Abstract
In a progesterone withdrawal (PWD) model of premenstrual anxiety, we have previously demonstrated that increased hippocampal expression of the α4 subunit of the GABAA receptor (GABAA-R) is closely associated with higher anxiety levels in the elevated plus maze. However, several studies indicate that sex differences in regulation of the GABAA-R in specific brain regions may be an important factor in the observed gender differences in mood disorders. Thus, we investigated possible sex differences in GABAA-R subunit expression and anxiety during PWD. To this end, we utilized the acoustic startle response (ASR) to assess anxiety levels in male and female rats undergoing PWD as the ASR is also applicable to the assessment of human anxiety responses. We also investigated GABAA-R α4 subunit expression in the amygdala, as the amygdala directly regulates the primary startle circuit. Female rats exhibited a greater ASR during PWD than controls, indicating higher levels of anxiety and arousal. In contrast, male rats undergoing PWD did not demonstrate an increased ASR. The sex differences in the ASR were paralleled by sex differences in the expression of the GABAA-R α4 subunit in the amygdala such that α4 subunit expression was up-regulated in females during PWD whereas α4 levels in males undergoing PWD were not altered relative to controls. These findings might have implications regarding gender differences in human mood disorders and the aetiology of premenstrual anxiety.
Keywords: acoustic startle response, allopregnanolone, amygdala, anxiety, GABAA receptor, neurosteroid, premenstrual syndrome, progesterone withdrawal, sex differences, α4 subunit
Introduction
The regulation of anxiety is integrally associated with the function of the GABAA receptor (GABAA-R; Sanders & Shekhar, 1995; Sundström et al., 1998; Crestani et al., 1999). In fact, unique GABAA-R isoforms in specific brain regions may modulate anxiety differentially (Crestani et al., 1999; Menard & Treit, 1999). Furthermore, endogenous modulators of the GABAA-R, such as the neurosteroid metabolites of progesterone, regulate GABAA-R subunit expression and function (Smith et al., 1998a, b; Gulinello et al., 2001, 2002). There are several lines of evidence that indicate strongly that endogenous fluctuations of neurosteroids, such as those that occur during the human menstrual cycle and the rodent oestrous cycle, could play a role in mood and anxiety disorders.
Acute administration of neurosteroids is anxiolytic (Akwa et al., 1999; Bitran et al., 1995), consistent with their role as positive modulators of GABAA-R-gated chloride influx (Majewska et al., 1986). However, in contrast to acute administration, chronic administration of, and withdrawal from, 3α-5α-THP increases GABAA-R α4 subunit expression, decreases total GABA-gated current and alters the GABAA-R response to several classes of GABA modulatory drugs (Smith et al., 1998a, b; Gulinello et al., 2002). Chronic exposure to and/or withdrawal from chronically elevated neurosteroid levels is also correlated with hyperexcitability, anxiety and cognitive deficits (Smith et al., 1998a, b; Ladurelle et al., 2000; Reilly et al., 2000; Gulinello et al., 2002; Johansson et al., 2002). This is consistent with the withdrawal effects of other GABA modulators, such as benzodiazepines, barbiturates and alcohol (Rassnick et al., 1992; Suzuki et al., 1992; Rasmussen et al., 1993; Follesa et al., 2001;).
Many of the effects of progesterone withdrawal (PWD) are observed in both male and female rats (Gulinello et al., 2002). These data are consistent with the body of evidence suggesting that neurosteroids are relevant modulators of behaviour in males in addition to females (Uzunova et al., 1998; Ladurelle et al., 2000). However, other groups have demonstrated gender differences in GABAA-R function (Wilson & Biscardi, 1997), in the anxiolytic effects of 3α-5α-THP (Wilson & Biscardi, 1997; Zimmerberg et al., 1999) and in animal models of anxiety and mood disorders (Johnston & File, 1991; Lehmann et al., 1999; Zimmerberg et al., 1999). Although apparent gender differences in anxiety behaviours could be dependent on the paradigm utilized to assess anxiety (Johnston & File, 1991), the majority of these data corroborate the reports of gender differences in the prevalence and severity of human mood disorders (Shear, 1997; Pigott, 1999). We therefore further investigated gender-dependent responses of anxiety and GABAA-R subunit expression in the PWD model.
To this end, we utilized the acoustic startle reflex to assess anxiety levels after PWD in male and female rats. The startle reflex is a whole body response to auditory stimuli which has a similar circuitry and pharmacology in humans as it does in animals (Davis, 1980; Lang et al., 1990; Davis et al., 1994; Koch, 1999; Schachinger et al., 1999). Altered acoustic startle responses (ASR) have been demonstrated in anxiety and depressive disorders in humans (Morgan et al., 1996; Allen et al., 1999; Medina et al., 2001) and in animal models of these disorders (Davis et al., 1997; Schwegler et al., 1997; Stohr et al., 1999; Plappert & Pilz, 2002). Furthermore, the ASR is relevant to PWD as chronic exposure and withdrawal from other GABAA-R modulators (such as alcohol or benzodiazepines) also increases the ASR (Rassnick et al., 1992; Rasmussen et al., 1993; (Krystal et al., 1997; Ponomarev & Crabbe, 1999) and can alter the pharmacological effects of GABAA-R modulators (Davis & Gallager, 1988).
Sensorimotor gating was also assessed by measuring prepulse inhibition (PPI) of the startle response. In this protocol, the magnitude of the response to a target startle stimulus is reduced by the prior presentation of a low-intensity, nonstartling stimulus (Ison et al., 1973). The PPI component of the ASR is thought to reflect an individual’s ability to screen or ‘gate’ sensory stimuli.
Both the magnitude of the ASR and sensorimotor gating of the ASR are subject to regulation by GABAA-R in several relevant brain regions, notably, the amygdala (Davis, 1980; Davis et al., 1994; Fendt et al., 2000). We also therefore determined GABAA-R subunit immunoreactivity in the amygdala after PWD.
