Abstract
The onset of puberty defines a developmental stage when some learning processes are diminished, but the mechanism for this deficit remains unknown. We found that, at puberty, expression of inhibitory α4βδ γ-aminobutyric acid type A (GABAA) receptors (GABAR) increases perisynaptic to excitatory synapses in CA1 hippocampus. Shunting inhibition via these receptors reduced N-methyl-D-aspartate receptor activation, impairing induction of long-term potentiation (LTP). Pubertal mice also failed to learn a hippocampal, LTP-dependent spatial task that was easily acquired by δ−/− mice. However, the stress steroid THP (3αOH-5α[β]-pregnan-20-one), which reduces tonic inhibition at puberty, facilitated learning. Thus, the emergence of α4βδ GABARs at puberty impairs learning, an effect that can be reversed by a stress steroid.
Certain learning and cognitive processes decline at the onset of puberty (1–3). The pubertal process that shapes this developmental decline is unknown but is likely to involve the hippocampus, which is widely regarded as the site for learning (4–6). In addition to excitatory input, the inhibitory GABAergic (GABA, γ-aminobutyric acid) system plays a pivotal role in shaping developmental plasticity, as in the visual cortex (7), where drugs that target the γ-aminobutyric acid type A (GABAA) receptor (GABAR) alter the timing of the critical period. The GABAR mediates most central nervous system inhibition and consists of diverse subtypes with distinct properties. Of these, α4βδ GABARs increase at pubertal onset in the mouse hippocampus (8), suggesting that they may shape plasticity here.
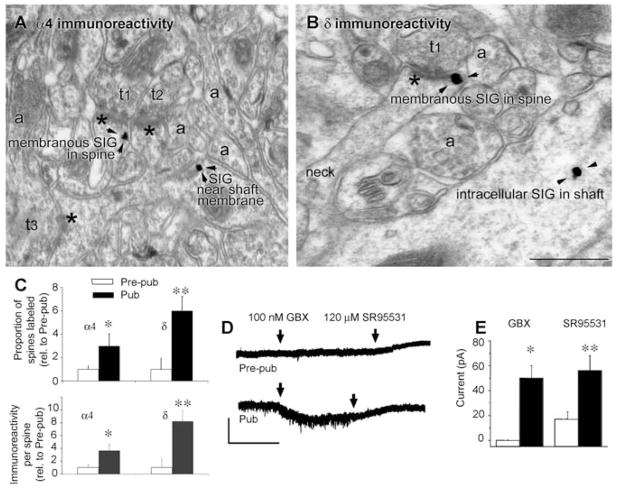
We employed immunocytochemical, electron microscopic techniques (9) to localize and quantify α4 and δ GABAR subunits on CA1 hippocampal pyramidal cells across the pubertal state of female mice, because females exhibit greater deficits in learning at puberty than males (10, 11). We detected immunostaining of both subunits perisynaptic to asymmetric synapses on the plasma membrane of spines of the apical dendrite, which increased up to 700% at puberty (Fig. 1, A to C, and fig. S1; α4, P = 0.0048; δ, P = 0.00091) (9). In contrast, α4 and δ immunoreactivity on the dendritic shaft increased by less than 100% at puberty (fig. S2). Functional expression of δ-containing GABAR at puberty was demonstrated by robust responses of pyramidal cells at puberty to 100 nM gaboxadol, which, at this concentration, is selective for this receptor (Fig. 1, D and E) (12). Gaboxadol had no effect before puberty and only a modest effect in the adult hippocampus (Fig. 1, D and E), where α4 and δ expression is lower than at puberty (fig. S3).
Fig. 1.
