Abstract
Background
Ribavirin, a nucleoside analogue, is administered in combination with interferon to patients with chronic hepatitis C. To evaluate the feasibility of ribavirin therapeutic drug monitoring, we investigated the influence of blood collection and pre-analytical conditions on ribavirin concentrations and compared the results obtained from inter-laboratory blind tests by three laboratories using different analytical techniques.
Material and Methods
On three occasions, blank serum samples spiked with ribavirin and pooled serum samples from patients on ribavirin were sent to the three laboratories. Two analytical techniques were based on LC-MS/MS and one on HPLC-UV, with protein precipitation or solid-phase extraction, all validated according to international guidelines.
Results
Inter-batch and intra-batch mean relative errors ranged from −7.4 to +10.3 % and from −10.3 to +7.4%, respectively. Relative standard deviations were <13.5% and <10.6%, respectively. Linearity, assessed blindly, between 125 and 4550 ng/mL was excellent (r >0.991) for all three methods. The two LC-MS/MS techniques were slightly less precise and accurate than HPLC-UV, perhaps because the internal standard used was not a ribavirin isotope.
Conclusion
Accurate and precise LC-MS/MS and HPLC-UV methods developed in three different laboratories provided excellent and consistent results to blind tests for ribavirin determination in spiked serum samples and pools of serum samples from patients with chronic HCV.
Keywords: Antiviral Agents; therapeutic use; Chromatography, High Pressure Liquid; Drug Monitoring; Drug Stability; Feasibility Studies; Hepacivirus; drug effects; growth & development; Hepatitis C; drug therapy; metabolism; Hepatitis C, Chronic; drug therapy; metabolism; Humans; Ribavirin; pharmacokinetics; therapeutic use; Spectrometry, Mass, Electrospray Ionization
Keywords: Hepatitis C, ribavirin, HPLC, LC-MS/MS, proficiency testing, therapeutic drug monioring.
1. Introduction
The combination of peginterferon and ribavirin [1,2] is the standard approved treatment of patients with chronic hepatitis C virus (HCV). Half of the patients with this combined therapy relapse or remain virological non responders. The main predictive factors of a poor sustained virological response (SVR) are an HCV genotype other than 2 or 3, a high baseline viral load and a dose of ribavirin <10.6 mg/kg of body weight (BW) [1]. Indeed, two clinical trials [1,2] suggested that ribavirin dose adjustment for BW may be beneficial, while two other studies in patients with chronic HCV receiving combination therapy [3,4] found no correlation between ribavirin dose adjusted for BW and a single ribavirin serum concentration time point at week 4 (W4) or at steady state (W12), i.e. there was a poor dose-concentration relationship. A better concentration-effect than dose-effect relationship was reported by several groups [4–6]. In a recent cohort study [5], patients’ global exposure to ribavirin was widely distributed, despite dose-adjustment for BW.
Ribavirin has a large distribution volume, a long half-life and large inter-individual pharmacokinetic variability [7]. Following a single oral dose, the plasma concentration of ribavirin exhibits a three-phase profile: rapid absorption, with a mean time to the maximum concentration (tmax) of about 1.5 h; rapid distribution (half life of approximately 3.7 h); and a long terminal elimination, the last measurable concentration time point being at approximately 100 h post-dose [7,8]. Recently, it has been suggested that the gastro-intestinal tract is the major site of ribavirin first-pass elimination [8]. High fat meals can increase ribavirin bioavailability by 46% relative to the fasting state [9], suggesting low oral bioavailability. Metabolism accounts for most of ribavirin elimination, but the exact mechanisms and sites involved are unknown. Glue et al [10] showed that hepatic dysfunction had no substantial influence on the apparent clearance of ribavirin, which would mainly be affected by BW, gender, age and serum creatinine, although these four covariates only explained 27 to 40% of its inter-individual variability [3, 11].