Materials and methods
Animals
Male and female Long-Evans rats (Charles River) were housed in single gender pairs under a 14 h light : 10 h dark cycle (lights on at 0700 h) with food and water available ad libitum. All animals were tested during the light portion of the circadian cycle between 09.00 h and 14.00 h. In female rats, the stage of the oestrous cycle was determined by microscopic examination of the vaginal lavage and by measures of vaginal impedance, as described previously (Gulinello et al., 2002), throughout one entire cycle before testing. Male rats were handled for the same amount of time. Only females in dioestrus were used as subjects. Following the appropriate treatment (see below), animals were either tested behaviourally or were killed by decapitation, the amygdala removed and frozen on dry ice for isolation of plasma membrane fractions and subsequent Western blot analysis. Both female and male rats weighed 250 g (females ≈70–80 days old; males ≈60–70 days old) at the time of testing. All protocols were approved by the institutional Animal Care and Use Committee.
Hormone administration
Progesterone was administered rather than 3α,5α-THP because it is known that elevated circulating levels of progesterone, such as those found during the oestrous (or menstrual) cycle or after stress (Purdy et al., 1991; Frye et al., 2000; Girdler et al., 2001) are readily converted to 3α,5α-THP in the brain and result in 3α-5α-THP levels sufficient to potentiate GABAergic inhibition (Bitran et al., 1995) and modulate GABAA-R subunit expression (Smith et al., 1998a, b). Progesterone implants were made from silicone tubing as described previously and implanted subcutaneously under halothane anaesthesia in the abdominal area of the rat (Smith et al., 1998b) for 21 days. This method has been shown to result in central nervous system levels of 3α-5α-THP in the high physiological range (6–12 ng/g hippocampal tissue; Moran & Smith, 1998). Control animals were implanted with empty (sham) silicone capsules. Animals were tested 24 h after removal of the implant (progesterone withdrawal).
Acoustic startle
Acoustic startle magnitude was assessed using an S-R Laboratory (San Diego Instruments, San Diego, CA, USA). Rats were placed in a 20 cm × 32 cm plexiglass cylinder attached to a piezoelectric transducer platform to detect the motion of the rat. Movement of the platform results in a voltage change in the transducer, which was digitized and analysed by the S-R Laboratory program on an attached computer. After a 5 min period of acclimatization to 65 dB background noise, rats were presented with nine consecutive trials of 120 dB sound pulses of 40 ms duration in a habituation trial. Immediately thereafter startle magnitude and threshold were assessed by presentation of broadband noise of varying intensities (0, 90, 110 or 120 dB) presented a total of five times in random order with random time intervals separating each trial. Startle magnitude was defined as an average of responses to each intensity of stimulus and is illustrated in the Results sections either as the maximum or the total integrated startle response. Prepulse inhibition was then assessed by presentation of a low intensity, nonstartling pulse immediately (80 ms) preceding a target 120 dB pulse. Prepulses of 2, 4, 8, and 16 dB above background were presented a total of five times each in random order in addition to five target pulses of 120 dB not preceded by prepulses. Results from PPI trials are illustrated as mean percentage inhibition compared with the mean response to 120 dB target pulses (%PPI). In addition, the absolute startle magnitude (total Integral) to the target pulse following the prepulse is also represented.
Western blot α4 levels were measured in amygdala crude membrane fractions using Western blot procedures explained in detail elsewhere (Smith et al., 1998b). Immunoreactivity of the α4 band (67 kDa) was probed with an antibody developed against a peptide sequence of the rat α4 subunit (amino acids 517–523) using ECL (enhanced chemiluminescence) detection and quantified using One-Dscan software (Smith et al., 1998a). The results were standardized to a glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 36 kDa) control protein and were then expressed as a ratio of the average optical density of control values (Gulinello et al., 2002).
Materials
Chemicals were obtained from Sigma (St Louis, MO, USA) unless otherwise indicated. The α4 antibody was produced by Sigma Genosys (The Woodlands, TX, USA), and the GAPDH antibody Chemicon (Temecula, CA, USA). ECL supplies were provided by Pierce Biotechnology (Rockford, IL, USA). Silicone tubing and adhesive were obtained from Nalgene Co. (Rochester, NY, USA) and Dow Corning Corp. (Midland, MI), respectively.
Statistical analysis
Differences between groups in Western blots were assessed using an unpaired Student’s t-test (two-tailed). Data from the startle test and PPI were analysed in a two-way analysis of variance (ANOVA) with weighted means (condition × sex) followed by post hoc t-tests (Bonferonni/Dunnet). Statistical significance for each analysis is indicated in the relevant Results section. Slopes of the startle habituation curves were also analysed following linear regression and are indicated in the Results.
