Abstract
Background
OXR1 is an ancient gene, present in all eukaryotes examined so far that confers protection from oxidative stress by an unknown mechanism. The most highly conserved region of the gene is the carboxyl-terminal TLDc domain, which has been shown to be sufficient to prevent oxidative damage.
Methodology/Principal Findings
OXR1 has a complex genomic structure in the mosquito A. gambiae, and we confirm that multiple splice forms are expressed in adult females. Our studies revealed that OXR1 regulates the basal levels of catalase (CAT) and glutathione peroxidase (Gpx) expression, two enzymes involved in detoxification of hydrogen peroxide, giving new insight into the mechanism of action of OXR1. Gene silencing experiments indicate that the Jun Kinase (JNK) gene acts upstream of OXR1 and also regulates expression of CAT and GPx. Both OXR1 and JNK genes are required for adult female mosquitoes to survive chronic oxidative stress. OXR1 silencing decreases P. berghei oocyst formation. Unexpectedly, JNK silencing has the opposite effect and enhances Plasmodium infection in the mosquito, suggesting that JNK may also mediate some, yet to be defined, antiparasitic response.
Conclusion
The JNK pathway regulates OXR1 expression and OXR1, in turn, regulates expression of enzymes that detoxify reactive oxygen species (ROS) in Anopheles gambiae. OXR1 silencing decreases Plasmodium infection in the mosquito, while JNK silencing has the opposite effect and enhances infection.
Introduction
Reactive Oxygen Species (ROS) are highly reactive oxygen-derived molecules that include free radicals and peroxides. They are formed, in part, as natural byproducts of mitochondrial respiration and it is estimated that as much as 0.1 to 5% of all oxygen consumed by mitochondria is converted into ROS [1]. High levels of ROS can also be generated abruptly, as part of the immune response to pathogens [2], and the intracellular redox state is a key determinant of cell survival, proliferation, differentiation, and apoptosis [3].
ROS produced in living organisms have the potential to damage key cellular components including lipids, proteins and DNA. ROS-mediated DNA damage contributes to spontaneous mutagenesis that can lead to various functional disorders, including premature aging and cancer [4]. Cells protect themselves from ROS by preventing cell damage through detoxification of these chemicals, and by repairing ROS-induced damage once it takes place. For example, superoxide dismutases (SODs), convert superoxide anion into hydrogen peroxide, a less reactive species, while catalase detoxifies hydrogen peroxide into water and oxygen. In mammals, the nuclear factor kappa B (NF-κB) is actively involved in the induction of catalase and glutathione peroxidase (Gpx) expression in response to oxidative stress [5].
ROS act as signaling molecules and a number of transcription factors that regulate expression of antioxidant genes in response to ROS have been characterized in prokaryotes and in yeast [6] [7]. Hydrogen peroxide (H2O2) is one of the most stable and diffusible ROS signaling molecule and has been shown to activate the Jun N-terminal Kinase (JNK) [8] [9] and NF-κB [8] [5] signaling cascades. In Drosophila, the Jun N-terminal Kinase (JNK) pathway activates the transcription factor Activator Protein-1 (AP-1) and induces expression of stress-response genes [10].
The Oxidation Resistance 1 (OXR1) gene is present in all eukaryote genomes sequenced so far and is known to protect yeast and human cells from oxidative damage, through an unknown mechanism [11]. When human OXR1 is expressed in Escherichia coli mutants that are unable to repair oxidative damage, it has a protective effect, reducing the accumulation of mutations in the bacterial genome [12]. The OXR1 protein is localized in the mitochondria in yeast and human cells [12] [11], but nuclear localization has also been reported in mouse and rat cell lines [13] [14]. The most highly conserved region of the gene is the carboxyl-terminal TLDc domain, which has been shown to be sufficient to reduce the mutation rate in E. coli [15]. The TLDc domain is restricted to eukaryotes, and is commonly found in proteins that also contain lysin motifs (LysM) (IPR002482).
In Drosophila melanogaster, the OXR1 gene (CG32464) has 19 different transcripts that are generated by alternative splicing of 25 different exons (Entrez Gene and FlyBase). Eighteen of these transcripts share a common 195 aa C-terminal region (exons 24 and 25) that codes for the TLDc domain. This conserved region can splice with at least 6 different exons. The short isoforms splice a unique exon (either exon 19, 20, 21, 22 or 23) to exons 24–25, giving rise to five different OXR1 transcripts (isoforms A, C, H, I and Q). Twelve longer transcripts share exons 14–18 which are also spliced to exons 24–25 (E18 group) and only differ in the exon they splice at the 5′ of the mRNA. Genetic studies revealed that OXR1 (L82 gene) is a late puff gene with an essential developmental role. Deletion of this locus results in lethality due to the inability of adult flies to eclose [16]. OXR1 was also identified in a recent genetic screen for genes that affect the survival of Plasmodium gallinaceum parasites injected into the Drosophila hemocele [17]. Furthermore, OXR1 silencing reduces Plasmodium berghei infection in the mosquito A. gambiae [17]. In this study, we examined the participation of A. gambiae OXR1 in protecting mosquitoes from oxidative stress and on the survival of early stages of P. berghei parasites in the mosquito. The role of Jun Kinase (JNK) in the regulating the expression of OXR1 and ROS detoxification enzymes, and in limiting Plasmodium infection was also explored.
