Abstract
In this study, 48 h administration of 3α-OH-5β-pregnan-20-one (3α,5β-THP) or 17β-estradiol (E2)+progesterone (P) to female rats increased expression of the δ subunit of the GABAA receptor (GABAR) in CA1 hippocampus. Coexpression of α4 and δ subunits was suggested by an increased response of isolated pyramidal cells to the GABA agonist 4,5,6,7- tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP), following 48 h steroid treatment, and nearly complete blockade by 300 μM lanthanum (La3+). Because α4βδ GABAR are extrasynaptic, we also recorded pharmacologically isolated GABAergic holding current from CA1 hippocampal pyramidal cells in the slice. The La3+-sensitive THIP current, representative of current gated by α4βδ GABAR, was measurable only following 48 h steroid treatment. In contrast, the bicuculline-sensitive current was not altered by steroid treatment, assessed with or without 200 nM gabazine to block synaptic current. However, 48 h steroid treatment resulted in a tonic current insensitive to the benzodiazepine agonists lorazepam (10 μM) and zolpidem (100 nM). These results suggest that 48 h steroid treatment increases expression of α4βδ GABAR which replace the ambient receptor population. Increased anxiolytic effects of THIP were also observed following 48 h steroid treatment. The findings from the present study may be relevant for alterations in mood and benzodiazepine sensitivity reported across the menstrual cycle.
Keywords: Neurosteroid, δ subunit, GABAA receptor, THIP, CA1 hippocampus, Tonic current
1. Introduction
The GABA-modulatory steroid 3α-OH-5α-pregnan-20-one (3α,5α-THP) or allopregnanolone and its active isomer, pregnanolone (3α,5β-THP), are metabolites of the steroid progesterone (P). Unlike most steroids, the THP isomers are potent positive modulators of the GABAA receptor (GABAR) when acutely applied (Belelli et al., 2002; Brown et al., 2002; Majewska et al., 1986; Wohlfarth et al., 2002), an effect which enhances tonic GABAergic current in areas such as dentate gyrus (Stell et al., 2003). As expected for GABA-modulatory compounds, such as benzodiazepines (BDZ) and barbiturates, these steroids possess dose-dependent anxiolytic and anticonvulsant properties (Bitran et al., 1999; Frye, 1995). However, prolonged exposure to these steroids across a period of 48–72 h, to mimic physiological fluctuations, produces alterations in GABAR subunit composition as well as reduced levels of inhibition. Increases in hippocampal expression of the GABAR α4 subunit are observed following 48 h 3α,5β-THP administration to female rats in association with increases in anxiety, assessed using two animal measures: the elevated plus maze (Gulinello et al., 2001) and the acoustic startle paradigm (Gulinello and Smith, 2003). As expected for an increase in α4-containing GABAR (Wafford et al., 1996; Wisden et al., 1991), hippocampal pyramidal cells are insensitive to BDZ modulation of GABA-gated current following 48 h steroid exposure, assessed using whole cell patch clamp techniques (Gulinello et al., 2001). A similar insensitivity to the anxiolytic effects of the BDZ lorazepam (LZM) is also observed at this time (Gulinello et al., 2001).
The α4 subunit can coexpress with either γ2 or δ subunits (Sur et al., 1999), which both produce receptors insensitive to BDZ-modulation (Brown et al., 2002; Wisden et al., 1991). However, the other pharmacological characteristics, biophysical properties, and sub-cellular localization of α4βγ2 and α4βδ GABAR are distinct (Bianchi et al., 2002; Brown et al., 2002; Peng et al., 2002). For instance, the GABA partial agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) or gaboxadol generates a current greater than maximum concentrations of GABA only at α4[6]βδ GABAR (Brown et al., 2002) but not at other αβγ2 subtypes (Ebert et al., 1997). In addition, the trivalent cation lanthanum (La3+) is a potent inhibitor of current gated by α4[α6]βδ GABAR (Brown et al., 2002; Saxena et al., 1997; Zhu et al., 1998), while it produces minimal inhibition of α4[6]βγ2 GABAR, and potentiates α1βγ2 and α1βδ GABAR (Saxena et al., 1997; Zhu et al., 1998).
In contrast to γ2-containing GABAR, δ-containing GABAR are localized exclusively to extrasynaptic sites (Nusser et al., 1998; Wei et al., 2003), where they produce a tonic current (Rossi and Hamann, 1998; Wei et al., 2003). In cerebellar and dentate gyrus granule cells this tonic current is believed to act as a resistive shunt to limit excitability of the neuron (Brickley et al., 2001). Recent studies have reported that for neurosteroids and sedative drugs such as anesthetics, BDZs and ethanol, effects on tonic current are greater in magnitude than observed at the synapse (Bai et al., 2000; Belelli and Herd, 2003; Stell et al., 2003; Wei et al., 2004; Yeung et al., 2003). Therefore, the tonic current may have important implications for the behavioral effects of these GABA-modulatory compounds, as well as for establishing cellular mechanisms.
Although our previous work suggests that α4βγ2 GABAR expression increases following 48 h steroid administration (Hsu et al., 2003), levels of δ subunit expression have yet to be tested. This is especially warranted because we have recently reported that a steroid withdrawal paradigm which increases hippocampal expression of the α4 GABAR subunit results in α4βδ coexpression (Sundstrom-Poromaa et al., 2002). Therefore, in the present study, both molecular and pharmacological techniques were employed to determine both δ subunit expression and α4βδ GABAR coexpression following 48 h treatment of female rats with 3α,5β-THP. In addition, because the steroid 17β-estradiol (E2) can act in a permissive fashion to produce the in vitro BDZ insensitivity associated with steroid withdrawal (Costa et al., 1995), similar studies were carried out to quantify α4 and δ subunit expression following combined administration of E2+P for 48 h. Finally, in order to demonstrate a physiologically relevant outcome for these pharmacological changes related to α4βδ GABAR expression, the anxiolytic effects of THIP were tested using the elevated plus maze following 48 h steroid exposure. The results of the present study may be important in furthering our understanding of the homeostatic mechanisms resulting from sustained exposure to endogenous GABA modulators such as 3α,5α[β]-THP.
2. Materials and methods
2.1. Experimental animals
Adult, female Long Evans rats (Charles River, 140–200 g), housed in groups of three, were used for all protocols. Animals were maintained under controlled conditions of light (14 h light:10 h dark) and temperature (21 °C) with free access to food and water. Animals were used during the light phase of the circadian cycle, 1 h following steroid or vehicle injection. Control rats were tested on the day of diestrus–1, a low hormone stage of the cycle, verified by microscopic examination of the vaginal lavage, a routine procedure. All protocols were conducted under guidelines established by the Institutional Animal Care and Use Committee.
2.2. Steroid administration paradigm
Animals were injected intraperitoneally with either (i) 3α,5β-THP (3α-OH-5β-pregnan-20-one, 10 mg/kg), (ii) P (progesterone, 25 mg/kg), (iii) E2 (17β-estradiol, 8 μg/kg)+P (progesterone, 25 mg/kg) or (iv) oil vehicle. Injections were administered daily for 3 days beginning on diestrus–1, and the animals sacrificed 1 h following the final injection. These steroid administration paradigms have been shown to result in physiological levels of circulating steroids (Moran and Smith, 1998). Intact, non-ovariectomized rats were used for the study because ovariectomy has been shown to alter CNS neurosteroid metabolism following injection of exogenous P (Corpechot et al., 1993).