Results
PWD increases acoustic startle response in female but not male rats
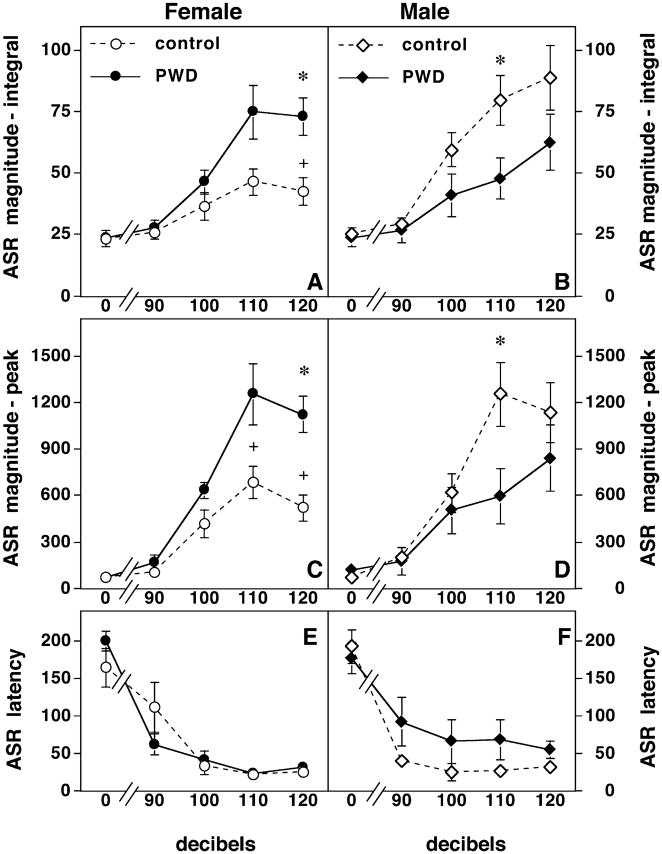
There were significant gender differences in anxiety as measured by the acoustic startle magnitude in male control rats compared with female controls, such that female rats startle less than their male counterparts (P <0.01, Fig. 1). In addition, female rats undergoing PWD had a significantly higher ASR compared to sham implanted females (Fig. 1) as assessed by an increased total integrated startle response (Fig. 1A) and by the maximum response (Fig. 1C, P <0.01). In contrast, male rats undergoing PWD had a significantly decreased ASR compared with sham implanted controls at one stimulus intensity (Fig. 1B and D; P <0.01). There were no significant differences in the latency of the startle response in any of the treatment groups (Fig. 1E and F). We have previously demonstrated that locomotor activity is not significantly affected by PWD (Gulinello et al., 2002). Additionally, there were no mean differences in body weight across gender or treatment conditions by MANOVA analysis of startle data [Female-control =250 g ±0.10; Female-PWD =260 g ±0.10; Male-control 262 g ±0.11; Male-PWD 273 g ±0.10: F(weight)condition × sex = 0.22, P <0.883].
Fig. 1.
Progesterone withdrawal results in sex differences in the acoustic startle response. This figure illustrates the sex differences in measure of acoustic startle response (ASR, vertical axis) to varying sound pulses (horizontal axis). (A and C) PWD increases the ASR in female rats. Both the total integrated ASR (A) and the peak ASR (C) is higher in female rats undergoing PWD (n =7 closed circles) than in sham implanted controls (n =7, open circles). Significant differences between PWD and control groups (P <0.01) are indicated by (*), whereas significant sex differences between controls (P <0.01) are indicated by (+) in this and the following graphs. (B and D) PWD decreases the ASR in male rats. Both the total integrated ASR (B) and the peak ASR (D) are lower in male rats undergoing PWD (n =7, closed triangles) than in sham implanted controls (n =7, open triangles). Furthermore, male control rats startle significantly more than control females and males undergoing PWD startle significantly less than control males. (E and F) Latency of the ASR is not affected by PWD. There are no significant differences in the latency of the ASR across treatment conditions or sex.
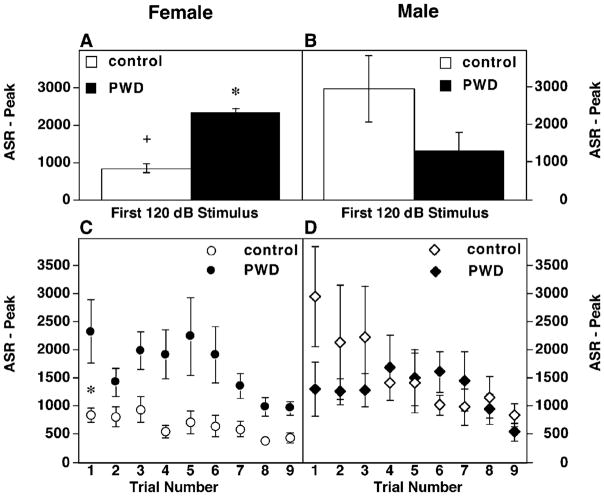
Similar anxiogenic effects of PWD in female rats were also evident when an analysis of the response to the first stimulus was performed (Fig. 2). This measure is generally assumed to be free of effects of sensitization and habituation (Plappert & Pilz, 2002). Female rats undergoing PWD demonstrated a roughly twofold increase in peak ASR to the first presentation of a 120 dB stimulus (Fig. 2A; P <0.02). Although males undergoing PWD were not significantly different relative to sham implanted males (Fig. 2B), control males had a higher peak ASR than control females (Fig. 2A and B).
Fig. 2.
Response to the first stimulus and habituation curves. (A and B) Peak ASR to the first presentation of an acoustic stimulus (120 dB) in female (A) or male (B) rats. Sample sizes are the same as the previous graph. Statistically significant increased ASR in PWD females relative to controls is indicated by (*, P <0.05). Increased ASR in control males relative to control females is indicated by (+, P <0.05). (C and D) Habituation curve of the peak ASR to nine consecutive presentations (trial no, horizontal axis) of a 120 dB stimulus in female (C) or male (D) rats.
The habituation responses to the first nine consecutive presentations of a 120 dB stimulus were also examined (Fig. 2C and D). The slope of the habituation curve in both PWD females (−33.6 ±49.35) and control females (−59.27 ±13.59) was significantly different than 0 (P <0.03 and P <0.003, respectively). Furthermore, the slopes between PWD and controls animals did not significantly differ in females (P <0.2) indicating that habituation occurs in both groups and is not impaired by PWD. In contrast the elevation, or y-intercept, of the slopes was significantly different between females undergoing PWD (2336 ±251.8) and controls (1015 ±76.48, P <0.001), indicating a higher startle magnitude during PWD throughout the habituation trials.
This pattern was different in males. Male controls had a significantly steeper habituation slope (−236.7 ±39.89, P <0.001) than male rats undergoing PWD (−62.15 ±43.05). Furthermore, male controls had a significantly steeper habituation slope than control females (P <0.001), a characteristic that has been reported previously (Rinaldi & Thompson, 1985; Blaszczyk & Tajchert, 1996; Lehmann et al., 1999).