Results
OXR1 gene structure and phylogenetic analysis
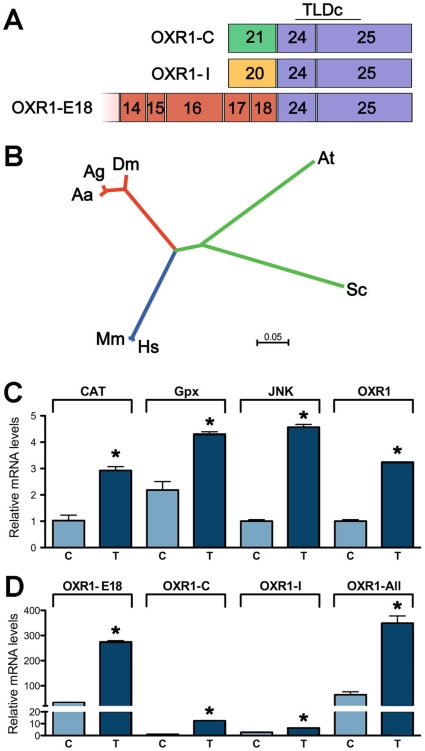
The A. gambiae OXR1 gene (AGAP001751-PA) has a complex structure, consisting of at least 17 exons that span a 45 kb region. Bioinformatic analysis, based on the assembly of available ESTs and Blast analysis of all Drosophila exons against the A. gambiae genome sequence, identified several putative OXR1 exons in A. gambiae. We confirmed, by PCR amplification with exon-specific primers, that at least three different types of OXR1 mRNA transcripts are expressed in adult mosquito females (Fig. 1A): those in which exon 24 (numbering based on the DmOxR1 gene) splices with exon 21 (homolog of Drosophila isoform C), with exon 20 (homolog of Drosophila isoform I), or with exon 18 (E18 group).
Figure 1. Genomic organization, phylogeny, and expression of the An. gambiae OXR1 gene.
(A) Genomic organization of AgOXR1 gene and splice forms transcript B, C, and I. (B) Phylogenetic tree based on the sequence alignment of the deduced amino acid sequence of members of the OXR1 family from different species. (Hs = Homo sapiens, Mm = Mus musculus, Dm = Drosophila melanogaster, Ae = Aedes aegypti, Ag = Anopheles gambiae, At = Arabidopsis thaliana and Sc = Saccharomyces cerevisiae). The sequence alignment used to build the dendrogram is shown in Figure S1. (C) Changes in Catalase, Glutathione peroxidase, JNK and OXR1 (TLDc domain region) mRNA levels 6 h after H2O2 injection (T) compared with water-injected control mosquitoes (C) in A. gambiae. Values were normalized using An. gambiae ribosomal protein S7 mRNA levels as an internal reference. Mean ± SEM. (D) Changes in mRNAs levels of specific OXR-1 isoforms (OXR1-C, OXR1-I and OXR1-E18) and of all OXR1 transcripts (OXR1-all, in which primers from the shared TLDc domain were used) were determined 6 h after injection of H2O2 (T = treated group) or water control (C = control group) in An. gambiae (G3 strain). Values were normalized using An. gambiae ribosomal protein S7 mRNA levels as an internal reference. Mean ± SEM. * indicates significant differences (p<0.05) by ANOVA.
The deduced amino acid sequence of the TLDc domain of AgOXR1 was aligned with that of Aedes aegypti and Culex quinquefasciatus mosquitoes, Drosophila melanogaster, Human, Mouse, Arabidopsis and Yeast (Fig. S1) and their phylogeny was analyzed (Fig. 1B). The AgOXR1 deduced amino acid sequence is most similar to that of its putative orthologs in A. aegypti and D. melanogaster (96% and 87% identity, respectively), and has diverged extensively from that of mammals, plants and yeast (52%, 36% and 28% identity, respectively); indicating that OXR1 is an ancient gene that was probably already present in the common ancestor of yeast and humans.
Oxidative stress induces expression of multiple OXR1 isoforms
To examine the involvement of OXR1 in the resistance of A. gambiae to oxidative stress, adult mosquito females were challenged by injecting H2O2 directly into the hemocele. As previously shown [18], this challenge induced expression of Catalase and Glutathione peroxidase (Gpx), two enzymes that detoxify H2O2, by 3 and 2 fold, respectively, relative to the water-injected control group (P = 0.018 and P = 0.024) (Fig. 1C). Expression of all OXR1 forms (using primers from the TLDc domain) and JNK also increases by 3 fold (P = 0.01 and P = 0.001, respectively); indicating oxidative stress also activates transcription of these two genes. Interestingly, OXR1 mRNA levels do not change significantly in response to a bacterial challenge with a mixture of E. coli and Micrococcus luteus (Fig. S2).
