Abstract
The explosion of genetic information from recent advances in sequencing technologies, bioinformatics and genomics, highlights the importance of understanding mechanisms involved in gene expression and regulation. Over the last decade it has become clear that small RNAs are a central component of the cellular gene regulatory network. MicroRNAs (miRNAs) are a family of endogenous, small, non-coding single-stranded RNA of ~22 nucleotides in length that act as post-transcriptional gene regulatory elements. MiRNAs can inhibit de novo protein synthesis by blocking translation through base-pairing with complementary mRNA and also suppress translation by promoting degradation of target messenger RNA (mRNA). MiRNAs are intimately involved in a variety of biologic processes including development, hematopoietic cell differentiation, apoptosis, and proliferation. To date over 800 human miRNAs have been identified, though the biologic function of only a fraction of miRNAs have been elucidated. Here we discuss how miRNAs are produced, identified, and quantitated, and focus on several key miRNA that govern expression of genes relevant to allograft rejection, tolerance induction, and post-transplant infection. Finally, we discuss potential ways in which the miRNA network can be modulated that ultimately may offer new strategies to promote long-term graft survival.
1. Biogenesis and Function of miRNAs
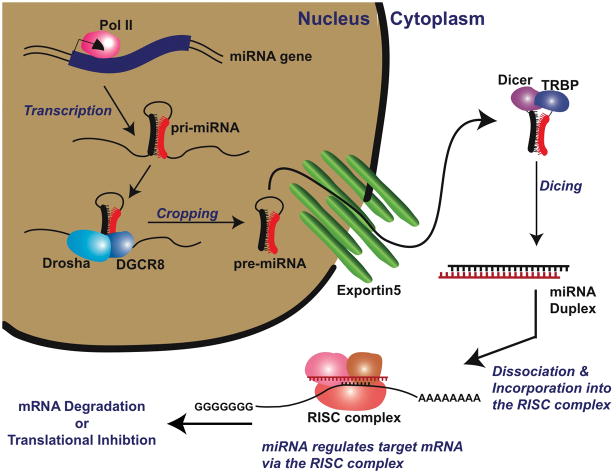
MicroRNAs (miRNAs) are encoded by specific genes, expressed in the nucleus, and undergo several processing steps before reaching their mature 19–25 nucleotide form (Fig. 1). First, long primary miRNA transcripts (pri-miRNAs), containing characteristic hairpin structures, are transcribed by RNA polymerase II and processed in the nucleus into ~70nt stem loop precursors (pre-miRNAs) (1) by the RNase III endonuclease Drosha and its partner Parsha/DGCR8 (DiGeorge syndrome critical region gene-8). Pre-miRNAs are then transported into the cytoplasm via exportin 5, where they are further processed into ~22nt small RNA duplexes by the RNase III enzyme Dicer and its partner transactivating response, TAR, RNA binding protein (TRBP). The two fragments then separate and the functional, mature miRNA strand is incorporated into the RNA-induced silencing complex (RISC), composed of multiple proteins including those of the Argonaute family, where it guides the silencing machinery to the target mRNA to either inhibit translation or promote mRNA degradation.
Figure 1. MicroRNA Biogenesis.
Pri-microRNAs are transcribed by RNA polymerase II (Pol II) and then processed in the nucleus into ~70nt stem loop precursors (pre-microRNAs) by RNase III endonuclease Drosha and its partner DGCR8/Parsha. The pre-microRNAs are then transported into the cytoplasm via the nuclear export receptor family member, Exportin5, for further processing into ~22nt small RNA duplexes by the RNase III enzyme Dicer and its partner TRBP. The duplex then separates and the functional miRNA strand is incorporated into the RNA-induced silencing complex (RISC) to either inhibit translation or promote mRNA degradation. Animations that demonstrate miRNA activity can be found at: http:www.nature.com/focus/rnai/animations/index.html and http://www.nature.com/ng/supplements/micrornas/rosetta_video.mpg.