2.3. Western blot procedures
Crude membranes from microdissected CA1 hippocampus were first normalized according to protein content using standard techniques; then, proteins were separated using SDS gel electrophoresis, transferred to nitrocellulose membranes and probed with selective antibodies for the rat α4 (67 kDa) (Smith et al., 1998), δ (54 kDa), α1 (51 kDa) or γ2 (44–46 kDa) subunits. Because of the low expression of α4 and δ subunits in CA1 hippocampus, these bands were detected with a highly sensitive chemiluminescence substrate (Pierce Supersignal West Femto substrate) for visualization and quantified using a Umax scanner and One-Dscan software. Probing with the α4 antibody yielded a double band, as has been reported (Kern and Sieghart, 1994). Because both bands were proportionately darker with steroid treatment, the optical density of the lower, darker band was quantified for each sample. The results were standardized to a glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 36 kDa) control protein. (The γ2 and α1 antibodies were obtained from Abcam, Inc., Cambridge, MA and Upstate Pharmaceuticals, Waltham, MA, respectively).
2.4. In vitro slice preparation
Animals were rapidly decapitated, and the brains removed and cooled using an ice cold solution of artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 124, KCl 5, CaCl2 2, KH2PO4 1.25, MgSO4 2, NaHCO3 26, and glucose 10, saturated with 95% O2, 5% CO2 and buffered to a pH of 7.4. The hippocampi were then rapidly removed and cut into 400 μm coronal slices with a Leica oscillating microtome. Hippocampal slices were initially incubated at physiological temperature (37 °C) in aCSF with 95% O2, 5% CO2 for 1–2 h. The slices were then transferred to the electrophysiology rig, where they were held between two nylon nets in a tissue chamber on the stage of the microscope and perfused with aCSF (2 ml/min).
2.5. Electrophysiological recording and analysis
Current was recorded from visually identified pyramidal cells in CA1 hippocampus using whole cell patch clamp procedures (Hsu et al., 2003) and low-pass filtering (2 kHz 4-pole Bessel filter) at a holding potential of −60 mV with an Axopatch 200B amplifier (Axon Instruments). Patch pipets were fabricated from borosilicate glass using a Flaming-Brown puller to yield open tip resistances of 2–4 MΩ (internal solution (in mM): CsCl 130, MgCl2, 2, HEPES 10, BAPTA 0.2, QX-314 5, Mg-ATP 2, pH 7.2, 290 mOsm). The bath solution contained aCSF with 2–5 mM kynurenic acid added to block currents gated by excitatory amino acid transmitters. The GABAergic nature of the recorded current was verified by blockade with bicuculline methiodide (20 μM) and reversal at ECl−. Data were recorded at room temperature (20–25 °C) at a 10 kHz sampling frequency using pClamp 8.2. Post-hoc analysis of synaptic current, shifts in the holding current and alterations in background noise during pharmacological tests was accomplished with pClamp 9.0. Recordings were made at room temperature to increase receptor sensitivity to agonists as has been shown (Perrais and Ropert, 1999), as well as to compare with results from recombinant receptors (Brown et al., 2002) and our work with acutely isolated neurons. Previous work in the field has established tonic recordings can be made effectively at room temperature (Brickley et al., 2001).
2.6. Pharmacological tests
In some cases, current was evoked using 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP) or gaboxadol, 5 and 30 μM), a GABA partial agonist. In order to minimize endogenous GABA release, recordings were carried out in the presence of 0.5–1.0 μM TTX. In these recordings, lanthanum (La3+, 30 or 300 μM) was also used to selectively block THIP-evoked current gated by α4βδ GABAR. In separate studies, recordings were carried out without TTX to permit action potential-generated spillover in order to quantify the level of tonic current present across experimental groups as the bicuculline-sensitive change in the holding current, in the presence or absence of 200 nM gabazine to block synaptic current (Stell and Mody, 2002). Responses of the holding current to the BDZ agonists lorazepam (LZM, 10 μM) or zolpidem (100 nM), as well as to the BDZ partial inverse agonist RO15-4513 (0.3 and 10 μM), were also determined to verify the presence of α4 and α1-containing GABAR, and the relative expression of α4βγ2 GABAR. All drugs were obtained from Sigma Chemical Co., except Cs-BAPTA and Qx-314 (from Calbiochem) and 3α,5β-THP, E2 and P (from Steraloids).
2.7. Acute isolation of hippocampal neurons and recording procedures
Pyramidal neurons were acutely dissociated from CA1 hippocampus and THIP-gated Cl− currents analyzed using whole cell patch clamp procedures as previously described (Smith et al., 1998). Briefly, tissue was digested at 32 °C for 50–60 min under 100% O2 in PIPES-buffered saline containing (in mM): NaCl 120, KCl 5, CaCl2 1, MgCl2 1, D-glucose 25, PIPES 20 and trypsin (type XI, Sigma) or pronase 0.8 mg/ml, pH 7.0. Following a 1 h enzyme-free incubation at room temperature, tissue was dissociated by trituration in 1 ml of 20 mM HEPES-buffered DMEM which was replaced by recording medium following transfer to the recording chamber. GABA-gated currents were recorded at room temperature (20–25 °C) at a holding potential of −50 mV in a bath containing (in mM): NaCl 120, CsCl 5, CaCl2 2, MgCl2 1, HEPES 10 and glucose 25, pH 7.4, 320 mOsm/kg H2O. The pipet solution contained (in mM): N-methyl-D-glucamine chloride 120, Cs4BAPTA 5 and Mg-ATP 5. The ATP regeneration system containing Tris phosphocreatine (20 mM) and creatine kinase was added to minimize GABA rundown.
Agonist application was achieved with a rapid concentration jump method. To this end, a piezo-controlled double-barreled theta tube (Sutter Instruments, 80–100 μm diameter tip) containing agonist and bath solution was positioned within 50–100 μm of the cell/patch to achieve brief (<100–300 μs) transitions to the agonist stream for 400 ms (Burleigh Instruments, LSS-3100). Exposure times were verified at the conclusion of the experiment by measurements of the tip potential. Multiple concentrations of THIP (1–105 μM) or GABA (5 mM) were sequentially applied, and current was recorded using an Axopatch 1D amplifier (Axon Instruments) filtered at 2 kHz (four-pole Bessel filter) and detected at an 8–10 kHz sampling frequency (pClamp 5.1). Peak currents were calculated for all THIP concentrations as % of the maximum GABA-gated current. Concentration–response curves were generated using non-linear curve fitting routines with a logistic equation (Origin, Microcal), and the maximum current, EC50 and Hill slope determined.
2.8. Elevated plus maze
The elevated plus maze is a device consisting of four 10×50 cm arms at 90° angles, elevated 50 cm above the floor. Two arms are enclosed by 40 cm walls, and two arms have no walls (“open arms”). The open arms are also partially bordered by small rails extending to the proximal half of the arm, and the floor of the maze is marked with grid lines every 25 cm. Initially, animals were acclimated to the maze for 30–45 min. Then, each animal was placed in the center of the maze 30 min after THIP administration, and exploratory activity recorded for 10 min. The time spent in the open and closed arms was tabulated, as were the number of grid crossings, a measure of general activity level. To be considered an open arm entry, the animal had to cross the line of the open platform with all four paws. An increase in time spent in the open arm is considered to be a measure of decreased anxiety, as we have described (Gulinello et al., 2001).
2.9. Statistical analysis
Average values for changes in current were evaluated across steroid-treatment groups by one way analysis of variance (ANOVA) with a post-hoc Tukey’s test. In addition, the statistical significance of EC50 values from the concentration–response curves were assessed using the log of the arithmetic means by ANOVA with a post-hoc Tukey’s test. Data were then converted to the geometric means. Data from the plus maze were analyzed in an ANOVA followed by post hoc t-tests (Fisher’s PLSD). A P value <0.05 was used as an indication of statistical significance.