The individual response of the three types of OXR1 transcripts to oxidative stress was also analyzed in adult females. The OXR1-exon18 group is the predominant form present in adult females (Fig. 1D) and expression of all AgOXR1 isoforms (Isoforms I, C and the Exon18 group) increases significantly in response to H2O2 injection (P<0.01) (Fig.1D). In all subsequent experiments, primers to quantitate OXR1 mRNA expression levels and dsRNA constructs to silence gene expression were designed from the TLDc domain, which is common to all OXR1 isoforms.
OXR1 regulates expression of ROS detoxification enzymes through the Jun kinase (JNK) pathway
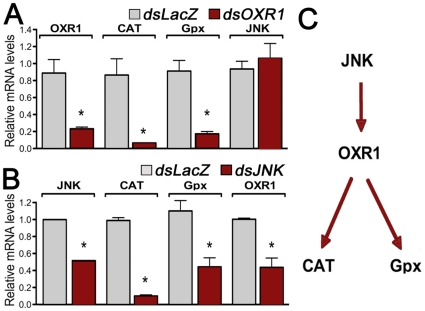
In spite of the well-defined role of OXR1 in protection against ROS damage in several organisms, the mechanism of this protective effect has not been established. The hypothesis that OXR1 regulates expression of ROS detoxification enzymes in A. gambiae was investigated. OXR1 silencing decreases endogenous Catalase and Gpx mRNAs by 92% and 82% respectively (p<0.001) but does not affect JNK mRNA levels, relative to the dsLacZ group (Fig. 2A). OXR1 silencing also decreases expression of SOD1, SOD2 and SOD3 but, in general, the effect is less striking (38%, 50% and 46% reduction, respectively, p<0,01) (Fig. S3).
Figure 2. Effect of OXR1 silencing on JNK and ROS detoxification enzymes mRNA levels and effect of JNK silencing on OXR1 and ROS detoxification enzymes mRNA levels.
(A) Changes in OXR1, Catalase, Glutathione peroxidase, JNK mRNA levels in dsOXR1 injected mosquitoes compared to dsLacZ control injected mosquitoes. (B) Changes in JNK, Catalase, Glutathione peroxidase, OXR1 mRNA levels in dsJNK-injected mosquitoes compared to dsLacZ control injected mosquitoes All transcript measurements were performed using qRT-PCR. Data are shown as Mean + SE. * indicates significant differences (p<0.05) by ANOVA.(C) Diagram of the proposed organization of the OXR1 signaling cascade in A. gambiae adult females.
In Drosophila, the JNK pathway has been shown to activate expression of stress-response genes [10]. We investigated whether JNK could be involved in regulating expression of OXR1, Catalase or Gpx in A. gambiae. Injection of dsJNK RNA reduced JNK mRNA levels by 50% relative to the dsLacZ control. Furthermore, JNK silencing significantly decreases Catalase, Gpx and OXR1 mRNA levels by 90%, 60% and 56%, respectively p<0.0001) (Fig. 2B). Taken together, these gene-silencing experiments indicate that JNK and OXR1 regulate the basal mRNA levels of ROS detoxification enzymes and that JNK acts upstream of OXR1 (Fig. 2C).
OXR1 and JNK are essential for mosquitoes to survive chronic oxidative stress
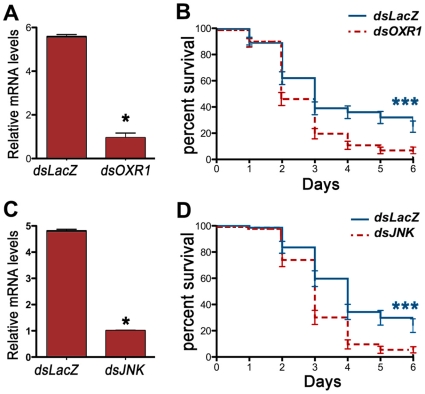
To determine whether OXR1 and JNK are required for mosquitoes to withstand chronic oxidative stress, groups of 3 day-old females were injected with dsLacZ, dsOXR1 or dsJNK and the efficiency of midgut silencing was tested 36 h post-infection (hpi). Endogenous levels of midgut OXR1 and JNK mRNA were reduced by 83 and 80%, respectively, relative to the dsLacZ control group (Fig. 3A and 3C). The effect of feeding mosquitoes water containing 1% H2O2 on daily survival was evaluated in triplicate groups for each treatment. This high dose of hydrogen peroxide caused substantial mortality in all experimental groups, but the mortality was significantly higher (P<0.0001, Log rank test) when either dsOXR1 (Fig. 3B) or dsJNK (Fig. 3D) were silenced. These experiments indicate that OXR1 and JNK play a critical role in protecting adult females mosquitoes from chronic oxidative stress.
Figure 3. Effect of OXR1 midgut silencing on A. gambiae G3 adult females survival when challenged by H2O2 chronic oral administration.