The first attempts at ribavirin pharmacokinetic (PK)-pharmacodynamic (PD) studies were based on single point sampling strategies. Jen et al reported that low ribavirin serum concentrations (with no precision on timing) at “steady state” (W4) were associated with a low SVR rate, but were less influential than the genotype or viral load [6]. Larrat et al also found an association between SVR and a single serum concentration taken 2–4 h after the morning dose of ribavirin at W12 (n=24; p=0.03) and W24 [4]. Taking into account ribavirin concentration-time profiles, a recent study in 24 patients with genotype 1 HCV showed that after the first ribavirin dose (D0), patients with SVR had a significantly higher AUC0–12h (p=0.03) and AUC0–4h (p=0.03) than non-responders, with an AUC0–4h threshold of 1755 μg.h/L. Also, ribavirin may cause anemia in a dose-dependent manner. Therefore, monitoring ribavirin plasma concentrations could be useful for tailoring individual dosing of ribavirin. Several analytical methods have been developed for the determination of ribavirin in biological fluids (for review see [12]) including radioimmunoassays, capillary electrophoresis, or liquid chromatography coupled with UV [13, 14] or tandem mass-spectrometric detection [15, 16]. The reported sample preparation procedures were either based on protein precipitation with various solvents [12] or on solid-phase extraction [4–5, 13, 14]. However, these techniques were not compared head-to-head and, in the absence of an inter-laboratory blind test, their applicability to routine therapeutic drug monitoring (TDM) of ribavirin is unknown.
The aim of the present study was to evaluate the feasibility of ribavirin TDM by comparing the results obtained at repeated inter-laboratory blind tests by three laboratories employing different HPLC methods, never compared or harmonized before (two with MS/MS and one with UV detection).
2. Materials and methods
a. Description of the analytical methods
The organic solvents, reagents, all of analytical grade, and the pure compounds were supplied by different retailers (Sigma Aldrich, Saint-Quentin Fallavier, France; Merck, Darmstadt, Germany; Prolabo, Fontenay-sous-Bois, France; Carlo Erba, Val-de-Reuil, France). The analytical methods applied by the three participating laboratories are described in Table 1. Briefly, serum or plasma samples were extracted using either solid-phase extraction with 100 mg, 1 mL Bond-Elut Phenyl Boronic Acid cartridges (Varian, Harbor City, CA, USA), or a simple protein precipitation procedure. The LC-MS/MS methods were carried out with a 2000 QTRAP™ and a 3200 QTRAP™ LC-MS/MS system, respectively (Applied-Biosystem/Sciex, Foster City, CA, USA), equipped with a Turbolon-Spray™ and a Turbo V™ ionization source, respectively. HPLC-UV employed a UV 6000LP detector (Thermo Fisher Scientific, Les Ulis, France) operated at 214 and 240 nm.
Table 1.
Description of the three analytical methods developed for the analysis of ribavirin
| Method 1 | Method 2 | Method 3 | |
|---|---|---|---|
| Volume sample | 200 μL | 5 μL | 500 μL |
| Extraction procedure |
|
|
|
| Column | Atlantis HILIC Silica, 5 μm (150 × 2.1 mm) (Waters) | Luna HILIC, 3 μm (100 × 2.0 mm) (Phenomenex) | Uptisphere HDO-QS, 3 μm (150 × 4.6mm) (Interchim) |
| Mobile phase | Aqueous ammonium formate and acetonitrile | Aqueous ammonium acetate and acetonitrile | Aqueous orthophosphoric acid and acetonitrile |
| Detection mode | MS/MS, ESI, positive ionization mode. Ribavirin (ammonium adduct): m/z 262.1/113.2 (quantification); m/z 262.1/96.2, m/z 245.1/113.2 (confirmation); IS: m/z 282.2/150.1. | MS/MS, ESI, positive ionization mode. Ribavirin: m/z 245.2/113.1 (quantification); m/z 245.2/96.2 (confirmation); IS, m/z 258.1/126.1. | UV detection at 214 and 240 nm. |
| Analytical range | 10–5000 ng/mL | 100–7000 ng/mL | 100–10000 ng/mL |
| Validation results |
Intra-assay accuracy (n=5): CV<7.8%, MRE<6.5% Inter-assay accuracy (n=5): CV < 13.3 %, MRE < 5.7 % Recovery (n=3): > 92.8 % |
Intra-assay accuracy (n=6): CV<3.9%, MRE < 3.1 % Inter-assay accuracy (n= 6): CV < 10.7 %, MRE < 6.7 % Recovery (n=3): > 92.9% |
Intra-assay accuracy (n=6): CV <6.7 %, MRE <7.5 % Inter-assay accuracy (n=23): CV < 6.0 %, MRE < 1.8% Recovery (n= 5):> 55.3 % |
The three methods were validated in-house in terms of linearity, inter- and intra-assay precision and accuracy, and extraction recovery. Linearity was evaluated using least-square quadratic regression of ribavirin-to-internal standard (IS) peak-area ratios versus theoretical concentrations for the laboratories using MS/MS detection, and linear regression for HPLC-UV. Accuracy was expressed as the bias relative to the theoretical concentrations, and precision as the percent root mean square error (RMSE%) of back-calculated concentrations. Intra-assay precision and accuracy were assessed at different concentration levels by extraction and analysis on the same day of replicates of fortified serum samples.
