Abstract
Withdrawal from the endogenous steroid progesterone (P) after chronic administration increases anxiety and seizure susceptibility via declining levels of its potent GABA-modulatory metabolite 3α-OH-5α-pregnan-20-one (3α,5α-THP). This 3α,5α-THP withdrawal also results in a decreased decay time constant for GABA-gated current assessed using whole cell patch-clamp techniques on pyramidal cells acutely dissociated from CA1 hippocampus. The purpose of this study was to test the hypothesis that the decreases in total integrated GABA-gated current observed at the level of the isolated pyramidal cell would be manifested as a reduced GABA inhibition at the circuit level following hormone withdrawal. Toward this end, adult, female rats were administered P via subcutaneous capsule for 3 wk using a multiple withdrawal paradigm. We then evaluated paired-pulse inhibition (PPI) of pyramidal neurons in CA1 hippocampus using extracellular recording techniques in hippocampal slices from rats 24 h after removal of the capsule (P withdrawal, P Wd). The population spike (PS) was recorded at the stratum pyramidale following homosynaptic orthodromic stimulation in the nearby stratum radiatum. The threshold for eliciting a response was decreased after P Wd, and the mean PS amplitude was significantly increased compared with control values at this time. Paired pulses with 10-ms inter-pulse intervals were then applied across an intensity range from 2 to 20 times threshold. Evaluation of paired-pulse responses showed a significant 40–50% reduction in PPI for PS recorded in the hippocampal CA1 region after P Wd, suggesting an increase in circuit excitability. At this time, enhancement of PPI by the benzodiazepine lorazepam (LZM; 10 µM) was prevented, while pentobarbital (10 µM) potentiation of PPI was comparable to control levels of response. These data are consistent with upregulation of the α4 subunit of the GABAA receptor (GABAR) as we have previously shown. Moreover, the reduced PPI caused by P Wd was prevented by suppression of GABAR α4-subunit expression following intraventricular administration of specific anti-sense oligonucleotides (1 µg/h for 72 h). These results demonstrating a reduction in PPI following P Wd suggest that GABAergic-mediated recurrent or feed-forward inhibition occurring at the circuit level were decreased following P Wd in female rats, an effect at least partially attributable to alterations in the GABAR subunit gene expression.
INTRODUCTION
The GABAA receptor (GABAR) mediates the majority of fast (1–100 ms) synaptic inhibition in the mammalian brain. This receptor is a member of a ligand-gated ion-channel superfamily that includes nicotinic and 5-HT3 receptors (Hevers and Luddens 1998). Structurally and pharmacologically GABARs are characterized as chloride ion channel–associated receptors composed of heteropentameric subunits (Hevers and Luddens 1998; Macdonald and Olsen 1994). Many distinct subunit subtypes (α1 – α6, β1 – β4, γ1 – γ3, δ, ε, π, ρ1 – ρ3, and θ) have been identified (reviewed by Barnard et al. 1998), which can be assembled into GABAR isoforms with distinct pharmacological and physiological properties. These properties include unique conductance states, kinetics of decay, time course of desensitization, and responses to modulators, such as benzodiazepines (BDZs) and barbiturates (Benke et al. 1997; Bianchi et al. 2001; Fisher and Macdonald 1997; Gingrich et al. 1995; Lavoie et al. 1997; Wafford et al. 1996; Wisden et al. 1991). In pyramidal cells of the rat CA1 hippocampus, the predominant GABAR subtypes expressed are α1β1/3γ2 and α1β1/3γ2, which are sensitive to modulation by BDZs and barbiturates, with moderate expression of α3- and α5-containing GABARs also observed (Endo and Olsen 1993; Nusser et al. 1996; Wisden et al. 1992).
P is a circulating steroid of ovarian and adrenal origin that is readily converted into 3α,5α-THP (or allopregnanolone) peripherally or within the CNS (Bitran et al. 1993; Corpechot et al. 1993; Purdy et al. 1991). 3α,5α-THP is a potent positive modulator of GABAR at physiological concentrations (10–100 nM), where it acts in a barbiturate-like fashion to prolong the open time of the GABA-gated Cl− channel (Callachan et al. 1987; Majewska et al. 1986; Twyman and Macdonald 1992), an effect that would enhance GABAergic inhibition at the circuit level assessed in vitro (Harrison et al. 1987; Patenaude et al. 2001) or in vivo (Smith et al. 1987a,Smith et al. 1987b). Behaviorally, this steroid acts like the BDZs and barbiturates in that it is anxiolytic (Bitran et al. 1991, 1999), anti-convulsant (Belelli et al. 1989; Devaud et al. 1995; Frye 1995; Wilson 1992), sedative (Lancel et al. 1997), and at high doses, can act as an anesthetic (Bixo and Backstrom 1990; Korneyev and Costa 1996).
In a manner similar to other GABA-modulatory agents (File 1990; Gallager et al. 1984; Kang et al. 1996), 3α,5α-THP also exhibits withdrawal properties following chronic (3 wk) administration of its parent compound, P (Costa et al. 1995; Smith et al. 1998a). This withdrawal state is characterized by increases in anxiety, assessed using a number of animal models including the defensive burying paradigm (Gallo and Smith 1993), light:dark transition (Gallo and Smith 1993), and the elevated plus maze (Smith et al. 1998b), as well as increases in seizure susceptibility (Moran and Smith 1998; Reddy et al. 2001; Smith et al. 1998a).
Our previous findings suggest that the behavioral excitability observed following withdrawal from the GABA-modulatory steroid 3α,5α-THP is due to a reduction in GABA-mediated inhibition, as a result, at least in part, of increased expression of α4-containing GABAR in the hippocampus (Concas et al. 1999; Smith et al. 1998a). However, our prior studies have utilized a postsynaptic model to examine GABAergic function in isolated cells. In evaluating the effect of P Wd on neuronal excitability, it is necessary to also consider other factors those may affect the hippocampal excitability at the circuit level. Factors such as presynaptic release of GABA, excitatory responses to glutamate, and altered effects of endogenous modulators may exert compensatory effects on the apparent decreases in GABAergic current we have observed in isolated cells following hormone withdrawal. The present study made use of a model of hippocampal circuit excitability, the PPI paradigm, which tests the percentage change in neuronal responses to the second of paired electrical stimuli delivered 10 ms apart. The inhibition of this second response normally observed is due to GABAergic feedback by interneurons innervating the pyramidal cell layer (Karnup and Stelzer 1999; Lacaille et al. 1989; Rogers and Hunter 1992), and has been used extensively to demonstrate increased excitability of the hippocampal circuitry following withdrawal from GABA-modulatory drugs, such as alcohol (Kang et al. 1998; Rogers and Hunter 1992), as well as following kindling in various seizure models (Barkai et al. 1994; Fathollahi et al. 1997; Kamphuis et al. 1992; Sloviter 1987).
The goal of this study was to examine the effects of P Wd on PPI of hippocampal pyramidal neurons in CA1 hippocampus as a general measure of hippocampal excitability to compare this parameter with other withdrawal hyperexcitability states and models of seizure activity. In addition, we evaluated the role of α4 GABAR subunit upregulation in mediating altered hippocampal excitability with the use of in vivo antisense oligonucleotide administration (Smith et al. 1998a). The results from these studies will have implications for altered behavioral excitability and seizure susceptibility observed across naturally occurring fluctuations of 3α,5α-THP, such as reported during the premenstrual period (Blumer et al. 1998; Endicott et al. 1999; Rapkin et al. 1997; Schmidt et al. 1994).
METHODS
Subjects
Female Long-Evans rats (110–130 g, Long Evans, Charles River) were housed in groups of three under a constant light/dark cycle (14/10 h light/dark). Food and water were available continuously in an environmentally controlled animal facility. The stage of the estrous cycle was assessed by microscopic evaluation of the vaginal lavage as described by Smith and Chapin (1996). Animal care and use were in accordance with approved university IACUC guidelines.
Progesterone administration and withdrawal paradigm
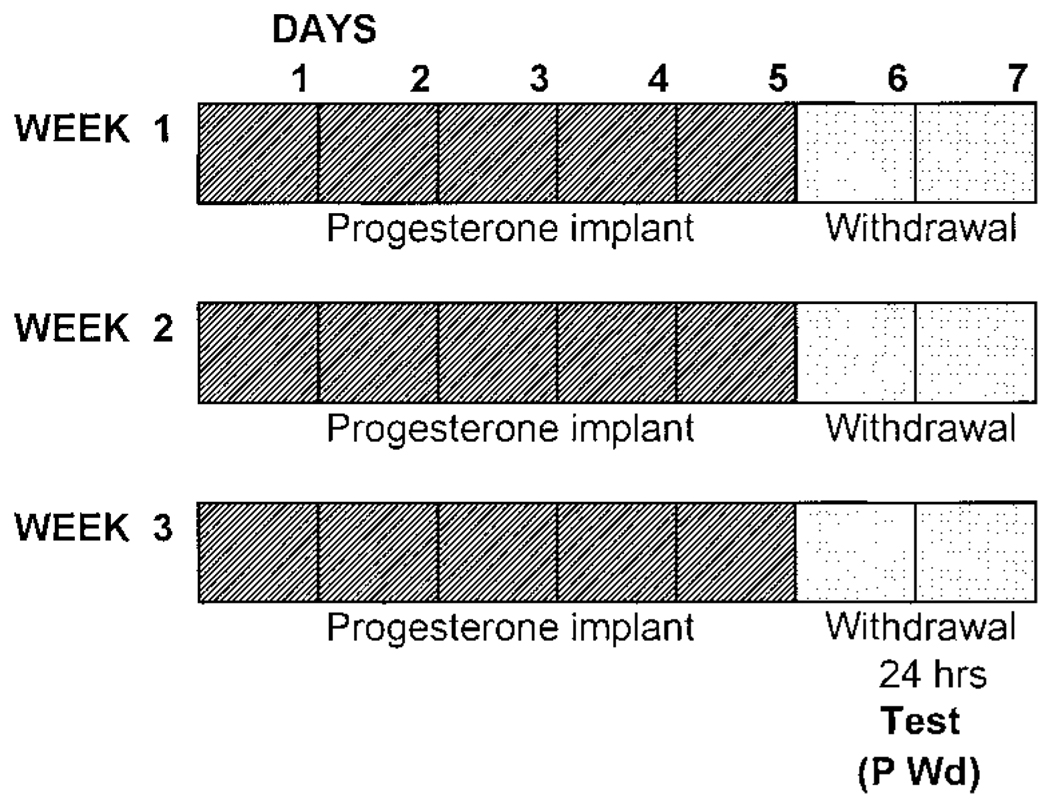
The use of multiple cycles to test the withdrawal properties of progesterone (P) is physiologically relevant as a model for premenstrual syndrome (PMS), where multiple hormone cycles produce altered mood (Endicott et al. 1999). A similar multiple-cycle withdrawal protocol also has been used as a model for ethanol withdrawal (Mahmoudi et al. 1997). Briefly, a P (Sigma, crystalline)-filled capsule of silicone tubing (1/16 × 1/8 in OD, 10 mm/100 g body weight; Nalgene) was implanted subcutaneously in the lower back after induction of halothane anesthesia (3% in O2, 1 l/min). This paradigm leads to physiological circulating levels of P as determined by radioimmunoassay (Moran and Smith 1998). Administration of P was undertaken for 5 days (2 days withdrawal/wk) for three weekly cycles (Fig. 1). Rats were tested 1 day after removal of the implant (“P withdrawal”) following this 3-wk paradigm. Controls received sham implants and/or vehicle and were tested on the day of diestrus.
FIG. 1.
Progesterone withdrawal protocol. A 3-wk multiple-withdrawal paradigm (P Wd) was conducted via subcutaneous implantation of a silastic capsule containing crystalline P or an empty capsule (sham control). Animals were tested 24 h after implant removal (“withdrawal”, P Wd).