(A) Relative amounts of midgut OXR1 mRNA after injection with dsOXR1 compared with dsLacZ injected control mosquitoes. (B) Relative amounts of midgut JNK mRNA after injection with dsJNK compared with dsLacZ injected control mosquitoes. (C) % Survival of control dsLacZ and dsOXR1-injected mosquitoes after 1% H2O2 chronic feeding over 6 days. (D) % Survival of control dsLacZ and dsJNK-injected mosquitoes after 1% H2O2 chronic feeding over 6 days. Significance was determined by Logrank test. * * * significance(p<0.001).
OXR1 silencing limits Plasmodium oocyst formation in A. gambiae
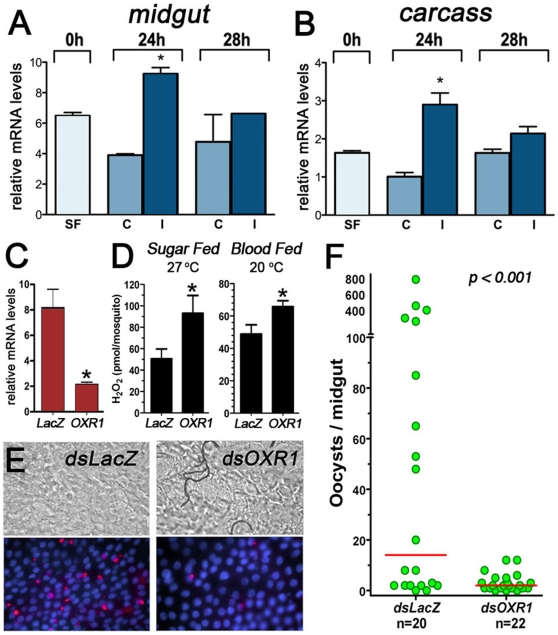
We have previously shown that OXR1 silencing decreases the number of Plasmodium berghei mature oocysts present six days post-infection (dpi) [17]. To better define the stage of the parasite that is affected by OXR1 silencing, we analyzed OXR1 expression in the midgut and carcass (rest of the mosquito without midgut) at early stages of infection, 24 and 28 h hpi. We found that Plasmodium infection induces OXR1 mRNA expression in the midgut and systemically 24 hpi, at the time when ookinetes are invading the midgut epithelium (Fig. 4A). This transcriptional response is transient, as it is no longer observed by 28 hpi (Fig. 4B). To investigate whether OXR1 silencing reduces the number of Plasmodium ookinetes that complete invasion and transform into oocysts, early oocyst formation was analyzed two days PI using immunofluorescence staining. Injection of dsOXR1 decreases OXR1 mRNA levels by 78%, relative to the dsLacZ control (Fig. 4C) and results in a significant increase in hemolymph H2O2 levels both in sugar-fed females (kept at 27°C) and in blood-fed females 24 hpi (Fig. 4D). OXR1 silencing also reduces the median number of early oocysts present 2dpi by 7 fold, relative to the dsLacZ-injected control (p<0.001, KS test) (Fig. 4 E–F).
Figure 4. AgOXR1 expression in response to P. berghei infection and effect of AgOXR1 silencing on parasite survival in Plasmodium-infected An. gambiae (G3 Strain) females.
(A) Changes in OXR1 mRNA levels in response to P. berghei infection (0, 24, 28 h hr PI) in midgut samples. (B) Changes in OXR1 mRNA levels in response to P. berghei infection (0, 24, 28 h hr PI) in carcass samples (whole body without the midgut). (C) Effect of dsOXR1 injection on OXR1 mRNA levels in G3 mosquitoes relative to a control group injected with LacZ dsRNA (dsLacZ). All transcript determinations were performed using qRT-PCR. (D) Effect of OXR1 silencing on H2O2 hemolymph levels of individual female mosquitoes (n = 10) that were either sugar-fed and kept at 27°C (left panel) or blood-fed on a healthy mouse and kept at 20°C (right panel), the temperature at which Plasmodium-infected mosquitoes are kept. Data are shown as Mean + SE. The asterisk indicates significant differences (p<0.05) by ANOVA. (E–F) Effect of dsRNA-mediated knockdown of An. gambiae OXR1 in P. berghei midgut infection after 48 h post-infection in dsOXR1 or dsLacZ injected mosquitoes. (D) Representative fields of immunofluorescence stainings of early P. berghei oocysts 48 h post-infection. Nuclei are in blue and P. berghei oocysts are in red. Scale bar 10 µm. (E) The dots represent the number of parasites present on individual midguts and the median number of oocysts is indicated by the horizontal line. The distributions are compared using the Kolmogorov-Smirnov test (KS test), n = number of mosquitoes, * significantly different with respect to dsLacZ injected controls (Kolgomorof-Smirnoff test, p<0.05).