3. Results
3.1. Forty-eight h steroid administration and δ GABAR subunit expression
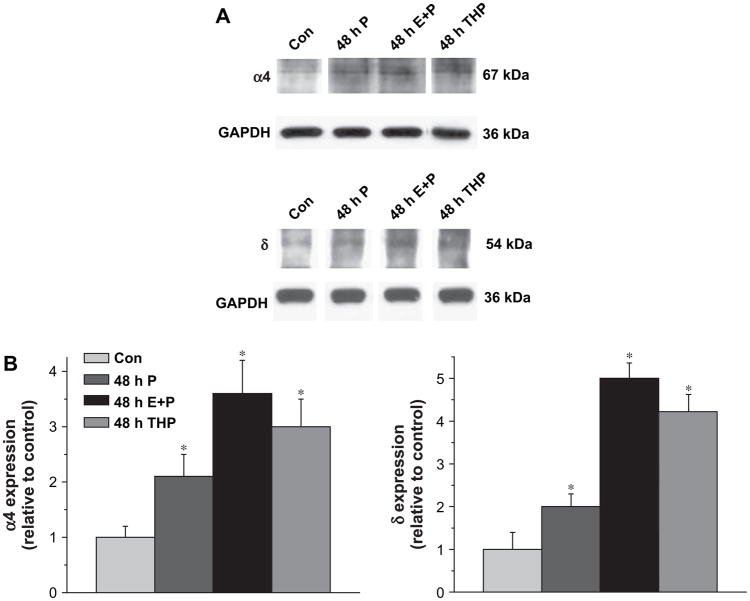
Forty-eight h administration of either 3α,5β-THP or E2+P produced significant four- to five-fold increases in expression of the GABAR δ subunit in CA1 hippocampus as evidenced by an increase in band density compared to control (Fig. 1). The increases in δ expression were accompanied by corresponding increases in expression of the α4 subunit following 48 h E2+P administration (Fig. 1), as well as following 48 h 3α,5β-THP exposure, as we have demonstrated previously (Gulinello et al., 2001). In addition, combined administration of E2+P for 48 h produced significantly (P<0.05) higher levels of both α4 and δ subunit expression than administration of P alone (Fig. 1). In all cases, subunit expression levels were normalized to levels of the GAPDH control protein.
Fig. 1.
Forty-eight hour steroid exposure increases expression of the GABAR δ subunit in CA1 hippocampus. (A) Representative Western blots with α4 or δ subunit levels detected in membranes from CA1 hippocampus following 48 h i.p. treatment of female rats with 3α,5β-THP (THP, 10 mg/kg), P (25 mg/kg) or E2 (8 μg/kg)+P (25 mg/kg) (E+P). All steroid treatment protocols increased expression of both α4 and δ subunits compared to control (Con). (B) Averaged data. Increases in δ subunit levels produced by steroid treatment were paralleled by increases in α4 levels of similar magnitude (n=6 rats/each, performed in triplicate). *P<0.05 versus the control for each group.
3.2. THIP and La3+ effects on acutely isolated neurons
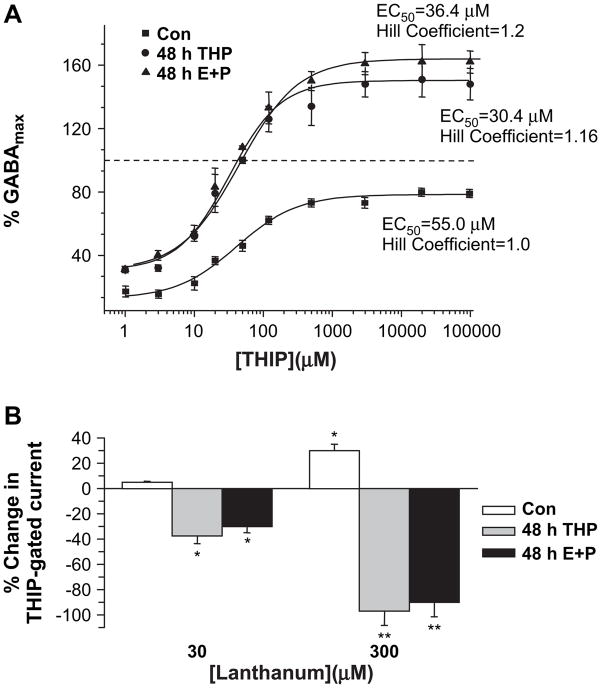
THIP is a GABA partial agonist which generates a maximum current greater than the maximum current gated by GABA only at δ-containing GABAR (Brown et al., 2002; Ebert et al., 1997). Therefore, in order to pharmacologically characterize GABAR current following steroid treatment, responses to THIP were recorded from pyramidal neurons acutely isolated from CA1 hippocampus and compared to the maximum GABA-gated current. The results indicate that the EC50 for THIP-gated current is lower (P<0.05) following 48 h treatment with E2+P or 3α,5β-THP compared to control. Because EC50 values do not display a normal distribution, the geometric means (mean−SEM, mean+ SEM) were calculated for each group: 48 h E2+P, 30.4 μM (29.2, 31.7); 48 h THP, 36.4 μM (34, 39); Con, 55 μM (49.6, 60.9). Additionally, THIP was more efficacious than GABA, resulting in ~50% greater current (P<0.05) in patches from steroid treated animals, where maximal THIP-gated current exceeded (P<0.001) control values (48 h E2+P, 147.3±5.14 μM; 48 h THP, 161.1±4.21 μM; Con, 85.4±1.17 μM). In contrast, under control conditions, THIP yielded a maximum current equivalent to only 80% of the maximum GABA-gated current. Thus, the pharmacological findings are consistent with upregulation of α4βδ GABAR in the hippocampus following both steroid protocols.
La3+ effects were also altered by steroid administration. Bath application of 30 μM La3+ decreased by 20–30% the amplitude of THIP-gated current recorded from pyramidal neurons acutely isolated following 48 h E2+P treatment (Fig. 2B), while a higher concentration of La3+ (300 μM) produced a nearly complete (85–90%) reduction in peak GABA-gated current. Similar results were observed after 48 h 3α,5β-THP exposure (Fig. 2B). In contrast, under control conditions, 30 μM La3+ had no effect, while application of 300 μM La3+ increased the amplitude of GABA-gated current by 30%, an effect consistent with a lack of α4 and δ subunits under control conditions in this region of hippocampus. Thus, these results are consistent with increased expression of hippocampal α4βδ GABAR following 48 h exposure to E2+P or 3α,5β-THP.
Fig. 2.
Steroid treatment alters the pharmacology of THIP and lanthanum. (A) Forty-eight hour treatment of female rats with E2+P (E+P) or 3α,5β-THP (THP) increased both the efficacy and potency of THIP, a GABA partial agonist with greatest effects at δ-containing GABAR. THIP-evoked current was recorded with whole cell patch clamp techniques using pyramidal cells acutely isolated from CA1 hippocampus. In all cases, peak responses to THIP were expressed as a percent of the maximum GABA-gated current (GABAmax), tested for each patch. Dashed line indicates maximum GABA-gated current. The EC50 for THIP was significantly lower following 48 h steroid treatment compared to control (n=6–8 points/group, the geometric means for the EC50 values are plotted). (B) The trivalent cation lanthanum (La3+) decreased THIP-gated current recorded from acutely isolated pyramidal neurons following 48 h in vivo administration of THP or E+P in a concentration-dependent manner. This inhibitory effect of La3+ is consistent with increased expression of α4βδ GABAR (n=11–14 cells/group; *P<0.05, **P<0.001 vs. the pre-La3+ control for each group).
