are conditions such as premenstrual dysphoric disorder (PMDD) where increases in THP levels can be associated with adverse mood.
Objectives
In order to test for conditions where THP might be anxiogenic, we developed a mouse model of THP withdrawal. Because δ-containing GABAR are highly sensitive to THP modulation, results were compared in wild-type and δ knockout mice.
Methods
Finasteride, a 5α-reductase blocker, was administered for 3 days to female wild-type or δ knockout mice. Then, animals were tested in the elevated plus maze, following acute administration of THP, lorazepam, flumazenil, or 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP), and results compared to vehicle-injected controls. CA1 hippocampal GABAR α4 subunit levels were assessed by Western blot.
Results
After THP withdrawal, THP produced anxiogenic effects, decreasing open arm entries on the elevated plus maze, following a brief shock, in contrast to its expected anxiolytic effects. As we have shown in rats, THP withdrawal also resulted in increased expression of the α4 subunit in mouse CA1 hippocampus. As expected for increases in α4-containing GABAR, THP withdrawn mice were relatively insensitive to the benzodiazepine (BDZ) lorazepam and had atypical responses to the BDZ antagonist flumazenil when tested on the plus maze. In contrast, they showed a greater anxiolytic response to THIP, which has greater efficacy at α4βδ than other GABAR. Although THP withdrawal in δ knockout mice also increased the α4 GABAR subunit, the anxiogenic effects of THP and the anxiolytic effects of THIP were not observed, implicating α4βδ GABAR in these effects.
Conclusions
Based on these behavioral and pharmacological findings, we suggest that THP withdrawal in the mouse may serve as a rodent model of PMDD.
Keywords: PMDD, Alpha-4, Delta, GABAA receptor, THIP, Allopregnanolone, Pregnanolone, Mouse, Elevated plus maze, Neurosteroid
Introduction
The GABAA receptor (GABAR) mediates most of the fast inhibition in the CNS. This receptor is a pentameric structure composed of varying combinations of α (1–6), β (1–3) and γ (1–3), δ or ε subunits (Hevers and Luddens 1998). Although the most prevalent GABAR isoform expressed in the brain is α1β2γ2, other possible subunit combinations exist, which vary in their response to steroids. δ-Containing GA BAR are reported to have the greatest steroid sensitivity (Wohlfarth et al. 2002; Belelli et al. 2002; Brown et al. 2002), suggesting that these receptors may serve as a primary target for steroid effects.
Positive modulators of the GABAR include most sedative, anxiolytic drugs, such as benzodiazepines (BDZ), barbiturates, ethanol, and the neuroactive metabolite of pro-gesterone (P), 3α-OH-5α[β]-pregnan-20-one (THP or [allo]pregnanolone) (Majewska et al. 1986). Behaviorally, THP produces anxiolytic (Bitran et al. 1995; Akwa et al. 1999), anticonvulsant (Frye 1995) and sedative effects (Korneyev and Costa 1996) in a dose-dependent fashion. Although the site of action of this steroid may be distributed throughout brain circuits, anxiolytic actions of the steroid have been reported after local application of THP to the dorsal hippocampus (Bitran et al. 1999) similar to BDZs (Menard and Treit 2001).
Across the menstrual cycle, circulating levels of P and THP are elevated during the 10–11 days of the luteal phase before declining to low levels in the late luteal phase and follicular phase (Schmidt et al. 1994; Rapkin et al. 1997). Therefore, recent animal studies have investigated the effects of chronic exposure to and withdrawal from THP in some cases subsequent to administration of the parent compound P to female rats (Follesa et al. 2000; Reddy et al. 2001; Smith et al. 1998a, b). Steroid withdrawal in the rat produces a state of anxiety (Gallo and Smith 1993; Smith et al. 1998b), accompanied by a GABAR subunit switch, leading to increased expression of α4-containing GABAR in the hippocampus (Smith et al. 1998a), midbrain (Griffiths and Lovick 2005), and cerebellar granule cell (Follesa et al. 2000). Consistent with the BDZ insensitivity reported for recombinant α4-containing GABAR (Wafford et al. 1996; Wisden et al. 1991), rats tested following P withdrawal are relatively insensitive to the anxiolytic effects of the BDZ lorazepam (LZM) (Moran et al. 1998). A similar BDZ insensitivity is also noted for GABA-gated current, recorded with whole-cell patch clamp techniques on pyramidal cells acutely isolated from CA1 hippocampus, following P withdrawal (Smith et al. 1998a). This BDZ insensitivity is prevented when α4 expression is suppressed using antisense oligonucleotide administration intraventricularly (Smith et al. 1998a), suggesting that BDZ responsiveness can reflect the levels of α4 expression.
Alterations in mood and BDZ responsiveness have been reported for human females across the menstrual cycle (Endicott et al. 1999). There are reports that mood changes can be observed late in the luteal phase (i.e., “premenstrual syndrome”) when endogenous THP levels are declining (Rapkin et al. 1997; Pearlstein et al. 2005), suggesting that steroid withdrawal in the rat may have clinical relevance. However, a severe form of this disorder is classified in the DSM-IV as premenstrual dysphoric disorder (PMDD) (Angst et al. 2001; Backstrom et al. 2003a,b; Freeman 2003; Rapkin 2003). PMDD has an onset early to midluteal phase, when P and THP levels are increased (Angst et al. 2001). Dysphoric mood at this time can encompass anxiety (Yonkers 1997), irritability, aggression, depression, and emotional reactivity, suggesting a diverse array of symptoms (Angst et al. 2001; Endicott et al. 1999; Freeman 2003; Pearlstein et al. 2005; Backstrom et al. 2003a; Smith et al. 2003b; Steiner et al. 1999).
The role of progesterone in the etiology of PMDD is not clear. Therapeutic administration of progesterone to women with PMDD has been shown to be no better than placebo (Elliott 2002; Kouri and Halbreich 1998; Freeman 2004). Although some studies report that women with PMDD do not respond differently to progesterone than nonaffected individuals (Freeman 2004), other studies suggest that administration of progesterone to women with PMDD increases dysphoric mood, especially anxiety (Schmidt et al. 1998; Tiemstra and Patel 1998). Consistent with this are other recent reports suggesting that women with PMDD display higher THP levels than normal subjects (Girdler et al. 2001). In another study (Freeman et al. 2002), improvement in PMDD symptoms was paradoxically associated with a decline in THP levels, rather than an increase, as would be expected based on its anxiolytic potential. Although other studies suggest conflicting results (Rapkin et al. 1997; Bicikova et al. 1998; Monteleone et al. 2000; Hsiao et al. 2004), the possibility exists that under some conditions, THP may paradoxically exert anxiogenic effects in women with PMDD in contrast to its well-established anxiolytic action.