JNK silencing enhances Plasmodium infection
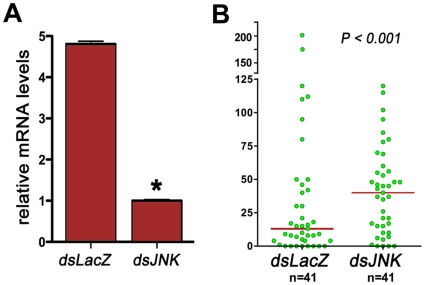
As JNK silencing reduces OXR1 expression (Fig. 3B), we hypothesized that JNK silencing would have a similar effect as OXR1 depletion, and would decrease Plasmodium infection (Fig. 2C). JNK silencing reduced whole body mRNA levels by 82% (Fig. 5A). However, this reduction in JNK expression has the opposite effect as OXR1 silencing, increasing the mean number of oocysts present 6 days PI by three fold (P<0.001) (Fig. 5B).
Figure 5. Effect of AgOXR1 silencing on parasite survival in Plasmodium-infected A. gambiae (G3 Strain) females.
(A) Relative amounts of JNK mRNA in G3 mosquitoes after injection with JNK dsRNA (dsJNK) compared with controls injected with LacZ dsRNA (dsLacZ). (B) Effect of dsRNA-mediated knockdown of An. gambiae JNK in P. berghei midgut infection after 6 days post-infection in dsJNK or dsLacZ injected mosquitoes. The dots represent the number of parasites present on individual midguts and the median number of oocysts is indicated by the horizontal line. The distributions are compared using the Kolmogorov-Smirnov test (KS test), n = number of mosquitoes, * significantly different with respect to dsLacZ injected controls (Kolgomorof-Smirnoff test, p<0.05).
Discussion
ROS are critical for A. gambiae to mount effective immune responses against bacteria and Plasmodium [18]. Previous studies showed that a mosquito line selected to be refractory (R) to Plasmodium infection has higher systemic levels of H2O2 than unselected (G3) or susceptible (S) females [19]. High H2O2 levels in the R strain are also associated with increased survival to bacterial injection. Conversely, mortality is higher in the S strain, which has the lowest level of H2O2. Reduction of ROS by dietary supplementation of Vitamin C or Uric Acid (strong antioxidants), dramatically decreases survival following a bacterial challenge [18].
We have previously shown that catalase silencing results in higher systemic levels of H2O2, increased ookinete lysis and reduced oocyst formation [18]. Because OXR1 silencing reduces catalase expression (Fig. 2A) and increases systemic levels of H2O2 (Fig. 4D), it is not surprising that it also decreases P. berghei infection. However, the unexpected finding that JNK silencing has the opposite effect on Plasmodium and enhances infection suggests that, besides its role in ROS balance, this cascade may also mediate an antiparasitic response, yet to be defined. In Drosophila, tissue damage activates the JNK signaling, which induces expression of cytokines that activate the STAT pathway [20]. In A. gambiae, the STAT pathway mediates antiplasmodial responses that target the oocysts stage by activating expression of nitric oxide synthase [21]. It is thus possible that JNK signaling participates in the activation of the STAT pathway during the late-phase antiparasitic response in A. gambiae.
A recent study revealed that P. berghei parasites are particularly vulnerable to oxidative stress in mosquito stages of their life cycle; as disruption of the glutamylcysteine synthetase (c-GCS) gene, the first enzyme in the glutathione biosynthesis pathway, has no effect in asexual stages, but results in stunted oocysts unable to produce infective sporozoites [22]. Different species (or strains) of Plasmodium may also differ in their sensitivity to oxidative stress. For example, OXR1 depletion in A. gambiae does not affect infection with the African Plasmodium falciparum 3D7 strain [23]. This raises the question of whether P. falciparum is less sensitive to ROS than P. berghei or whether it generates less ROS. Previous expression analysis revealed that 28 genes linked to mitochondrial, oxido-reductive or stress responses are induced in A. gambiae midguts infected with P. berghei, while only 14 genes are induced in P. falciparum-infected midguts [24]; suggesting that infection with P. falciparum, a well-adapted system, may be less stressful to the mosquito.
Although OXR1 has been shown to confer resistance against oxidative stress in several eukaryote organisms [12] [11] [14], the mechanism mediating this protective effect has not been elucidated. Our studies confirmed that OXR1 is also important to protect mosquitoes from oxidative stress and revealed that OXR1 mRNA levels are induced in response to systemic injection of hydrogen peroxide. Furthermore, we found that OXR1 regulates the basal levels of catalase and Gpx expression, two protective enzymes involved in the detoxification of hydrogen peroxide, providing new insight into the mechanism of action of OXR1. Previous studies showed that the JNK pathway regulates expression of stress-response genes in Drosophila, such as heat shock proteins, and that it is critical for flies to tolerate to chronic oxidative stress [10]. Our data provide direct experimental evidence that JNK acts upstream and regulates OXR1 expression. One can envision a regulatory cascade in which high systemic levels of hydrogen peroxide activate JNK signaling, which in turn activates transcription factors by phosphorylating them (probably AP-1 transcription factor complex, consisting of a Jun and Fos dimer) which, in Drosophila, are known to activate expression of JNK-target genes [10]. Our data suggest that both the JNK and OXR1 genes are potential targets genes of this cascade. Our studies indicated that OXR1 also regulates expression of Catalase and Gpx, but the molecular mechanism of actions of OXR1 remains to be defined. The role of OXR1 could be a direct one, as a cofactor that modulates transcriptional activation of Catalase and Gpx. If this were the case, one would expect the OXR1 protein to have a nuclear localization. Alternatively, the effect of OXR1 on Catalase and Gpx expression could be and indirect one. In some studies the OXR1 protein has been localized to the mitochondria, suggesting that OXR1 could also modulate ROS generation or signaling by this organelle. OXR1 is a complex gene that is expressed as multiple splice forms. It is possible that the different OXR1 isoforms localize to different subcellular compartments and carry out diverse functions. Silencing expression of specific variants and generating antibodies to isoform-specific exons would be required understand the functional significance of this complex differential splicing. We conclude that both JNK and OXR1 are critical components of the ROS-response system in A. gambiae and that one of the mechanism by which they protect mosquitos from oxidative damage is by regulating expression of Catalase and GPX, and thus modulating their ability to detoxify ROS.