Unraveling the miRNA translational silencing network remains a challenge in part because a single miRNA can inhibit multiple mRNA targets and because a single mRNA can be regulated by several distinct miRNAs that act cooperatively. Relatively little is known regarding the regulation of miRNA, although dysregulation of miRNA expression and function is associated with a variety of human diseases including cancer and autoimmunity.
2. Detection of miRNAs and Analysis of miRNA Function
The three main techniques to identify miRNAs are sequencing of total small RNA, array detection for total miRNA expression, and direct measurement of putative miRNA candidates by quantitative real-time PCR (qPCR). Sequencing approaches are the most sensitive and are required for identification of novel miRNAsConventional cDNA cloning followed by sequencing, as well as deep sequencing techniques (2) have been used to identify novel miRNA. A variety of miRNA array methods exist such as miRNA microarras and qPCR arrays although these approaches are limited to detection of previously known miRNAs (3–6) Commercially available kits can be effectively used for hybridization-based and qPCR-based miRNA expression profiling. Finally, direct qPCR measurement of individual miRNAs is a rapid means to determine expression of known miRNAs of interest. Approaches incorporating stem loop reverse transcriptase primers have enhanced specificity and sensitivity of miRNA (7). The reader is referred to Berezikov et al (8) for more extensive discussion on mRNA detection and discovery.
Determination of miRNA function often involves modulation of miRNA expression and analysis of subsequent effects on target mRNA expression. Lentiviral or retroviral vectors can be used to ectopically over-express miRNAs in cells followed by analysis of expression of putative target mRNA (9). A useful strategy is to introduce luciferase reporter constructs encoding the miRNA target sequence to measure effects on target expression when miRNA levels are increased by overexpression of pri-miRNA or decreased by shRNA or siRNA. Other methods to decrease expression of miRNAs include miRNA gene knockouts and sequence specific miRNA antagonists (10).
The mRNA targets of most miRNAs are unknown. Definitive determination of specific miRNA target genes and their binding sites remains a major challenge in the field. Array platforms and target prediction software can be use to identify mRNA which are putatively regulated by miRNAs (11, 12). It is estimated that at least half of all expressed mRNA may be regulated by miRNAs (13). These mRNA targets must then be confirmed, for example, by determining if their untranslated regions (UTRs) can be regulated by the identified miRNA using a luciferase reporter construct (14). However, the ability of a miRNA to regulate an expression construct or even a putative mRNA in an artificial system does not demonstrate that the mRNA is a functional relevant in vivo target of that miRNA (15).
3. Regulation of Immune-related Genes by miRNAs
We now know that miRNAs constitute a key regulatory component of immune system development and function. Specific miRNAs have been identified that dramatically impact B cell and T cell differentiation and cellular processes required for innate and adaptive immunity including inflammation, T cell receptor (TCR) signaling, toll-like receptor (TLR) signaling, cytokine production, T regulatory (Treg) cell function and antigen presentation. Thus, miRNAs are critically involved in immune events that govern allograft rejection and tolerance induction though miRNA studies in transplant models are needed to confirm this. Here we highlight specific miRNAs that impact lymphocyte development or function relevant to transplantation.
MicroRNAs and B Cell Development
Strategies to decipher the role of miRNAs in the immune system have primarily focused on ablating expression of Dicer or Drosha (Figure 1) or using loss or gain of function genetic approaches for specific miRNAs in lymphoid cells. Ablation of Dicer in early B cell progenitors resulted in a block at the pre- to pro-B cell transition and enhanced apoptosis (16, 17) and was linked to an increased expression of Bim, a target of the miR-17~92 cluster in pro-B cells. Bim, a pro-apoptotic gene, is important in regulation of lymphocyte survival. In contrast, mice that overexpress miR-17~92 in lymphoid cells have increased proliferation, diminished activation-induced cell death, and reduced expression of both Bim and the tumor suppressor, phosphatase and tensin homolog (PTEN) (18). These mice developed a lymphoproliferative disease and autoimmunity resulting in early death.