Antisense oligodeoxynucleotide administration
Antisense or missense oligodeoxynucleotides (18 mer) specific for the GABAR α4 subunit (+3 relative to the ATG for initiating translation) were phosphorothioated at all positions and HPLC purified (Genosys), as described previously (Smith et al. 1998a). The α4 antisense (ACCTTCTGGACAGAAACC) was used for the experimental group of animals and the α4 missense (ACCATCTAGACTGAAGCC) was used as a control. Three days before P implant removal, antisense oligos were delivered via a subcutaneously implanted osmotic minipump (Alza) at a concentration of 1 µg/day (vehicle, 0.35% BSA/0.15 M saline) at a rate of 1 µl/h for 72 h through 29-gauge tubing attached to an implanted cannula terminating within the right lateral ventricle (−0.8 mm A-P; 1.5 LAT; 3.2 DOWN). (Correction for size: bregma-lambda distance/9 times stereotaxic coordinates). Controls received missense oligonucleotides (1 µg/day) using the same procedure.
Western blot protocol
Successful downregulation of α4 subunit expression in hippocampus was determined with standard Western blot procedures, as described previously (Smith et al. 1998b). To this end, hippocampal membranes were first normalized according to protein content and probed with an antibody developed against a peptide of rat α4 (amino acids 517–523, with an amino terminal cysteine), from a protocol originally described by Kern and Sieghart (1994), which has been fully characterized (Smith et al. 1998a). The α4 band (67 kDa) was detected with enhanced chemiluminescence visualization and quantified using a Umax scanner and One-Dscan software. The results were standardized to a GAPDH (36 kDa) control protein.
In vitro slice preparation
Animals were rapidly decapitated, and the brains were removed and cooled using an ice cold solution of artificial cerebrospinal fluid (aCSF) containing (in mM) 124 NaCl, 5 KCl,2 CaCl2, 1.25 KH2PO4, 2 MgSO4, 26 NaHCO3, and 10 glucose, which was saturated with 95% O2-5% CO2 and buffered to a pH of 7.4. The hippocampi were then rapidly removed and cut into 400-µm coronal slices with a McIlwain-type Tissue Chopper. Hippocampal slices were held between two nylon nets in a tissue chamber on the stage of the microscope and perfused with aCSF (2 ml/min) at 34 ± 0.5°C. The slices were allowed to incubate in an oxygenated chamber for at least 1 h prior to the electrophysiological recording described below.
Extracellular recording methods: PPI paradigm
Extracellular recordings in the CA1 stratum pyramidale were made with NaCl (3 M)-filled glass electrodes with a resistance of 3–7 MΩ. A bipolar electrode was positioned approximately 150 µm away from the recording electrode in the stratum radiatum to maximize the activation of inhibitory inputs to the CA1 pyramidal neurons. The tip of the recording electrode was positioned at a depth of 60–160 µm from the slice surface. Square wave pulse stimuli (200 µs duration) were delivered at 0.02 Hz with a range of 10–1,000 µA, depending on the threshold intensity for each slice. Field potentials were amplified (Axon Instruments), stored, and analyzed off-line by computer with pCLAMP 5.1 software (Axon Instruments). Slices with maximal field potential amplitudes <3 mV were excluded from the study. PS threshold was determined as the stimulus intensity required to result in a 0.5-mV deflection from baseline. Paired pulses were applied through the same stimulating electrode (homosynaptic stimulation) at interpulse intervals of 10 ms, after initial pilot recordings revealed maximal PPI at this inter-pulse interval (data not shown). A 10-ms interpulse interval has been used previously to investigate inhibition mediated by postsynaptic GABAR (Davies et al. 1990). PSs were routinely evoked with a range of stimulus intensities ranging from threshold to maximal response. Three to six paired responses were obtained at each intensity with a 50-s interval between every paired stimulation, and a 1- to 2-min interval between the sampling at each intensity.
Drug application
GABA modulatory drugs, such as the benzodiazepine lorazepam (LZM, 10 µM) and the barbiturate pentobarbital (10 µM), were bath applied in the slice chamber to test the pharmacology of the PPI response in different experimental groups. These doses were selected because they result in robust GABA-modulatory effects of hippocampal CA1 pyramidal cells recorded from control rats at room temperature (Smith et al. 1998a). To reach equilibrium, the compounds were applied more than 10 min before recording. All compounds were obtained from Sigma Chemical.
Data analysis
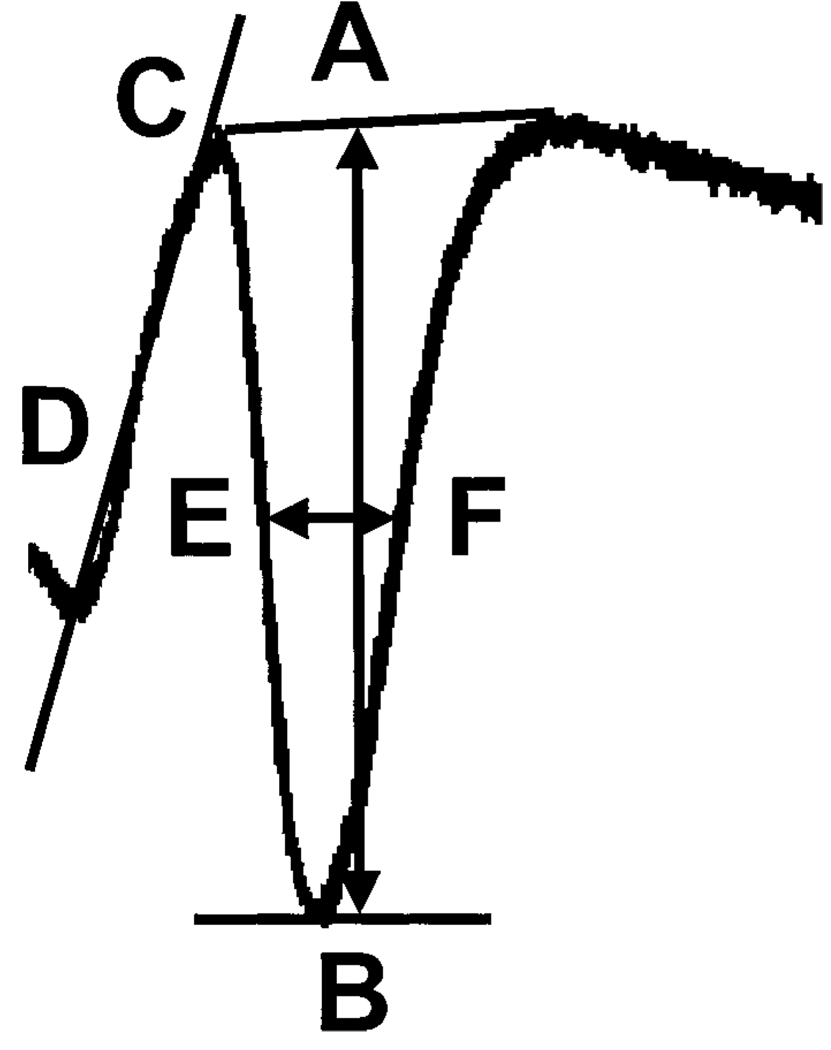
For this PPI paradigm, PS amplitude (Fig. 2, A and B) and area were measured and compared between the second (test) and the first (conditioning) PS responses using Origin software (Microcal). For comparison, the data obtained from three to six responses were averaged to minimize the variability between slices and each sampling of PS. In addition, the threshold for eliciting a PS, as well as the excitatory postsynaptic potential (EPSP) slope (Fig. 2, C and D) and half-width (Fig. 2, E and F) of the PS were evaluated across the range of stimulus intensities tested. The paired-pulse response was defined as the second evoked response of the indicated parameter (e.g., amplitude of PS) expressed as a percentage decrease of the first evoked response and is described by the following equation
Comparisons between slices from control and P Wd animals were analyzed using the Student’s t-test (2 groups). One-way ANOVA with repeated measures and Student-Newman-Keuls post hoc procedures were used to assess the differences when more than two groups were compared. Significance was determined when P < 0.05. Numerical and graphed data (Sigmaplot, Jandel Scientific) were presented as mean ± SE.
FIG. 2.
Schematic diagram of population spike analysis. The population spike (PS) parameters analyzed included the following: 1) PS amplitude (mV; A and B), 2) excitatory postsynaptic potential (EPSP) slope (mV/ms; C and D), and 3) PS half-width (ms; E and F), as indicated. In some cases, the PS area was also determined for P Wd and control groups across a range of stimulus intensities (2–20T).
RESULTS
PS threshold
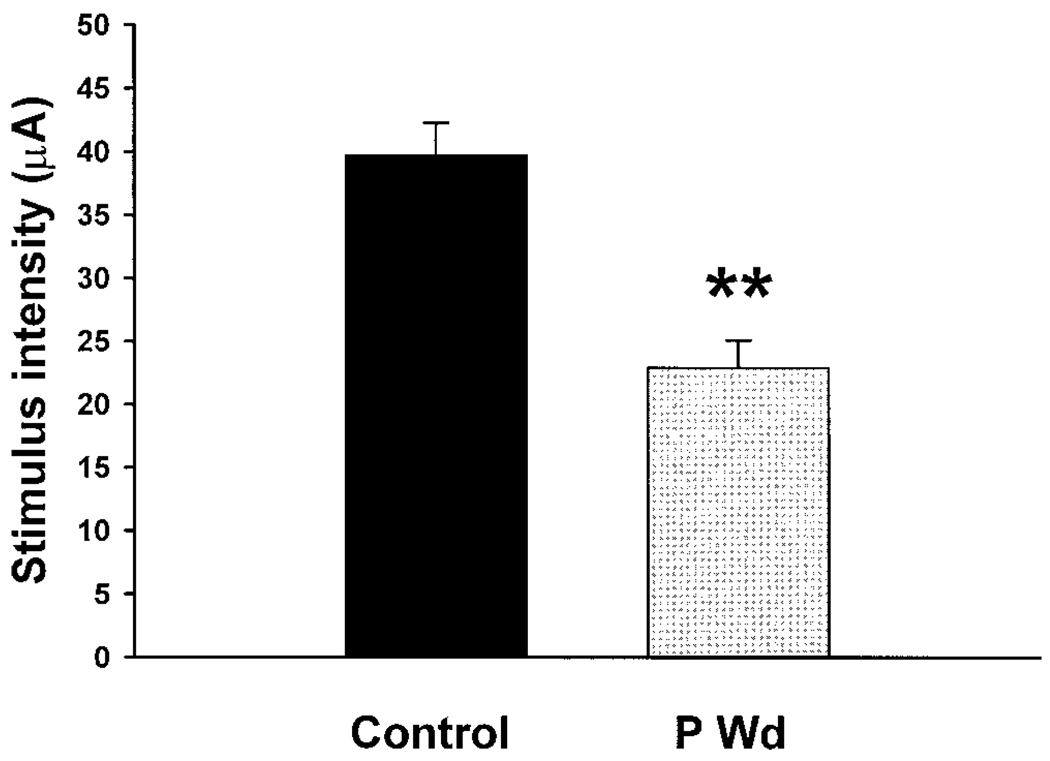
An initial measure of neuronal excitability is the threshold stimulus for eliciting a PS. Following P Wd, the PS-evoked threshold was significantly reduced (P < 0.001) compared with control values (Fig. 3). The minimum stimulus intensity to evoke a PS in hippocampal slices from rats undergoing P Wd was 22.9 ± 2.17 µA (n = 48) versus 39.7 ± 2.57 µA in the control (n = 66), a 42.3% decrease. (For this and the following figures, data are represented as mean ± SE.)
FIG. 3.
Threshold for evoking a PS is reduced after progesterone withdrawal. For this and the following studies, hippocampal slices were prepared to investigate hippocampal excitability following progesterone withdrawal (P Wd, see Fig. 1). Threshold for eliciting a PS was significantly reduced (**P < 0.001) following removal of the P implant (P Wd): 39.7 ± 2.57 (SE) µA (n = 66) and 22.9 ± 2.17 µA (n = 48) were the minimum intensities to evoke a PS in control and P Wd slices, respectively.