3.3. THIP and La3+ effects on hippocampal current in the slice
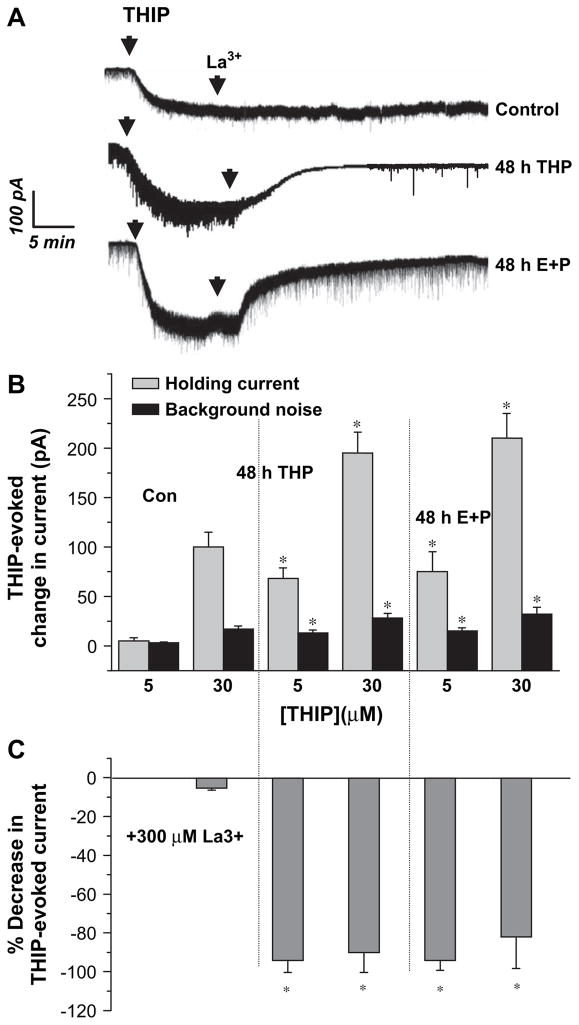
Because α4βδ GABAR are localized exclusively to the extrasynaptic GABAR population (Wei et al., 2003), changes in the holding current of CA1 hippocampal pyramidal cells were evaluated following bath application of THIP using the slice preparation. This current would necessarily be generated via both synaptic and extrasynaptic GABAR, which would include putative α4βδ GABAR increased by 48 h exposure to 3α,5β-THP or E2+P. An EC50 concentration of THIP under steroid treatment conditions (30 μM) produced a 170–200 pA shift in the holding current (400 pS/100 s) and a 30 pA increase in the background noise (a six-fold increase, Fig. 3A,B). These are values approximately two-fold greater than observed under control conditions (P<0.001), similar to previous reports (Liang et al., 2004). A lower concentration of THIP (5 μM) produced a 70–80 pA current following steroid treatment, while the THIP-generated current recorded under control conditions was barely detectable.
Fig. 3.
Steroid treatment increases the lanthanum-sensitive THIP-evoked current recorded from the hippocampal slice. In order to assess effects of THIP on both synaptic and extrasynaptic GABAR populations, changes in the holding current were recorded from CA1 hippocampal pyramidal cells in the presence of 1 μM TTX using whole cell patch clamp procedures in the slice following bath application of 30 μM THIP. This concentration of THIP produced a greater effect on both the holding current and level of background noise following 48 h treatment with E2+P compared to control. In addition, La3+ produced a near complete block of this effect following steroid treatment but was ineffective in control slices. (A) Representative traces. (B) Averaged data, THIP application. (C) Averaged data, La3+ inhibition (n=15–20 cells/group). *P<0.05 versus the corresponding control value.
La3+blocks current gated by α4[6]βδ GABAR (Brown et al., 2002; Saxena et al., 1997; Zhu et al., 1998). Therefore, the level of La3+-sensitive THIP-evoked current would be an estimate of current generated by α4βδ GABAR in the slice preparation. Although La3+ is also a potent calcium channel blocker, where it would be expected to alter transmitter release, for this study recordings were carried out in the presence of TTX to minimize GABA release. La3+ (300 μM) produced an 80–90% blockade of THIP-generated current following treatment with either 3α,5β-THP or E2+P, with minimal (<5%) effects on THIP-generated current recorded under control conditions (Fig. 3C).
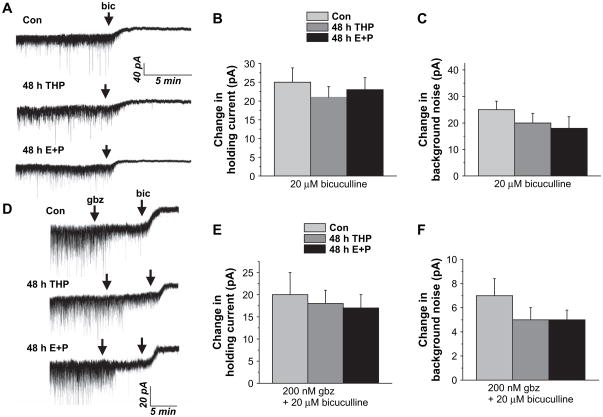
3.4. Bicuculline-sensitive tonic current
In order to quantify the level of tonic GABAergic current present under conditions of spontaneous transmitter release, the holding current of CA1 hippocampal pyramidal neurons was recorded from the slice under conditions where action potentials could be generated (i.e., without TTX). Under these conditions, spillover of transmitter from neighboring synapses would gain access to the extrasynaptic receptor pool or be taken up by transporters for later release. Under these conditions, the spontaneously generated tonic current was not different between steroid-treated groups versus control (Fig. 4A–C). This bicuculline-sensitive tonic current, tested as the change in the holding current (24.8±4 pA, con; 20.8±3 pA, 3α,5β-THP; 22±3.5 pA, E2+P) and the background noise (25±2 pA; 20.3±2.2 pA 3α,5β-THP; 18.7±2.7 pA, E2+P), was similar for all groups tested. Bicuculline-sensitive changes were also observed under conditions where the synaptic current was selectively blocked (Fig. 4D–F) using a 200 nM concentration of the competitive antagonist gabazine (Stell and Mody, 2002; Yeung et al., 2003). Thus, these results suggest that the level of spontaneously generated GABAergic tonic current is not altered by steroid exposure.
Fig. 4.
GABAergic tonic current is unchanged following steroid exposure. Pharmacologically isolated GABAergic current was recorded from CA1 hippocampal pyramidal cells with whole cell patch clamp techniques in the presence of excitatory amino acid and GABAB receptor blockers. (A) Representative traces reveal a 20–25 pA change in the holding current in response to bath application of 20 μM bicuculline (Bic) for both groups. Averaged data reveal no significant difference in holding current (B) or background noise (C) produced by bicuculline in steroid-treated versus control groups. (D) Representative currents reveal that the bicuculline-sensitive current was of similar magnitude for both groups under conditions where synaptic current was blocked with 200 nM gabazine. Averaged changes in holding current (E) and background noise (F) (20.5±3.2, Con; 15.6±3.5, THP; 17.8±2.6, E+P; 4–8 cells/group).
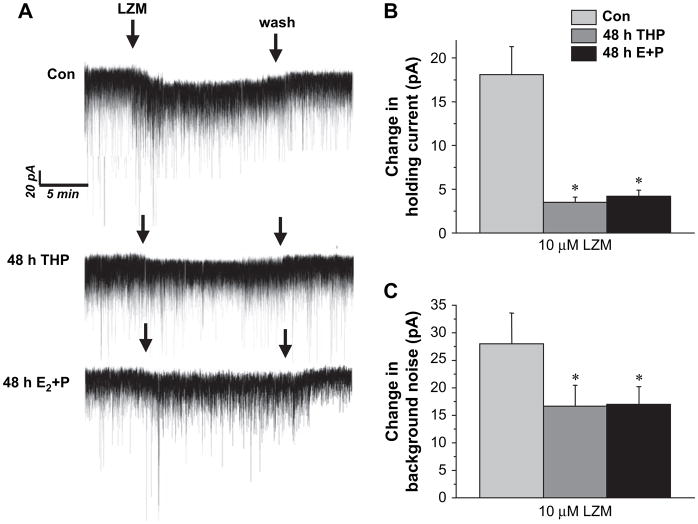
3.5. LZM potentiation of GABAergic tonic current
Because α4 and δ-containing GABAR are insensitive to modulation by BDZs, we tested the response of tonic current to the BDZ LZM at a concentration (10 μM) we have previously shown to produce maximal effects in isolated pyramidal neurons under control conditions (Smith et al., 1998). Bath application of LZM produced a 15–20 pA change in the holding current under control conditions and a 30 pA change in background noise (Fig. 5A–C). In contrast, current recorded following either of the two steroid treatments was relatively insensitive to modulation by this compound which yielded only a 3–4 pA change in the holding current, significantly less than under control conditions (P<0.01). LZM also produced a greater effect on the background noise level in the control recordings compared to the steroid-treated groups. These data are consistent with increased expression of GABAR which are less sensitive to LZM modulation, such as α4βδ GABAR, following steroid exposure.