Therefore, we conducted the present study to determine whether we could observe anxiogenic effects of THP under conditions of THP cyclicity relevant for the menstrual cycle. Because any potentially “dysphoric” effects of THP in the luteal phase would occur, following a period of relative “THP withdrawal” in the follicular phase, we used a mouse model of THP withdrawal to test this possibility. Mice display high nocturnal surges of THP (20–30 nM) in brain (Corpechot et al. 1997), which exceed those found in rat (10–12 nM) (Corpechot et al. 1993). Because of this, withdrawal can be accomplished by blocking formation of these endogenously high levels of THP with finasteride, a 5α-reductase blocker (Frye and Walf 2004). Because PMDD symptoms include anxiety and irritability (Angst et al. 2001), we distinguished between effects of the steroid on baseline anxiety and emotional response to an aversive stimulus.
Our earlier work indicated that steroid withdrawal could increase α4-containing GABAR (Smith et al. 1998a). Therefore, we also assessed α4 levels in CA1 hippocampus across steroid state. The α4 subunit can coexpress with either the δ or γ2 subunit (Sur et al. 1999), both of which have a distinctive pharmacological response (Wafford et al. 1996; Belelli et al. 2002; Brown et al. 2002). δ-Containing GABAR have an increased responsiveness to steroids (Wohlfarth et al. 2002), but variable responses have been reported (Zhu et al. 1996) suggesting that the functional role of steroids at this receptor may be modifiable. Therefore, it was important to compare the effects of steroid withdrawal on both wild-type and δ knockout mice to determine the role of δ-containing GABAR in mediating behavioral responses to THP, following steroid withdrawal.
Methods
Animals
Prepubertal wild-type female mice (C57BL6, Jackson Labs) and δ knockout mice were housed in a reverse light/dark cycle (lights on 2330 hours/lights off 1130 hours). These animals do not exhibit estrous cyclicity and were used in order to isolate selective effects of THP withdrawal on behavioral state. Circulating levels of estradiol at this time are increasing to within the high diestrous range (Overpeck et al. 1978) and are elevated above levels earlier in development. Additional δ knockout mice were bred in-house, and tails genotyped after experimental procedures were completed. Mice were housed at an ambient temperature of 21–25°C in groups of three with access to food and water. Their pre-pubertal status was verified by an imperforate vagina. Mice were injected on a daily basis for 3 days with finasteride (50 mg/kg, i.p.) or vehicle 1–1.5 h before dark onset. This 3-day THP “withdrawal” was used to simulate the THP withdrawal in the human, where more prolonged fluctuations in steroids occur. In all cases, the “Principles of laboratory animal care” were followed, as were the guidelines from the Institutional Animal Care and Use Committee.
Radioimmunoassay for 3α,5α-THP
Animals were killed 1 h after dark onset in order to determine hippocampal levels of THP which have been shown to peak to 207–35 nM at this time (Corpechot et al. 1997), values two to three times higher than peak levels during the light period. Brains were removed and stored on dry ice, and serum was collected on ice. 3α,5α-THP levels in plasma and hippocampus were determined by radioimmunoassay according to previously published methods (Frye et al. 1998; Frye and Bayon 1999; Smythe et al. 1994).
Extraction of 3α,5α-THP
Following incubation with water and 800 cpm of tritiated 3α,5α-THP (NET-1047: specific activity=65.0 Ci/mmol; New England Nuclear, Boston, MA), 3α,5α-THP was extracted from plasma with ether. Hippocampal tissues were homogenized with a glass/glass homogenizer in 5 ml of methanol, 1% acetic acid and 800 cpm of tritiated 3α,5α-THP. Homogenates were centrifuged at 3,000×g for 15 min. Supernatants were chromatographed on Sepak cartridges with increasing concentrations of methanol. Samples were dried, and fractions were reconstituted in phosphate assay buffer (pH 7.4).
3α,5α-THP radioimmunoassay
The standard curve was prepared in duplicate with a range of nine concentrations from 50 to 8,000 pg/ml. The total volume of the assay was 950 μl. The standards were added to phosphate assay buffer, followed by addition of the 3α,5α-THP (921412-5) antibody (purchased from Dr. Robert Purdy, Veteran’s Medical Center, La Jolla, CA) in a concentration of 1:5,000 and 3H-steroid. The assay was incubated overnight at 4°C.
Termination of binding
Separation of bound and free 3α,5α-THP was accomplished by the rapid addition of dextran-coated charcoal. Following incubation with charcoal, samples were centrifuged at 1,200×g, and the supernatant was pipetted into a glass scintillation vial with 6 ml scintillation cocktail. Sample tube concentrations were calculated using the logit-log method (Rodbard and Hutt 1974), interpolation of the standards, and correction for recovery. The minimum detectable limit of the assay was 50 pg. The intra-assay and inter-assay coefficients of variance were 0.12 and 0.15.
Western blot procedure
Crude membranes from microdissected CA1 hippocampus were first normalized according to protein content using standard techniques (Smith et al. 1998b); then, proteins were separated using SDS gel electrophoresis, transferred to nitrocellulose membranes, and probed with a selective antibody for the rat α4 (67 kDa) subunit (Smith et al. 1998a). Because of the low expression of α4 subunit in CA1 hippocampus, these bands were detected with a highly sensitive chemiluminescence substrate (Pierce Supersignal West Femto substrate) for visualization and quantified using a Umax scanner and One-Dscan software. The results were standardized to a glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 36 kDa) control protein.
Elevated plus maze
The elevated plus maze is a device consisting of four 8×35 cm arms at 90° angles, elevated 57 cm above the floor. Two arms are enclosed by 33-cm walls, and two arms have no walls (“open arms”). The open arms are also partially bordered by small rails (5×15 cm) extending to the proximal half of the arm, and the floor of the maze is marked with grid lines every 25 cm. Each animal was initially acclimated to the room for 30 min to 1 h before being placed in the center of the maze, and exploratory activity was recorded for 5 min. Background white noise was used for most tests, except for the flumazenil experiment where a 30-kHz auditory signal was used to increase the aversive qualities of the experimental conditions (Wavetek 186 5 MHz Phaselock generator). The time spent in the open and closed arms was tabulated, as were the entries. The number of total entries is a measure of general activity level. To be considered an open arm entry, the animal had to cross the line of the open platform with all four paws. An increase in time spent in the open arm is considered to be a measure of decreased anxiety (Pellow et al. 1995), as we have described (Smith et al. 1998b). For the shock-plus maze paradigm, mice were administered a 400-μA shock for 1–2 s immediately before timing in the maze. Open and closed arm data were tabulated as described above.