PS amplitude
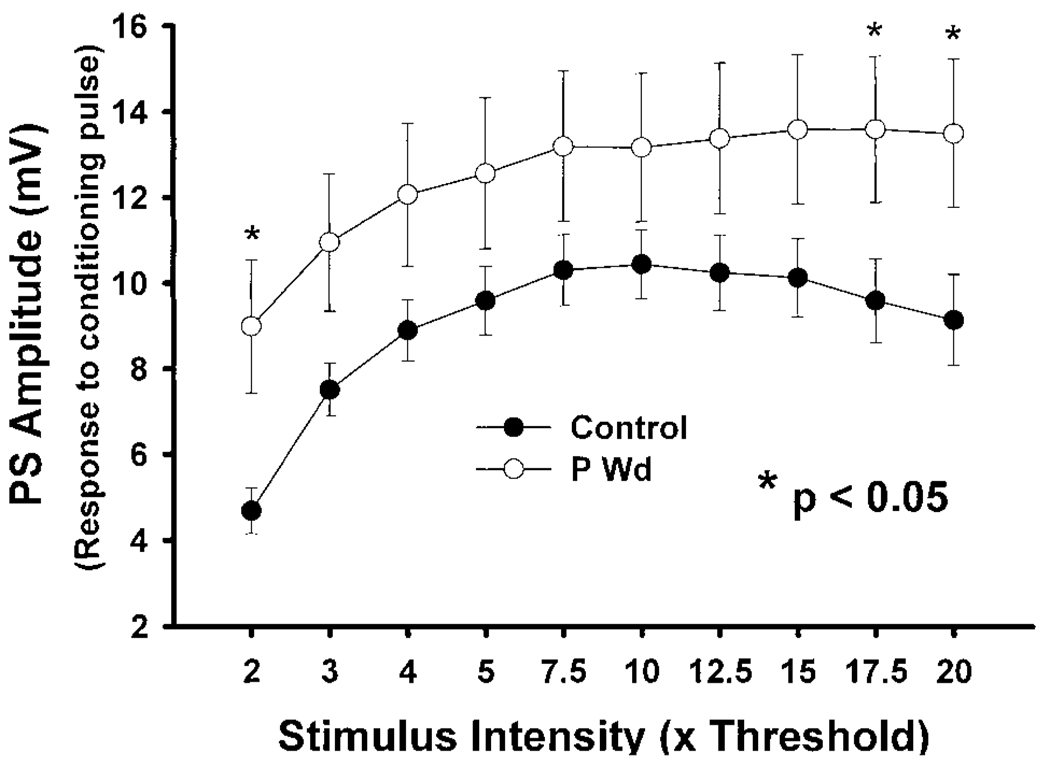
The mean amplitude of the PS in response to the initial (conditioning) stimulus, assessed 1 day after P Wd, was increased significantly above control values (Fig. 4; *P < 0.05). At this time, the PS amplitude was twofold higher than control at the lowest stimulation intensity (2T, 8.99 ± 1.56 mV, P Wd vs. 4.68 ± 0.53 mV, control, P < 0.05) and increased by 47.7% (P < 0.05) at the highest stimulation intensity (20T) following P Wd (13.50 ± 1.73 mV) compared with control responses (9.14 ± 1.07 mV).
FIG. 4.
PS amplitude is increased after P Wd. For this and the following figures, data were normalized with the minimal intensity (Threshold, “T”) to evoke the lowest amplitude PS. PS amplitude in response to the conditioning pulse increases as a function of stimulus intensity up to a plateau phase. A significant (*P < 0.05) increase in PS amplitude was observed following P Wd both at the lowest and the highest stimulus intensities (2T: 8.99 ± 1.56 mV; 20T: 13.50 ± 1.73 mV) compared with those of controls (2T: 4.68 ± 0.53 mV; 20T: 9.14 ± 1.07 mV). n = 12–13 slices/group.
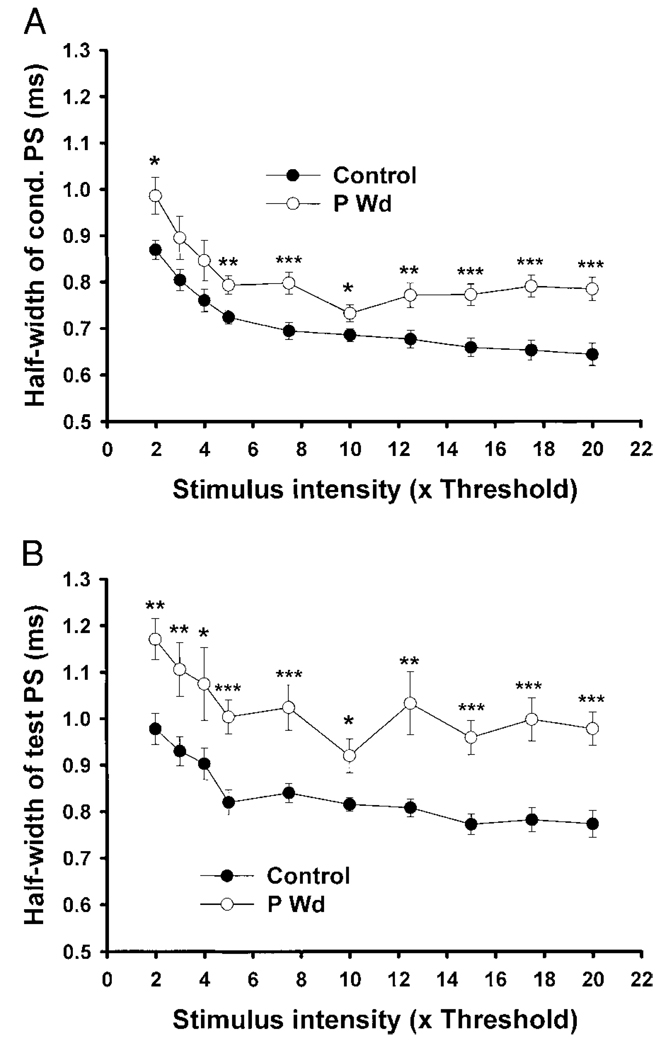
PS half-width
In addition to increasing the relative and absolute amplitudes of the test and conditioning PS, P Wd also resulted in a marked widening of the PS, especially evident in the test PS (Fig. 5B) where values for PS half-width are 25–35% greater than control across the range of stimulus intensities tested. To a lesser extent, the half-width of the conditioning PS was increased by 10–20% at increasing stimulus intensities following P Wd compared with control (Fig. 5A).
FIG. 5.
PS half-width is increased following P Wd. Both the conditioning (1st) PS (A) and test (2nd) PS (B) half-width values were increased following P withdrawal (P Wd) compared with control across the range of stimulus intensities tested. *P < 0.05, **P < 0.01, ***P < 0.001, and n = 12–13 slices/group.
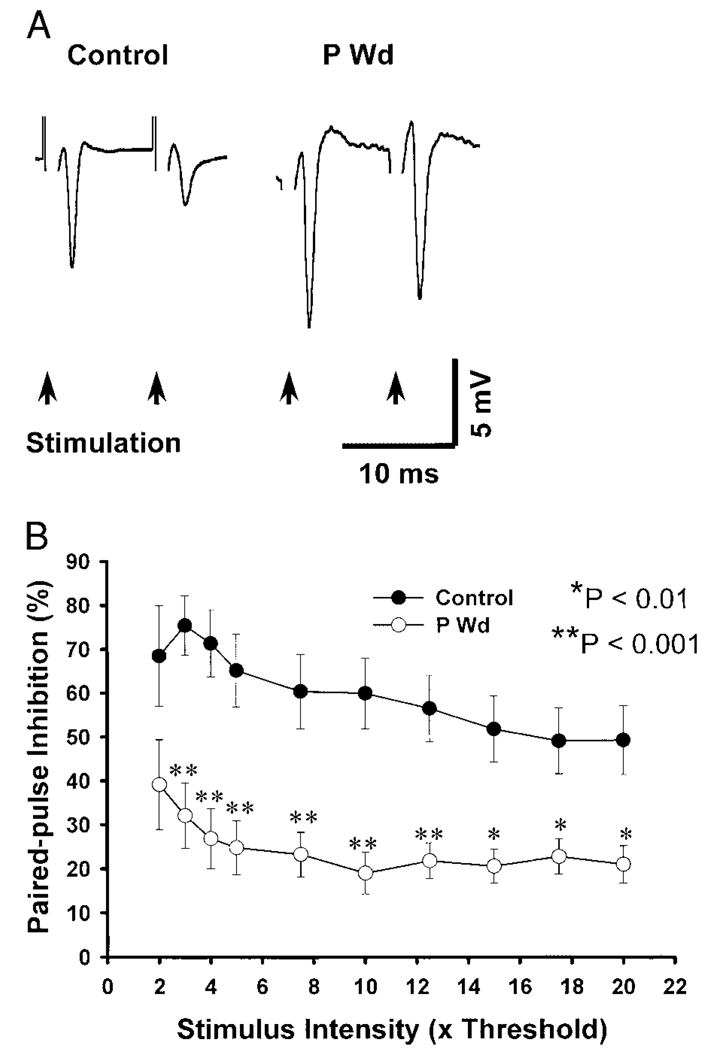
PPI
Under control conditions, the population response to the second (test) of two paired stimuli (PStest) delivered 10 ms apart is routinely of smaller amplitude than the response to the initial (conditioning) stimulus (PScond), an effect referred to as PPI (Davies et al. 1990). The degree of this inhibition was significantly decreased (40–60%; *P < 0.01 and **P < 0.001) after P Wd (Fig. 6, A and B) compared with control. For this and the following figures, PPI is expressed as a percentage change in the peak amplitude for the second PS response versus the initial response: [(PScond amplitude – PStest amplitude)/PScond amplitude] × 100%. At a stimulus intensity of five times threshold (5T), PPI was 24.78 ± 6.13% following P Wd, a 61.98% decrease in this parameter compared with control (65.18 ± 8.28%). This result is consistent with the hypothesis that increased neuronal excitability is observed after P Wd (Smith et al. 1998a).
FIG. 6.
Paired-pulse inhibition (PPI) is decreased following P Wd. For this and the following figures, paired stimuli were applied through the same stimulating electrode (homosynaptic stimulation) at a 10-ms inter-pulse interval. A: representative traces from paired-pulse recordings of hippocampal slice activity from control and P Wd animals. B: PS response to the 2nd stimulus expressed as a percentage decrease of the initial (conditioning) stimulus: [(PScond – PStest)/PScond] × 100%, is graphed as a function of the stimulus intensity. The data indicate that the degree of PPI was markedly decreased after P Wd (* P < 0.01, **P < 0.001; n = 12 slices/group).