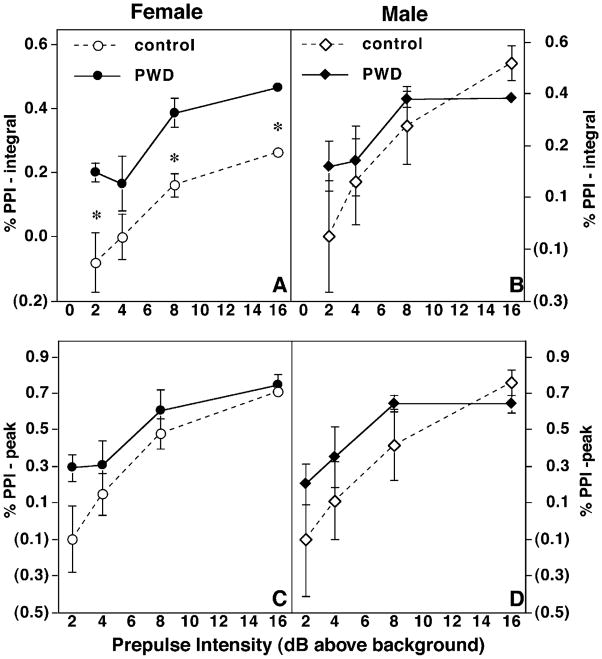
PWD increases sensorimotor gating in female but not male rats
Despite the fact that female rats undergoing PWD had a larger ASR, sensorimotor gating (as measured by prepulse inhibition) was preserved in these subjects (Fig. 3). Presentation of a nonstartling stimulus (2–16 dB above background) reliably results in a decreased startle response to a target pulse of 120 dB, known as prepulse inhibition (PPI). Female rats undergoing PWD had significantly increased PPI when these data were expressed as a percentage of the total integrated target pulse response (P <0.01, Fig. 3A). In contrast, male rats undergoing PWD did not differ in any measure of sensorimotor gating when compared to controls (Fig. 3). There were no significant differences in percentage PPI between male and female control rats. Female rats undergoing PWD had no significant differences in PPI compared with control rats as a percentage of the maximum response to the target pulse (Fig. 3).
Fig. 3.
PWD increases sensorimotor gating in female rats. Sensorimotor gating is indicated as percentage PPI (either percentage total integrated response or percentage peak response, vertical axis) following presentation of increasing decibels of acoustic stimuli (horizontal axis). (A and B) PWD increases percentage PPI in female rats. Sensorimotor gating is increased in female rats undergoing PWD when gating is assessed as a percentage of inhibition relative to the total integrated target pulse (A) or as a percentage of the peak response (B). Sample sizes and significance levels are the same as in Fig. 2. (C and D) PWD does not alter percentage PPI in males. Relative PPI is not affected in male rats undergoing PWD when expressed as percentage integrated response (C) or as percentage peak response (D), nor are there any significant sex differences in percentage PPI.
Sex differences in the GABAA-R α4 subunit expression in the amygdala following PWD
The levels of GABAA-R α4 subunit in the amygdala increased 2–3-fold after PWD in female rats. In contrast, there were no significant changes in α4 subunit expression in male rat amygdala membranes (Fig. 4). The levels of GAPDH control protein did not differ between treatment conditions. These data differ from our previously published results in the hippocampus, where the α4 subunit is upregulated in the hippocampus of both male and female rats during PWD (Gulinello et al., 2002)
Fig. 4.
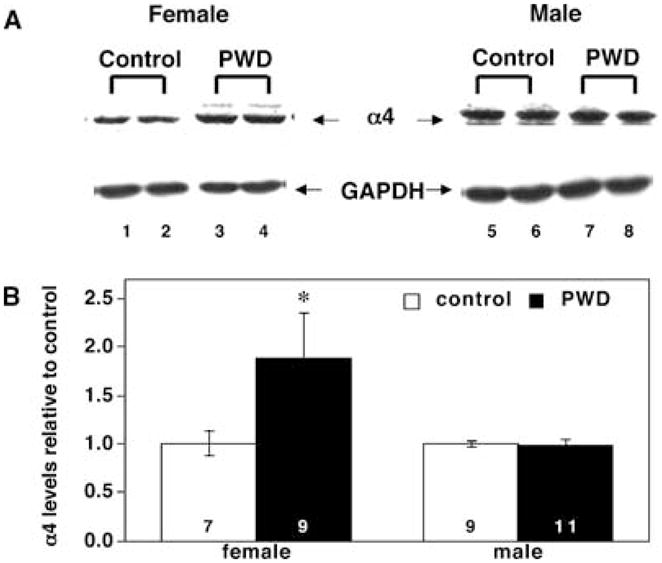
PWD increases the expression of the α4 subunit of the GABAA-R in the amygdala of female but not male rats. (A) Representative Western blot. This blot illustrates sex differences in expression of the α4 subunit in the amygdala following PWD. Lanes 1 and 2, female control; lanes 3 and 4, female PWD; lanes 5 and 6, male control; lanes 7 and 8, male PWD. α4 Subunit immunoreactivity is indicated by arrows. The levels of the GAPDH control protein (also indicated by arrows) do not change in any treatment condition. (B) Averaged data. Immunoreactivity of the α4 subunit of the GABAA-R, averaged data. The sample size for each group is indicated by the numbers at the base of each bar and statistical significance (*) is P <0.01.
Discussion
These data demonstrate increased, gender-dependent expression of the α4 subunit of the GABAA-R in the amygdala in association with increased anxiety (measured by increased acoustic startle response) in female rats undergoing PWD. In contrast, male rats exhibit neither increased ASR nor any alteration in α4 subunit expression in the amygdala during PWD. The results from this rodent model of pre-menstrual hormone fluctuations are consistent with the fact that there are corresponding gender differences in the prevalence and severity of mood disorders in humans, which are thought to be related to neuroendocrine factors (Shear, 1997; Pigott, 1999).