Pharmacological studies
In order to test the effect of various GABA-modulatory drugs, separate groups of wild-type or δ knockout mice were tested late in the morning prior to the lights-off period, with THP (10 mg/kg, i.p. in oil; 20 min before testing, n=8–10, unpaired plus maze; n=17–26, shock-paired plus maze), LZM (0.1 mg/kg, i.p. 10 min before testing, n=7), flumazenil (2 or 7 mg/kg, i.p., 10 min before testing, n=7/dose), 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP; 3 mg/kg, 30 min before testing, n=11–12), or vehicle for the indicated time period prior to plus maze testing. (These timings have been determined empirically to yield significant responses in the plus maze.) Because performance on the plus maze is altered by prior experience, each animal was tested only once in this task. Thus, the comparisons are between groups of animals tested on the same day.
Statistical analysis
THP levels
The statistical significance of differences in hippocampal and serum levels of THP between animals receiving finasteride vs vehicle was assessed with the Student’s t test.
α4 Subunit levels
Differences between groups were assessed with a one-way ANOVA followed by a Tukey’s post hoc analysis.
THP effects on plus maze behavior
For this study, a mixed linear model multifactorial ANOVA was used (SAS, Cary, NC) to test the effects of three fixed factors: THP withdrawal state, acute THP administration, and shock, on two dependent variables in the elevated plus maze: open arm time and open arm entries. In addition to main effects, two- and three-way interactions between effects were tested. Litter group was considered a random factor, and locomotor activity (assessed as the total number of arm entries) was used as a covariate. Individual post hoc comparisons with the Fisher’s test accounted for total arm entries. Because litter grouping was not a significant factor (P=0.398), it was not included in subsequent models. As described in the previous paragraphs, all comparisons were between animals.
Lorazepam and THIP effects on plus maze behavior
For this study, a simplified mixed linear model (SPSS) was employed to test the effect of LZM or THIP on two dependent variables, open arm time and open arm entries relative to their values under control conditions. Two fixed factors, THP withdrawal state and genotype, were examined, as well as two-way interactions between effects, with the total number of arm entries used as a covariate.
Flumazenil effects on plus maze behavior
Because the hypothesis to be tested was that flumazenil (Flz) dose reversed its effects from anxiolytic to anxiogenic in wild-type mice, separate ANOVA comparisons (SPSS) were made to examine the effects of either 2 or 7 mg/kg doses on open arm time and open arm entries. Results were compared in animals undergoing THP withdrawal relative to vehicle-injected controls. As stated above, the total number of arm entries was used as a covariate. In all comparisons, data are expressed as the mean±SEM, and a P level less than 0.05 was accepted as a level of significance.
Results
THP withdrawal
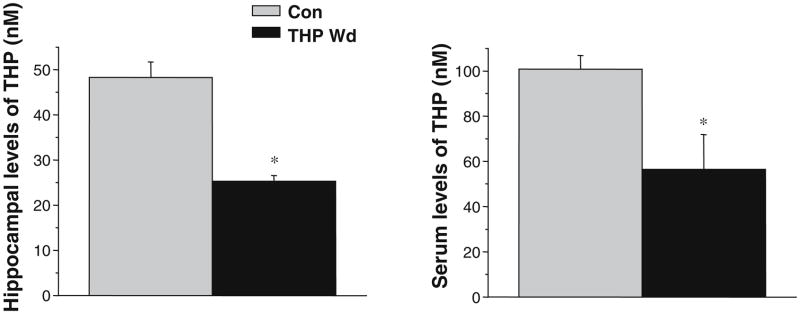
A state of THP withdrawal was produced with administration of the 5α-reductase blocker finasteride (50 mg/kg, i.p. for 3 days) to prepubertal female mice 1 h before dark onset. When tested 1 h after dark onset during the nocturnal surge in brain levels of THP (Corpechot et al. 1997), this regimen resulted in a 49±0.5% (P<0.05) decrease in hippocampal levels of THP for animals treated with finasteride (Fig. 1) compared to control animals injected with vehicle. Serum levels of THP, assessed at this time, were also reduced by finasteride by 44±2% (P<0.05, Fig. 1). These data suggest that finasteride administration significantly reduces the initial nocturnal surge of THP in hippocampus.
Fig. 1.
Finasteride administration decreases hippocampal levels of THP. Daily administration of finasteride (50 mg/kg, i.p.) or vehicle 1–1.5 h before dark onset to prepubertal female mice significantly decreased levels of THP in hippocampus and blood when assessed 1 h after dark onset. (P<0.05 vs Con, n=3–5 animals/group)
Hippocampal α4 GABAR subunit expression
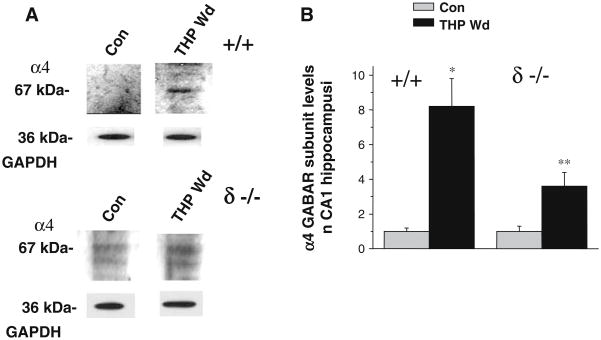
Because our earlier studies in rats (Smith et al. 1998a) suggest that neurosteroid withdrawal could increase expression of the GABAR α4 subunit, we assessed α4 levels in isolated CA1 hippocampal membranes. The THP withdrawal state produced by finasteride administration resulted in a highly significant eightfold increase in α4 expression (P<0.001) when assessed in wild-type (+/+) mice (Fig. 2). We also tested the effects of THP withdrawal in mice lacking expression of the δ GABAR subunit (δ knockout mice). Although less significant (P<0.05), THP withdrawal in δ knockout mice produced a threefold increase in α4 expression in CA1 hippocampus (Fig. 2).
Fig. 2.
Withdrawal from THP increases α4 subunit expression. THP withdrawal (THP Wd) in both +/+ and δ −/− mice increased expression of the α4 subunit in isolated CA1 hippocampal membranes, without altering levels of the GAPDH control protein. a Representative Western blot. b Group means. (*P<0.05 vs Con, n=9 animals, each)
Effects of THP in the elevated plus maze
Because clinical reports suggest that women with PMDD are most likely to experience increases in irritability or emotional reactivity to aversive stimuli in the luteal phase (Angst et al. 2001) when THP levels are increased, we assessed THP effects on behavior in the elevated plus maze with or without a preceding shock. In this case, mice were tested, following 3 days of finasteride administration to decrease hippocampal levels of THP (“THP Wd”, see Fig. 1), and compared with vehicle-administered control mice. Both open arm time and open arm entries were evaluated, as measures of anxiety, as well as the total number of arm entries, considered a measure of locomotion.