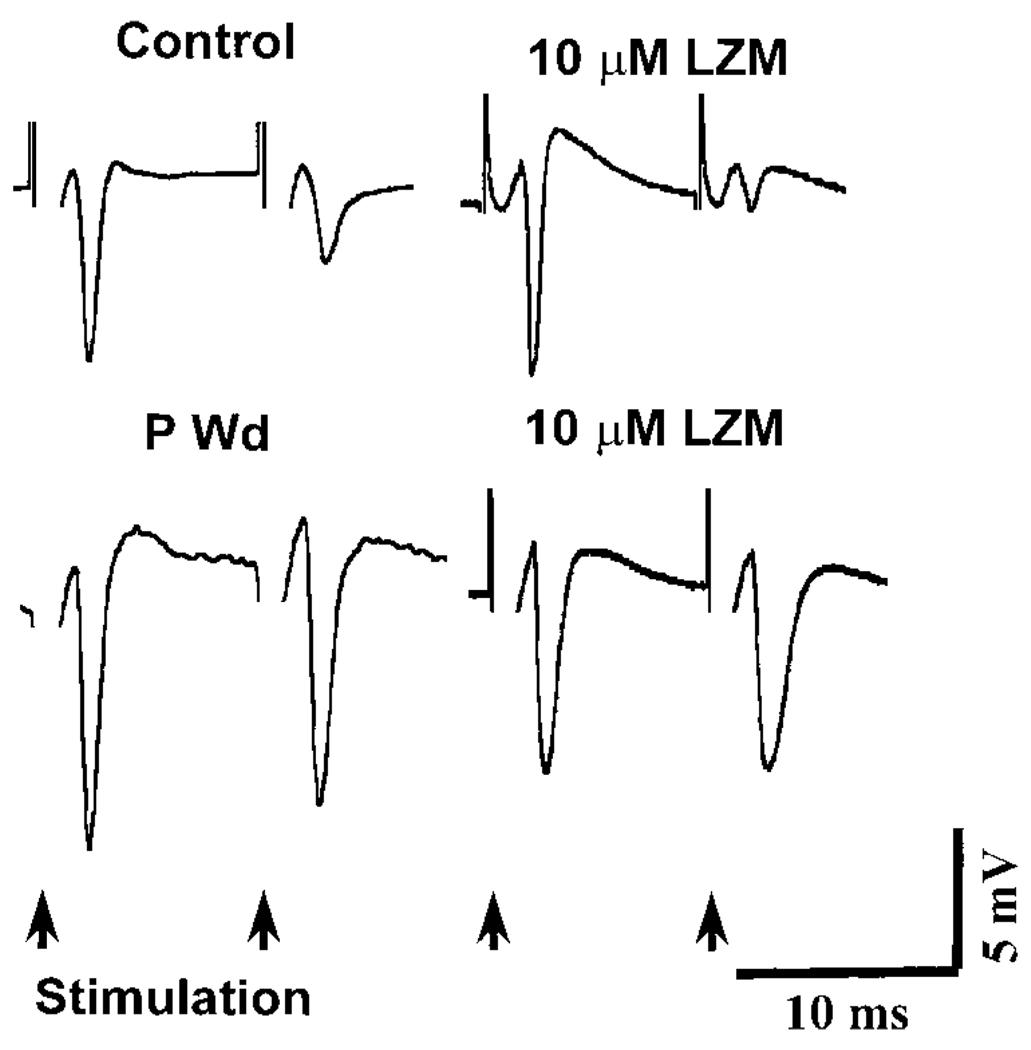
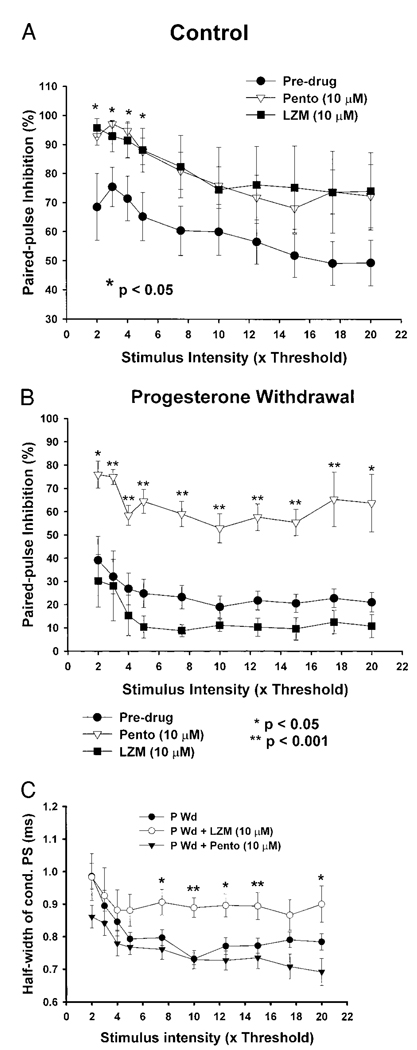
Pharmacology of PPI
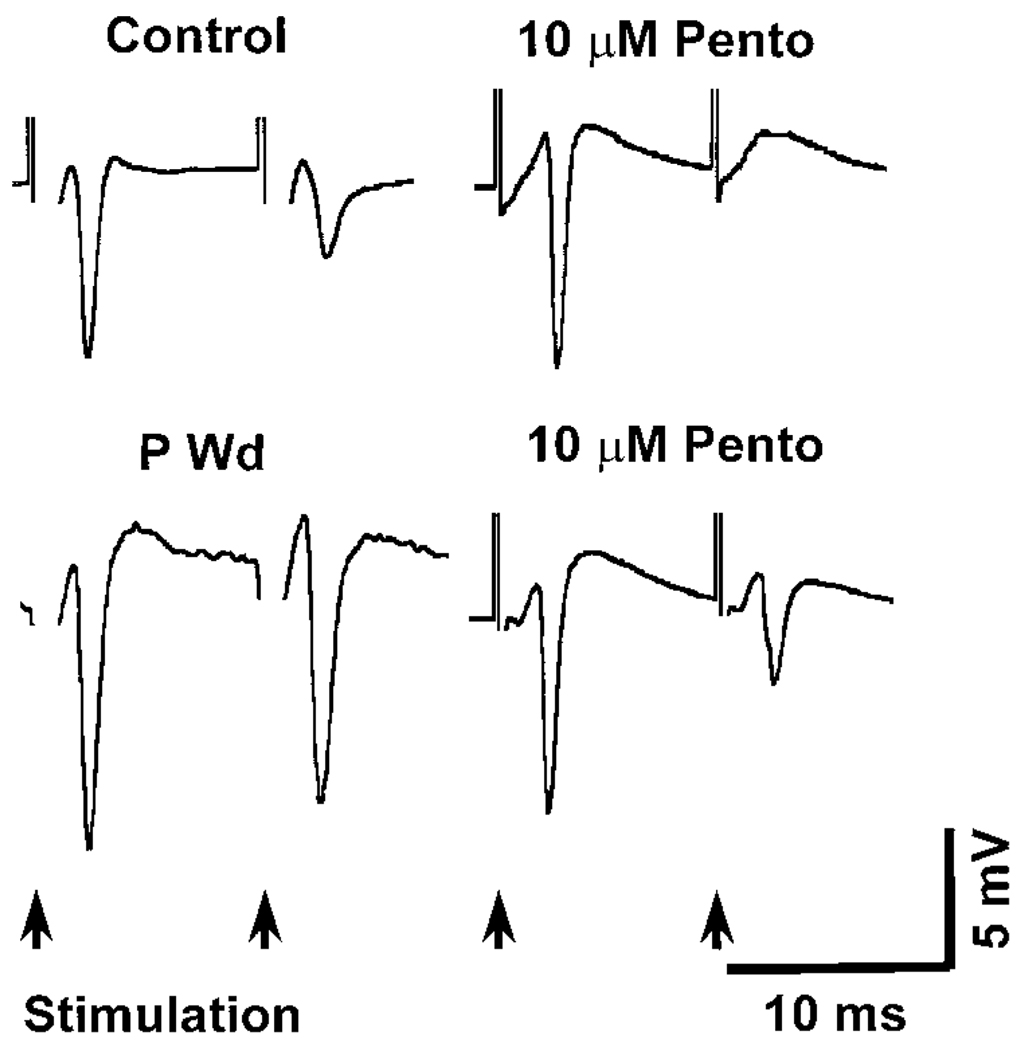
In control slices, PPI was strongly potentiated (PPI > 70%) following bath application of either the benzodiazepine LZM (10 µM, Figs. 7 and 9A) or the barbiturate pentobarbital (10 µM, Figs. 8 and 9A). In both cases, the test PS was almost totally inhibited (PPI > 95%) at a range of stimulus intensities (2–4T) following administration of these drugs (6 of 7 slices in each drug treatment group). At a 3T intensity stimulation, the degree of PPI was 97.13 ± 0.99% and 92.94 ± 5.40% following application of pentobarbital or LZM, respectively. In both cases, these values were significantly greater (P <0.05) than control predrug PPI values (75.40 ± 6.76%) by 25–30%. These results are consistent with the role of these compounds as positive modulators of the GABAR.
FIG. 7.
P Wd reduces the ability of the benzodiazepine lorazepam (LZM) to potentiate PPI in CA1 hippocampus. Representative traces indicate that LZM loses its ability to potentiate PPI (bottom right) following P Wd compared with its effect on control slices (top right). In control slices, PPI was strongly potentiated (PPI > 70%) by bath application of LZM (10 µM) as noted by the significant decrease in the PS response to the 2nd (test) stimulus compared with the response to the 1st (conditioning) stimulus. In contrast, LZM produced no significant effect on the amplitude of the 2nd (test) pulse following P Wd (bottom right).
FIG. 9.
Pharmacology of PPI: summary data. A: population data demonstrating the percentage PPI plotted as a function of stimulus intensity (XT) reveal significant effects (*P < 0.05) for both pentobarbital and LZM in enhancing PPI in control slices. B: in contrast, only pentobarbital, but not LZM, was effective in potentiating PPI in slices from P Wd animals (*P < 0.05, **P < 0.001). C: conditioning PS half-width was increased by LZM, but not pentobarbital administration, following P withdrawal (P Wd) at stimulus intensities from 7.5 to 20T. (*P < 0.05, **P < 0.01; n = 6–12 slices/group).
FIG. 8.
PPI is significantly potentiated by pentobarbital in CA1 hippocampus following P Wd. These representative traces demonstrate that pentobarbital (Pento) was still effective in potentiating PPI (bottom right) in slices from animals undergoing P Wd (>60% with 5T intensity) similar to control (top right, >90% with 5T intensity).
PPI pharmacology—P Wd
The robust potentiation of hippocampal PPI observed following administration of LZM under control conditions was completely prevented following P Wd. At a 5T stimulus intensity, bath application of 10 µM LZM did not significantly increase PPI above predrug values (10.34 ± 4.67% vs. 24.78 ± 6.13%, respectively, Figs. 7 and 9B). In contrast, pentobarbital (10 µM) was able to significantly (*P < 0.05 and **P < 0.001) potentiate PPI after P Wd (Figs. 8 and 9B) at this time. At a 4T stimulus intensity, pentobarbital increased the degree of PPI twofold above predrug values (58.06 ± 4.68%, P Wd + Pento vs. 26.83 ± 6.86%, P Wd without Pento). This degree of PPI potentiation represents an increase of 116.40%, a four- to fivefold greater potentiation than observed under control conditions (Fig. 9A).
Although PPI and PS amplitude were unaltered by LZM following P Wd, consistent with upregulation of the α4 subunit observed at this time, the half-width of the conditioning PS (as well as the test PS; data not shown) was significantly increased (by 13–20%) by LZM at stimulus intensities >7.5T (Fig. 9C). In contrast, pentobarbital did not alter the PS half-width, suggesting a specific effect of the withdrawal conditions on this parameter.