Experimental procedures
Ethics Statement: Animal care guidelines were followed according to the NIH Animal Care and Use Committee (ACUC).
Sequence and phylogenetic analysis
The predicted amino acid sequence of several members of the OXR1 family from different species (Ms = Mus musculus Hs = Homo sapiens, Dm = Drosophila melanogaster, Ae = Aedes aegypti, Ag = Anopheles gambiae, At = Arabidopsis thaliana and Sc = Saccharomyces cerevisiae) were aligned and dendrograms constructed using the ClustalW software (Thompson et al., 1994) and the results were visualized graphically with TreeviewX 0.5.0. The sequence alignment appears in Figure S1.
P. berghei Infection of Mosquitoes
Female mosquitoes (5 days old) were infected with P. berghei by feeding on anesthetized infected BALB/c mice. Mosquitoes were fed when mice reached 4–10% parasitemia and had at least 1 exflagellation per field, following standard procedures previously described [25]. Blood-fed infected and control mosquitoes were kept at 21°C and 80% humidity. P. berghei midgut infection was quantified 48 h post-infection by immunofluorescence using mouse anti Pbs21 antibody as previously described [26].
Quantitation of Gene Expression
A. gambiae midguts/carcasses/whole bodies were collected from 10 unfed or blood-fed females. All samples were collected in duplicate groups, frozen in liquid nitrogen and stored at −80°C. Poly(A) mRNA or total RNA was isolated using Oligotex-dT beads (Qiagen) or RNAeasy (Qiagen) respectively, following the manufacturer's instructions. First-strand cDNA was synthesized using QuantiTect reverse transcriptase (Qiagen). Gene expression was assessed by SYBR green quantitative real-time PCR (qPCR) (DyNAmo HS; New England Biolabs) in a Chromo4 system (Bio-Rad). PCR involved an initial denaturation at 95°C for 15 min, 44 cycles of 10 sec at 94°C, 20 sec at 58°C, and 30 sec at 72°C. Fluorescence readings were taken at 72°C after each cycle. A final extension at 72°C for 5 min was completed before deriving a melting curve (70–95°C) to confirm the identity of the PCR product. qRT-PCR measurements were made in duplicate. Primers used for qPCR amplify an OXR1 fragment nonoverlapping with the fragment used for RNAi. Relative quantitation results were normalized with A. gambiae ribosomal protein S7 as internal standard and analyzed by the 2–ΔΔCt method [27]. Primers used are provided in Table S1.
Bacterial Challenge of Mosquitoes
Three-day-old adult females were injected with a mixture of Escherichia coli and Micrococcus luteus. Bacterial cultures were grown to an optical density of 0.5 (600 nm) in LB broth, and 200 µl from each culture were mixed and centrifuged for 5 min at maximum speed. The supernatant was discarded, and the pellet was washed twice with PBS and resuspended in 125 µl of PBS. Mosquito survival was monitored daily for 8 days after intrathoracic injection of 0.138 µl of either PBS or bacterial suspension using a microinjection system and a micromanipulator (Nanoject II microinjection system; Drummond).
H2O2 Injection of Mosquitoes
Sugar-fed 4-day-old A. gambiae G3 females were injected as above with 18 nmol of H2O2 (in 69 nl); controls were injected with water. Mosquitoes were kept at 27°C, and 6 h after treatment, groups of 10 were collected as previously described [18].
Cloning and sequencing of OXR1 C-terminal domain
Adult females were fed on a healthy mouse and collected 24 h after blood feeding. Whole mosquitoes were collected and stored at –70°C until mRNA extraction. Poly(A) mRNA was isolated from groups of 10 whole mosquitoes using Oligotex-dT beads (Qiagen), following the manufacturer's instructions. First-strand cDNA was synthesized using random hexamers and Superscript II reverse transcriptase (Invitrogen). The AgOXR1 C-terminal cDNA fragment was cloned by using the cDNA obtained from the 10 whole mosquitoes as template.