The main effect of THP administration was highly significant in altering open arm time (P=0.0002, Table 1; Fig. 3) and open arm entries (P=0.0001), with significant two-way interactions between THP administration and THP withdrawal state (P=0.0001 and 0.007, for open arm time and entries, respectively) as well as THP administration and shock (P=0.03, open arm time). There was also a significant three-way interaction between THP administration, THP withdrawal state, and shock (P=0.0144 and 0.021, for open arm time and entries, respectively).
Table 1.
Summary of statistical results for three factors, THP Wd (withdrawal), THP, and shock for wild-type mice, plus two- and three-way interactions between experimental conditions
| Effect | Number df | Error df | Open time |
Open entries |
||
|---|---|---|---|---|---|---|
| F value | P | F value | P | |||
| THP Wd | 1 | 147 | 1.61 | 0.207 | 0.793 | 0.375 |
| THP | 1 | 144 | 15.07 | 0.0002 | 20.77 | 0.0001 |
| THP Wd*THP | 1 | 147 | 33.72 | <0.0001 | 7.6 | 0.007 |
| Shock | 1 | 19.8 | 10.77 | 0.0038 | 1.361 | 0.245 |
| THP Wd*shock | 1 | 147 | 0.85 | 0.3592 | 6.472 | 0.012 |
| THP*shock | 1 | 145 | 4.79 | 0.0302 | 0.090 | 0.765 |
| THP Wd*THP*shock | 1 | 147 | 6.13 | 0.0144 | 5.425 | 0.021 |
Comparisons were made using a mixed linear model. (Total number of entries was a covariate.) Significant effects are indicated in bold
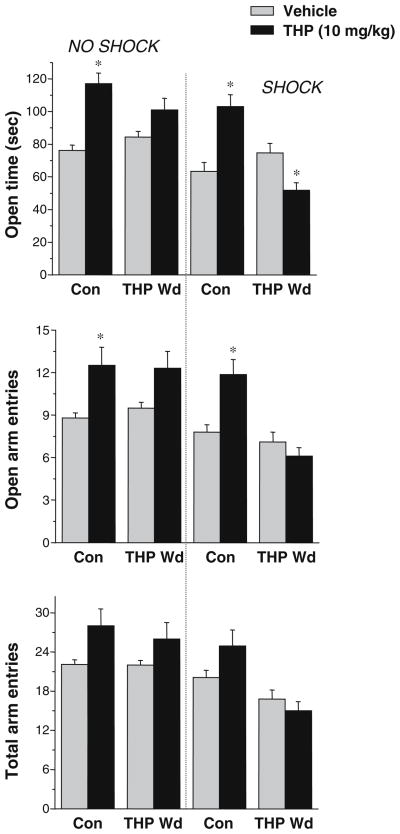
Fig. 3.
THP increases anxiety in a shock-paired plus maze paradigm, following THP withdrawal. When tested in the elevated plus maze without a preceding shock (left panel), an anxiolytic dose of THP (10 mg/kg, i.p.) increased time in the open arm and open arm entries in control mice, but not in mice undergoing THP Wd. (*P<0.05 vs Vehicle, n=8–10). However, when tested following a brief shock (right panel), THP decreased open arm time, following THP Wd, in contrast to its effect in control mice, where it increased this parameter. (*P<0.05 vs Vehicle, n=17–26). The total number of arm entries (lower panel) was not altered by any experimental treatment
An increase in time spent in the open arm of the maze (open arm time) and/or the number of open arm entries are considered to be measures of decreased anxiety (Pellow et al. 1995), and as expected, these parameters were increased by 54±5.2 and 42±4%, respectively, in control mice in response to THP administration (P<0.05, Fig. 3) compared to vehicle-treated mice, consistent with the well-known anxiolytic action of this steroid (Akwa et al. 1999; Bitran et al. 1995). The addition of a shock preceding the plus maze test did not alter the effect of THP in control mice. In no case was locomotor activity altered by THP, as evidenced by a lack of effect on the total number of arm entries.
However, in mice undergoing THP withdrawal, THP significantly (P<0.0001) decreased open arm time by 31±3.5% (P<0.05, Fig. 3) when a shock preceded the plus maze test, suggesting that under these conditions, THP possesses an anxiogenic effect. In contrast, when tested without the shock, THP did not produce significant effects on open arm time, suggesting that both the THP withdrawal state and the effect of an aversive stimulus are necessary to reverse the normally anxiolytic effect of THP to an anxiogenic action.
Effects of THP on δ knockout mice in the elevated plus maze
Because δ-containing GABAR are especially sensitive to modulation by steroids such as THP (Wohlfarth et al. 2002; Belelli et al. 2002; Brown et al. 2002), we tested the effects of THP on δ knockout mice using the plus maze paradigm with and without a preceding shock, as described above. As seen in wild-type mice, the main effect of THP administration was significant in the δ knockout mice for both open arm time (P=0.015, Table 2; Fig. 4) as well as open arm entries (P=0.025). A significant effect of THP withdrawal was also seen for open arm time (P=0.05), but no other main effect or interactions were significant.
Table 2.
Summary of statistical results for three factors, THP Wd, THP, and shock for δ knockout mice, plus two- and three-way interactions between experimental conditions
| Effect | Number df | Error df | Open time |
Open entries |
||
|---|---|---|---|---|---|---|
| F value | P | F value | P | |||
| THP Wd | 1 | 42 | 3.903 | 0.05 | 0.07 | 0.793 |
| THP | 1 | 33 | 6.521 | 0.015 | 5.475 | 0.025 |
| THP Wd*THP | 1 | 42 | 1.26 | 0.270 | 2.451 | 0.127 |
| Shock | 1 | 0.111 | 0.741 | 0.0001 | 0.984 | |
| THP Wd*shock | 1 | 42 | 0.241 | 0.627 | 1.530 | 0.225 |
| THP*shock | 1 | 41 | 0.005 | 0.942 | 2.175 | 0.150 |
| THP Wd*THP*shock | 1 | 42 | 0.032 | 0.858 | 0.069 | 0.795 |
Comparisons were made using a mixed linear model. (Total number of entries was a covariate). Significant effects are indicated in bold
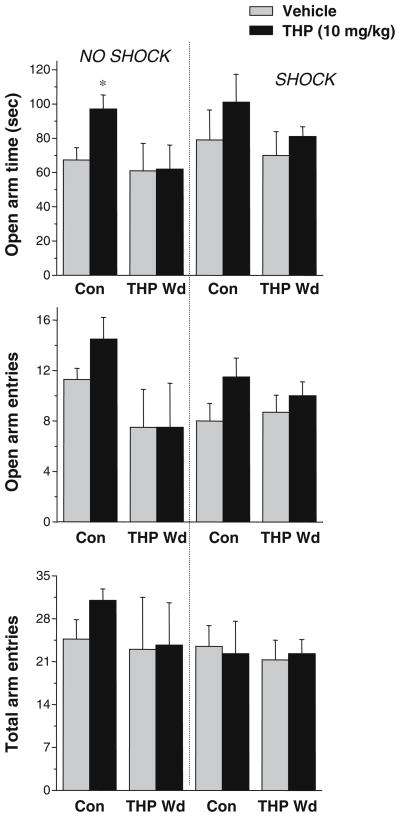
Fig. 4.