The increased anxiety demonstrated in female rats undergoing PWD occurs in close association with increased expression of the α4 subunit of the GABAA-R in several tasks (Gallo & Smith, 1993; Smith et al., 1998b; Gulinello et al., 2002). During PWD, female rats exhibit increased anxiety in the buried shock-probe paradigm (Gallo & Smith, 1993), and in the elevated plus maze (Smith et al., 1998b; Gulinello et al., 2002) in addition to the increased startle magnitude demonstrated here. Withdrawal from 3α-5α-THP after chronic exposure to its parent compound, progesterone, also decreases the sensitivity of the GABAA-R to several GABAA-R modulators (Smith et al., 1998a, b; Gulinello et al., 2001, 2002) and decreases total GABA-gated current (Smith et al., 1998a, b). Our recent studies (Sundstrom-Poromaa et al., 2002) also demonstrate that the ASR is uniquely sensitive to modulation by low deses of alcohol in female rats following PWD, an effect produced by increased expression of α4βδ GABAA-R at this time. These phenomena could contribute to relevant behavioural outcomes, such as changes in emotional behaviour, because the degree of sensitivity to GABAA-R modulators is correlated with the severity of negative symptoms in mood disorders (Sundström et al., 1998).
However, whereas males subjected to the PWD paradigm exhibit increased anxiety in the elevated plus maze (Gulinello et al., 2002) they do not demonstrate increased startle magnitude as do their female counterparts, suggesting that the effects of PWD are regulated in a gender-dependent way in specific brain regions. One possible explanation of these results is that neurotransmitter systems relevant to the modulation of anxiety might be regulated by neurosteroid withdrawal in a sexually dimorphic way in specific brain regions.
There are indeed sexually dimorphic features of limbic brain regions known to regulate anxiety, and these include the amygdala (Reisert et al., 1996; Wilson & Biscardi, 1997; Stefanova, 1998). The primary startle pathway consists of the auditory nerve, the cochlear root neurons, the nucleus of the lateral lemniscus, the pontine nuclei and spinal interneurons and motorneurons (Fendt & Fanselow, 1999; Koch, 1999). This primary circuit is regulated through a direct input from the amygdala (and other regions) (Davis et al., 1982; Rosenet al., 1991; Fendt & Fanselow, 1999; Koch, 1999). GABAA-R expression is also regulated in a sex-dependent manner (Stefanova, 1998; Wilson & Biscardi, 1997) which could be an important factor in the regulation of the ASR (Kellogg et al., 1991; Fendt, 1999; Koch, 1999; Fendt et al., 2000; Bast et al., 2001).
The significant sex differences in α4 subunit expression in the amygdala closely parallel sex differences in behaviour following PWD. In fact, although we have previously demonstrated that PWD increases expression of the α4 subunit in the hippocampus of both male and female rats, in the present study we show that PWD increases α4 subunit expression in the amygdala only in female rats. Several groups have noted similar sex differences after withdrawal from other GABAA-R modulators, such as benzodiazepines, barbiturates and ethanol (Devaud et al., 1999; Suzuki et al., 1992; Pesce et al., 1994). It is interesting to note in this context that alcohol withdrawal, which also increases α4 subunit expression in the hippocampus (Devaud et al., 1997; Mahmoudi et al., 1997), decreases expression of the α4 subunit of the GABAA-R in the amygdala of male rats (Papadeas et al., 2001).
In this context, it is also noteworthy that males have a higher baseline startle response than females. The increased startle response, in addition to other characteristic sex differences, such as the steeper habituation curve evident in males, which have been previously demonstrated by other groups (Rinaldi & Thompson, 1985; Blaszczyk & Tajchert, 1996; Lehmann et al., 1999), are reversed by chronic exposure to and withdrawal from progesterone in males. There are several variables that might contribute to this pattern of results. It has been suggested elsewhere that the apparently higher startle response in control males could be partially a function of a stronger muscular response in male animals (Blaszczyk & Tajchert, 1996). This hypothesis would be consistent with the lack of sex differences in control animals that we have reported previously in the elevated plus maze (Gulinello et al., 2002).
Alternatively, these sex differences in baseline startle in control animals could be the result of sexually dimorphic reactions to stressors (Akinci & Johnston, 1993; Farabollini et al., 1996; Wood et al., 2001; Figueiredo et al., 2002). It has been demonstrated elsewhere, for example, that chronic testosterone exposure has opposite effects on behaviour and GABA-ergic transmission as exposure to progesterone (Bitran et al., 1993), suggesting that testosterone might also play a role in the manifestation of anxiety. Furthermore, both oestrogen and testosterone regulate the sexually dimorphic response to stressors (Viau & Meaney, 1996; Shors et al., 1999; Wood et al., 2001). The higher oestrogen levels in females could also be important in the manifestation of anxiety, as oestrogen interacts with progesterone and 3α-5α-THP to regulate neuronal excitability and anxiety (Becker & Rudick, 1999; Chesler & Juraska, 2000; Cyr et al., 2000; Laconi et al., 2001) and might also have independent effects on both inhibitory and excitatory transmission (Woolley, 1999).
These factors could also be important in the striking differences between male and female rats undergoing PWD. Other groups have also demonstrated that withdrawal from GABA-modulators is more severe in females than males (Pesce et al., 1994). However, it is also possible that male startle responses have been ‘feminized’ by chronic exposure to progesterone. It has certainly been demonstrated elsewhere that exposure to progesterone in males can induce patterns of behaviour typical of females (Fraile et al., 1988). Although we have previously demonstrated that the PWD syndrome is not replicated by chronic exposure to progesterone in females (Smith et al., 1998b; Gulinello et al., 2001) we can not rule out that there are such effects in males.
Sensorimotor gating is preserved and even augmented in female rats undergoing PWD despite their elevated initial startle response. The increased startle magnitude and the preservation of intact sensorimotor gating in this rodent model of premenstrual dysphoria are consistent with the fact that the primary symptoms of this disorder tend to be affective and mood symptoms rather than other psychiatric deficits that are more closely associated with disrupted gating (Braff et al., 1978; Perry et al., 1999).