In contrast to the miR-17~92 cluster, miR-150 is expressed in mature B cells but not in their progenitors. A predicted target of miR-150 is c-Myb, a transcription factor that participates in lymphocyte development and generation of B1 cells. c-Myb is expressed at high levels in lymphocyte progenitors and is downregulated in mature B cells, thus displaying the opposite expression pattern of miR-150 (19). Further, miR-150 can control c-Myb expression in vivo to alter B cell development. Other studies found miR-150 to be differentially expressed in pro and pre-B cells (low levels) compared to mature B cells (high levels) (20). Ectopic expression of miR-150 in hematopoietic stem cells and progenitors transplanted into lethally irradiated mice resulted in significantly reduced repopulation of mature B cells in the periphery. A similar lethally irradiated bone marrow transplant model demonstrated that miR-181 expression promoted development and expansion of mature CD19+ B cells with a concomitant decrease in T cell development (9).
MiRNAs in T Cell Development and Function
Conditional deletion of Dicer in the T cell lineage in mice resulted in a significant reduction in mature CD8+ T cells and more modest reduction in CD4+ T cells (21). Moreover, CD4+ T cells were functionally impaired as they displayed diminished proliferation, increased apoptosis and were predisposed to adopt the Th1 lineage owing to a deficiency in repression of IFN-γ production.
Using miRNA profiling it was demonstrated that miRNAs are differentially expressed in distinct, functional T cell populations (22). While ninety-four known miRNAs were cloned from purified, antigen specific naïve, effector, and memory CD8+ T cell subsets, a dominant group of seven miRNAs was present in all subsets. Interestingly, the expression of these dominant miRNAs was dynamic and highly regulated depending on the state of activation and differentiation of the cells suggesting that miRNAs participate in regulation of genes that control CD8+ T cell function.
As discussed above, mice that express high levels of the miR-17~92 cluster in their lymphocytes developed lymphoproliferative disease and autoimmunity (18). Both CD4+ and CD8+ T cells were expanded in number in these mice, though the CD4+ T cell population was particularly elevated, had a recently activated profile, and produced elevated levels of IFN-γ and IL-10. Moreover, the CD4+ T cells were not dependent upon CD28 costimulation for proliferation or survival. miR-17~92 overexpression in these mice resulted in decreased expression of Bim and PTEN, known to be important in maintenance of central and peripheral tolerance.
MiRNAs have also been implicated in modulation of TCR signaling. Increased expression of miR-181 in T cells resulted in enhanced strength and sensitivity of the TCR response to peptide antigens, while decreased expression in immature T cells diminished sensitivity and interfered with both positive and negative selection (23). An important observation in this study is that miR-181 does not act as a switch in controlling TCR responses but rather provides more subtle regulation, or dampening, of the response. The ability of miR-181 to tune the threshold of TCR signaling was, at least in part, attributed to its ability to suppress expression of multiple cytoplasmic phosphatases, including SHP2, that negatively regulate the TCR signaling module. These findings have important implications for how TCR signals are translated into T cell responses with respect to effector function or tolerance induction.
MiR-155 plays an important role in adaptive immunity and T-dependent antibody responses (24). CD4+ T cells from mice lacking miR-155 showed a Th2 profile under non-polarizing conditions while their B cells were deficient in TNF and LT-α production. Further, these mice had reduced germinal center (GC) formation. Conversely, mice overexpressing miR-155 had increased fractions of GC B cells and enhanced antibody responses. Similarly, mice deficient in miR-155 could not generate protective immunity to Salmonella typhimurium (25). Defects in B cell antibody production and the ability of dendritic cells to activate T cells, along with an increased commitment to Th2 cytokine profiles, were measured in miR-155−/− mice. Thus, miR-155 appears to act broadly in a variety of lymphoid cell types to affect adaptive immune responses. Differences in miR-155 levels could potentially influence alloimmunity through effects on Th (Fig. 2).