Fig. 5.
GABAergic tonic current is benzodiazepine-insensitive following steroid exposure. The benzodiazepine lorazepam (LZM, 10 μM) produced a significant change in the holding potential and increase in background noise under control conditions. However, GABAergic tonic current recorded in slices from animals following 48 h treatment with either THP or E+P was minimally altered by LZM. (A) Representative traces. Averaged data for the LZM-induced change in the holding current (B) and background noise (C). These results are consistent with increased expression of α4-containing GABAR (n=6–8 cells/group). *P<0.05 vs. control.
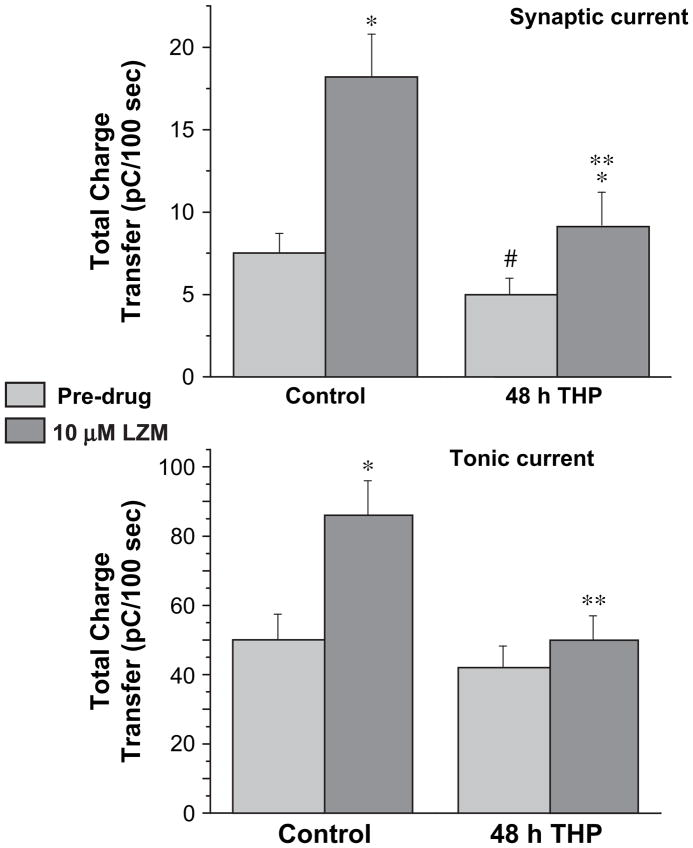
When the integrated current (total charge transfer) was evaluated across a 100 s time period, the magnitude of the tonic current was found to be about eight- to ten-fold greater than the synaptic current recorded concomitantly for both steroid treatment and control groups (Fig. 6), as has been reported by other investigators (Bai et al., 2000). In addition, tonic current recorded following steroid treatment was nearly insensitive to modulation by the BDZ LZM. In contrast, a significant LZM modulation of synaptic current was observed following steroid treatment, although of lower magnitude than control (P<0.05). These results suggest that alterations in GABAR produced by 48 h exposure to 3α,5β-THP may be localized more to the extrasynaptic than to the synaptic population of receptors.
Fig. 6.
Benzodiazepine-induced changes in total charge transfer produced by GABA: synaptic versus extrasynaptic current. The total charge transfer for GABA-gated current per 100 s was calculated for synaptic current (A), sIPSCs recorded concomitantly with tonic current (B) in the present study. Tonic current produced a significantly greater charge transfer than synaptic current (P<0.05). Following 48 h THP treatment, tonic current was less sensitive to LZM modulation than was synaptic current, consistent with increases in α4 expression in the extrasynaptic population of GABAR (n=15–20 cells/group). *P<0.05 vs. the pre-drug condition, **P<0.05 vs. the control LZM, #P<0.05 vs. control pre-drug.
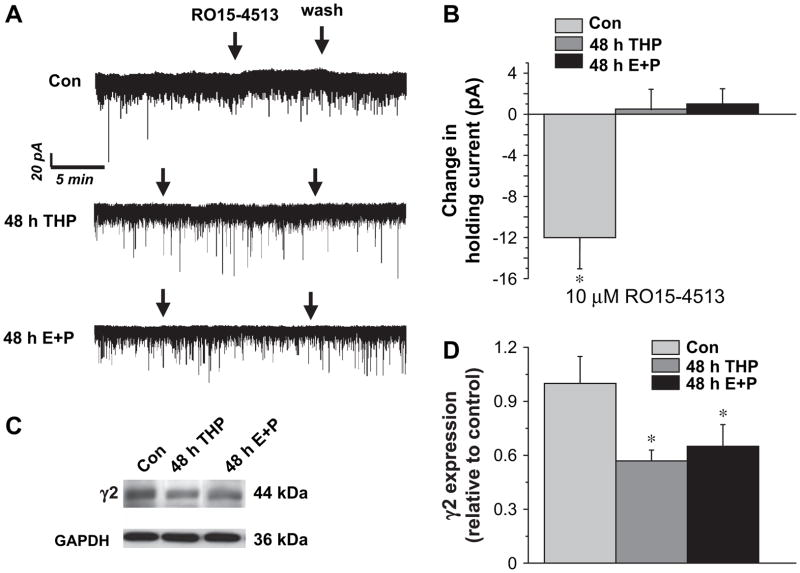
3.6. Effects of RO15-4513 on GABAergic tonic current
In order to determine whether the pharmacological responses of the tonic current were consistent with upregulation of α4βγ2 GABAR following steroid treatment, we tested the effects of the GABAR partial inverse agonist RO15-4513. In contrast to lorazepam, RO15-4513 slightly decreased tonic current recorded from pyramidal cells in control CA1 hippocampus, at doses of 10 μM (Fig. 7A,B) and 300 nM (data not shown). In contrast, RO15-4513 produced no effect on the tonic current following steroid treatment (Fig. 7A,B). These data suggest that α4βγ2 GABAR are not increased in the extrasynaptic domain of CA1 hippocampus. In order to directly test effects of the steroid paradigms, γ2 levels were also assessed in CA1 hippocampus using Western blot procedures. Both 48 h THP and 48 h E2+P decreased γ2 expression by 40–50% (P<0.05) compared to the diestrous control. Thus, both pharmacological and biochemical tests suggest that α4βγ2 GABAR are not increased by steroid treatment, and that in fact γ2 levels decrease in association with increases in δ expression in CA1 hippocampus.
Fig. 7.
Steroid treatment decreases γ2 expression and reduces responses of tonic current to RO15-4513. (A) Representative traces reveal no effect of bath applied RO15-4513 (10 μM) on pharmacologically isolated GABAergic tonic current recorded from steroid treatment groups. (B) Averaged data (n=5–6 cells/group). (C,D) Steroid treatment also reduced γ2 expression in CA1 hippocampus compared to control (n=4/group, performed in triplicate).