Extraction recoveries were determined at several concentration levels, by spiking a series of ribavirin-free serum samples with the appropriate amounts of ribavirin and the internal standard (IS), and another series with the IS alone. After extraction and evaporation, the dry extracts were reconstituted with the chromatographic mobile phase for samples of the first series, and with the mobile phase spiked with the nominal amount of ribavirin for samples of the second series.
For the LC-MS/MS methods, ion suppression was investigated by injecting 10 different blank human serum samples into the LC system while a solution of ribavirin was continuously infused in parallel in the ionization source through a PEEK tee.
b. Inter-laboratory blind tests
The inter-laboratory survey carried out at the university hospitals of Grenoble, Limoges and Lyon, France (referenced here as Laboratories 1, 2 and 3 in a different order), involved blank serum samples spiked with ribavirin at known concentrations (blinded to the three laboratories) and pools of patients’ serum. Blood from patients treated with ribavirin was repeatedly collected into tubes without an anticoagulant at day 1, week 12, week 12+1 day and week 24 of ribavirin treatment, as part of a pharmacokinetic study [5]. All patients gave their written informed consent for this study, which was approved by the regional ethics committee and authorized by the French Drug Agency (AFSSAPS).
After 15 minutes of centrifugation of blood samples at 1733 g and 4°C, serum was aliquoted and stored at −80°C in brown 1.5 mL polypropylene tubes until analysis. For this study, pools of serum were prepared by mixing 4 aliquots from 4 different patients sampled at the same time post-dose and treatment period. After vortex-mixing, these pools were aliquoted into brown 1.5 mL polypropylene tubes.
Outdated drug-free serum from healthy-volunteers provided by the Etablissement Français du Sang was aliquoted and spiked with working solutions of ribavirin in water at different concentrations between 125 and 4550 ng/mL.
All the samples were kept at −80°C until their shipment to the laboratories in dry ice.
Shipment 1 included 22 randomly distributed blank serum samples spiked at 10 different concentrations of ribavirin (125, 375, 775, 1400, 1750, 2150, 2650, 3150, 3750 and 4550 ng/mL), including 5 replicates of the samples at 775, 1750 and 3150 ng/mL for estimating the intra-assay precision and accuracy.
Shipment 2 was made of aliquots of the 3 spiked blank serum samples at 375, 2150 and 3750 ng/mL already distributed in shipment 1, and 20 samples from patients’ serum pools.
Shipment 3 contained aliquots of the same 3 spiked serum samples at 375, 2150 and 3750 ng/mL (for inter-assay accuracy and precision estimation), and 20 aliquots of patients’ serum pools.
3. Results
The main method validation results obtained by each laboratory in-house are reported in Table 1. Intra- and inter-assay accuracy and precision were all <15.0% over the calibration ranges used. The lower band of these ranges (which was not the actual LOQ for at least one of the LC-MS/MS methods) was between 10 ng/mL and 100 ng/mL, depending on the intended use, whether TDM at steady-state, or pharmacokinetic studies after a single, or the first dose. Examples of chromatograms obtained in each laboratory are presented in Figure 1. The results of the ion suppression study performed by laboratories 1 and 2, using MS/MS detection, are shown in Figure 2. For method 1, a slight decrease of the signal close to 14 min led to an increase in the chromatographic run time up to 18 min (though the retention time of ribavirin and IS were around 4 min) to eliminate all contaminations. For method 2, the signal remained stable during ribavirin and internal standard elution (between 1.5 and 2.1 min), then slightly decreased around 4 min but returned to initial conditions before the 6th minute.
Figure 1.