α4 and δ GABAA receptor subunit expression increases on dendritic spine membranes of CA1 hippocampal pyramidal cells at puberty. (A) α4 and (B) δ silver-intensified immunogold labeling (SIG) occurs along the plasma membrane of spines forming excitatory synapses. Shafts also exhibit immunoreactivity. Asterisks, postsynaptic density; t1 to t3, presynaptic axon terminals; a, nonsynaptic axons. In (B), the neck connects the labeled shaft with the spine. Scale bar, 500 nm. Arrowheads indicate SIG immunolabeling of the indicated GABAR subunits. (C) Proportion of labeled spines (upper panel: α4, *P < 0.018; δ, **P = 0.002) and immunoreactivity per spine (lower panel: α4, *P < 0.005, δ, **P = 0.00091) increase at puberty (Pub) relative to prepuberty (Pre-pub) (9). Error bars indicate SE of the mean. (D) Representative pyramidal-cell currents in response to the GABA agonist gaboxadol (applied continuously, arrows) and the GABAR antagonist SR95531 (applied continuously, arrows). Scale, 50 pA, 50 s. (E) Averaged data. *P = 0.0002, **P = 0.02, Pub versus Pre-pub.
Extrasynaptic α4β2δ GABARs on spines could impair voltage-triggered Mg++ unblock of N-methyl-D-aspartate (NMDA) receptors. Thus, we used whole-cell voltage clamp techniques with blockade of synaptic GABARs (13) to record evoked NMDA excitatory postsynaptic currents (EPSCs) from CA1 pyramidal cells. The threshold for triggering NMDA current was increased by 100 μA in CA1 hippocampal pyramidal cells at puberty (Fig. 2, A and B; P = 0.0009), whereas maximum current amplitudes decreased by 80% (Fig. 2A and fig. S4; P < 0.05). In contrast, NMDA EPSCs from the pubertal δ−/− hippocampus were similar to those from the prepubertal hippocampus (Fig. 2B), as were NMDA EPSCs under complete GABAR blockade (fig. S5).
Fig. 2.
NMDA current is decreased at puberty in CA1 hippocampal pyramidal cells: reversal by THP. (A) (Left) Representative traces, evoked (100 μA) NMDA current (0.05 Hz; Pre-pub, above; Pub, below) recorded at −60 mV. Scale, 15 pA, 100 ms. (Right) 300 μA stimulation; Pub +/+, above; Pub δ−/−, below. Scale for +/+, 20 pA, 100 ms; for −/−, 20 pA, 250 ms. Amplitudes decreased at puberty, but were restored by THP and δ knock-out. (B) NMDA EPSC amplitude with increasing stimulation intensities (Pre-pub, black squares; +THP, open squares; Pub, black triangle; +THP, open triangle; Pub δ−/−, orange; +THP, blue). *P < 0.05, Pre-pub versus Pub. Error bars indicate SE of the mean. (C to F) Representative traces [(C) and (E)] and summary data [(D) and (F)] of evoked EPSPs (whole-cell current clamp, black; +THP, red) and NMDA EPSPs (blue; +THP, yellow). Scale, 1mV, 100 ms. With synaptic GABAergic current blockade [200 nM SR95531 (13), (C) and (D)], the NMDA/AMPA ratio was reduced at puberty (*P < 0.05 versus all other groups, **P < 0.05 versus pre-Pub, Pub). (E and F) Complete GABAR blockade. (G) Representative EPSC responses to paired stimuli were unaltered across groups. (H) Summary, paired pulse ratio. Scale, 50 pA, 100 ms.
With whole-cell current clamp recordings under synaptic GABAR blockade, the NMDA/AMPA ratio (Fig. 2) was markedly reduced at puberty (0.02), as compared with adult (0.09) and prepubertal (0.14) values (P = 0.007). However, under complete GABAR blockade, nearly identical NMDA/AMPA ratios of excitatory postsynaptic potentials (EPSPs) or EPSCs were observed before and after puberty (Fig. 2, E and F, and fig. S5).
Stress steroids (14, 15) such as THP enhance inward current at α4β2δ GABAR (12, 16–19), while reducing outward current (8) by polarity-dependent desensitization (8, 16). Therefore, THP should facilitate NMDA EPSCs in CA1 at puberty, when GABAergic current is outward (8), but not before puberty, when it is inward (fig. S6).