Administration of α4-subunit antisense oligonucleotides
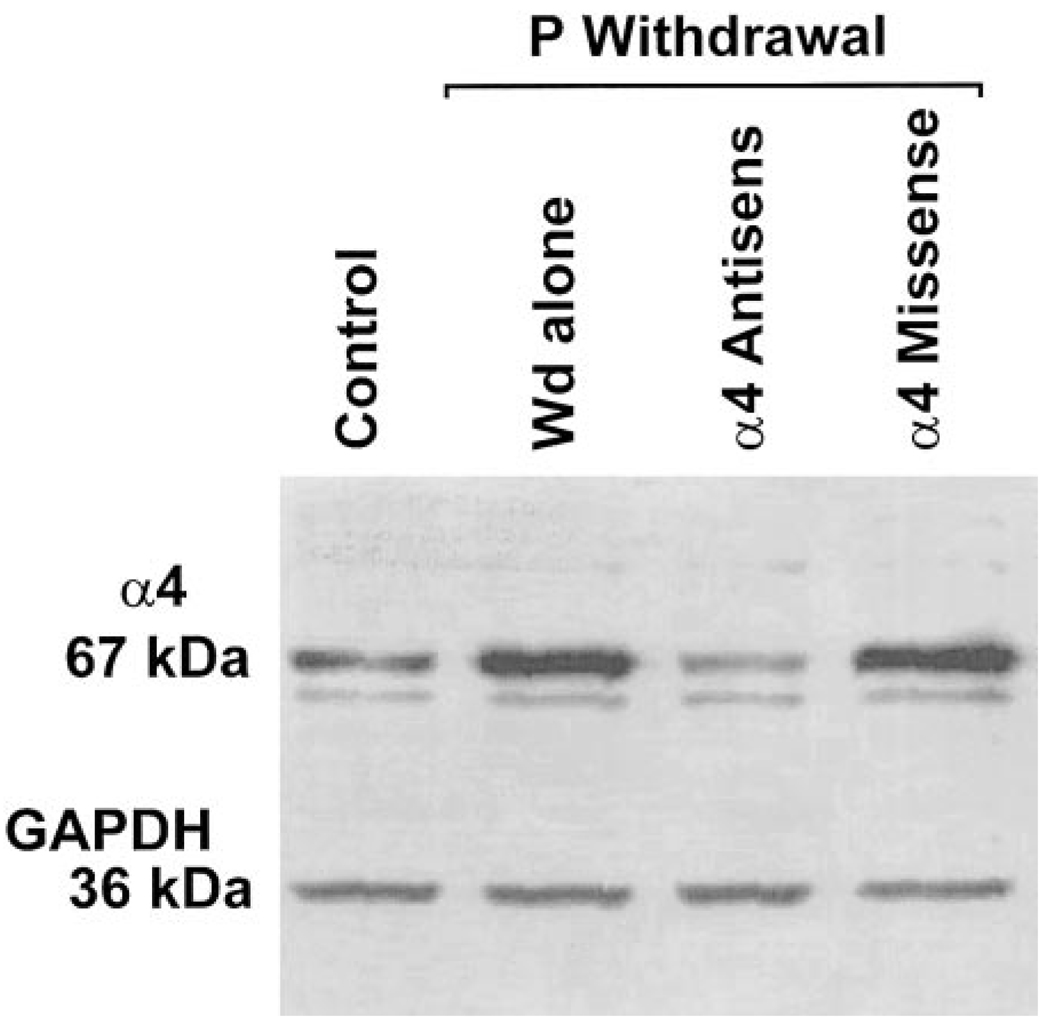
Based on our previous findings (Smith et al. 1998a) that suggest that withdrawal from P upregulates the α4 subunit of the GABAR, we tested the possibility that increases in α4-containing GABAR contribute to the observed decrease in the degree of PPI following P Wd. To this end, antisense oligonucleotides specific for the GABAR α4 subunit were administered intraventricularly for the final 72 h of the P Wd paradigm. This treatment successfully prevented increased expression of the α4 subunit normally observed following P Wd in six of seven rats tested (Fig. 10).
FIG. 10.
Administration of α4-antisense oligonucleotide into the lateral ventricle prevents upregulation of the GABAR α4 subunit normally observed following P Wd. Western Blot analysis of α4-subunit levels from hippocampi of animals treated with α4-subunit antisense oligonucleotides during the final 72 h of the P Wd paradigm indicates a significant reduction in α4-subunit levels (67 kDa) compared with the results obtained after α4 missense treatment to P Wd animals. In contrast, P Wd (Wd alone) resulted in marked threefold increases in α4 levels compared with values from sham-implanted untreated animals (Control). Results are normalized to levels of the housekeeping protein, GAPDH (36 kDa).
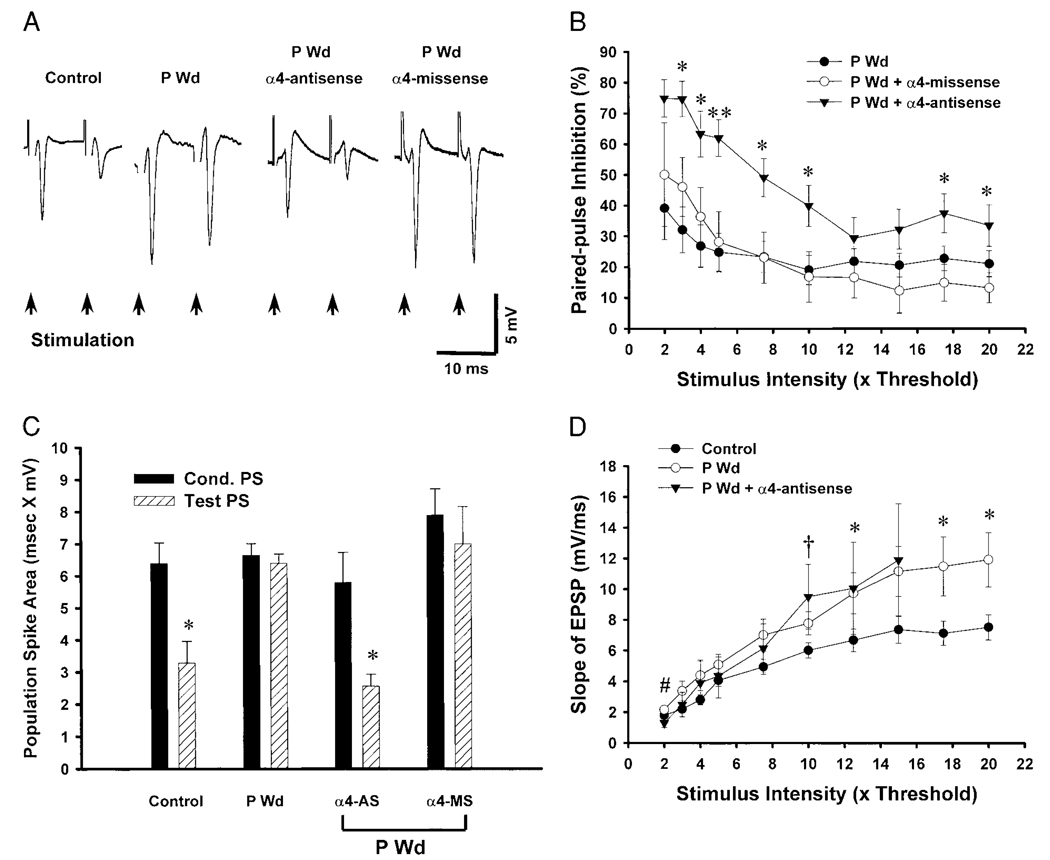
Antisense-induced suppression of expression of α4 subunit levels prevented the reduction in PPI normally observed following hormone withdrawal across a range of stimulus intensities (3–10T, Fig. 11B), suggesting that upregulation of GABAR containing this subunit reduces inhibition in CA1 hippocampal circuits. Under reduced levels of α4 subunit expression, P Wd resulted in an average PPI of 61.95 ± 5.97% at a 5T stimulus intensity (Fig. 11, A and B), similar to the original control value of PPI (65.18 ± 8.28%, see Fig. 6B). In contrast, missense treatment failed to prevent the reduction in PPI normally observed following P Wd compared with control (20–30% PPI). At higher stimulus intensities (12.5–20T), however, antisense treatment either failed to alter PPI (12.5–15T) or produced more modest changes in PPI (30–40%) compared with changes observed at the lower stimulus intensities (60–80%), suggesting an effect dependent on the level of stimulation.
FIG. 11.
Suppression of GABAA receptor (GABAR) α4-subunit expression prevents the decrease in PPI induced by P Wd. A: representative paired PS traces reveal that the reduction in PPI normally observed after P Wd is prevented by the prior intraventricular administration of GABAR α4 antisense oligonucleotide. In contrast, administration of GABAR α4 missense oligonucleotide does not prevent the decreased PPI produced by P Wd. B: averaged data for PPI across the range of stimulus intensities reveal significant increases in the PPI following antisense administration to P Wd rats (P Wd + α4 antisense) compared with PWD and PWD + α4 missense groups. The most significant changes were observed at lower (3–10T) ranges of stimulus intensities. C: averaged data graphically depict the changes in PStest area compared with PScond area, across hormone state as a function of α4-subunit regulation. The robust decrease in PStest area normally observed under control conditions compared with PScond area (5T stimulus) was not observed following P Wd, when these values were not significantly different. However, concomitant suppression of α4 GABAR subunit expression during the final 72 h of the withdrawal period (α4-AS) produced a robust decrease in the PStest area compared with PScond area, a measure of PPI. In contrast, α4 missense (α4-MS) treatment did not alter these parameters following P Wd (*P < 0.01 vs. control PS). D: increased EPSP slope observed at 10–20T stimulus intensities following P withdrawal (P Wd) was not prevented by prior treatment with α4 antisense to reduce α4 subunit expression. (†P < 0.05, PWD + antisense vs. control; * P < 0.05, PWD alone vs. control; # P < 0.05, PWD alone vs. PWD + antisense; n = 5–13 slices/group).
In addition to measures of amplitude, the PS area gives a more accurate representation of the excitatory response (Kang et al. 1998), in that it incorporates both the spike amplitude as well as the current decay. Following P Wd, the mean PS area for conditioning and test responses, assessed at a 5T stimulus intensity, were almost identical (Fig. 11C), such that no significant PPI would result. In contrast, under control conditions, a significant (P < 0.01) 48.59% decrease in area was observed for the test versus the conditioning PS, suggesting as robust a PPI as seen using values of PS amplitude alone. Suppression of expression of α4-subunit expression during P Wd restored PPI to a level not significantly different from the original control value (55.86%), while missense administration did not significantly alter the relative areas for test versus conditioning PS. Thus these data suggest that upregulation of GABAR containing the α4 subunit contributes to increased excitability of CA1 hippocampal circuits following P Wd.
PS amplitude—α4 antisense treatment
Suppression of expression of the GABAR α4-subunit restored PScond amplitude following P Wd to values similar to control (data not shown). In this case, the mean PScond amplitude was 6.41 ± 0.96 mV (P Wd + α4 antisense, 5T), a value not significantly different from sham control (9.59 ± 0.80 mV, P > 0.1), but markedly reduced (P < 0.01) from the value obtained after P Wd alone at a 5T intensity stimulation (12.56 ± 1.76 mV). In contrast, intraventricular application of missense oligonucleotide did not prevent the increase in conditioning PS amplitude following P Wd. However, the reduction in threshold for producing the conditioning PS following P Wd was unaltered by antisense or missense treatment (data not shown), suggesting a mechanism not dependent on α4 upregulation.
EPSP slope—α4 antisense treatment
In addition to altering the PS amplitude and PPI, P Wd also produced a significant increase in the slope of the EPSP (by 12–60%) at stimulus intensities of 10T and greater (Fig. 11D). In this case, administration of α4 antisense to suppress expression of the α4 subunit failed to correct this change, suggesting that this parameter is not due to upregulation of GABAR containing the α4 subunit.
LZM modulation—α4 antisense treatment
Because α4-containing GABARs are insensitive to modulation by BDZs such as LZM, we tested the hypothesis that suppression of α4 expression with antisense administration would restore BDZ sensitivity of hippocampal responses to afferent stimulation following P Wd. To this end, conditioning and test PS amplitudes were evaluated following bath application of 10µM LZM in slices from antisense or missense treated animals following P Wd. While LZM treatment reduced conditioning PS amplitudes only by 5% in control slices (from 9.59 ± 0.80 to 9.13 ± 0.76 mV, 5T, data not shown), this compound produced a 65% decrease in PS amplitude (from 6.41 ± 0.96 to 2.25 ± 0.26 mV, 5T, P < 0.05) after antisense treatment in the P Wd animals, but had no effect in slices from P Wd animals treated with missense oligonucleotide, suggesting that antisense treatment increases BDZ sensitivity above control levels. Further, the test PS was undetectable (<0.5 mV) at a 5T stimulus intensities after antisense treatment compared with PS amplitudes of 7.93 ± 1.41 mV after missense treatment, again suggesting that antisense treatment increases pyramidal cell sensitivity to LZM-induced inhibition to levels greater than observed under control conditions.