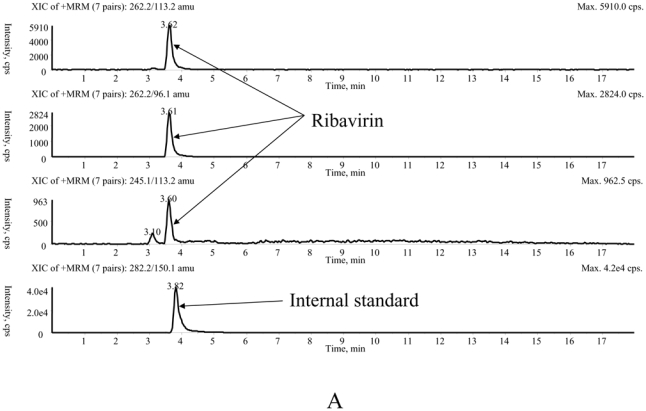
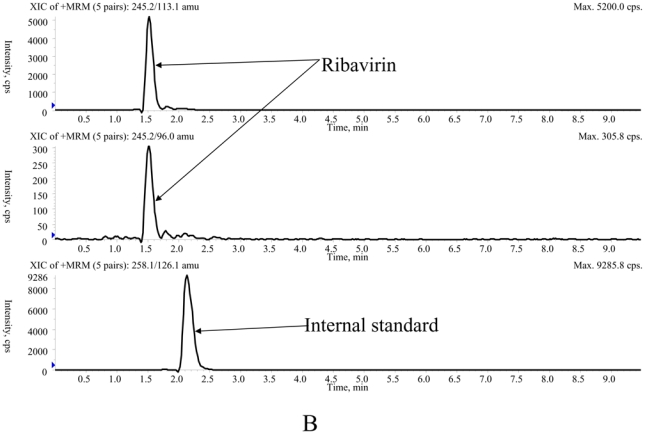
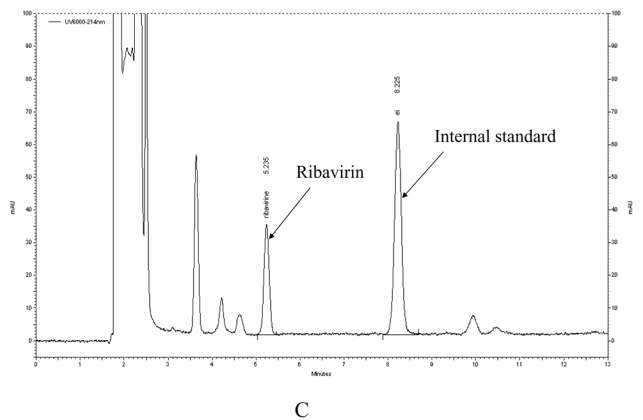
Typical chromatograms of extracted patients' samples analysed with method 1 (A), method 2 (B) and method 3 (C).
Figure 2.
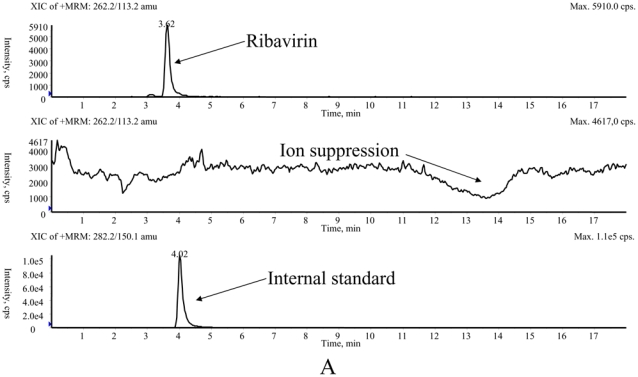
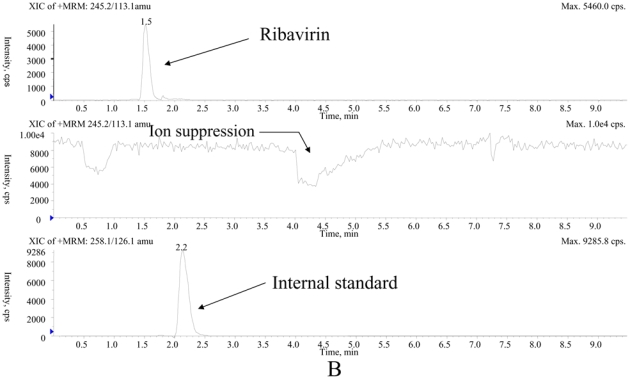
Investigation of the ion suppression phenomenon by means of a continuous infusion of ribavirin at 1 μg/mL and parallel injection of extracts of blank samples applying method 1 (A) and method 2 (B).