Figure 2. Effect of mir-155 on T Helper Cell Differentiation and Impact on Alloimmune Responses.
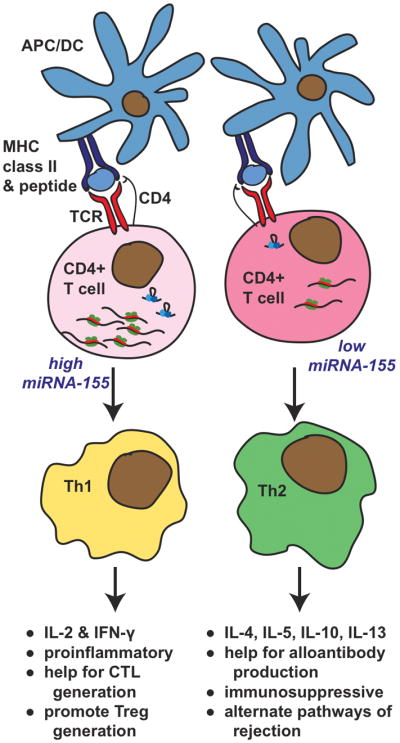
CD4+ T cells recognize donor allogeneic class II molecules on donor APC or allopeptides presented by self APC. T cell receptor stimulation is known to induce upregulation of miR-155. CD4+ T cells with high miR-155 levels are prone to develop into Th1 cells, while CD4+ T cells with a lack of, or low, miR-155 levels develop into Th2 cells. The relative balance of Th1/Th2 cells can a have significant, but complex, impact on graft outcome. Th1 cells produce IFN-γ and IL-2 that can have pro-inflammatory effects and promote CTL generation, while IL-2 can also promote Treg generation. In contrast, Th2 cells can produce IL-4, IL-5, IL-10, and IL-13. IL-4 and IL-10 in some circumstances can be immunosuppressive, however, Th2 cytokines can also faciliate Ig class switching and alloantibody production. Finally, multiple alternate mechanisms of graft rejection can be induced by Th2 cytokines.
Role of microRNAs in T Regulatory Cell Generation and Function
Natural Treg cells have miRNA profiles distinct from conventional CD4+CD25− cells and the Foxp3 transcription factor contributes to the miRNA profile characteristic of Tregs (26). Several studies in which Dicer and Drosha have been conditionally ablated implicate miRNAs in Treg development and function. Deletion of Dicer at the double positive (DP) stage impaired thymic differentiation of Tregs leading to markedly reduced numbers of natural Tregs and development of inflammatory bowel disease (26). Deletion of Dicer at the time of Foxp3 expression showed no effects on Treg development, proliferation or survival (27). However, these mice developed systemic, fatal autoimmune disease that phenotypically copied mice lacking Foxp3. Detailed analysis of peripheral Tregs indicated that the lineage was not stable as a significant proportion of the Dicer−/− cells had lost Foxp3 expression and adapted a T effector-like phenotype marked by high levels of IFN-γ production, as well as IL-4 and IL-10 production, and expression of memory cell markers. A third study in which Dicer was deleted in Tregs also resulted in fatal autoimmunity (28). Finally, Chong et al (29) showed that deletion of Dicer or Drosha in Treg cells resulted in an early, fatal lymphoproliferative disease. Mice with Drosha deficient T cells had markedly reduced numbers of mature CD8+ T cells in the periphery and developed spontaneous inflammatory disease characterized by high frequencies of IFN-γ and IL-17A secreting CD4+ T cells. The levels of CD4+Foxp3+ cells were reduced in Drosha-deficient mice and their ability to suppress in vitro T cell proliferation was blunted. The induction of Foxp3 in naïve CD4 T cells by TGF-β and retinoic acid was also impaired. In contrast, Drosha deficiency did not affect the differentiation of Th1 and Th2 cells. Together, these studies provide strong evidence that miRNAs are indispensable for preservation of self-tolerance as mediated by Treg cells. However, limited information is available on the particular miRNAs that are involved in Treg development and function. MiR-155 is of particular interest since Foxp3 binds to the promoter of its gene, bic. A recent report (30) shows that miR-155−/− mice have reduced numbers of CD4+CD25+FoxP3+ Tregs in the thymus and spleen. This defect appears attributable to a requirement for miR-155 in Treg thymic development rather than a requirement for maintenance or proliferation in the periphery. Understanding the contribution of miRNAs to Treg generation, stability, and function will offer new opportunities to harness Treg activity in transplantation.