THP withdrawal reduces the anxiolytic effects of acute THP administration in δ knockout mice. (Left panel) Although reduced, anxiolytic effects of THP in Con δ −/− mice in the unpaired plus maze were reflected by an increase in open arm time and a decrease in open arm entries. (*P<0.05 vs Vehicle, n=4–6). After THP Wd, THP had no effect in δ −/− mice. When the elevated plus maze test was preceded by a shock (right panel), the anxiolytic effects of THP were not significant are pharmacologically consistent with an increase in δ-containing GABAR, following THP withdrawal.
As reported by other labs (Mihalek et al. 1999), THP was less effective as an anxiolytic in δ knockout mice than in wild-type mice (Fig. 4), producing a 44±2% increase in open arm time (P<0.05) and a 28±6.1% increase in open arm entries, effects which were dampened in the shock-paired plus maze. Following THP withdrawal, however, THP produced no significant effect on either open arm time or open arm entries. These results suggest that the anxiogenic effect of THP in the shock-paired plus maze test is absent in mice which lack expression of the δ GABAR subunit.
THP withdrawal results in a BDZ insensitivity
GABAR containing the α4 subunit are characterized by a relative insensitivity to modulation by BDZ agonists (Wafford et al. 1996; Wisden et al. 1991). Therefore, because THP withdrawal in both wild-type and δ knockout mice increased expression of the α4 GABAR subunit (see Fig. 2), we tested the effects of LZM (0.1 mg/kg, i.p.) on performance in the shock-paired plus maze. For both wild-type and δ knockout mice, the main effect of THP withdrawal was significant for LZM effects on open arm time (P=0.048, Table 3) and open arm entries (P=0.03). There was no significant effect of genotype on these parameters nor were there significant interactions between effects.
Table 3.
Summary of values (mean±SEM) from the elevated plus maze following acute administration of LZM (0.1 mg/kg) or THIP (3 mg/kg)
| Group | Open arm time (relative to control) | df | Effect | F | P | Open entries/total entries (relative to control) | F | P | |
|---|---|---|---|---|---|---|---|---|---|
| LZM | |||||||||
| +/+ | Con | 1.46±0.17 | 1, 21 | THP Wd | 4.49 | 0.048 | 1.1±0.1 | 5.709 | 0.03 |
| THP Wd | 0.56±0.22 | Geno | 0.182 | 0.675 | 0.47±0.03 | 0.015 | 0.905 | ||
| δ −/− | Con | 1.77±0.12 | Geno*THP Wd | 0.323 | 0.577 | 1.27±0.08 | 4.157 | 0.058 | |
| THP Wd | 1.00±0.28 | 0.8±0.16 | |||||||
| THIP | |||||||||
| +/+ | Con | 1.47±0.09 | 1, 37 | THP Wd | 0.002 | 0.961 | 1.06±0.04 | 0.415 | 0.525 |
| THP Wd | 1.76±0.16 | Geno | 25.926 | 0.0001 | 1.21±0.06 | 4.523 | 0.043 | ||
| δ −/− | Con | 1.00±0.14 | Geno *THP Wd | 5.296 | 0.029 | 0.85±0.13 | 1.900 | 0.180 | |
| THP Wd | 0.76±0.11 | 0.86±0.11 | |||||||
In each case, two main factors were compared: THP Wd and genotype (Geno), as well as interactions between effects. The total number of arm entries was used as a covariate. Significant findings for main drug effect or group interactions are indicated in bold
Under control conditions, LZM significantly (P<0.05, Table 3) increased open arm time by 46±8% and increased open arm entries relative to the total entries by 15±3%, accompanied by a 10% increase in total entries. In contrast, after THP withdrawal, LZM reduced open arm time by 44± 0.2% and open arm entries/total entries by 53±0.03%. A similar insensitivity to LZM was also observed, following THP withdrawal in δ knockout mice. Surprisingly, the anxiolytic effect of LZM was greater in control δ knockout mice than in wild-type mice, as evidenced by a 77±10% increase in open arm time and a 27±3% increase in open arm entries/total (P<0.001).
Effects of THIP on the elevated plus maze
Because δ-containing GABAR have increased sensitivity to the GABA partial agonist THIP (Brown et al. 2002), we tested the effect of THIP on behavior in the elevated plus maze. Consistent with this, genotype was a significant factor in determining the THIP effect on open arm time (P=0.0001, Table 3) and open arm entries (P=0.043), with δ knockout mice exhibiting a 15–47% reduction in response to THIP compared to wild-type mice. There was also a significant interaction between THP withdrawal and genotype on THIP effects on open arm time (P=0.029, Table 3).
A dose of THIP subthreshold for its sedative effect (3 mg/kg, i.p.) significantly (P<0.001) increased open arm time by 76±7%, following THP withdrawal only in wild-type mice (Table 3), a significantly greater effect than that observed in control animals (P<0.05). This dose of THIP also increased open arm entries/total entries by 20±1.5% (P<0.05) compared to 6±2% in controls. Thus, these results are pharmacologically consistent with an increase in δ-containing GABAR, following THP withdrawal.
Effects of flumazenil on the elevated plus maze
GABAR containing the α4 subunit have atypical responses to the BDZ antagonist flumazenil (Dunn et al. 2003; Wafford et al. 1996). Therefore, we tested the effects of flumazenil (2 and 7 mg/kg, i.p.) in the shock-paired plus maze. The main effect of THP withdrawal was significant for both the higher dose (P=0.002, Table 4) as well as the lower dose of flumazenil (P=0.03). The lower dose of flumazenil tested (2 mg/kg), which had no significant effect in vehicle-treated animals, produced a 50% reduction in open arm time (P<0.05), following THP withdrawal in wild-type mice, suggesting that it has an anxiogenic effect under these conditions. In contrast, this dose of flumazenil increased both open arm time and open arm entries/total in δ knockout mice, following THP withdrawal.
Table 4.