As part of the inter-laboratory survey, the intra-assay precision and accuracy were assessed blindly at three concentrations (775, 1750 and 3150 ng/mL), showing mean relative errors between −10.3 and +7.4% and RSD% always <10.6% (Figure 3). Similarly, inter-assay precision and accuracy were evaluated using three other blank serum samples spiked at 375, 2150 and 3750 ng/mL, repeatedly sent with shipments 1, 2 and 3. The mean relative error ranged between −7.4 and +10.3% and the RSD% was <13.5% (Figure 4). The linearity of the three analytical methods was assessed from the results reported for the blank serum samples spiked at 10 different concentrations between 125 and 4550 ng/mL (Figure 5). The calculated coefficients of correlation were all >0.991, with the best correlation being obtained by the laboratory using HPLC-UV (lab 2). The mean relative error (MRE) of back-calculated concentrations varied between −10.0 and +16.0% with the highest MRE for the lowest spiked concentrations, and the inter-laboratory RSD ranged from 0.3 to 14.6%.
Figure 3.
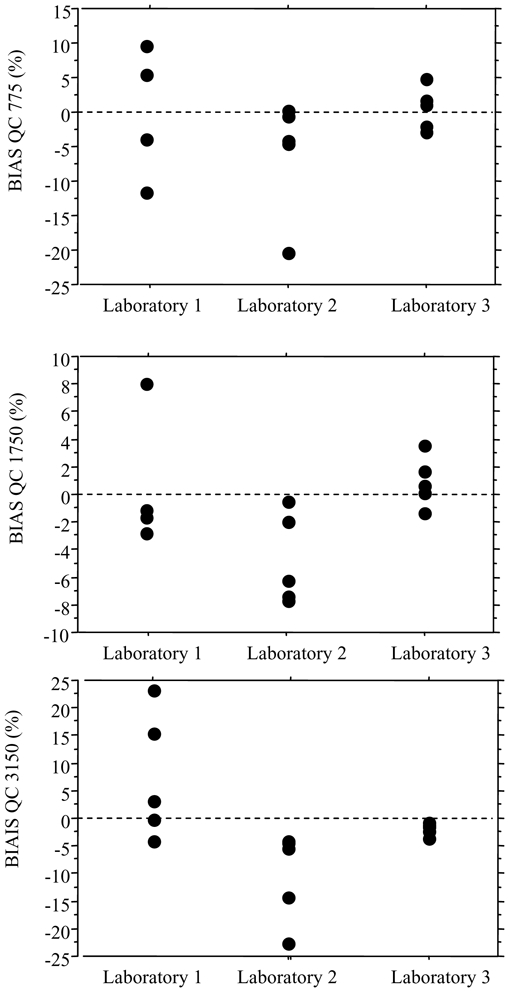
Intra-series precision and accuracy: relative bias obtained by the three laboratories at 775 ng/mL (A), 1750 ng/mL (B) and 3150 ng/mL (C).
Figure 4.
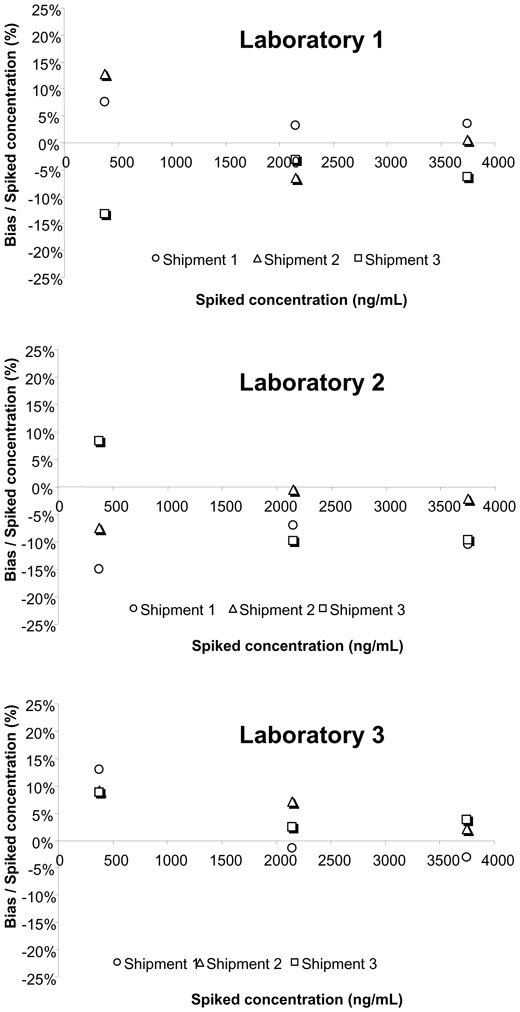
Inter-series precision and accuracy: relative bias obtained by the three laboratories at 375, 2150 and 3750 ng/mL.