Although these data might initially appear paradoxical, it has been demonstrated previously that PPI and startle magnitude can be regulated differentially (Bullock et al., 1997; Stohr et al., 1999) and we suggest that these results might be consistent with alterations in GABA-gated current resulting from increased α4 subunit expression during PWD (Smith et al., 1998a; Smith et al., 1998b). The increased ASR during PWD in females is certainly consistent with the body of literature demonstrating that the ASR is reliably enhanced by GABAA-R antagonists and is conversely decreased by GABA-ergic anxiolytics (Davis & Gallager, 1988; Hijzen & Slangen, 1989; Kellogg et al., 1991; Davis et al., 1994; Davis, 1998; Guscott et al., 2000; Walker & Davis, 2002).
This, of course, does not account for the fact that PPI is preserved during PWD despite the increased ASR, although there are precedents for such responses. Anticipatory anxiety and greater attention to threat cues can increase PPI (Grillon & Davis, 1997). Furthermore, GABAB receptor mutant mice also show increased behavioural excitability in conjunction with increased PPI as the result of their decreased receptor function (Prosser et al., 2001). However, the literature regarding the pharmacology and mechanisms of the regulation of PPI is not always consistent, and is a function of the specific brain region investigated (Kodsi & Swerdlow, 1995; Bast et al., 2001; Swerdlow et al., 2001). This could be an important factor, as the expression of the atypical α4 subunit is also restricted to specific brain regions (Pirker et al., 2000).
Several groups have demonstrated that that PPI is disrupted by agents that increase GABAergic inhibition, such as benzodiazepines (Depoortere et al., 1997; Schachinger et al., 1999) and high levels of neurosteroids during proestrus (Koch, 1998). Furthermore, blockade of GABAA-R in the superior colliculus, where there is α4 expression, also increases PPI (Fendt, 1999; Pirker et al., 2000). Conversely, activation of GABAA-R in regions with strong α4 expression reduces PPI (Kodsi & Swerdlow, 1997). In contrast, blocking GABAA-R function by picrotoxin in the ventral striato-pallidal pathway reduces PPI. However, α4 subunits are not expressed there substantially, and are unlikely to affect PPI through these pathways (Pirker et al., 2000). Furthermore, whereas GABAA-R antagonists injected into the amygdala reduce PPI, so do glutamate receptor antagonists (Bakshi et al., 1999; Fendt et al., 2000), which strongly indicates that PPI is a network property, regulated by specific cell types in specific brain regions, and not a simple function of general excitability (Bakshi & Geyer, 1998; Swerdlow et al., 2001).
In addition, the characteristics of PPI are also regulated by several other major neurotransmitter systems (Fendt et al., 2000, 2001; Braff et al., 2001) which have not been investigated here. It has, for example, been demonstrated previously that both 3α-5α-THP and progesterone have neuroleptic properties that are mediated through non-GABAA-R mechanisms (Maurice et al., 1999; Rupprecht et al., 1999).
In summary, in a rodent model of premenstrual anxiety, we have demonstrated sex differences in expression of the α4 subunit of the GABAA-R in the amygdala which occur in association with sex differences in the manifestation of anxiety in a task that is influenced by the amygdala. Further investigation of sex differences in the regulation of GABAA-R subunit expression has important implications for the underlying aetiology and gender differences in human mood disorders.
Acknowledgments
This work was supported by NIH grants DA09618 and AA 12958 and contracts from Merck to S.S.S. We are grateful to Diana Dow-Edwards and Sue Melnick for the use of their startle equipment and to Yevgeniy Ruderman and Cristianne Toussaint for technical assistance.
Abbreviations
- 3α-5α-THP
3α-hydroxy-5α-pregnan-20-one or allopregnanolone
- ASR
acoustic startle response
- dB
decibel
- ECL
enhanced chemiluminescence
- GABAA-R
GABAA receptor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- PPI
prepulse inhibition
- PWD
progesterone withdrawal
References
- Akinci M, Johnston G. Sex differences in acute swim stress-induced changes in the binding of MK-801 to the NMDA subclass of glutamate receptors in mouse forebrain. J Neurochem. 1993;61:2290–2293. doi: 10.1111/j.1471-4159.1993.tb07472.x. [DOI] [PubMed] [Google Scholar]
- Akwa Y, Purdy R, Koob G, Britton K. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–125. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: preliminary findings. Biol Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J Neurosci. 1998;18:8394–8401. doi: 10.1523/JNEUROSCI.18-20-08394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, Tricklebank M, Neijt HC, Lehmann-Masten V, Geyer MA. Disruption of prepulse inhibition and increases in locomotor activity by competitive N-methyl-D-aspartate receptor antagonists in rats. J Pharmacol Exp Ther. 1999;288:643–652. [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Hyperactivity, decreased startle reactivity, and disrupted prepulse inhibition following disinhibition of the rat ventral hippocampus by the GABA (A) receptor antagonist picrotoxin. Psychopharmacology (Berl) 2001;156:225–233. doi: 10.1007/s002130100775. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Blaszczyk J, Tajchert K. Sex and strain differences of acoustic startle reaction development in adolescent albino Wistar and hooded rats. Acta Neurobiol Exp. 1996;56:919–925. doi: 10.55782/ane-1996-1199. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Bullock AE, Slobe BS, Vazquez V, Collins AC. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav Neurosci. 1997;111:1353–1360. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Juraska JM. Acute administration of estrogen and progesterone impairs the acquisition of the spatial morris water maze in ovariectomized rats. Horm Behav. 2000;38:234–242. doi: 10.1006/hbeh.2000.1626. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent J, Belzung C, Fritschy J, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. J Neuroendocrinol. 2000;12:445–452. doi: 10.1046/j.1365-2826.2000.00471.x. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurochemical modulation of sensory-motor reactivity: acoustic and tactile startle reflexes. Neurosci Biobehav Rev. 1980;4:241–263. doi: 10.1016/0149-7634(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Davis M. Anatomic and physiologic substrates of emotion in an animal model. J Clin Neurophysiol. 1998;15:378–387. doi: 10.1097/00004691-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Davis M, Gallager DW. Continuous slow release of low levels of diazepam produces tolerance to its depressant and anxiolytic effects on the startle reflex. Eur J Pharmacol. 1988;150:23–33. doi: 10.1016/0014-2999(88)90746-7. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann NY Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Perrault G, Sanger DJ. Potentiation of prepulse inhibition of the startle reflex in rats: pharmacological evaluation of the procedure as a model for detecting antipsychotic activity. Psychopharmacology (Berl) 1997;132:366–374. doi: 10.1007/s002130050357. [DOI] [PubMed] [Google Scholar]
- Devaud L, Fritschy J, Sieghart W, Morrow A. Bidirectional alterations of GABA (A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Matthews DB, Morrow AL. Gender impacts behavioral and neurochemical adaptations in ethanol- dependent rats. Pharmacol Biochem Behav. 1999;64:841–849. doi: 10.1016/s0091-3057(99)00164-1. [DOI] [PubMed] [Google Scholar]
- Farabollini F, Fluck E, Albonetti M, File S. Sex differences in benzodiazepine binding in the frontal cortex and amygdala of the rat 24 hours after restraint stress. Neurosci Lett. 1996;218:177–180. doi: 10.1016/s0304-3940(96)13158-x. [DOI] [PubMed] [Google Scholar]
- Fendt M. Enhancement of prepulse inhibition after blockade of GABA activity within the superior colliculus. Brain Res. 1999;833:81–85. doi: 10.1016/s0006-8993(99)01525-5. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schwienbacher I, Koch M. Amygdaloid N-methyl-D-aspartate and γ-aminobutyric acid A receptors regulate sensorimotor gating in a dopamine-dependent way in rats. Neuroscience. 2000;98:55–60. doi: 10.1016/s0306-4522(00)00086-5. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, Massa F, Speranza Desole M, Carta M, Busonero F, Sanna E, Biggio G. Increase in expression of the GABA (A) receptor alpha (4) subunit gene induced by withdrawal of, but not by long-term treatment with, benzodiazepine full or partial agonists. Brain Res Mol Brain Res. 2001;92:138–148. doi: 10.1016/s0169-328x(01)00164-4. [DOI] [PubMed] [Google Scholar]
- Fraile IG, McEwen BS, Pfaff DW. Comparative effects of progesterone and alphaxalone on aggressive, reproductive and locomotor behaviors. Pharmacol Biochem Behav. 1988;30:729–735. doi: 10.1016/0091-3057(88)90091-3. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha- THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Gallo M, Smith S. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology. 1997;34:511–517. doi: 10.1111/j.1469-8986.1997.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha4 GABA (A) receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong Q, Smith S. Progesterone withdrawal increases the alpha4 subunit of the GABAA receptor in male rats in association with anxiety and altered pharmacology – A comparison with female rats. Neuropharmacology. 2002;43:702–715. doi: 10.1016/s0028-3908(02)00171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guscott MR, Cook GP, Bristow LJ. Contextual fear conditioning and baseline startle responses in the rat fear-potentiated startle test: a comparison of benzodiazepine/gamma-aminobutyric acid-A receptor agonists. Behav Pharmacol. 2000;11:495–504. doi: 10.1097/00008877-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Hijzen TH, Slangen JL. Effects of midazolam, DMCM and lindane on potentiated startle in the rat. Psychopharmacology. 1989;99:362–365. doi: 10.1007/BF00445558. [DOI] [PubMed] [Google Scholar]
- Ison JR, McAdam DW, Hammond GR. Latency and amplitude changes in the acoustic startle reflex of the rat produced by variation in auditory prestimulation. Physiol Behav. 1973;10:1035–1039. doi: 10.1016/0031-9384(73)90185-6. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Birzniece V, Lindblad C, Olsson T, Backstrom T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Johnston A, File S. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Kellogg CK, Sullivan AT, Bitran D, Ison JR. Modulation of noise-potentiated acoustic startle via the benzodiazepine – gamma-aminobutyric acid receptor complex. Behav Neurosci. 1991;105:640–646. doi: 10.1037//0735-7044.105.5.640. [DOI] [PubMed] [Google Scholar]
- Koch M. Sensorimotor gating changes across the estrous cycle in female rats. Physiol Behav. 1998;64:625–628. doi: 10.1016/s0031-9384(98)00098-5. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Ventral pallidal GABA-A receptors regulate prepulse inhibition of acoustic startle. Brain Res. 1995;684:26–35. doi: 10.1016/0006-8993(95)00372-w. [DOI] [PubMed] [Google Scholar]
- Kodsi MH, Swerdlow NR. Regulation of prepulse inhibition by ventral pallidal projections. Brain Res Bull. 1997;43:219–228. doi: 10.1016/s0361-9230(96)00440-6. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, 3rd, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m- chlorophenylpiperazine (mCPP) Psychopharmacology (Berl) 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Laconi MR, Casteller G, Gargiulo PA, Bregonzio C, Cabrera RJ. The anxiolytic effect of allopregnanolone is associated with gonadal hormonal status in female rats. Eur J Pharmacol. 2001;417:111–116. doi: 10.1016/s0014-2999(01)00865-2. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Eychennea B, Dentonb D, Blair-Westb Schumachera J, Robela MP, Baulieu E. Prolonged intracerebroventricular infusion of neurosteroids affects cognitive performances in the mouse. Brain Res. 2000;858:371–379. doi: 10.1016/s0006-8993(00)01953-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–395. [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Feldon J. Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav Brain Res. 1999;104:113–117. doi: 10.1016/s0166-4328(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang M, Tillakaratne N, Tobin A, Olsen R. Chronic intermittent ethanol treatment in rats increases GABA (A) receptor alpha4-subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Majewska M, Harrison N, Schwartz R, Barker J, Paul S. Steroid hormone metabolites are barbiturate like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- Medina AM, Mejia VY, Schell AM, Dawson ME, Margolin G. Startle reactivity and PTSD symptoms in a community sample of women. Psych Res. 2001;101:157–169. doi: 10.1016/s0165-1781(01)00221-9. [DOI] [PubMed] [Google Scholar]