MiRNAs, Inflammation and Innate Immunity
Microbial products are important pro-inflammatory stimuli and several TLR ligands have been shown to modulate miRNA. MiR-155 was upregulated in macrophages by the synthetic triacylated lipopeptide Pam3CSK4, the synthetic double stranded RNA analog poly(I:C), lipopolysaccharide (LPS) and CpG oligonucleotides, indicating that several TLR ligands can induce miR-155 expression (31). The induction of miR-155 by LPS has also been demonstrated in vivo and is accompanied by a decrease in miR-125b expression (32). It has been proposed that these miRNAs coordinately regulate translation of TNF–α mRNA in response to LPS stimulation. Finally, miR-146 is also induced by LPS (TLR4), as well as by Pam3CSK4 (TLR2) and flagellin (TLR5) (33).
4. MiRNAs and Viruses
It is well-established that viruses can co-opt host gene expression machinery to promote their own propagation and survival. Similarly viruses employ miRNAs that are capable of modulating both viral and host gene expression. To date, more than 50 viral miRNAs have been identified in the pathogenic herpesviruses, including human cytomegalovirus (hCMV), herpes simplex virus types I and II (HSV-1/2), Karposi’s Sarcoma associated herpesvirus (KSHV) and Epstein-Barr virus (EBV) (34). Emerging studies suggest that virus-derived miRNAs function to regulate viral and host gene expression specifically to enhance survival of the virus. However, in contrast to cellular miRNAs whose sequence and mRNA targets are conserved, viral miRNA sequences are poorly conserved, hampering identification of miRNA targets by computational means (35). An algorithm that calculates the binding energy of miRNAs predicted target sites for human cytomegalovirus (hCMV) miR-UL112 and identified the mRNA encoding the major immediate early (MIE) trans-activating protein IE72, a viral protein, as a target of miR-UL112. Since miR-UL112 accumulates during viral infection, it may function to inhibit IE72 expression thereby promoting the transition to a latent CMV infection (35). Quite surprisingly, hCMV mIR-UL112 also targets, and downregulates, the MHC class I-related chain B (MICB) protein (36). MICB is a stress-induced ligand of the NK cell activating receptor NKG2D (35) and a decrease in expression of NKG2D ligands, like MICB, protects virally-infected target cells from NK cell mediated killing. Thus, the hCMV miR-112 targets both viral and cellular targets. MICB translation is also targeted by EBV miR-BART2-5p (37). Decreased expression of MICB protects the virally-infected host cell from NK cell-mediated killing. These studies suggest a functional conservation among herpesviruses (KSHV miR-K12-7 also targets MICB) in their ability to evade the immune system. EBV also expresses viral miRNAs. EBV miR-BART2 can induce degradation of the viral transcript for the EBV DNA polymerase BALF5 which is transcribed antisense to miR-BART2 (35). Latent membrane protein 1 (LMP1), which contributes to EBV tumorigenesis, can be decreased by the expression of EBV miRNAs, miR-BARt-1-5p, miR-BART-16 and miR-BART-17-p5 (35). It is apparent that EBV viral miRNAs can target both cellular and viral transcripts. The functional relationships between viral miRNAs, viral and cellular gene expression, viral infection and transformation and the host immune response to virally-infected cells remain to be elucidated.