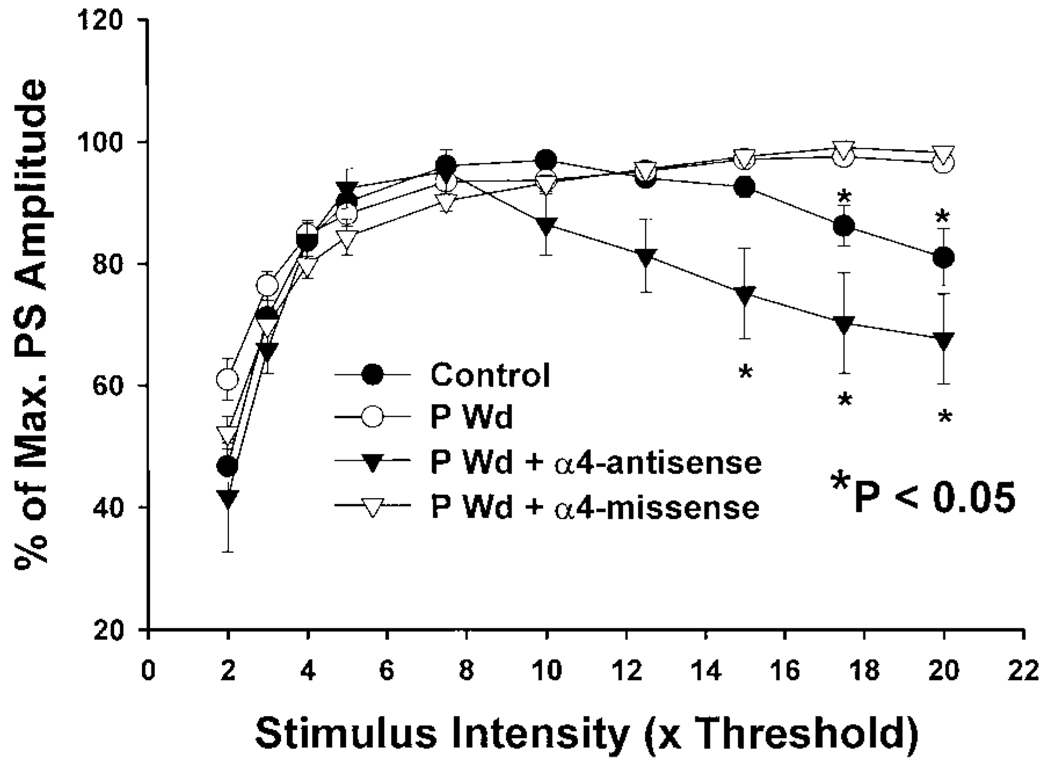
High-intensity stimulation—α4 antisense treatment
In addition to the above effects, suppression of α4 expression during P Wd restored the decrease in PScond amplitude normally observed under control conditions at high (17.5–20T) stimulation intensities (Fig. 12). After normalizing the PScond amplitude as a percentage of the maximum value, decreases in PS amplitude were observed by 10T and became significant by 17.5–20T stimulation intensities under control conditions (*P < 0.05). This decrease in amplitude may be the result of recruitment of feed-forward inhibitory pathways or the desensitization of excitatory responses at high levels of stimulation (Karnup and Stelzer 1999). This reduction in PS amplitude at high-intensity stimulation was completely prevented following hormone withdrawal, when the relative PS amplitude continued to increase as a function of stimulation intensity. Anti-sense, but not missense, oligonucleotide administration restored the decrease in PS amplitude normally observed at high-intensity stimulations seen in control slices (Fig. 12, *P < 0.05). It became evident at a lower stimulation intensity (7.5T) and became significant at a lower stimulation intensity (15T) than observed under control conditions. These data suggest that increases in α4-containing GABARs produced by P Wd may also alter feed-forward GABAergic pathways to produce changes in hippocampal excitability.
FIG. 12.
The decrease in PS amplitude produced by high-intensity stimulation is prevented following P Wd. The conditioning PS amplitude is expressed as a percentage of the maximum PS for each slice in all groups (ordinate) and is plotted as a function of stimulus intensity (abscissa, from 2 to 20T). Under control conditions, the maximum value for PS amplitude peaks at a 10T stimulus intensity and then declines at higher stimulus intensities (17.5–20T, P < 0.05) perhaps as a result of feed-forward inhibition. This attenuation in PS amplitude at high stimulus intensities was not observed following P Wd except under conditions where α4 subunit expression was suppressed with antisense treatment (α4-AS). Missense treatment (α4-MS) did not alter this input-output relationship following P Wd (n = 5, MS, n = 6, AS, n = 12, P Wd, n = 13, Control; *P < 0.05).
DISCUSSION
The results from this study suggest that PPI, one measure of circuit excitability, is significantly attenuated in CA1 hippocampus following withdrawal from the endogenous steroid P. The decrease in PPI would be indicative of an increase in hippocampal excitability, an effect consistent with the increase in seizure susceptibility (Frye and Bayon 1998, 1999; Moran and Smith 1998; Reddy et al. 2001; Smith et al. 1998a) and anxiety (Gallo and Smith 1993; Smith et al. 1998b) observed during the hormone withdrawal period. Further, the alterations in this circuit property normally observed after P Wd were prevented by suppression of expression of the GABAR α4 subunit. These results suggest that the upregulation of α4-containing GABARs we have shown to result from hormone withdrawal (Smith et al. 1998a) produce a decreased level of inhibitory control of pyramidal cell excitability.
PPI and neuronal excitability
The PPI paradigm measures the attenuated response of hippocampal pyramidal cells to the second (test) of paired stimuli delivered 10 ms apart. As such, it is a homeostatic process primarily reflecting the degree of inhibitory tone within the hippocampal circuitry (Davies et al. 1990). In fact, PPI has been used as a model paradigm to assess hippocampal circuit excitability in different seizure models that employ electrical kindling (Kamphuis et al. 1992; Sloviter 1987; Zhao and Leung 1991) as well as chemical kindling (Barkai et al. 1994; Bragin et al. 2002; Corda et al. 1990; Fathollahi et al. 1997; Psarropoulou et al. 1994). The PPI paradigm has also been used to evaluate hippocampal excitability after GABA with-drawal (Garcia-Ugalde et al. 1992), ethanol withdrawal (Kang et al. 1996; Rogers and Hunter 1992), and chronic BDZ administration (Xie and Tietz 1991). In the majority of these studies, the PPI in CA1 hippocampus is reduced, indicating in many cases impairment of recurrent inhibition (Denslow et al. 2001; Doherty and Dingledine 2001; Kamphuis et al. 1992; Kang et al. 1996; Kapur et al. 1989; Rogers and Hunter 1992), although in some kindling paradigms, increased synaptic GABAR density is reported (Nusser et al. 1998) and PPI of dentate gyrus granule cells is robust (Bragin et al. 2002). Thus the decrease in PPI observed following P Wd in the present study compares well with most of these other known states of behavioral excitability. Although multiple factors influence PPI outcome, potentiation of postsynaptic GABAR has been suggested as a mechanism by a number of studies, demonstrating modifiability of PPI amplitude with GABA-modulatory drugs, such as anesthetics (Albertson et al. 1992) and BDZs (Higashima et al. 1998), consistent with the results from the present study.
PPI in CA1 hippocampus—circuit properties
Inhibitory pathways contributing to PPI in hippocampal slice have been well characterized and can involve both feed-forward and feed-back inhibitory circuits (Karnup and Stelzer 1999; Rogers and Hunter 1992; Turner 1990) with transmission via both GABAA and GABAB receptors. However, in the present study, the use of a 10-ms inter-pulse interval is selective for GABAA- versus GABAB-mediated events, because the latter exhibits a longer latency onset of response following an excitatory stimulus and is optimally observed with inter-pulse intervals of 200 ms (Davies et al. 1990; Karlsson and Olpe 1989; Williams et al. 1994; Zeng and Tietz 1997).
Feed-forward inhibition and PPI
Feed-forward inhibition is the result of activity from interneurons in the lacunosum-moleculare (L-M), with peak inhibitory postsynaptic potentials (IPSPs) that coincide with the rising phase of the pyramidal cell EPSP, or from interneurons in the alveus-oriens (A-O), with peak IPSPs that coincide with the decay phase of the pyramidal cell EPSP (Karnup and Stelzer 1999). In this way it is believed that the feed-forward interneuron system can directly regulate excitability of the pyramidal cell, even for the response to a single stimulus. Thus dendritic inhibition by L–M interneurons is mediated by GABAR-mediated inhibitory postsynaptic currents (IPSCs) (Bertrand and Lacaille 2001). A second mechanism of GABAergic control of pyramidal cell excitability is through increases in GABA-gated conductance (gGABA), which are shorter latency events than either the GABAergic IPSC or IPSP (Karnup and Stelzer 1999). This increase in conductance, which precedes membrane hyperpolarization, would also serve to limit excitability of the pyramidal cell by acting as a resistive shunt for input current (Karnup and Stelzer 1999).
Both of these mechanisms may have been attenuated under conditions of P Wd, when increases in the amplitude of the PS response to the conditioning pulse were seen in conjunction with a significantly lower threshold for activation of this short-latency response to the first stimulus compared with control slices. The fact that the increase in PS amplitude following P Wd was prevented by α4 antisense administration suggests that upregulation of GABAR containing this subunit mediates changes in feed-forward inhibitory pathways. Further, the decrease in PS amplitude seen at very high stimulation intensities under control conditions is most probably also an effect of feed-forward inhibition. This decline in PS amplitude at high-intensity stimulation was prevented also as a result of α4 GABAR subunit upregulation following P Wd and reversed with antisense suppression of α4 expression, suggesting that α4-containing GABARs regulate the inhibitory efficacy of this feed-forward inhibitory pathway.
However, the excitatory amino acid receptor population may also play a role in mediating the effects of P Wd because α4 antisense administration did not prevent the increase in EPSP slope produced by P Wd, nor did it prevent the lowered threshold for PS activation, thus implicating the glutamatergic system in addition to the GABAergic system. Furthermore, the PPI observed at high stimulus intensities (>12T) was also not altered by antisense treatment, implicating other non-α4 mechanisms.
Recurrent inhibition and PPI
The temporal properties of recurrent inhibition, mediated via pyramidal cell activation of adjacent interneurons, have also been characterized and are most relevant as a mechanism explaining the observed differences in the degree of PPI across experimental groups. Stimulation of the Schaeffer collateral pathway elicits an action potential in the pyramidal cells with latencies in the range of approximately 2–5 ms in our stimulation preparation. This pyramidal cell then activates interneurons, such as bistratified cells, with a latency of 6.3 ms (Karnup and Stelzer 1999). Across a range of stimulation intensities, the resulting recurrent IPSP assessed in the pyramidal cell would then occur from 6–28 ms following its response to the first stimulus (Karnup and Stelzer 1999). This time course fits well with the 10-ms inter-pulse interval used in the PPI paradigm for the current study, which demonstrated a reduced PS amplitude in response to the test pulse. That GABAR activation mediated via this recurrent inhibitory pathway is responsible for this reduction in PS amplitude is further suggested by its modifiability by GABA-modulatory drugs such as BDZs, anesthetics, and picrotoxin (Albertson et al. 1992; Higashima et al. 1998).
Presynaptic versus postsynaptic GABAR mechanisms after withdrawal from other GABA-modulatory drugs
Presynaptic GABAergic mechanisms cannot be ruled out, however, as mediating factors producing the decrease in PPI observed following hormone withdrawal in the present study. Decreased presynaptic GABAergic function in the hippocampus may contribute to BDZ withdrawal hyperexcitability, as demonstrated by decreases in the sIPSC frequency after chronic BDZ exposure (Zeng and Tietz 1999). Furthermore, presynaptic mechanisms have also been invoked as mediating factors to explain paired pulse depression in unitary inhibitory synapses, i.e., a reduction in quantal content due to transmitter depletion following the initial response (Kraushaar and Jonas 2000; Lambert and Wilson 1994; Waldeck et al. 2000; Wilcox and Dichter 1994).
However, postsynaptic GABAR mechanisms have also been shown to play a role in mediating BDZ withdrawal hyperexcitability (Zeng and Tietz 1999), as several groups have demonstrated reduced quantal size (Lambert and Wilson 1994; Wilcox and Dichter 1994) of GABAergic synapses assessed by 7 days following BDZ withdrawal, as well as “silent synapses” (Poisbeau et al. 1997). These data suggest that a decrease in the total number of GABARs present at postsynaptic sites is a result of chronic exposure to this GABA-modulatory compound.