- Menard J, Treit D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev. 1999;23:591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- Moran M, Smith S. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Morgan CA, Grillon C, Southwick SM, Davis M, Charney DS. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. Am J Psychiatry. 1996;153:64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA (A) receptor alpha1 and alpha4 subunit peptide expression and GABA (A) receptor-mediated 36 Cl (−) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry. 1999;56:277–281. doi: 10.1001/archpsyc.56.3.277. [DOI] [PubMed] [Google Scholar]
- Pesce M, Acevedo X, Pinardi G, Miranda H. Gender differences in diazepam withdrawal syndrome in mice. Pharmacol Toxicol. 1994;75:353–355. doi: 10.1111/j.1600-0773.1994.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Pigott TA. Gender differences in the epidemiology and treatment of anxiety disorders. J Clin Psychiatry. 1999;60:4–15. [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA (A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PKD. Difference in anxiety and sensitization of the acoustic startle response between the two inbred mouse strains BALB/cAN and DBA/2N. Genes Brain Behav. 2002;1:176–186. doi: 10.1034/j.1601-183x.2002.10306.x. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. Genetic association between chronic ethanol withdrawal severity and acoustic startle parameters WSP and WSR mice. Alcohol Clin Exp Res. 1999;23:1730–1735. [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epilepto-genesis and enhanced prepulse inhibition in GABA (b1)-deficient mice. Mol Cell Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- Purdy P, Morrow A, Moore P, Paul S. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K, Helton DR, Berger JE, Scearce E. The CCK-B antagonist LY288513 blocks effects of diazepam withdrawal on auditory startle. Neuroreport. 1993;5:154–156. doi: 10.1097/00001756-199311180-00015. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Reilly MT, Crabbe JC, Rustay NR, Finn DA. Acute neuroactive steroid withdrawal in withdrawal seizure-prone and withdrawal seizure-resistant mice. Pharmacol Biochem Behav. 2000;67:709–717. doi: 10.1016/s0091-3057(00)00416-0. [DOI] [PubMed] [Google Scholar]
- Reisert I, Lieb K, Beyer C, Pilgrim C. Sex differentiation of rat hippocampal GABAergic neurons. Eur J Neurosci. 1996;8:1718–1724. doi: 10.1111/j.1460-9568.1996.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi PC, Thompson RF. Age, sex and strain comparison of habituation of the startle response in the rat. Physiol Behav. 1985;35:9–13. doi: 10.1016/0031-9384(85)90164-7. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJ, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behav Neurosci. 1991;105:817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Koch M, Montkowski A, Lancel M, Faulhaber J, Harting J, Spanagel R. Assessment of neuroleptic-like properties of progesterone. Psychopharmacology (Berl) 1999;143:29–38. doi: 10.1007/s002130050916. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schachinger H, Muller BU, Strobel W, Langewitz W, Ritz R. Midazolam effects on prepulse inhibition of the acoustic blink reflex. Br J Clin Pharmacol. 1999;47:421–426. doi: 10.1046/j.1365-2125.1999.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegler H, Pilz PK, Koch M, Fendt M, Linke R, Driscoll P. The acoustic startle response in inbred Roman high- and low-avoidance rats. Behav Genet. 1997;27:579–582. doi: 10.1023/a:1021465217299. [DOI] [PubMed] [Google Scholar]
- Shear MK. Anxiety disorders in women: gender-related modulation of neurobiology and behavior. Semin Reprod Endocrinol. 1997;15:69–76. doi: 10.1055/s-2008-1067969. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Pickett J, Wood G, Paczynski M. Acute stress persistently enhances estrogen levels in the female rat. Stress. 1999;3:163–171. doi: 10.3109/10253899909001120. [DOI] [PubMed] [Google Scholar]
- Smith S, Gong Q, Hsu F, Markowitz R, ffrench-Mullen J, Li X. GABA (A) receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith S, Gong Q, Li X, Moran M, Bitran D, Frye C, Hsu F. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N. Gamma-aminobutyric acid-immunoreactive neurons in the amygdala of the rat – sex differences and effect of early postnatal castration. Neurosci Lett. 1998;255:175–177. doi: 10.1016/s0304-3940(98)00735-6. [DOI] [PubMed] [Google Scholar]
- Stohr T, Szuran T, Pliska V, Feldon J. Behavioural and hormonal differences between two Lewis rat lines. Behav Brain Res. 1999;101:163–172. doi: 10.1016/s0166-4328(98)00148-x. [DOI] [PubMed] [Google Scholar]
- Sundström I, Andersson A, Nyberg S, Ashbrook D, Purdy R, Bäckström T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. 1998;67:126–138. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nature Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Koike Y, Yanaura S, George FR, Meisch RA. Sex differences in physical dependence on pentobarbital in four inbred strains of rats. Gen Pharmacol. 1992;23:487–492. doi: 10.1016/0306-3623(92)90116-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 2002;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- Wilson M, Biscardi R. Influence of gender and brain region on neurosteroid modulation of GABA responses in rats. Life Sci. 1997;60:1679–1691. doi: 10.1016/s0024-3205(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Rackow SH, George-Friedman KP. Sex-dependent behavioral effects of the neurosteroid allopregnanolone (3α,5α-THP) in neonatal and adult rats after postnatal stress. Pharmacol Biochem Behav. 1999;64:717–724. doi: 10.1016/s0091-3057(99)00149-5. [DOI] [PubMed] [Google Scholar]