Our group has shown that EBV infection of B cells can induce expression of miR-221 and miR-222, and that this effect can be attributed to signaling through the viral protein LMP1. Moreover, these miRNAs are increased in EBV+ B cell lymphoma lines from patients with post-transplant lymphoproliferative disease (38). Future studies will determine whether expression of these cellular miRNAs affect host cell survival or growth. Nevertheless, virally expressed and virally induced miRNAs may constitute new therapeutic targets in post-transplant infection.
5. Monitoring of miRNA Expression in Transplant Recipients
While we are only beginning to understand how specific miRNAs participate in immune responses, there is good evidence that miRNAs are integrally involved in maintaining immune homeostasis and self-tolerance. Further, miRNA expression patterns and levels are highly regulated in concert with lymphocyte differentiation and activation. As specific gene expression programs may be hallmarks of graft rejection or tolerance, it is reasonable to propose that changes in miRNA expression may underlie these patterns. Suthanthiran’s group profiled the expression pattern of 365 miRNAs in 7 renal allograft biopsies and analyzed selected miRNAs in 23 additional renal allograft biopsies (12 acute rejection (AR) and 21 normal) (39). Clustering analysis showed that the AR samples grouped separately from the normal samples. Seventeen miRNAs were differentially expressed in the AR biopsies and normal controls, with 10 underexpressed in AR biopsies and 7 miRNAs overexpressed in AR biopsies compared to control biopsies. Three microRNAs, miR-142-5p, miR-155, and miR-223, were highly predictive of AR and their levels were strongly linked to intragraft levels of CD3 mRNA. As we have reviewed above, miR-155 has been linked to antibody production, cytokine production, TLR signaling and Treg generation, all of which are relevant to allograft rejection.
6. Therapeutic Strategies to Target miRNAs
It is clear that a major focus in miRNA biology in the coming years will be to elucidate target genes and biologic significance of specific miRNAs. Progress in this area would undoubtedly advance our understanding of the immune response and provide potential targets for novel approaches in transplantation. Indeed, strategies have already been developed to target and silence miRNAs in vivo using ‘“antagomirs”’, cholesterol-conjugated single stranded RNA oligonucleotides that are complementary for miRNAs (40). Another approach includes vector-based delivery of miRNA target sites to function as decoys for endogenous target mRNAs (41). Alternately, replacement of miRNAs through adeno-associated virus delivery has been shown to provide therapeutic benefit in a murine hepatocellular carcinoma model (42). Future advances in understanding miRNA expression and function in regulating immune responses will undoubtedly provide new opportunities for modulating alloreactivity and improving long-term graft survival in transplant recipients.
Table 1.
Effect of MicroRNAs on Immune Function
| MicroRNA | B Cell | CD4+ T Cell Subsets |
|||||
|---|---|---|---|---|---|---|---|
| Th1 | Th2 | Treg | CD8+ T Cell | Innate Immunity | Ref | ||
| 17–92 Cluster | Promotes survival | Promotes CD4+ T Cell survival | Promotes CD8+ T cell survival | 16–19 | |||
| 150 | Suppresses B cell development | 19,20 | |||||
| 181 family | Promotes B cell maturation | -------------Modulates Sensitivity to TCR Signaling-------- | 9,23 | ||||
| 155 family | Supports GC formation and B cell differentiation | Regulates polarization | Required for Treg thymic development | Induced by TLR signaling, regulates TNFα | 24,25, 30,32 | ||
| 125b | Regulates TNFα | 32 | |||||
| 146 | Induced by bacterial ligands of TLR | 33 | |||||
Acknowledgments
The authors thank Chris Arnold for helpful discussion.
Funding Sources: This work was supported by NIH RO1AI41769 (OMM).