Summary of the dose-dependent effects of flumazenil (Flz) in the elevated plus maze (Mean±SEM)
| Group | Open arm time (relative to control) | df | Effect | F | P | Open arm entries/total entries (relative to control) | F | P | |
|---|---|---|---|---|---|---|---|---|---|
| Flz, 2 mg/kg | |||||||||
| +/+ | Con | 0.9±0.16 | 1, 19 | THP Wd | 4.625 | 0.03 | 1.01±0.09 | 0.441 | 0.525 |
| THP Wd | 0.51±0.12 | 1.11±0.09 | |||||||
| δ −/− | Con | 1.08±0.5 | 1, 12 | THP Wd | 4.281 | 0.077 | 0.76±0.3 | 0.629 | 0.454 |
| THP Wd | 1.51±.3 | 1.33±0.3 | |||||||
| Flz, 7 mg/kg | |||||||||
| +/+ | Con | 1.19±0.19 | 1, 24 | THP Wd | 13.446 | 0.002 | 1.01±0.15 | 0.511 | 0.483 |
| THP Wd | 2.36±0.36 | Geno | 9.7 | 0.005 | 0.96±0.10 | 0.145 | 0.708 | ||
| δ −/− | Con | 1.24±0.27 | THP Wd*Geno | 5.115 | 0.035 | 0.86±0.18 | 1.280 | 0.271 | |
| THP Wd | 1.61±0.16 | 1.16±0.13 | |||||||
Flz, 2 mg/kg: Significant findings for the main effect, THP Wd, are indicated for each dose and genotype tested. Flz, 7 mg/kg: Significant findings for each of two main effects, THP Wd and genotype, as well as the interaction between factors. The total number of arm entries was used as a covariate. Significant effects are indicated in bold
A higher dose of flumazenil (7 mg/kg) produced modest increases in open arm time in control mice (20–30%), which were not significant (Table 4). However, 7 mg/kg flumazenil significantly increased the open arm time by more than twofold in wild-type mice (P<0.001) compared to vehicle-treated mice, following THP withdrawal. A less dramatic increase in open arm time was observed in δ knockout mice undergoing THP withdrawal (P<0.05). Thus, these results suggest that the effect of flumazenil is dose-dependent and is affected by steroid state, as well as by the ability to express the GABAR δ subunit.
Discussion
The results from the present study suggest that a 48-h withdrawal from THP in female mice produces a paradoxical anxiogenic response to THP, assessed with a well-established model of anxiety paired with an aversive stimulus. Further, this THP withdrawal state produced a significant increase in hippocampal expression of the α4 subunit of the GABAR, as we have previously demonstrated in the rat (Smith et al. 1998a). Consistent with increased expression of α4-containing GABAR (Wafford et al. 1996; Wisden et al. 1991), mice undergoing THP withdrawal were insensitive to a BDZ agonist. The behavioral and pharmacological profile observed, following this withdrawal state, is comparable in many respects to that reported for women with PMDD.
While multiple symptoms of PMDD have been reported, the most common affective component is an increase in irritability or emotional reactivity to aversive stimuli (Endicott et al. 1999; Angst et al. 2001; Smith et al. 2003b), as well as increased anxiety and tension (Yonkers 1997; Endicott et al. 1999). Emotional reactivity can be further subclassified into anxious, depressive, or aggressive components, depending on individual susceptibility (Angst et al. 2001; Endicott et al. 1999; Backstrom et al. 2003a; Freeman 2003; Pearlstein et al. 2005; Steiner et al. 1999). Therefore, the present study employed a shock-paired plus maze paradigm to test emotional reactivity in response to an aversive stimulus to better compare with the most prevalent PMDD symptom.
Both the onset and the hormonal milieu associated with dysphoric symptoms is highly variable (Pearlstein et al. 2005) in women with this disorder. However, cyclicity of steroid levels appears to be necessary for PMDD symptom onset, as drugs which prevent ovulation, and thus prevent the increase in circulating levels of P and THP, prevent PMDD symptoms (Schmidt et al. 1998). Conversely, high steady-state levels of P and THP in pregnancy are also associated with reduction of PMDD symptoms (Cloitre et al. 2004). With our THP withdrawal model in the mouse, suppression of the elevated hippocampal levels of THP for 3 days would produce a sustained THP withdrawal state to better simulate the low THP conditions occurring during the follicular phase of the human cycle, which precedes the onset of PMDD symptoms in the luteal phase.
Clinical studies have only indirectly established the effect of THP in PMDD, but there is considerable evidence to suggest that this steroid may reverse to increase anxiety rather than to act as an anxiolytic agent at least under some conditions in susceptible individuals. This possibility was first suggested by a paper reporting that women with PMDD have an abnormal response to administration of the parent compound P, which triggered PMDD symptoms after cessation of steroid cyclicity (Schmidt et al. 1998). Two other studies have noted a positive correlation between circulating levels of THP and adverse mood, one in PMDD subjects (Freeman et al. 2002) and the other in postmenopausal women with E+P replacement therapy (Andreen et al. 2004). In the latter study (Andreen et al. 2004), high luteal phase levels of THP were associated with dysphoric mood compared to lower and higher levels of the steroid. Further, women with PMDD show increased cortical excitability in the luteal phase (Smith et al. 2003a) in contrast to normal subjects who show increased cortical inhibition at this time. Taken together, this body of data suggests that the effect of THP may reverse to trigger excitability and emotional reactivity in PMDD. The results from the present study suggest that in a state of THP withdrawal as seen following the follicular phase, acute administration of THP produces an anxiogenic response to an aversive stimulus.
In contrast to these studies, there are reports which suggest that PMDD is in fact correlated with lower circulating levels of THP or progesterone (Rapkin et al. 1997; Bicikova et al. 1998; Monteleone et al. 2000) or rather showed no correlation at all (Hsiao et al. 2004). The lack of consistency for correlations between THP levels and PMDD symptoms may be due to the fact that the extent or rate of change of these levels is more important than absolute levels of the steroid, that steroid metabolism in these women is altered, or that additional steroid metabolites complicate comparisons. Conversely, women with PMDD may have an abnormal response to steroid fluctuations.
These apparently conflicting reports may so be due to the diverse etiology of the syndrome or to the conditions under which progesterone was administered. In fact, our results suggest that THP produces no significant effect on plus maze behavior when administered without an aversive stimulus. Insensitivity to the GABA-modulatory effect of THP has been reported for women with PMDD (Sundstrom et al. 1998), using both subjective and objective measures of sedation. Thus, these results suggest that the effect of THP is dependent upon the behavioral context.
Increases in circulating levels of estradiol have also been implicated in the etiology of PMDD, where administration of the steroid has even been shown to trigger dysphoric symptoms (Schmidt et al. 1998; Seippel and Backstrom 1998). Although we used 5-week-old mice in the peripubertal period to minimize the hormonal variability, these animals have somewhat increased circulating levels of estradiol in the high diestrous range (Overpeck et al. 1978), and this may have played a role in triggering the paradoxical anxiogenic effect of THP. Further, the results from the present study may also have relevance for mood swings and irritability, commonly reported at the onset of puberty in association with fluctuations in steroid hormones (Buchanan et al. 1992; Cameron 2004).