Figure 5.
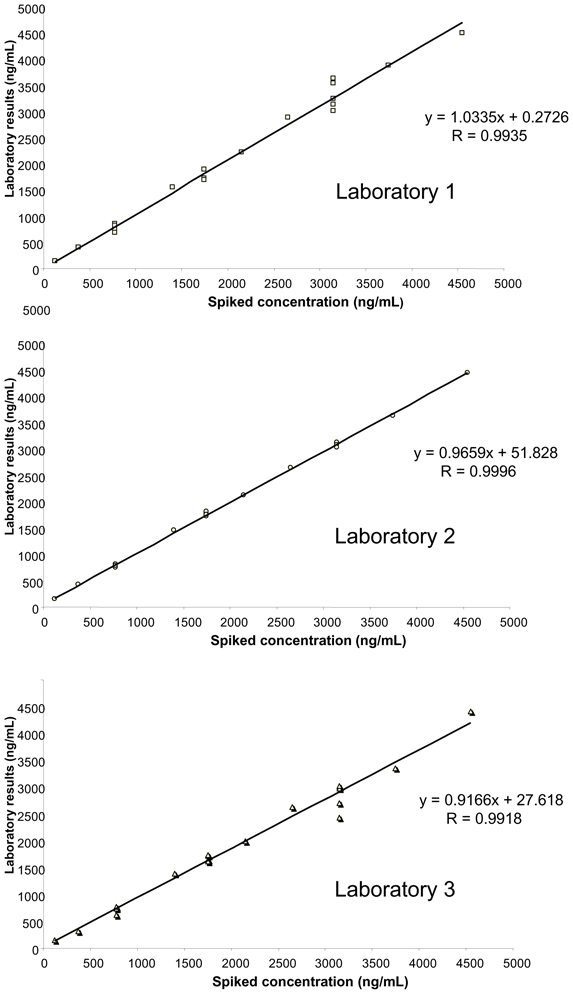
Blind linearity evaluation of the three methods.
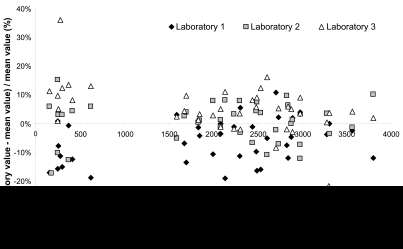
The applicability of the three methods was also investigated through the analysis of pools of samples collected from patients with hepatitis C at day 0, week 12 and week 24 after the beginning of the treatment with ribavirin. Each pool corresponded to a given sampling time, between 0.5 hour and 10 hours post-dose, resulting in a large range of concentrations. Among the 40 pools analyzed, in only three instances was inter-laboratory RSD >30%, due to outlier results reported by any one of the laboratories with regards to the two others. For all the other patients’ pools, the reported concentrations varied from 157 to 3572 ng/mL, the inter-laboratory RSD% was 8.7% on average and always < 17.0% (Figure 6).
Figure 6.
Analysis of pools of patients' samples: relative differences of ribavirin concentrations found by each laboratory with respect to the mean measured values.
4. Discussion
This study shows that HPLC-UV or LC-MS/MS methods developed in three different laboratories and employing different types of sample extraction procedures gave excellent and equivalent results for ribavirin determination in blinded tests. The data were from both spiked and patients’ serum samples, suggesting that routine ribavirin monitoring is technically feasible, despite the absence of commercial assays.