30 nM THP reduced the threshold and increased amplitudes of NMDA EPSCs and EPSPs at puberty (Fig. 2, A, C, and D, and fig. S4; P = 0.05). In contrast, THP modestly reduced NMDA currents in the prepubertal and adult hippocampus (Fig. 2, A and B), where THP is inhibitory (fig. S7) (8). Importantly, THP had no effect on the NMDA/AMPA ratio under total GABAR blockade (Fig. 2, E and F) or in the pubertal δ−/− hippocampus (Fig. 2, A and B). The paired pulse ratio was unchanged by THP at puberty (Fig. 2, G and H), indicating that THP was not altering glutamate release.
Because NMDA receptors are essential for long-term potentiation (LTP), an in vitro model of learning (6, 20, 21), we examined whether puberty onset impaired LTP induced by theta-burst stimulation (TBS) (figs. S8 and S9) of the Schaffer collaterals (20). TBS induced NMDA receptor–dependent LTP (Fig. 3A and fig. S10) in both the prepubertal and adult hippocampus, with more success before puberty (Fig. 3A; P = 0.00018). However, LTP was not induced at puberty (Fig. 3A; P = 0.002 versus prepuberty). In contrast, LTP was robustly produced under complete GABAR blockade (Fig. 3C), as well as in the pubertal δ−/− hippocampus (Fig. 3B). In adults, induction of LTP was of similar magnitude in wild-type (WT) and δ−/− mice (Fig. 3, A and D).
Fig. 3.
LTP induction is attenuated at puberty: reversal by the stress steroid THP. (A) TBS (dashed line) induced LTP (black) before puberty (Pre-pub, left) and in adult (right), but not in the pubertal (Pub, middle) CA1 hippocampus. THP (red, 30 nM) permitted LTP induction at puberty. (Inset) Representative field EPSPs. TBS, arrow. Scale, 0.5 mV, 50 ms. (B) Pubertal δ−/−. (C) Complete GABAR blockade (Pre-pub, black; Pub, blue). (D) Adult δ−/−. (E) Local application of THP (arrow) to stratum radiatum during TBS under synaptic GABA blockade. (F) THP (arrows) applied before TBS and after TBS (Pub).
Because THP facilitated NMDA receptor activation at puberty, we predicted it would also facilitate LTP. Indeed, 30 nM THP restored LTP at puberty (Fig. 3A), whereas it reduced LTP before puberty. In contrast, its inactive βOH-isomer (8), which blocks THP’s effects (8), prevented LTP induction when administered before THP (fig. S11).
Synaptic GABAR blockade did not reverse the deficit in LTP induction at puberty, nor did it prevent LTP induction by local dendritic application of THP during TBS (Fig. 3D). Application of THP 5 min after LTP induction had no effect (Fig. 3E), verifying that THP was facilitating LTP induction rather than maintenance.
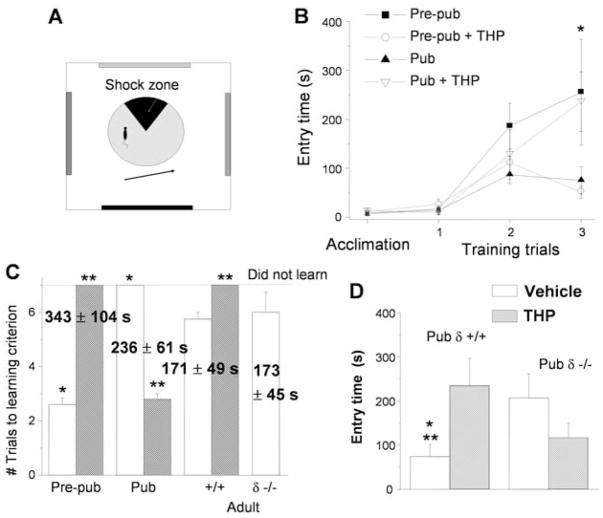
We tested whether spatial learning would be impaired at puberty using a hippocampus-dependent spatial learning task that requires LTP for memory storage (6, 22) and produces minimal stress compared with other tasks (23). Mice were trained across three sessions to avoid a moving zone (0.3 mA; Fig. 4A), which delivered a minimal foot-shock subthreshold for stress steroid release (24). The time to first enter the zone was recorded as a measure of learning.