Results from the present study suggest that one outcome of THP withdrawal is an increase in expression of the α4 subunit of the GABAR in the hippocampus. In fact, the pharmacological results from the plus maze are consistent with increased expression of both α4βδ and α4βγ2 subunit combinations. These include a relative insensitivity to the anxiolytic effects of the BDZ agonist LZM (both α4βδ and α4βγ2; Brown et al. 2002), anxiolytic responses to the BDZ antagonist flumazenil (α4βγ2; Wafford et al. 1996), and increased anxiolytic responses to the GABA partial agonist THIP (α4βδ; Brown et al. 2002). Because THIP has greater efficacy at δ-containing GABAR (Brown et al. 2002), it was almost ineffective as an anxiolytic in δ knockout mice. The anxiogenic effect of the lower dose of flumazenil may also be due to effects at α4βδ GABAR, where it has been shown to act as a BDZ inverse agonist (Dunn et al. 2003). Further, this anxiogenic effect of flumazenil was not observed in the δ knockout mouse, suggesting that α4βδ GABAR mediate this atypical effect of the BDZ antagonist. Both the BDZ insensitivity (Sundstrom et al. 1997) and anxiogenic effect of flumazenil (Le Melledo et al. 2000) have been reported for women with PMDD, suggesting an additional comparison with the THP withdrawal model in the present study.
The anxiogenic effect of THP observed, following THP withdrawal in the present study, was not observed in δ knockout mice, implicating actions at δ-containing GABAR. These receptors are extrasynaptic (Wei et al. 2003), where they mediate a tonic inhibition via activation by ambient levels of GABA or via spillover from adjacent synapses (Wei et al. 2003). Furthermore, fluctuations in circulating levels of THP have been shown to alter expression of the δ subunit (Sundstrom-Poromaa et al. 2002; Lovick et al. 2005; Maguire et al. 2005). Although αβδ GABAR have been shown to exhibit an increased sensitivity to modulation by steroids (Wohlfarth et al. 2002; Stell et al. 2003), conflicting reports exist (Zhu et al. 1996), and posttranslational mechanisms, such as receptor phosphorylation, are required for steroid modulation (Fancsik et al. 2000; Harney et al. 2003; Leidenheimer and Chapell 1997; Vicini et al. 2002). In addition, steroid potentiation of α4βδ GABAR increases receptor efficacy (Bianchi and Macdonald 2003), which increases the rate and extent of receptor desensitization. Such an effect would therefore eventually decrease inhibition and theoretically could lead to increased behavioral reactivity.
In conclusion, THP withdrawal in the mouse results in paradoxical, anxiogenic effects of THP in response to an aversive stimulus. Although the array of symptoms and hormonal correlations reported for PMDD suggests a diverse etiology, the mouse model presented here suggests that anxiogenic effects of THP are possible but are dependent upon the hormonal milieu and behavioral context. In this model, underlying factors may include the increase in α4 expression and, in particular, increased α4βδ GABAR expression, which subserve tonic inhibition in many areas of the limbic system.
Acknowledgments
The authors wish to thank Ellen Freeman, M. D. and Peter Schmidt, M. D. for comments on the manuscript. We are grateful to Jeremy Weedon for assistance with the statistical tests. This work was supported by NIH grants DA09618 and AA12958 and a contract from Lundbeck Pharmaceuticals (Copenhagen, Denmark) to SSS. All experiments performed complied with the current laws of the US.
Contributor Information
Sheryl S. Smith, Email: Sheryl.smith@downstate.edu, Department of Physiology and Pharmacology, SUNY Downstate Medical Center, 450 Clarkson Ave., Box 31 Brooklyn, NY 11203, USA
Yevgeniy Ruderman, Department of Physiology and Pharmacology, SUNY Downstate Medical Center, 450 Clarkson Ave., Box 31 Brooklyn, NY 11203, USA.
Cheryl Frye, Department of Psychology, SUNY at Albany, 1400 Washington Ave., Albany, NY 12222, USA.
Gregg Homanics, Department of Anesthesiology, University of Pittsburgh, W1356 Biomedical Science Tower, Pittsburgh, PA 15261, USA.
Maoli Yuan, Department of Physiology and Pharmacology, SUNY Downstate Medical Center, 450 Clarkson Ave., Box 31 Brooklyn, NY 11203, USA.
References
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Andersson A, Nyberg S, Backstrom T. Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology. 2004;30:212–224. doi: 10.1016/j.psyneuen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Angst J, Sellaro R, Merikangas KR, Endicott J. The epidemiology of premenstrual psychological symptoms. Acta Psychiatr Scand. 2001;104:110–116. doi: 10.1034/j.1600-0447.2001.00412.x. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Andreen L, Birzniece V, Bjorn I, Johannson IM, Nordenstam-Haghjo M, Nyberg S, Sundstrom-Poromaa I, Wahlstrom G, Wang M, Zhu D. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. 2003a;17:325–342. doi: 10.2165/00023210-200317050-00003. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Andersson A, Andreen L, Birzniece V, Bixo M, Bjorn I, Haage D, Isaksson M, Johansson IM, Lindblad C, Lundgren P, Nyberg S, Odmark IS, Stromberg J, Sundstrom-Poromaa I, Turkmen S, Wahlstrom G, Wang M, Wihlback AC, Zhu D, Zingmark E. Pathogenesis in menstrual cycle-linked CNS disorders. Ann N Y Acad Sci. 2003b;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA (A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA (A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicikova M, Dibbelt L, Hill M, Hampl R, Starka L. Allopregnanolone in women with premenstrual syndrome. Horm Metab Res. 1998;30:227–230. doi: 10.1055/s-2007-978871. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABA-A receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and luteal septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha (4)beta (3)delta GABA (A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan CM, Eccles JS, Becker JB. Are adolescents the victims of raging hormones: evidence for activational effects of hormones on moods and behavior at adolescence. Psychol Bull. 1992;111:62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Ann N Y Acad Sci. 2004;1021:110–123. doi: 10.1196/annals.1308.012. [DOI] [PubMed] [Google Scholar]
- Cloitre M, Yonkers KA, Pearlstein T, Althemus M, Davidson KW, Pigott TA, Shear MK, Pine D, Ross J, Howell H, Brogan K, Rieckmann N, Clemow L. Women and anxiety disorders: implications for diagnosis and treatment. CNS Spectr. 2004;9:1–16. [PubMed] [Google Scholar]
- Corpechot C, Young J, Clavel M, Wehrey C, Veltz JN, Touter G, Mouren M, Prasad VV, Banner C, Sjovall J, Baulieu E-E, Robel P. Neurosteroids: 3α-OH-5α-pregnan-20-one and its precursors in the brain plasma and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Collins B, Carey M, Tsouros A, Robel P, Fry J. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- Dunn SMJ, Tancowny B, Martin IL. Characterisation of GABA-A receptors containing alpha4-beta1-delta subunits. J Physiol. 2003;548P:5P. [Google Scholar]