All OXR1 sequences available in EST databases were aligned using the Sequencher 4.8 (Mac) program. Primers were designed to amplify the conserved C-terminal domain (exons 24–25 in Fig. 1A) and the PCR product was cloned and sequenced. The nucleotide and deduced amino acid sequences are appear in Figure S3.
RNAi gene-silencing assays
Sense and antisense RNAs were synthesized from PCR-amplified gene fragments using the T7 Megascript kit (Ambion). dsRNA was further purified with water and concentrated to 3 µg/µl using a Microcon YM-100 filter (Millipore). The sequences of the primers are listed in Table S2. The gene specific amplicon is cloned into the pCR_IITOPO vector (Invitrogen). T7 polymerase promoter sites were incorporated onto both ends of this fragment by amplification with the following vector primers: forward 5′-CTCGAGTAATACGACTCACTATAGGGCTAGTAACGGCCGCCAGTGT-3′ and reverse 5′-CTCGAGTAATACGACTCACTATAGGGGCCAGTGTGATGGATATCTGC-3′. The PCR product was used as template to synthesize double-stranded of the target gene RNA in vitro using the MEGAscript RNA-mediated interference kit (Ambion). About 69 nl of dsRNAs (207 ng of dsRNA) in water was introduced into the thorax of cold-anesthetized 1-day old (for systemic silencing) or 3–4 days-old (midgut silencing) female mosquitoes using a nano-injector (Nanoject; Drummond Scientific, Broomall, Pennsylvania,United States) with a glass capillary needle according to established methodology [28]. For gene-silencing assays, 1-day-old or 3-day old female mosquitoes were injected, in parallel, with LacZ dsRNA as a control group or with target gene–specific dsRNA for the experimental group. Gene silencing was verified 24 h after dsRNA injection by real-time quantitative RT-PCR, with the A. gambiae ribosomal S7 gene as the internal control for normalization. The primers for silencing verification are listed in Table S1.
H2O2 chronic feeding of Mosquitoes
Adult females fed on a 10% sugar solution were injected with dsRNA at 3 days post emergence and fed overnight on the same 10% sugar solution. On day 4 PE they were fed on a sugar cube and water separately, to ensure that the crop was empty. On day 5 PE the treatment to induce chronic oxidative stress was initiated by feeding them on a 10% sugar solution containing 1% H2O2 until they died. Mortality was recorded over a period of 6 days. Two groups of 50 mosquitoes were used for each treatment. Significance was determined by Log rank test (p<0.005).
Hemolymph H2O2 quantitation
Mosquito hemolymph was collected by flushing the hemocoel. The cuticle from the last abdominal segment was torn open, 8 µl of PBS containing 2 mg/ml of catalase inhibitor (3-amino-triazole) were injected into the thorax and 5 µl of flushed hemolymph collected from the aperture in the posterior end. Levels of H2O2 in the hemolymph of 10 individual mosquitoes was determined using the Amplex red assay fluorimetric test (Molecular probes) for each experimental treatment, following the manufacturer instructions. The results are shown as the Mean ± SE of each experimental treatment.
Supporting Information
Sequence alignment of the TLDc Domain of the OXR1 genes from different species (Hs = Homo sapiens, Mm = Mus musculus, Dm = Drosophila melanogaster, Ae = Aedes aegypti, Ag = Anopheles gambiae, At = Arabidopsis thaliana and Sc = Saccharomyces cerevisiae).
(0.03 MB DOC)
OXR1 mRNA expression in sugar-fed adult females challenged with bacteria. OXR1 mRNA in whole body at different times after mosquitoes were challenged with a mixture of heat-killed E. coli and M. luteus. All transcript measurements were performed using qRT-PCR. Data are shown as Mean + SE. * indicates significant differences (p<0.05) by ANOVA.
(0.82 MB TIF)
Effect of OXR1 silencing on JNK and ROS detoxification enzymes mRNA induction upon blood feeding. Effect of OXR1 silencing on mRNA expression levels of (A) OXR1,(B) SOD1, (C) SOD2, (D) SOD3a in 24 h uninfected blood fed females (whole body) compared to dsLacZ control injected mosquitoes. All transcript measurements were performed using qRT-PCR. Data are shown as Mean + SE.
(1.42 MB TIF)
Primers used to determine gene expression by qRT-PCR in An. gambiae.
(0.04 MB DOC)
Primers used to produce PCR Amplicons for dsRNA Synthesis in An. gambiae.