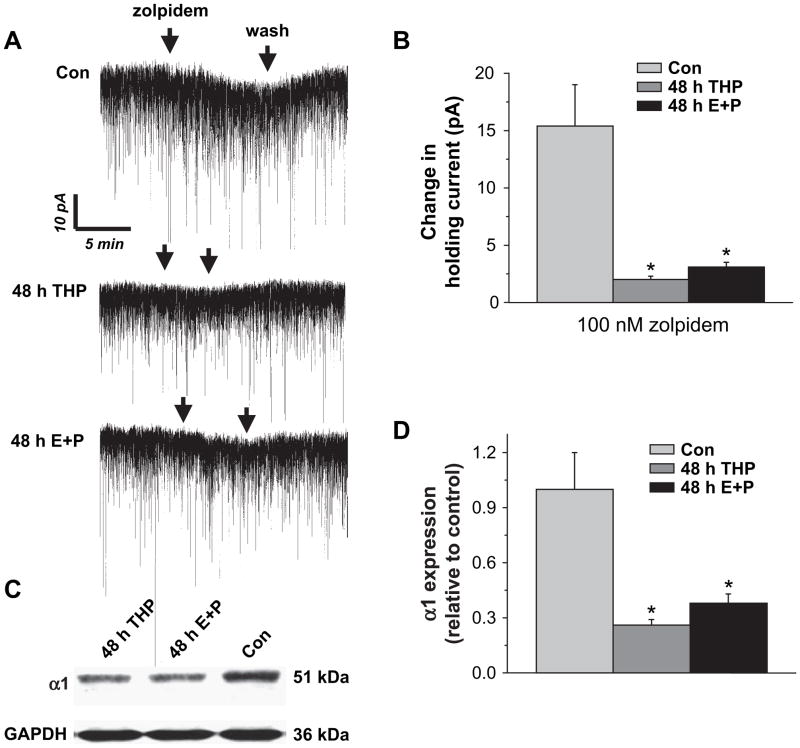
3.7. Zolpidem responsiveness of GABAergic tonic current
In order to further examine the steroid-induced change in BDZ pharmacology, we examined responsiveness of pharmacologically isolated GABAergic tonic current to bath application of 100 nM zolpidem, a concentration selective for α1-containing GABAR (Vicini et al., 2001). Under control conditions, this BDZ agonist produced a 15 pA shift in the holding current (Fig. 8A,B). However, following treatment with either 3α,5β-THP or E2+P, this α1-selective compound produced only a ~3 pA shift in the holding current, suggesting that α1-containing GABAR are decreased in the extrasynaptic domain. Therefore, we also directly tested whether steroid treatment altered α1 expression in hippocampal membranes using Western blot techniques. In fact, 48 h in vivo treatment with either 3α,5β-THP or E2+P produced a 60–70% decrease in α1 expression compared to the diestrous–1 control (P<0.05, Fig. 8C,D).
Fig. 8.
Zolpidem sensitivity is decreased following steroid exposure. 100 nM zolpidem produced a significantly greater shift in the holding current under control conditions than after treatment with THP or E+P. (A) Representative traces of pharmacologically isolated GABAergic tonic current. (B) Averaged population data (n=5 cells/group, P<0.05). (C) Western blot results and (D) averaged data demonstrating significant decreases in hippocampal α1 expression following 48 h in vivo treatment with THP or E+P compared to the diestrous control. Results are normalized to the GAPDH control and expressed relative to the control (n=6 rats/group, performed in duplicate, *P<0.05).
3.8. Anxiolytic effects of THIP in the elevated plus maze
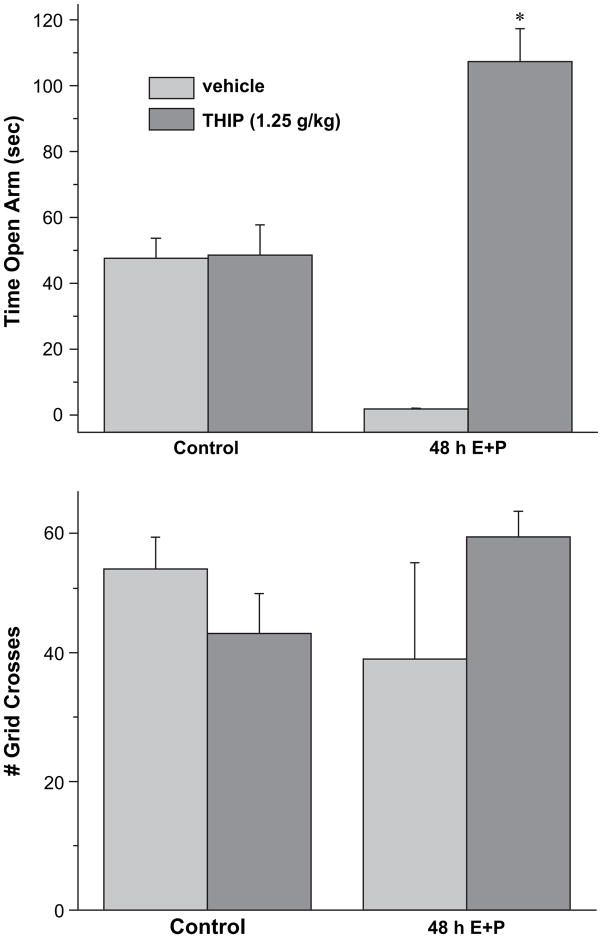
Because the THIP-generated current was increased following steroid treatment, most likely as a result of increased expression of α4βδ GABAR, we tested the effects of THIP on anxiety measures using the elevated plus maze. For this test, exploration of the open arm is evaluated in animals 30 min after i.p. injection of vehicle (saline) or THIP at a dose sub-threshold (Gulinello et al., 2003) for its sedative effect (1.25 g/kg). THIP increased the time spent in the open arm more than 20-fold after 48 h E2+P treatment (Fig. 9). Increases in anxiety in steroid-treated animals receiving only vehicle were evidenced by minimal (5 s) time in the open arm of the maze. Thus, the anxiolytic effect of THIP is highly significant (P<0.001). In comparison, this dose of THIP had no effect on open arm time in control animals nor did it produce any significant effect on locomotor activity, assessed as the number of grid crosses in the maze. Thus, these results suggest that a low dose of THIP is anxiolytic following 48 h steroid exposure where it has no effect under control conditions.
Fig. 9.
THIP is anxiolytic following 48 h E+P treatment. A dose of THIP sub-threshold for its sedative effect (1.25 g/kg) produced a significant increase in the time in the open arm (A) assessed using the elevated plus maze following 48 h steroid treatment. In contrast, this dose of THIP was ineffective in altering open arm time in vehicle-treated controls. In neither case did THIP alter locomotor activity, assessed as the number of grid crosses (B) (n=6–8 animals/group). *P<0.05 vs. vehicle or control/THIP.
4. Discussion
In the present study, 48 h exposure of female rats to the steroids 3α,5β-THP or E2+P increased expression of the GABAR δ subunit in CA1 hippocampus, which resulted in pharmacological changes characteristic of α4βδ GABAR expression in pyramidal cells of this region. Because these changes were observed in the absence of changes in the total bicuculline-sensitive tonic current, they suggest that α4βδ GABAR may substitute for, rather than add to, the existing extra-synaptic pool of GABAR. These results may have implications for naturally occurring fluctuations in steroids across the menstrual cycle when alterations in GABAR pharmacology have been reported (Sundstrom et al., 1997).
One pharmacological consequence of α4βδ GABAR expression is an increased response to the GABA partial agonist THIP. This compound has a greater potency and efficacy at δ-containing GABAR (Brown et al., 2002) than other subtypes (Ebert et al., 1997), and consistent with this, following 48 h steroid treatment, the EC50 for THIP was lower, and the maximal current generated by THIP was greater than recorded from control neurons. In addition, the maximal THIP-gated current was greater than the maximal GABA-gated current following 48 h steroid treatment, another unique pharmacological characteristic of α4[6]βδ GABAR (Brown et al., 2002) but not other GABAR subunit combinations, where THIP is a partial GABA agonist. In addition, La3+ produced a nearly complete inhibition of THIP-generated current following steroid treatment, which is also characteristic of α4[6]βδ GABAR (Brown et al., 2002; Saxena et al., 1997; Zhu et al., 1998). Although La3+ effects are not known at all GABAR subtypes, potent inhibition by La3+ is not observed at α1βδ, α1βγ2 or α4βγ2 subtypes (Brown et al., 2002; Saxena et al., 1997; Zhu et al., 1998), and thus allows us to partially distinguish between these receptor subtypes. We have reported (Sundstrom-Poromaa et al., 2002) similar pharmacological characteristics of CA1 hippocampal pyramidal cells after withdrawal from P, when α4βδ GABAR expression is increased in hippocampus.