References
- 1.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 2.Friedlander MR, Chen W, Adamidi C, Maaskola J, Einspanier R, Knespel S, et al. Discovering microRNAs from deep sequencing data using miRDeep. Nat Biotechnol. 2008;26(4):407–415. doi: 10.1038/nbt1394. [DOI] [PubMed] [Google Scholar]
- 3.Castoldi M, Benes V, Hentze MW, Muckenthaler MU. miChip: a microarray platform for expression profiling of microRNAs based on locked nucleic acid (LNA) oligonucleotide capture probes. Methods. 2007;43(2):146–152. doi: 10.1016/j.ymeth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Shingara J, Keiger K, Shelton J, Laosinchai-Wolf W, Powers P, Conrad R, et al. An optimized isolation and labeling platform for accurate microRNA expression profiling. Rna. 2005;11(9):1461–1470. doi: 10.1261/rna.2610405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson PT, Baldwin DA, Scearce LM, Oberholtzer JC, Tobias JW, Mourelatos Z. Microarray-based, high-throughput gene expression profiling of microRNAs. Nat Methods. 2004;1(2):155–161. doi: 10.1038/nmeth717. [DOI] [PubMed] [Google Scholar]
- 6.Liu CG, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci U S A. 2004;101(26):9740–9744. doi: 10.1073/pnas.0403293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33(20):e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat Genet. 2006;38 (Suppl):S2–7. doi: 10.1038/ng1794. [DOI] [PubMed] [Google Scholar]
- 9.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303(5654):83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 10.Fabani MM, Gait MJ. miR-122 targeting with LNA/2′-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. Rna. 2008;14(2):336–346. doi: 10.1261/rna.844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2(11):e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115(7):787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen CP, Bordeleau ME, Pelletier J, Sharp PA. Short RNAs repress translation after initiation in mammalian cells. Mol Cell. 2006;21(4):533–542. doi: 10.1016/j.molcel.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Mao TK, Chen CZ. Dissecting microRNA-mediated gene regulation and function in T-cell development. Methods Enzymol. 2007;427:171–189. doi: 10.1016/S0076-6879(07)27010-7. [DOI] [PubMed] [Google Scholar]
- 16.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132(5):860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao C, Srinivasan L, Calado DP, Patterson HC, Zhang B, Wang J, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9(4):405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131(1):146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci U S A. 2007;104(17):7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202(2):261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, et al. miRNA profiling of naive, effector and memory CD8 T cells. PLoS One. 2007;2(10):e1020. doi: 10.1371/journal.pone.0001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129(1):147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316(5824):604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cobb BS, Hertweck A, Smith J, O’Connor E, Graf D, Cook T, et al. A role for Dicer in immune regulation. J Exp Med. 2006;203(11):2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205(9):1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205(9):1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205(9):2005–2017. doi: 10.1084/jem.20081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol. 2009;182(5):2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- 31.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104(5):1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 33.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolken L, Jonjic S. All for one and one for all: herpesviral microRNAs close in on their prey. Cell Host Microbe. 2009;5(4):315–317. doi: 10.1016/j.chom.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Gottwein E, Cullen BR. Viral and cellular microRNAs as determinants of viral pathogenesis and immunity. Cell Host Microbe. 2008;3(6):375–387. doi: 10.1016/j.chom.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern-Ginossar N, Gur C, Biton M, Horwitz E, Elboim M, Stanietsky N, et al. Human microRNAs regulate stress-induced immune responses mediated by the receptor NKG2D. Nat Immunol. 2008;9(9):1065–1073. doi: 10.1038/ni.1642. [DOI] [PubMed] [Google Scholar]
- 37.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5(4):376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Harris A, Lambert SL, Krams SM, Martinez O. Epstein-Barr Virus latent membrane protein 1 modulates host microRNAs in B Cell Lymphomas. Clin Immunology. 2009;131(Supplement):S40. [Google Scholar]
- 39.Anglicheau D, Sharma VK, Ding R, Hummel A, Snopkowski C, Dadhania D, et al. MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci U S A. 2009;106(13):5330–5335. doi: 10.1073/pnas.0813121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 41.Brown BD, Naldini L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet. 2009;10(8):578–585. doi: 10.1038/nrg2628. [DOI] [PubMed] [Google Scholar]
- 42.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]