The three analytical techniques presented were set up for different clinical trials, with the aim of measuring either ribavirin morning trough concentrations at steady-state (labs 2 and 3) or ribavirin pharmacokinetic profiles after the first and repeated doses (lab 1), which explains the differences in terms of LOQ and linearity range, in particular between the two LC-MS/MS techniques. All three fulfilled the international requirements for analytical method validation. The previously published sample preparation procedures were either based on protein precipitation with various solvents [12] or solid-phase extraction with cartridges containing phenyl boronic acids with a high affinity for compounds containing diol moieties, due to reversible boronate formation [4–5, 11–14]. Recently, Morello et al. [14] have used another type of extraction cartridge (Microsep 3K Omega) and liquid chromatography with UV detection, yielding similar results. As recently reviewed [12], other LC-MS/MS methods for ribavirin determination have been published [15–17], some of which yielded shorter turnaround times. However, the absence of the ion suppression phenomenon, frequently observed when protein precipitation procedures are employed, was not documented in these papers. Different analytical approaches have been employed here to minimize this phenomenon, i.e., in method 2, dilution of the sample before precipitation and in method 1 solid-phase extraction, which is theoretically cleaner and tends to prevent or minimize ion suppression. However, according to the results presented in figure 2, this phenomenon could not be totally avoided, though no ion suppression was visible at the retention times of ribavirin and the internal standards. The use of an isotope-labelled analog of ribavirin as internal standard could help to compensate for any residual ion suppression and, above all, improve precision. UV detection was the most frequently employed technology for ribavirin, owing to the accessible analytical ranges involved [4, 11, 13, 14, 18–21], but the sample volumes required were generally higher and the extraction procedure longer than those for most of the LC-MS/MS methods.
The results obtained from the inter-laboratory blind tests in the present study were excellent for all three methods, and even better in terms of precision and linearity for the HPLC-UV technique, although the in-house method validation results were almost equivalent (Table 1). LC-MS/MS is used increasingly for TDM [22] in clinical laboratories. However, its variability can be somewhat higher than that of “classical” HPLC in the absence of isotope-labelled internal standards, and LC-MS/MS techniques need to be thoroughly validated, including the investigation of ion suppression or potential interferences, before they can be applied in a clinical setting [23]. Otherwise, quantification or even identification errors can occur, and the advantages of mass-spectrometry can be lost.
The recently reported relationship between early exposure to ribavirin (plasma AUC0–4h) and clinical outcome in hepatitis C patients, [5] together with the results of the present study, suggest that HPLC-UV or LC-MS/MS techniques can be used for the routine TDM of ribavirin as well as to conduct prospective multicentre clinical trials to validate the clinical benefit of such monitoring. The setting up of a larger and regular inter-laboratory proficiency testing scheme, involving more laboratories, using different detection modes, would be of the utmost interest in both these aims.
Acknowledgments
This study was funded by Roche Pharma, France. We gratefully thank Ingrid Barthelemy, Jean-Louis Dupuy, Karine Delaune, Franck Giraudie, Karine Scalabrino, Cécile Girard and Chantal Nahum for their excellent technical assistance.