Both 3α,5β-THP and combined administration of E2 + P produced similar changes in THIP/La3+ pharmacology, in association with increased expression of the δ subunit. Our findings also suggest that co-administration of E2+P results in maximal increases in α4 and δ GABAR subunit expression in CA1 hippocampus compared to administration of P alone. Although the mechanism for this permissive effect of E2 is not clear, it may be mediated through increased production of 3α,5α-THP, via enhanced activity of 3α-hydroxysteroid-oxidoreductase (Compagnone and Mellon, 2000), or through post-translational modifications, via receptor phosphorylation (Qiu et al., 2003) which can alter GABAR levels (Kittler and Moss, 2003). Our previous findings demonstrate that increases in α4 expression produced by 48 h P exposure were due to its 3α,5α-THP metabolite, suggesting that the effect of E2+P in the present study are likely not due to classic hormone effects.
α4βδ GABARs are localized extrasynaptically (Wei et al., 2003) where they generate a tonic current. Therefore, traditional analyses of synaptic current cannot be used to pharmacologically characterize current generated by this receptor subtype in situ. Instead, the increase in La3+-sensitive THIP current, recorded from the hippocampal slice, reflected the increase in α4βδ GABAR expression following 48 h steroid current. This increase in THIP sensitivity electrophyisologically was associated with an increase in the anxiolytic effect of THIP following 48 h steroid treatment, thus suggesting that the steroid-mediated increase in α4βδ GABAR generates a tonic current which is behaviorally relevant. Several recent reports (Crestani et al., 2002; Mihalek et al., 1999) have described behavioral outcomes resulting from alterations in extrasynaptic GABAR populations underlying tonic current, further confirming their role in regulating behavioral excitability.
The receptors which have been shown to be extra-synaptic include α5-containing GABAR in CA1 hippocampus (Crestani et al., 2002) and δ-containing GABAR in granule cells in cerebellum (Nusser et al., 1998) and dentate gyrus (Wei et al., 2003), as well as in CA1 hippocampus following steroid treatment, as demonstrated here. These receptors generate a tonic current in CA1 hippocampus (Caraiscos et al., 2004), dentate gyrus (Stell and Mody, 2002) and the cerebellar granule cell (Brickley et al., 2001) by accessing GABA via spillover from adjacent synapses (Mitchell and Silver, 2000; Rossi and Hamann, 1998; Wei et al., 2003) or via reversal of the GABA transporters (Caraiscos et al., 2004; Wu et al., 2003). Additional factors such as anatomical barriers to diffusion as seen in the cerebellar glomerulus can influence levels of ambient GABA (Rossi and Hamann, 1998) as can alterations in the metabolic clearance rate via GABA transaminase (Overstreet and Westbrook, 2001). In fact, ambient levels of GABA appear to be closely controlled by these mechanisms to achieve mean levels close to 0.8–3 μM in the extracellular space (Lerma et al., 1986; Wu et al., 2003).
Both α4βδ and α5β3γ2 GABAR possess a high sensitivity to GABA, with EC50 s an order of magnitude lower than synaptically expressed α1β2γ2 GABAR (Brown et al., 2002; Caraiscos et al., 2004). They also exhibit lower rates and extent of desensitization (Bianchi et al., 2002; Brown et al., 2002; Caraiscos et al., 2004) than receptor combinations expressed subsynaptically, which together with their increased GABA sensitivity render them ideally suited for an extrasynaptic location. Because the GABA reversal potential is close to the membrane resting potential in many CNS areas, current generated by extrasynaptic receptors is believed to act as a shunting inhibition (Brickley et al., 2001). The importance of this inhibition to neuronal function is illustrated by the compensatory increase in expression of a novel non-inactivating K+ channel in cerebellar granule cells in mice lacking expression of the α6-containing GABAR which normally comprise the extrasynaptic population here (Brickley et al., 2001).
Several recent studies have demonstrated that the tonic current (Bai et al., 2000; Nusser and Mody, 2002; Stell et al., 2003) represents a greater fraction of the inhibitory component in dentate gyrus and hippocampus than the phasic component. This tonic current is also a major target of GABA-modulatory drugs such as BDZs, anesthetics, barbiturates, steroids and ethanol (Bai et al., 2000; Belelli and Herd, 2003; Liang et al., 2004; Stell et al., 2003; Wei et al., 2004). Our data as well suggest that the tonic component produces a greater response to BDZs recorded under control conditions. In contrast, 48 h steroid treatment resulted in a near complete BDZ insensitivity assessed by the change in the holding potential. By comparison, sIPSCs recorded at the same time exhibited only reduced BDZ sensitivity suggesting that increases in α4-containing GABAR expression in the extrasynaptic milieu may represent the primary outcome of this chronic steroid protocol. The decrease in BDZ responsiveness of synaptic current necessarily reflects increased expression of α4βγ2 GABAR by steroid treatment, as the γ2 subunit is required for tethering at synaptic sites. Indeed, our previous findings demonstrate that synaptic current is prolonged by RO15-4513 following 48 h steroid exposure (Hsu et al., 2003).
However, α4βγ2 GABAR were not increased in the extrasynaptic domain following steroid treatment, because the tonic current did not respond to application of RO15-4513, a BDZ partial agonist which acts as a typical BDZ agonist at α4βγ2 GABAR (Brown et al., 2002; Wafford et al., 1996). As we have shown for the synaptic current in an earlier study (Hsu et al., 2003), the tonic current recorded from control CA1 hippocampal neurons was slightly decreased by RO15-4513, consistent with its role as a partial inverse agonist at most GABAR subtypes (Wafford et al., 2004). However, our findings contrast with those of Liang et al. (2004) who report potent effects of RO15-4513 on tonic current recorded from CA1 hippocampal neurons. This apparent discrepancy is likely due to gender differences in GABAR subunit composition of this region, as the Liang study used adult male rats, while this study employed female rats in the diestrous–1 stage of the estrous cycle. Our pharmacological findings are supported by the Western blot data demonstrating almost undetectable levels of α4 expression in the CA1 hippocampus of diestrous female rats. Gender differences in both subunit composition and GABA pharmacology have been widely reported (Gulinello and Smith, 2003; Stock et al., 2000; Yee et al., 2004), so this pharmacological difference is not surprising.
The results from the present study suggest that the increase in α4βδ GABAR produced by 48 h steroid treatment represents a subunit switch rather than an increase in GABAR density in CA1 hippocampal pyramidal cells. First, the total bicuculline-sensitive tonic current was unchanged across steroid treatment groups, assessed under conditions when synaptic current was selectively blocked with a low concentration of gabazine (Stell and Mody, 2002). Secondly, 48 h steroid administration produced significant decreases in α1 and γ2 subunit expression in association with the increase in α4 and δ subunit expression in CA1 hippocampus. In the rat, unlike the mouse, a considerable percentage of the extrasynaptic GABAR population in the CA1 hippocampus is composed of α1-containing GABAR (Liang et al., 2004). In the present study, the steroid-induced decrease in α1 expression was reflected pharmacologically by a markedly reduced responsiveness of the GABAergic tonic current to zolpidem at a concentration selective for α1-containing GABAR (Horne et al., 1992; Vicini et al., 2001). Similar α4/α1 subunit substitutions have been reported following withdrawal from other GABA-modulatory compounds, such as ethanol (Kumar et al., 2003; Montpied et al., 1991; Liang et al., 2004) and BDZs (Chen et al., 2004), suggesting that this may represent a homeostatic mechanism common to GABA modulators. In fact, reduced responsiveness of the tonic current to these modulators has also been reported in the chronic intermittent model of ethanol intoxication (Liang et al., 2004).