α4 Subunit and CNS excitability
In this study, upregulation of α4-containing GABARs is implicated as a mechanism for the decrease in PPI observed following P Wd for two reasons. First, antisense-induced suppression of α4 upregulation prevented the decrease in PPI observed following P Wd. Second, pharmacological alterations in the PPI response following P Wd were characteristic of α4-containing GABARs (Wafford et al. 1996; Wisden et al. 1991) in that they were insensitive to BDZ modulation, an effect reversed by antisense treatment, but responsive to barbiturate modulation, as we have previously demonstrated in isolated cells (Smith et al. 1998a). Thus these findings suggest that the decrease in PPI we observe following P Wd is due to a reduction in GABAergic recurrent inhibition of pyramidal cell activity directly as a result of altered subunit composition.
α4 subunit upregulation is associated with CNS excitability, as suggested by studies demonstrating increased levels of this subunit following kindling paradigms (Clark 1998; Kapur 2000) or in seizure states (Brooks-Kayal et al. 1998) and drug-induced tremor (Frostholm et al. 2000). α4 subunit upregulation also accompanies the behavioral excitability associated with chronic treatment and withdrawal from ethanol (Mahmoudi et al. 1997) or BDZs (Follesa et al. 2001; Holt et al. 1996). Recent studies (Maric et al. 1999) suggest that α4-containing GABAR channels display shorter mean open times compared with α2-containing GABARs, and our preliminary data (Hsu et al. 1999) suggest that, following neuroactive steroid exposure, a subpopulation of synaptic GABARs pharmacologically characterized as α4-containing decay more quickly than BDZ-responsive synaptic currents. Conversely, states that decrease expression of the α4 subunit are characterized by increases in GABAR-mediated Cl− flux (Papadeas et al. 2001). In contrast, recent studies (Juttner et al. 2001) have correlated prolonged IPSC decays with increased PPI.
α4 GABAR subunit localization
The specific subcellular localization of GABAR mediating this change in PPI cannot be determined by the present study. Recent neuroanatomical studies have reported that GABAR containing α1 or α2 subunits are specifically localized subsynaptically on either the soma and proximal dendrites or the axon-initiating segment, respectively, of hippocampal pyramidal cells (Nusser et al. 1996). However, α4-containing GABARs have not yet been localized to synaptic or extrasynaptic sites, although our recent studies suggest that at least a population of these receptors is subsynaptic (Hsu et al. 1999).In addition, the tetrodotoxin-resistant mIPSCs recorded following P Wd are reduced in amplitude (Hsu et al. 1999), suggesting a reduced GABAR number postsynaptically. This effect would decrease inhibition and is consistent with the reduction in PPI we observed in the present study following P Wd.
Extrasynaptic GABAR
We have recently reported that P Wd results in increased expression of α4βδ GABAR (Sundstrom-Poromaa et al. 2002), a receptor isoform thought to be extrasynaptic (Nusser et al. 1998). An extrasynaptic site of action for α4-containing GABARs would alter the degree of tonic inhibition following increased activity of GABAergic afferents to the cell when “transmitter spillover” has been reported to occur (Isaacson et al. 1993), and extrasynaptic GABAR populations would have access to ligand. Extrasynaptic GABAR may act as a resistive shunt to limit excitability of the neuron, as demonstrated recently in dentate gyrus (Nusser and Mody 2002) and cerebellum (Brickley et al. 2001), as well as under recent theoretical analysis (Holmes and Levy 1997). Under these conditions, replacement of the ambient extrasynaptic GABAR population in CA1 hippocampus by α4-containing GABAR would result in current with faster decay kinetics, thus decreasing the “total charge transfer” following P Wd, an outcome that would decrease inhibitory input and thereby decrease PPI. A faster decay for these GABAergic currents would also impact on the duration of excitatory responses, which are prolonged following P Wd, as evidenced by the increased half-width of the PS. Prolongation of the half-width by LZM following P Wd, which seems paradoxical, may be due to disinhibition of pyramidal cell excitability via an effect on the interneuron population.
In fact, inhibition mediated by the extrasynaptic GABAR population may also be evidenced during activation of feed-forward inhibitory pathways. This would be most likely to occur at high stimulus intensities (>10T), which would favor spillover and were associated with a progessive decline in conditioning PS amplitude in control hippocampus. This phenomenon was only observed following P Wd (>7.5T) when α4-containing GABARs were downregulated by antisense treatment, again suggesting that α4-containing GABAR result in less inhibition than non-α4 containing receptors.
Behavioral correlations
The increased behavioral excitability observed following P Wd was evidenced both as increased seizure susceptibility (Moran and Smith 1998; Reddy et al. 2001; Smith et al. 1998a) and increased anxiety (Gallo and Smith 1993; Smith et al. 1998b), two outcomes that can be generated by alterations in excitability of the hippocampal circuitry as observed in the present study. Specifically, the CA1 hippocampus has been shown to be the major source of ictal-like activity (Namba et al. 1991; Taylor 1988), while the CA2-CA3 region may display interictal-like activity (Colom and Saggau 1994). In addition, alterations in the excitability of the dorsal hippocampus produced by local infusion of GABA-modulatory drugs have been shown to influence anxiety state (Bitran et al. 1999, 2000; Menard and Treit 2001).
Clinical correlations
Altered excitability associated with hormonal states has been reported across the menstrual cycle, specifically related to epilepsy (i.e., catamenial epilepsy) (Backstrom 1976; Herzog and Friedman 2001; Herzog et al. 1997; Reddy et al. 2001) and mood (PMS) (Backstrom and Carstensen 1974; Endicott et al. 1999; Rapkin et al. 1997; Schmidt et al. 1994, 1998), in many cases during the late luteal phase when P levels have declined. Recent studies have suggested that CNS excitability is also altered by ovarian hormones in women (Blumer et al. 1998; Smith et al. 1999). In one study, which used a paradigm analogous to the PPI paradigm described here (paired transcranial stimulation), the level of inhibition was positively correlated with the progesterone level (Smith et al. 1999). Overall, the clinical literature suggests that both CNS excitability and mood can fluctuate across naturally occurring changes in circulating neuroactive steroids.
CONCLUSIONS
This study demonstrated increased hippocampal excitability following P Wd using a model that is routinely used to demonstrate excitability of this circuit in other withdrawal and kindling paradigms. The results suggest that PPI is significantly attenuated following withdrawal from P, predominantly as a result of an increase in α4-containing GABARs in CA1 hippocampus. This increase in excitability of a limbic circuit may be relevant for alterations in mood and seizure susceptibility, which have been reported in women across the menstrual cycle.
Acknowledgments
The authors thank X. Li for expert technical assistance.
This work was supported by National Institutes of Health Grants DA-09618 and AA-12958 to S. S. Smith.
REFERENCES
- Albertson TE, Walby WF, Joy RM. Modification of GABA-mediated inhibition by various injectable anesthetics. Anesthesiology. 1992;77:488–499. doi: 10.1097/00000542-199209000-00014. [DOI] [PubMed] [Google Scholar]
- Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Carstensen H. Estrogen and progesterone in plasma in relation to premenstrual tension. J Steroid Biochem. 1974;5:257–260. doi: 10.1016/0022-4731(74)90139-3. [DOI] [PubMed] [Google Scholar]
- Barkai E, Grossman Y, Gutnick MJ. Long-term changes in neocortical activity after chemical kindling with systemic pentylenetetrazol: an in vitro study. J Neurophysiol. 1994;72:72–83. doi: 10.1152/jn.1994.72.1.72. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of GABA-A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Benke D, Michel C, Mohler H. GABAA receptors containing the α4-subunit: prevalence, distribution, pharmacology, and subunit architecture in situ. J Neurochem. 1997;69:806–814. doi: 10.1046/j.1471-4159.1997.69020806.x. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Lacaille JC. Unitary synaptic currents between lacunosum-moleculare interneurones and pyramidal cells in rat hippocampus. J Physiol (Lond) 2001;532:369–384. doi: 10.1111/j.1469-7793.2001.0369f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABA-A receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3-alpha-OH-5-beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Foley M, Audette D, Leslie N, Frye CA. Activation of peripheral mitochondrial benzodiazepine receptors in the hippocampus stimulates allopregnanolone synthesis and produces anxiolytic-like effects in the rat. Psychopharmacology. 2000;151:64–71. doi: 10.1007/s002130000471. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Bitran D, Purdy RH, Kellogg CK. Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol Biochem Behav. 1993;45:423–428. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- Bixo M, Backstrom T. Regional distribution of progesterone and 5α-pregnane-3, 20-dione in rat brain during progesterone-induced ‘anesthesia’. Psychoneuroendocrinology. 1990;15:159–162. doi: 10.1016/0306-4530(90)90025-5. [DOI] [PubMed] [Google Scholar]
- Blumer D, Herzog AG, Himmelhoch J, Salgueiro CA, Ling FW. To what extent do premenstrual and interictal dysphoric disorder overlap? Significance for therapy. J Affect Disord. 1998;48:215–225. doi: 10.1016/s0165-0327(97)00173-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA-A receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- Clark M. Sensitivity of the rat hippocampal GABA-A receptor alpha-4 subunit to electroshock seizures. Neurosci Lett. 1998;250:17–20. doi: 10.1016/s0304-3940(98)00422-4. [DOI] [PubMed] [Google Scholar]
- Colom LV, Saggau P. Spontaneous interictal-like activity originates in multiple areas of the CA2-CA3 region of hippocampal slices. J Neurophysiol. 1994;71:1574–1585. doi: 10.1152/jn.1994.71.4.1574. [DOI] [PubMed] [Google Scholar]
- Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- Corda MG, Giorgi O, Longoni B, Orlandi M, Biggio G. Decrease in the function of the γ-aminobutyric acid-coupled chloride channel produced by the repeated administration of pentylenetetrazol to rats. J Neurochem. 1990;55:1216–1221. doi: 10.1111/j.1471-4159.1990.tb03127.x. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VV, Banner C, Sjovall J, Baulieu EE, Robel P. Neurosteroids: 3α-OH-5 α-pregnan-20-one and its precursors in the brain, plasma, and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Costa AMN, Spence KT, Smith SS, Ffrench-Mullen JMH. Withdrawal from the endogenous steroid progesterone results in GABAA currents insensitive to benzodiazepine modulation in rat CA1 hippocampus. J Neurophysiol. 1995;74:464–469. doi: 10.1152/jn.1995.74.1.464. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol (Lond) 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow MJ, Eid T, Du F, Schwarcz R, Lothman EW, Steward O. Disruption of inhibition in area CA1 of the hippocampus in a rat model of temporal lobe epilepsy. J Neurophysiol. 2001;86:2231–2245. doi: 10.1152/jn.2001.86.5.2231. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Morrow AL. The neurosteroid, 3αOH-5α-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin Exp Res. 1995;19:350–355. doi: 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. Reduced excitatory drive onto interneurons in the dentate gyrus after status epilepticus. J Neurosci. 2001;21:2048–2057. doi: 10.1523/JNEUROSCI.21-06-02048.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewaart DE, Thys-Jacobs S. Is premenstrual dysphoric disorder a distinct clinical entity? J Women’s Health Gend Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Endo S, Olsen RW. Antibodies specific for alpha-subunit subtypes of GABA-A receptors reveal brain regional heterogeneity. J Neurochem. 1993;60:1388–1398. doi: 10.1111/j.1471-4159.1993.tb03300.x. [DOI] [PubMed] [Google Scholar]
- Fathollahi Y, Motamedi F, Semnanian S, Zardoshti M. Examination of persistent effects of repeated administration of pentylenetetrazol on rat hippocampal CA1: evidence from in vitro study on hippocampal slices. Brain Res. 1997;758:92–98. doi: 10.1016/s0006-8993(97)00164-9. [DOI] [PubMed] [Google Scholar]