Fig. 4.
Spatial learning is attenuated at puberty: reversal by the stress steroid THP. (A) Spatial learning platform (shock zone, black sector). (B) Times for first entry of the shock zone. Pre-pub mice attained the longest entry times. Pre-pub, black square; +THP, open circle; Pub, black triangle; +THP, open triangle (*Pre-pub versus Pre-pub +THP, Pub, P < 0.05; *Pub versus Pub +THP, P < 0.05, Tukey’s test). Error bars indicate SE of the mean. (C) Time to reach criterion (120 s) indicated for each group (numbers, best entry time). Vehicle, white bars; THP, hatched bars. *P < 0.05 Pre-pub versus Pub; **P < 0.05 versus vehicle. (D) Longest first entry time for Pub +/+ and δ−/− mice after vehicle or THP (vehicle, white bars; THP, hatched bars). *P < 0.05 versus δ−/− vehicle; **P < 0.05 versus +/+ THP.
We found that puberty impaired learning: The time to enter the shock zone decreased by 70% (Fig. 4B; P < 0.05), and fewer animals learned (fig. S12) compared with prepubertal WT and pubertal δ−/− mice (Fig. 4). THP (10 mg/kg intra-peritoneally) completely reversed the learning deficit at puberty (Fig. 4, B and C), whereas it impaired learning before puberty. In contrast, the number of shocks per entry was unaltered across groups (fig. S13), indicating that the shock was equally aversive for all animals. In contrast to pubertal mice, both WT and δ−/− adults learned shock avoidance, but not as well as did prepubertal mice (Fig. 4C).
Although effects of puberty on synaptic plasticity have not been studied previously, the development of LTP in the CA1 hippocampus is maximal at ~3 weeks of age (25–27). In the absence of GABAR blockade, LTP declines around 35 to 45 postnatal days (27), consistent with puberty onset. This developmental time course is also reflected behaviorally (11). Thus, increased expression of extrasynaptic α4βδ GABAR at puberty may represent the mechanism for this decline.
LTP induction requires voltage-triggered Mg++ unblock of the NMDA receptor (28), where local depolarization (29) has a greater effect on LTP induction than back-propagating action potentials. In this context, a GABAR shunting inhibition on spines, where we observe the greatest increase in α4βδ expression, would be more effective at impairing NMDA receptor activation than inhibition on the dendritic shaft. In the visual system, increased activity of fast-spiking basket cells targeting α1 receptors delimits the critical period (7). Taken together, these results suggest that diverse types of GABA inhibition shape plasticity during development.
In the adult, drugs that alter GABAR function also alter plasticity (30–33), probably mediated by dendritic α5-containing GABARs (31, 33), which localize at spines and modify learning (34, 35). α4βδ GABARs did not play a role in adult synaptic plasticity, when their expression is low (36), and learning and LTP induction in δ−/− mice were similar to that in WT animals.
The learning deficit at puberty is acutely reversed by the stress steroid THP via its inhibition of α4βδ GABAR, in contrast to its typical impairment of learning at other ages (30). THP effects are distinguishable from corticosterone, which alters learning after a delay (37, 38) but has no effect acutely (39). Thus, the stress steroid THP provides a novel means for rapid changes in synaptic plasticity at puberty.
Supplementary Material
Acknowledgments
We thank D. Lovinger for a critical reading of the manuscript; W. Sieghart for his generous gift of the δ antibody; and A. Kuver, L. Silva, and J. Molla for technical assistance. This work was supported by NIH grants DA09618 and AA12958 to S.S.S.