The consequences of increased expression of α4βδ GABAR expression produced by 48 h steroid exposure would be determined both by their biophysical properties as well as their pharmacological properties. δ-containing GABAR, and α4β2δ GABAR in particular, have faster macroscopic current deactivation rates (Smith and Gong, 2005; Bianchi et al., 2002), with shorter single channel mean open times and lower open probability than other subtypes, such as α1βγ2 (Akk et al., 2004; Fisher and MacDonald, 1997). Thus, they would generate less inhibitory current than the α1βγ2 GABAR they are replacing. This conclusion is strengthened by our behavioral findings demonstrating increased anxiety in rats following 48 h steroid exposure.
Expression of α4βδ GABAR exhibits a bimodal distribution, increasing both after short-term exposure to E2+P or 3α,5α[β]-THP, as well as during withdrawal from these steroids (Sundstrom-Poromaa et al., 2002). This bimodal pattern is well-correlated with increases in anxiety states (Gulinello et al., 2001). As such, these findings suggest that our rodent model of steroid exposure/withdrawal may be effective as a model of premenstrual dysphoria (PMDD) (Endicott et al., 1999), which often exhibits bimodal patterns of symptom exacerbation, early in the luteal phase after short-term steroid exposure (Schmidt et al., 1998) and during the decline in steroids during the late luteal phase, a time of steroid “withdrawal”. Interestingly, women with PMDD have a relative insensitivity to BDZs (Sundstrom et al., 1997), assessed both using anxiety measures, as well as modulation of eye saccade velocity, a sensitive measure of GABAergic tone.
In conclusion, the results from the present study suggest that short-term exposure of female rats to an endogenous GABA-modulatory steroid alters in the expression of α4βδ GABAR. These changes altered the pharmacology of tonic current recorded from hippocampus, which were also reflected behaviorally. These results may be relevant for naturally occurring fluctuations in steroids, when altered GABA pharmacology has been reported.
Acknowledgments
The authors are grateful to Xinshe Li, Maria Gulinello and Yevgeniy Ruderman for helpful technical assistance, and to Ruth McKernan (Merck) for supplying the δ antibody. This work was supported by NIH grants DA-09618 and AA-12958 and a pharmaceutical contract from Lundbeck (Copenhagen, Denmark) to SSS.
References
- Akk G, Bracamontes J, Steinbach JH. Activation of GABA(A) receptors containing the alpha4 subunit by GABA and pentobarbital. J Physiol. 2004;556:387–399. doi: 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2000;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABA(A) receptors containing the delta subunit. Neuropharmacology. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3alpha-OH-5beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateal septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford K, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Huang X, Zeng XJ, Sieghart W, Tietz EI. Benzodiazepine-mediated regulation of alpha-1, alpha-2, beta1-3, and gamma-2 GABA(A) receptor subunit proteins in the rat brain hippocampus and cortex. Neuroscience. 2004;93:33–44. doi: 10.1016/s0306-4522(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Young J, Clavel M, Wehrey C, Veltz JN, Touter G, Mouren M, Prasad VV, Banner C, Sjovall J, Baulieu EE, Robel P. Neurosteroids: 3α-OH-5α-pregnan-20-one and its precursors in the brain plasma and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Costa AMN, Spense KT, Smith SS, Ffrench-Mullen JMH. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha-5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Thompson S, Saounatsou K, McKernan R, Krogsgaard-Larsen P, Wafford KA. Differences in agonist/antagonist binding affinity and receptor transduction using recombinant human gamma-aminobutyric acid type A receptors. Mol Pharmacol. 1997;52:1150–1156. [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewaart D, Thys-Jacobs S. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Fisher JL, MacDonald RL. Single channel properties of recombinant GABAA receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse L929 cells. J Physiol. 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA. The neurosteroid 3α, 5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the anxiolytic actions of gaboxadol: role of alpha4betadelta GABA(A) receptors. Neuroreport. 2003;14:43–46. doi: 10.1097/01.wnr.0000050303.92401.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABA-A receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- Horne AL, Hadingham KL, Macaulay AJ, Whiting PJ, Kemp JA. The pharmacology of recombinant GABA-A receptors containing bovine alpha-1, beta-1, gamma-2L subunits stably transfected into mouse fibroblast L-cells. Br J Pharmacol. 1992;107:732–737. doi: 10.1111/j.1476-5381.1992.tb14515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol. 2003;89:1929–1940. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, Sieghart W. Polyclonal antibodies directed against an epitope specific for the alpha4-subunit of GABAA receptors identify a 67-kDa protein in rat brain membranes. J Neurochem. 1994;62:764–769. doi: 10.1046/j.1471-4159.1994.62020764.x. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABA-A receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. Chronic ethanol consumption enhances internalization of alpha1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem. 2003;86:700–708. doi: 10.1046/j.1471-4159.2003.01894.x. [DOI] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herrerras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABA-A receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Zhiwei L, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000;20:8651–8658. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montpied P, Morrow AL, Karanian JW, Ginns EI, Martin BM, Paul SM. Prolonged ethanol inhalation decreases gamma-aminobutyric acidA receptor alpha subunit mRNAs in the rat cerebral cortex. Mol Pharmacol. 1991;39:157–163. [PubMed] [Google Scholar]
- Moran MH, Smith SS. Progesterone withdrawal I: Proconvulsant effects. Brain Res. 1998;807:91–100. doi: 10.1016/s0006-8993(98)00781-1. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of differing GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. Paradoxical reduction of synaptic inhibition by vigabatrin. J Neurophysiol. 2001;86:596–603. doi: 10.1152/jn.2001.86.2.596. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABA-A receptors in central synapses. J Neurosci. 1999;19:575–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signalling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Neelands TR, Macdonald RL. Contrasting actions of lanthanum on different recombinant gamma-aminobutyric acid receptor isoforms expressed in L929 fibroblasts. Mol Pharmacol. 1997;51:328–335. doi: 10.1124/mol.51.2.328. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Nieman L, Danaceau M, Adams L, Rubinow D. Differential behavioral effects of gonadal steroids in women with premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, Ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–929. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH. Neurosteroid administration and withdrawal alter GABAA receptor kinetics in CA1 hippocampus of female rats. J Physiol (Lond) 2005;564:421–436. doi: 10.1113/jphysiol.2004.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock H, Foradori C, Ford K, Wilson MA. A lack of tolerance to the anxiolytic effects of diazepam on the plus-maze: comparison of male and female rats. Psychopharmacology (Berl) 2000;147:362–370. doi: 10.1007/s002130050004. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong Q, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith S. Hormonally regulated α4β2δ GABA-A receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Backstrom T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Sur C, Farrar S, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha-4 and delta subunits of the GABA-A receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford K, Macauley AJ, Fradley R, O’Meara GF, Reynolds DS, Rosahl TW. Differentiating the role of gamma-aminobutyric acid type A (GABA-A) receptor subtypes. Biochem Soc Trans. 2004;32:553–556. doi: 10.1042/BST0320553. [DOI] [PubMed] [Google Scholar]
- Wafford K, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABA-A receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg P. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson G. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol. 2003;89:2021–2034. doi: 10.1152/jn.00856.2002. [DOI] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Mohler H, Rudolph U, Feldon J. GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABA-A receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Wang JF, Corsi L, Vicini S. Lanthanum-mediated modification of GABA-A receptor deactivation, desensitization, and inhibitory synaptic currents in rat cerebellar neurons. J Physiol. 1998;511:647–661. doi: 10.1111/j.1469-7793.1998.647bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]