- File SE. The history of benzodiazepine dependence: a review of animal studies. Neurosci Biobehav Rev. 1990;14:135–146. doi: 10.1016/s0149-7634(05)80214-3. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. Single channel properties of recombinant GABAA receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse L929 cells. J Physiol (Lond) 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, Massa F, Desole MS, Carta M, Busonero F, Sanna E, Biggio G. Increase in expression of the GABA-A receptor alpha-4 subunit gene induced by withdrawal of, but not by long-term treatment with, benzodiazepine full or partial agonists. Brain Res Mol Brain Res. 2001;92:138–148. doi: 10.1016/s0169-328x(01)00164-4. [DOI] [PubMed] [Google Scholar]
- Frostholm A, Evans JE, Cummings SL, Rotter A. Harmaline-induced changes in gamma aminobutyric acid-A receptor subunit mRNA expression in murine olivocerebellar nuclei. Brain Res Mol Brain Res. 2000;85:200–208. doi: 10.1016/s0169-328x(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neurosteroid 3α, 5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3 alpha, 5 alpha-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Cyclic withdrawal from endogenous and exogenous progesterone increases kainic acid and perforant pathway induced seizures. Pharmacol Biochem Behav. 1999;62:315–321. doi: 10.1016/s0091-3057(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Gallager DW, Lakoski JM, Gonsalves SF, Rauch SL. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984;308:74–77. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- Gallo MA, Smith SS. Progesterone withdrawal decreases latency to and increases duration of electrified prod burial: a possible rat model of PMS anxiety. Pharmacol Biochem Behav. 1993;46:897–904. doi: 10.1016/0091-3057(93)90219-j. [DOI] [PubMed] [Google Scholar]
- Garcia-Ugalde G, Galarraga E, Bargas J, Brailowsky S. Hyperexcitability of hippocampal CA1 region in brain slices after GABA withdrawal. Neurosci Lett. 1992;147:229–232. doi: 10.1016/0304-3940(92)90602-4. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol (Lond) 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Friedman MN. Menstrual cycle interval and ovulation in women with localization-related epilepsy. Neurology. 2001;57:2133–2135. doi: 10.1212/wnl.57.11.2133. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Higashima M, Kinoshita H, Koshino Y. Differences in the effects of zolpidem and diazepam on recurrent inhibition and long-term potentiation in rat hippocampal slices. Neurosci Lett. 1998;245:77–80. doi: 10.1016/s0304-3940(98)00178-5. [DOI] [PubMed] [Google Scholar]
- Holmes WR, Levy WB. Quantifying the role of inhibition in associative long-term potentiation in dentate granule cells with computational models. J Neurophysiol. 1997;78:103–116. doi: 10.1152/jn.1997.78.1.103. [DOI] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Waldeck R, Faber DS, Smith SS. Estrous hormones alter the kinetics of miniature IPSCs of pyramidal cells in CA1 hippocampus. Soc Neurosci Abstr. 1999;25:1223. 491.5. [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Juttner R, Meier J, Grantyn R. Slow IPSC kinetics, low levels of alpha 1 subunit expression and paired pulse depression are distinct properties of neonatal inhibitory GABAergic synaptic connections in the superior colliculus. Eur J Neurosci. 2001;13:2088–2098. doi: 10.1046/j.0953-816x.2001.01587.x. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Gorter JA, Wadman WJ, Lopes da Silva FH. Hippocampal kindling leads to different changes in paired-pulse depression of local evoked field potentials in CA1 area and in fascia dentata. Neurosci Lett. 1992;141:101–105. doi: 10.1016/0304-3940(92)90344-7. [DOI] [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW. Alteration in the sensitivity of GABA-A receptors to allosteric modulatory drugs in rat hippocampus after chronic intermittent ethanol treatment. Alcohol Clin Exp Res. 1998;22:2165–2173. [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Sapp DW, Olsen RW. Persistent reduction of GABA-A receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Kapur J. Hippocampal neurons express GABA-A receptor insensitive to diazepam in hyperexcitable conditions. Epilepsia. 2000;41:S86–S89. doi: 10.1111/j.1528-1157.2000.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Kapur J, Michelson HB, Buterbaugh GG, Lothman EW. Evidence for a chronic loss of inhibition in the hippocampus after kindling: electrophysiological studies. Epilepsy Res. 1989;4:90–99. doi: 10.1016/0920-1211(89)90013-2. [DOI] [PubMed] [Google Scholar]
- Karlsson G, Olpe HR. Inhibitory processes in normal and epileptic-like rat hippocampal slices: the role of GABA-B receptors. Eur J Pharmacol. 1989;163:285–290. doi: 10.1016/0014-2999(89)90197-0. [DOI] [PubMed] [Google Scholar]
- Karnup S, Stelzer A. Temporal overlap of excitatory and inhibitory afferent input in guinea-pig CA1 pyramidal cells. J Physiol (Lond) 1999;516:485–504. doi: 10.1111/j.1469-7793.1999.0485v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, Sieghart W. Polyclonal antibodies directed against an epitope specific for the alpha 4-subunit of GABA-A receptors identify a 67-kDa protein in rat brain membranes. J Neurochem. 1994;62:764–769. doi: 10.1046/j.1471-4159.1994.62020764.x. [DOI] [PubMed] [Google Scholar]
- Korneyev A, Costa E. Allopregnanolone (THP) mediates anesthetic effects of progesterone in rat brain. Horm Behav. 1996;30:37–43. doi: 10.1006/hbeh.1996.0006. [DOI] [PubMed] [Google Scholar]
- Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J Neurosci. 2000;20:5594–5607. doi: 10.1523/JNEUROSCI.20-15-05594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille JC, Kunkel DD, Schwartzkroin PA. Electrophysiological and morphological characterization of hippocampal interneurons. In: Chan-Palay V, Kohler C, editors. Neurology and Neurobiology, The Hippocampus—New Vistas. vol. 52. New York: Liss AR, Inc; 1989. pp. 287–305. [Google Scholar]
- Lambert NA, Wilson WA. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. J Neurophysiol. 1994;72:121–130. doi: 10.1152/jn.1994.72.1.121. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Schiffelholz T, Romeo E, Di Michele F, Holsboer F, Rupprecht R. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282:1213–1218. [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABAA receptor α4 subunit expression: possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Maric D, Maric I, Wen X, Fritschy JM, Sieghart W, Barker JL, Serafini R. GABA-A receptor subunit composition and functional properties of Cl- channels with differential sensitivity to zolpidem in embryonic rat hippocampal cells. J Neurosci. 1999;19:4921–4937. doi: 10.1523/JNEUROSCI.19-12-04921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard J, Treit D. The anxiolytic effects of intra-hippocampal midazolam are antagonized by intra-septal L-glutamate. Brain Res. 2001;888:163–166. doi: 10.1016/s0006-8993(00)03046-8. [DOI] [PubMed] [Google Scholar]
- Moran MH, Smith SS. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- Namba H, Iwasa H, Kubota M, Hagihara Y, Yamaura A. Changes of hippocampal glucose utilization subsequent to amygdaloid-kindled generalized seizures. Epilepsia. 1991;32:27–32. doi: 10.1111/j.1528-1157.1991.tb05606.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA-A receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. Chronic ethanol consumption differentially alters GABA-A receptor α1 and α4 subunit peptide expression and GABA-A receptor-mediated 36 Cl(−) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res. 2001;25:1270–1275. [PubMed] [Google Scholar]
- Patenaude C, Nurse S, Lacaille JC. Sensitivity of synaptic GABA-A receptors to allosteric modulators in hippocampal oriens-alveus interneurons. Synapse. 2001;41:29–39. doi: 10.1002/syn.1057. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Williams SR, Mody I. Silent GABAA synapses during flurazepam withdrawal are region-specific in the hippocampal formation. J Neurosci. 1997;17:3467–3475. doi: 10.1523/JNEUROSCI.17-10-03467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarropoulou C, Matsokis N, Angelatou F, Kostopoulos G. Pentylenetetrazol-induced seizures decrease gamma-aminobutyric acid-mediated recurrent inhibition and enhance adenosine-mediated depression. Epilepsia. 1994;35:12–19. doi: 10.1111/j.1528-1157.1994.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Rogers CJ, Hunter BE. Chronic ethanol treatment reduces inhibition in CA1 neurons of the rat hippocampus. Brain Res Bull. 1992;28:587–592. doi: 10.1016/0361-9230(92)90107-9. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Purdy RH, Moore PH, Jr, Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N EnglJ Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of inter-neurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Menstrual cycle effects on cortical excitability. Neurology. 1999;53:2069–2072. doi: 10.1212/wnl.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Smith SS, Chapin JK. The estrous cycle and the olivo-cerebellar circuit. I. Contrast enhancement of sensorimotor-correlated cerebellar discharge. Exp Brain Res. 1996;111:371–384. doi: 10.1007/BF00228726. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, Ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-one withdrawal using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Chapin JK, Woodward DJ. Progesterone alters GABA and glutamate responsiveness: a possible mechanism for its anxiolytic action. Brain Res. 1987a;400:353–359. doi: 10.1016/0006-8993(87)90634-2. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied progesterone metabolites alter neuronal responsiveness in the cerebellum. Brain Res Bull. 1987b;18:739–747. doi: 10.1016/0361-9230(87)90209-7. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Li X, Sabado T, Light A, Wiedmann M, Williams K, Smith SS. Hormonally-regulated α4β52δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP. How do seizures begin? Clues from hippocampal slices. Trends Neurosci. 1988;11:375–378. doi: 10.1016/0166-2236(88)90070-7. [DOI] [PubMed] [Google Scholar]
- Turner DA. Feed-forward inhibitory potentials and excitatory interactions in guinea-pig hippocampal pyramidal cells. J Physiol (Lond) 1990;422:333–350. doi: 10.1113/jphysiol.1990.sp017987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor-single channel kinetic properties of mouse spinal cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABA-A receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- Waldeck RF, Pereda A, Faber DS. Properties and plasticity of paired-pulse depression at a central synapse. J Neurosci. 2000;20:5312–5320. doi: 10.1523/JNEUROSCI.20-14-05312.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KS, Dichter MA. Paired pulse depression in cultured hippocampal neurons is due to a presynaptic mechanism independent of GABAB auto-receptor activation. J Neurosci. 1994;14:1775–1788. doi: 10.1523/JNEUROSCI.14-03-01775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Samulack DD, Beaulieu C, Lacaille JC. Membrane properties and synaptic response of interneurons located near the stratum lacu-nosum-moleculare/radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices. J Neurophysiol. 1994;71:2217–2235. doi: 10.1152/jn.1994.71.6.2217. [DOI] [PubMed] [Google Scholar]
- Wilson MA. Influences of gender, gonadectomy, and estrous cycle on GABA/DZ receptors and benzodiazepine responses in rats. Brain Res Bull. 1992;29:165–172. doi: 10.1016/0361-9230(92)90022-p. [DOI] [PubMed] [Google Scholar]
- Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor α4 subunit. FEBS Lett. 1991;289:227–230. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I.Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie XH, Tietz EI. Chronic benzodiazepine treatment of rats induces reduction of paired-pulse inhibition in CA1 region of in vitro hippocampus. Brain Res. 1991;561:69–76. doi: 10.1016/0006-8993(91)90750-p. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tietz EI. Depression of early and late monosynaptic inhibitory postsynaptic potentials in hippocampal CA1 neurons following prolonged benzodiazepine administration—role of a reduction in C1-driving force. Synapse. 1997;25:125–136. doi: 10.1002/(SICI)1098-2396(199702)25:2<125::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Zeng XJ, Tietz EI. Benzodiazepine tolerance at GABAergic synapses on hippocampal CA1 pyramidal cells. Synapse. 1999;31:263–277. doi: 10.1002/(SICI)1098-2396(19990315)31:4<263::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Zhao D, Leung LS. Effects of hippocampal kindling on paired-pulse response in CA1 in vitro. Brain Res. 1991;564:220–229. doi: 10.1016/0006-8993(91)91457-c. [DOI] [PubMed] [Google Scholar]