Footnotes
References and Notes
- 1.Johnson JS, Newport EL. Cognit Psychol. 1989;21:60. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- 2.Subrahmanyam K, Greenfield P. J Appl Dev Psychol. 1994;15:13. [Google Scholar]
- 3.McGivern RF, Andersen J, Byrd D, Mutter KL, Reilly J. Brain Cogn. 2002;50:73. doi: 10.1016/s0278-2626(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 4.Bannerman DM, et al. Neurosci Biobehav Rev. 2004;28:273. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Burgess N, Maguire EA, O’Keefe J. Neuron. 2002;35:625. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 6.Pastalkova E, et al. Science. 2006;313:1141. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 7.Fagiolini M, et al. Science. 2004;303:1681. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 8.Shen H, et al. Nat Neurosci. 2007;10:469. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Materials and methods and complete statistics are available as supporting material on Science Online.
- 10.Hassler M. Int J Neurosci. 1991;58:183. doi: 10.3109/00207459108985434. [DOI] [PubMed] [Google Scholar]
- 11.Krasnoff A, Weston LM. Dev Psychobiol. 1976;9:261. doi: 10.1002/dev.420090310. [DOI] [PubMed] [Google Scholar]
- 12.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Br J Pharmacol. 2002;136:965. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stell BM, Mody I. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Proc Natl Acad Sci USA. 1991;88:4553. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Biol Psychol. 2001;49:788. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi MT, Haas KF, Macdonald RL. Neuropharmacology. 2002;43:492. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- 17.Belelli D, Casula A, Ling A, Lambert JJ. Neuropharmacology. 2002;43:651. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- 18.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Proc Natl Acad Sci USA. 2003;100:14439. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maguire JL, Stell BM, Rafizadeh M, Mody I. Nat Neurosci. 2005;8:797. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 20.Larson J, Wong D, Lynch G. Brain Res. 1986;386:347. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 21.Bliss TV, Collingridge GL. Nature. 1993;361:31. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 22.Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Proc Natl Acad Sci USA. 2001;98:3531. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison FE, Hosseini AH, McDonald MP. Behav Brain Res. 2009;198:247. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman SB, Ader R, Grota LJ, Larson T. Psychosom Med. 1967;29:323. doi: 10.1097/00006842-196707000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Dudek SM, Bear MF. J Neurosci. 1993;13:2910. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi Y, Zorumski CF. Synapse. 1995;20:19. doi: 10.1002/syn.890200104. [DOI] [PubMed] [Google Scholar]
- 27.Meredith RM, Floyer-Lea AM, Paulsen O. J Neurosci. 2003;23:11142. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herron CE, Lester RA, Coan EJ, Collingridge GL. Nature. 1986;322:265. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- 29.Hardie J, Spruston N. J Neurosci. 2009;29:3233. doi: 10.1523/JNEUROSCI.6000-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews DB, Morrow AL, Tokunaga S, McDaniel JR. Alcohol Clin Exp Res. 2002;26:1747. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- 31.Cheng VY, et al. J Neurosci. 2006;26:3713. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wigström H, Gustafsson B. Nature. 1983;301:603. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- 33.Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. Psychopharmacology. 2006;188:619. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- 34.Brünig I, Scotti E, Sidler C, Fritschy JM. J Comp Neurol. 2002;443:43. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- 35.Crestani F, et al. Proc Natl Acad Sci USA. 2002;99:8980. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng Z, et al. J Comp Neurol. 2002;446:179. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 37.Hodes GE, Shors TJ. Horm Behav. 2005;48:163. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luine V, Martinez C, Villegas M, Magariños AM, McEwen BS. Physiol Behav. 1996;59:27. doi: 10.1016/0031-9384(95)02016-0. [DOI] [PubMed] [Google Scholar]
- 39.Sadowski RN, Jackson GR, Wieczorek L, Gold PE. Behav Brain Res. 2009;205:19. doi: 10.1016/j.bbr.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.