Abbreviations
- UV
ultraviolet
- LC
liquid chromatography
- HPLC
high-performance liquid chromatography
- GTP
guanosine triphosphate
- TDM
Therapeutic Drug Monitoring
References
- 1.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW, Shiftman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 3.Jen JF, Glue P, Gupta S, et al. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit. 2000;22:555–565. doi: 10.1097/00007691-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Larrat S, Stanke-Labesque F, Plages A, et al. Ribavirin quantification in combination treatment of chronic hepatitis C. Antimicrob Agents Chemother. 2003;47:124–129. doi: 10.1128/AAC.47.1.124-129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loustaud-Ratti V, Alain S, Rousseau A, et al. Ribavirin exposure after the first dose is predictive of sustained virological response in chronic hepatitis C. Hepatology. 2008;47:1453–1461. doi: 10.1002/hep.22217. [DOI] [PubMed] [Google Scholar]
- 6.Jen J, Laughlin M, Chung C, et al. Ribavirin dosing in chronic hepatitis C: application of population pharmacokinetic-pharmacodynamic models. Clin Pharmacol Ther. 2002;72:349–361. doi: 10.1067/mcp.2002.127112. [DOI] [PubMed] [Google Scholar]
- 7.Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis. 1999;19:17–24. [PubMed] [Google Scholar]
- 8.Dixit NM, Perelson AS. The metabolism, pharmacokinetics and mechanisms of antiviral activity of ribavirin against hepatitis C virus. Cell Mol Life Sci. 2006;63:832–842. doi: 10.1007/s00018-005-5455-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khakoo S, Glue P, Grellier L, et al. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol. 1998;46:563–570. doi: 10.1046/j.1365-2125.1998.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glue P, Schenker S, Gupta S, et al. The single dose pharmacokinetics of ribavirin in subjects with chronic liver disease. Br J Clin Pharmacol. 2000;49:417–421. doi: 10.1046/j.1365-2125.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruchfeld A, Lindahl K, Schvarcz R, et al. Dosage of ribavirin in patients with hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit. 2002;24:701–708. doi: 10.1097/00007691-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bosch ME, Sánchez AJ, Rojas FS, et al. Ribavirin: analytical determinations since the origin until today. J Pharm Biomed Anal. 2007;45:185–193. doi: 10.1016/j.jpba.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Loregian A, Scarpa MC, Pagni S, et al. Measurement of ribavirin and evaluation of its stability in human plasma by high-performance liquid chromatography with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:358–364. doi: 10.1016/j.jchromb.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Morello J, Rodriguez-Nóvoa S, Cantillano AL, et al. Measurement of ribavirin plasma concentrations by high-performance liquid chromatography using a novel solid-phase extraction method in patients treated for chronic hepatitis C. Ther Drug Monit. 2007;29:802–806. doi: 10.1097/FTD.0b013e31815bddf3. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Yeh LT, Lau JY. Specific, sensitive and accurate liquid chromatographic-tandem mass spectrometric method for the measurement of ribavirin in rat and monkey plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;779:241–248. doi: 10.1016/s1570-0232(02)00379-3. [DOI] [PubMed] [Google Scholar]
- 16.Shou WZ, Bu HZ, Addison T, et al. Development and validation of a liquid chromatography/tandem mass spectrometry (LC/MS/MS) method for the determination of ribavirin in human plasma and serum. J Pharm Biomed Anal. 2002;29:83–94. doi: 10.1016/s0731-7085(02)00003-1. [DOI] [PubMed] [Google Scholar]
- 17.Lin CC, Luu K, Lourenco D, et al. Pharmacokinetics and metabolism of [(14)C]ribavirin in rats and cynomolgus monkeys. Antimicrob Agents Chemother. 2003;47:2458–2463. doi: 10.1128/AAC.47.8.2458-2463.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paroni R, Sirtori CR, Borghi C, et al. High-performance liquid chromatographic determination of ribavirin in serum and urine and of its urinary metabolite 1,2,4-triazole-3-carboxamide. J Chromatogr. 1987;420:189–196. doi: 10.1016/0378-4347(87)80172-x. [DOI] [PubMed] [Google Scholar]
- 19.Granich GG, Krogstad DJ, Connor JD, et al. High-performance liquid chromatography (HPLC) assay for ribavirin and comparison of the HPLC assay with radioimmunoassay. Antimicrob Agents Chemother. 1989;33:311–315. doi: 10.1128/aac.33.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svensson JO, Bruchfeld A, Schvarcz R, et al. Determination of ribavirin in serum using highly selective solid-phase extraction and high-performance liquid chromatography. Ther Drug Monit. 2000;22:215–218. doi: 10.1097/00007691-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Homma M, Matsuzaki Y, et al. Liquid chromatography assay for routine monitoring of cellular ribavirin levels in blood. Antimicrob Agents Chemother. 2004;48:3813–3816. doi: 10.1128/AAC.48.10.3813-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saint-Marcoux F, Sauvage FL, Marquet P. Current role of LC-MS in therapeutic drug monitoring. Anal Bioanal Chem. 2007;388:1327–1349. doi: 10.1007/s00216-007-1320-1. [DOI] [PubMed] [Google Scholar]
- 23.Sauvage FL, Gaulier JM, Lachâtre G, et al. Pitfalls and prevention strategies for liquid chromatography-tandem mass spectrometry in the selected reaction-monitoring mode for drug analysis. Clin Chem. 2008;54:1519–1527. doi: 10.1373/clinchem.2008.105478. [DOI] [PubMed] [Google Scholar]