- Elliott H. Premenstrual dysphoric disorder. A guide for the treating clinician. N C Med J. 2002;63:72–75. [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewaart D, Thys-Jacobs S. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G. Allopregnanolone synthesis in cerebellar granules cells: roles in regulation of GABA (A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol. 2000;57:1262–1270. [PubMed] [Google Scholar]
- Freeman EW. Premenstrual syndrome and premenstrual dysphoric disorder: definitions and diagnosis. Psychoneuroendocrinology. 2003;28:25–37. doi: 10.1016/s0306-4530(03)00099-4. [DOI] [PubMed] [Google Scholar]
- Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453–468. doi: 10.2165/00023210-200418070-00004. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–520. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neurosteroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Cyclic withdrawal from endogenous and exogenous progesterone increases kainic acid and perforant pathway induced seizures. Pharmacol Biochem Behav. 1999;62:315–321. doi: 10.1016/s0091-3057(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 2004;41:306–315. doi: 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Griffiths J, Lovick T. Withdrawal from progesterone increases expression of alpha4, beta1 and delta GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol. 2005;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- Harney SC, Frenguelli BG, Lambert JJ. Phosphorylation influences neurosteroid modulation of synaptic GABA (A) receptors in rat CA1 and dentate gyrus neurones. Neuropharmacology. 2003;45:873–883. doi: 10.1016/s0028-3908(03)00251-x. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Hsiao CC, Liu CY, Hsiao MC. No correlation of depression and anxiety to plasma estrogen and progesterone levels in patients with premenstrual dysphoric disorder. Psychiatry Clin Neurosci. 2004;58:593–599. doi: 10.1111/j.1440-1819.2004.01308.x. [DOI] [PubMed] [Google Scholar]
- Korneyev A, Costa E. Allopregnanolone (THP) mediates anesthetic effects of progesterone in rat brain. Horm Behav. 1996;30:37–43. doi: 10.1006/hbeh.1996.0006. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Halbreich U. Hormonal treatments for premenstrual syndrome. Drugs Today. 1998;34:603–610. doi: 10.1358/dot.1998.34.7.485258. [DOI] [PubMed] [Google Scholar]
- Le Melledo JM, Van Driel M, Coupland NJ, Lott P, Jhangri GS. Response to flumazenil in women with premenstrual dysphoric disorder. Am J Psychiatry. 2000;157:821–823. doi: 10.1176/appi.ajp.157.5.821. [DOI] [PubMed] [Google Scholar]
- Leidenheimer NJ, Chapell R. Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABA (A) receptors. Brain Res Mol Brain Res. 1997;52:173–181. doi: 10.1016/s0169-328x(97)00255-6. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Griffiths JL, Dunn SM, Martin IL. Changes in GABA(A) receptor subunit expression in the midbrain during the oestrous cycle in Wistar rats. Neuroscience. 2005;131:397–405. doi: 10.1016/j.neuroscience.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D. The anxiolytic effects of intra-hippocampal midazolam are antagonized by intra-septal L-glutamate. Brain Res. 2001;888:163–166. doi: 10.1016/s0006-8993(00)03046-8. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi Z-P, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Zhiwei L, DeLorey TM, Olsen RW, Homanics G. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. PNAS. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- Moran MH, Goldberg M, Smith SS. Progesterone withdrawal II: insensitivity to the sedative effects of a benzodiazepine. Brain Res. 1998;807:91–100. doi: 10.1016/s0006-8993(98)00781-1. [DOI] [PubMed] [Google Scholar]
- Overpeck JG, Colson SH, Hohmann JR, Applestine MS, Reilly JF. Concentration of circulating steroids in normal prepubertal and adult male and female humans, chimpanzees, rhesus monkeys, rats, mice, and hamsters: a literature survey. J Toxicol Environ Health. 1978;4:785–803. doi: 10.1080/15287397809529700. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord. 2005;85:275–282. doi: 10.1016/j.jad.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods. 1995;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Rapkin A. A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology. 2003;28:39–53. doi: 10.1016/s0306-4530(03)00096-9. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Rodbard D, Hutt DM. International Atomic Energy Agency. Statistical analysis of radioimmuno-assay and immunoradiometric assays: a generalized, weighted iterative, least squares method for logistic curve fitting. 1974. pp. 209–223. (Ref Type: Generic) [Google Scholar]
- Schmidt P, Purdy RH, Moore PH, Jr, Paul S, Rubinow D. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Nieman L, Danaceau M, Adams L, Rubinow D. Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Seippel L, Backstrom T. Luteal-phase estradiol relates to symptom severity in patients with premenstrual syndrome. J Clin Endocrinol Metab. 1998;83:1988–1992. doi: 10.1210/jcem.83.6.4899. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu F-C. Withdrawal from 3α-OH-5α-pregnan-20-one withdrawal using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wasserman EJ. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry. 2003a;54:757–762. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Schmidt PJ, Rubinow DR. Operationalizing DSM-IV criteria for PMDD: selecting symptomatic and asymptomatic cycles for research. J Psychiatr Res. 2003b;37:75–83. doi: 10.1016/s0022-3956(02)00053-5. [DOI] [PubMed] [Google Scholar]
- Smythe JW, McCormick CM, Rochford J, Meaney MJ. The interaction between prenatal stress and neonatal handling on nociceptive response latencies in male and female rats. Physiol Behav. 1994;55:971–974. doi: 10.1016/0031-9384(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Steiner M, Streiner DL, Steinberg S, Stewart D, Carter D, Berger C, Reid R, Grover D. The measurement of premenstrual mood symptoms. J Affect Disord. 1999;53:269–273. doi: 10.1016/s0165-0327(98)00121-9. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Backstrom T. Patients with pre-menstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, Backstrom T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. 1998;67:126–138. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Farrar S, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha-4 and delta subunits of the GABA-A receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Tiemstra JD, Patel K. Hormonal therapy in the management of premenstrual syndrome. J Am Board Fam Pract. 1998;11:378–381. doi: 10.3122/15572625-11-5-378. [DOI] [PubMed] [Google Scholar]
- Vicini S, Losi G, Homanics GE. GABA (A) receptor delta subunit deletion prevents neurosteroid modulation of inhibitory synaptic currents in cerebellar neurons. Neuropharmacology. 2002;43:646–650. doi: 10.1016/s0028-3908(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA (A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg P. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA (A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA. Anxiety symptoms and anxiety disorders: how are they related to premenstrual disorders? J Clin Psychiatry. 1997;58:62–69. [PubMed] [Google Scholar]
- Zhu WJ, Wang JF, Krueger KE, Vicini S. Delta subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]