(0.03 MB DOC)
Acknowledgments
We thank André Laughinghouse, Kevin Lee, Tovi Lehman, and Robert Gwadz for insectary support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 2.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Gowen LC, Avrutskaya AV, Latour AM, Koller BH, Leadon SA. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science. 1998;281:1009–1012. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- 5.Zhou LZ, Johnson AP, Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]
- 6.Storz G, Polla BS. Transcriptional regulators of oxidative stress-inducible genes in prokaryotes and eukaryotes. EXS. 1996;77:239–254. doi: 10.1007/978-3-0348-9088-5_16. [DOI] [PubMed] [Google Scholar]
- 7.Kuge S, Arita M, Murayama A, Maeta K, Izawa S, et al. Regulation of the yeast Yap1p nuclear export signal is mediated by redox signal-induced reversible disulfide bond formation. Mol Cell Biol. 2001;21:6139–6150. doi: 10.1128/MCB.21.18.6139-6150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer M, Pahl HL, Baeuerle PA. Regulation of the transcription factors NF-kappa B and AP-1 by redox changes. Chem Biol Interact. 1994;91:91–100. doi: 10.1016/0009-2797(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 9.Iordanov MS, Magun BE. Different mechanisms of c-Jun NH(2)-terminal kinase-1 (JNK1) activation by ultraviolet-B radiation and by oxidative stressors. J Biol Chem. 1999;274:25801–25806. doi: 10.1074/jbc.274.36.25801. [DOI] [PubMed] [Google Scholar]
- 10.Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- 11.Elliott NA, Volkert MR. Stress induction and mitochondrial localization of Oxr1 proteins in yeast and humans. Mol Cell Biol. 2004;24:3180–3187. doi: 10.1128/MCB.24.8.3180-3187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkert MR, Elliott NA, Housman DE. Functional genomics reveals a family of eukaryotic oxidation protection genes. Proc Natl Acad Sci U S A. 2000;97:14530–14535. doi: 10.1073/pnas.260495897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer H, Zhang XU, O'Brien KP, Kylsten P, Engvall E. C7, a novel nucleolar protein, is the mouse homologue of the Drosophila late puff product L82 and an isoform of human OXR1. Biochem Biophys Res Commun. 2001;281:795–803. doi: 10.1006/bbrc.2001.4345. [DOI] [PubMed] [Google Scholar]
- 14.Natoli R, Provis J, Valter K, Stone J. Expression and role of the early-response gene Oxr1 in the hyperoxia-challenged mouse retina. Invest Ophthalmol Vis Sci. 2008;49:4561–4567. doi: 10.1167/iovs.08-1722. [DOI] [PubMed] [Google Scholar]
- 15.Durand M, Kolpak A, Farrell T, Elliott NA, Shao W, et al. The OXR domain defines a conserved family of eukaryotic oxidation resistance proteins. BMC Cell Biol. 2007;8:13. doi: 10.1186/1471-2121-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stowers RS, Russell S, Garza D. The 82F late puff contains the L82 gene, an essential member of a novel gene family. Dev Biol. 1999;213:116–130. doi: 10.1006/dbio.1999.9358. [DOI] [PubMed] [Google Scholar]
- 17.Brandt SM, Jaramillo-Gutierrez G, Kumar S, Barillas-Mury C, Schneider DS. Use of a Drosophila Model to Identify Genes Regulating Plasmodium Growth in the Mosquito. Genetics: genetics.108.089748. 2008 doi: 10.1534/genetics.108.089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, et al. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem. 2008;283:3217–3223. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, et al. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci U S A. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1:144–154; discussion 153. doi: 10.1242/dmm.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, et al. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vega-Rodriguez J, Franke-Fayard B, Dinglasan RR, Janse CJ, Pastrana-Mena R, et al. The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog. 2009;5:e1000302. doi: 10.1371/journal.ppat.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaramillo-Gutierrez G, Rodrigues J, Ndikuyeze G, Povelones M, Molina-Cruz A, et al. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009;9:154. doi: 10.1186/1471-2180-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y, Aguilar R, Xi Z, Warr E, Mongin E, et al. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billker O, Shaw MK, Margos G, Sinden RE. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology. 1997;115 (Pt 1):1–7. doi: 10.1017/s0031182097008895. [DOI] [PubMed] [Google Scholar]
- 26.Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. Embo J. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, et al. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of the TLDc Domain of the OXR1 genes from different species (Hs = Homo sapiens, Mm = Mus musculus, Dm = Drosophila melanogaster, Ae = Aedes aegypti, Ag = Anopheles gambiae, At = Arabidopsis thaliana and Sc = Saccharomyces cerevisiae).
(0.03 MB DOC)
OXR1 mRNA expression in sugar-fed adult females challenged with bacteria. OXR1 mRNA in whole body at different times after mosquitoes were challenged with a mixture of heat-killed E. coli and M. luteus. All transcript measurements were performed using qRT-PCR. Data are shown as Mean + SE. * indicates significant differences (p<0.05) by ANOVA.
(0.82 MB TIF)
Effect of OXR1 silencing on JNK and ROS detoxification enzymes mRNA induction upon blood feeding. Effect of OXR1 silencing on mRNA expression levels of (A) OXR1,(B) SOD1, (C) SOD2, (D) SOD3a in 24 h uninfected blood fed females (whole body) compared to dsLacZ control injected mosquitoes. All transcript measurements were performed using qRT-PCR. Data are shown as Mean + SE.
(1.42 MB TIF)
Primers used to determine gene expression by qRT-PCR in An. gambiae.
(0.04 MB DOC)
Primers used to produce PCR Amplicons for dsRNA Synthesis in An. gambiae.
(0.03 MB DOC)