Abstract
The approach to the pediatric patient with membranous nephropathy (MN) can be challenging to the practitioner. The clinical presentation of the child with this histologic entity usually involves some degree of proteinuria ranging from persistent, subnephrotic-ranged proteinuria to overt nephrotic syndrome. Patients often have accompanying microscopic hematuria and may have azotemia or mild hypertension. Children presenting with nephrotic syndrome are often steroid resistant; as such, their biopsy for steroid-resistant nephrotic syndrome results in the diagnosis of MN. The practitioner treating MN in the pediatric patient must weigh the risks of immunosuppressive therapy against the benefits. In general, the child with subnephrotic proteinuria and normal renal function can likely be treated conservatively with angiotensin blockade (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) without the need for immunosuppressive therapy. Those with nephrotic syndrome are usually treated with steroids initially and often followed by alkylating agents (cyclophosphamide or chlorambucil). Calcineurin inhibitors may also be useful, but the relapse rate after their discontinuation remains high. The absence of controlled studies in children with MN makes treatment recommendations difficult, but until they are available, using the patient’s clinical presentation and risk of disease progression appears to be the most prudent approach.
Keywords: Membranous glomerulopathy, Membranous glomerulonephritis, Nephrotic syndrome, Cyclophosphamide, Cyclosporine, Pediatrics
Introduction
Membranous nephropathy (MN) is a rare histologic entity in children, which usually presents as nephrotic syndrome or asymptomatic proteinuria [1, 2]. Whereas it is one of the most common causes of primary nephrotic syndrome in adults, it contributes to <5% of cases in children [2–5]. It is characterized histologically by the uniform thickening of the glomerular capillary wall on light microscopy (Figs. 1 and 2). This thickening is associated with subepithelial immune complex deposits that appear as granular deposits of immunoglobulin (Ig) G on immunofluorescence and as electron-dense deposits on electron microscopy. In children, secondary causes of MN have been associated with conditions such as systemic lupus erythematosus (SLE), hepatitis B or C infection, secondary and congenital syphilis, malaria, and Ebstein Barr Virus (EBV) infection [1, 6, 7]. Other rare underlying causes are C4 deficiency, selective IgA deficiency, or antitubular basement membrane antibodies [8–10].
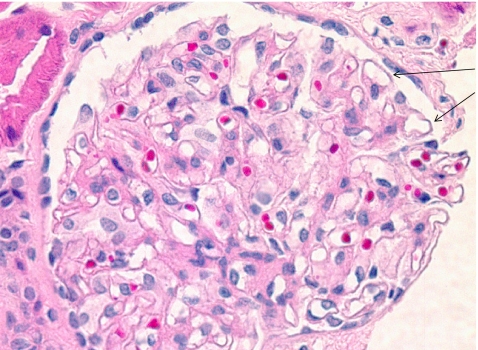
Fig. 1.
Primary membranous nephropathy (MN) (hematoxylin and eosin; x200). Glomerular capillary walls are uniform with mild thickening (arrows)
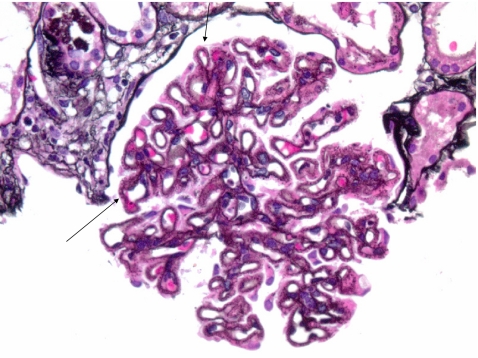
Fig. 2.
Primary membranous nephropathy (MN) (periodic acid-methenamine silver-Jones stain; x400). Characteristic spike-like epimembranous projections of basement membrane material on capillary walls (arrows)
Epidemiology
Data from the International Study for Kidney Disease in Children (ISKDC) demonstrated an incidence of MN in 1.5% in children with nephrotic syndrome [5]. Another report by Moxey-Mims et al. showed that whereas the incidence of MN was 1% in children 1–12 years of age, it increased to 22% in children between the ages of 13 and 19 years [11]. Other studies have reported incidence of idiopathic MN in children of 1.2−4.5% [4, 6, 12]. The median age at presentation has ranged from 7 to 12 years in various studies [2, 6, 12–14]. There is no specific gender distribution, with the boy:girl ratio ranging from 3:1 to 1:1 [6, 13]. The 2008 report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) reports the incidence of MN in children with chronic kidney disease (CKD) to be 0.5% [15]. According to the United States Renal Data System (USRDS) 2008 Annual Data Report, MN contributes to 0.6% of cases of pediatric end-stage renal disease (ESRD), with a median age at onset of ESRD being 16 years [16].
Clinical features
Children with MN most commonly present clinically with proteinuria, which may be nonselective and associated with microscopic hematuria. Approximately 40−75% of patients with MN present with nephrotic syndrome [2, 4]. Asymptomatic, nephrotic-range proteinuria has been reported in 16−38% [2, 4]. Proteinuria may be associated with microscopic hematuria in a majority of the patients [12]. Lee et al. reported an incidence of macroscopic hematuria of almost 40% in their series of 19 children with idiopathic MN from South Korea [13]. Hypertension may also be seen in a small subset of patients at presentation.
Histology
The characteristic feature of MN on light microscopy is a thickened glomerular capillary wall showing spikes on silver and periodic acid-Schiff stains with granular staining for IgG and complement component 3 along the capillary wall on immunofluorescence [17]. The hallmark is the presence of multiple, finely granular, electron-dense deposits exclusively along the subepithelial surface of the glomerular capillary wall between podocyte foot processes. Based on the location of the deposits on electron microscopy, Ehrenreich and Churg proposed a four-stage classification for MN [17, 18]. Stage 1 is characterized by small, sparsely distributed electron-dense deposits on the epithelial side without thickening of the glomerular basement membrane (GBM). In stage 2, there are more extensive and larger subepithelial deposits, with formation of basement membrane spikes between the deposits and thickening of the GBM. Stage 3 lesions show a combination of stage 2 along with larger deposits completely surrounded by basement membrane (intramembranous deposits); and in stage 4, there is incorporation of deposits in the GBM and irregular thickening and dissolution of the GBM.
Secondary causes
The overall prevalence of secondary causes of MN from various adult series is believed to be close to 20% of all patients with MN [10]. However, secondary causes appear to be more common in children. Kleinknecht et al. reported a prevalence of secondary causes in 30 of 85 children with MN [6]. In another study from Korea, almost 75% cases of MN were associated with a secondary cause [13]. This was, however, very likely related to the high prevalence of hepatitis B infection and hepatitis-B-related MN in this region. The other conditions associated with MN include hepatitis C, secondary and congenital syphilis, malaria, SLE, sickle-cell hemoglobinopathy, and medications such as D-penicillamine and gold salts [6, 7, 10]. Secondary MN has also been seen following a hematopoietic cell transplant and as a de novo glomerulopathy following renal transplantation [7] (Table 1).
Table 1.
Secondary causes of membranous nephropathy
| General causes | Specific causes |
|---|---|
| Infections | Hepatitis B |
| Hepatitis C | |
| Streptococcal | |
| Malaria | |
| Schistosomiasis | |
| Syphilis | |
| Leprosy | |
| Tuberculosis | |
| Cytomegalovirus | |
| Drugs | Captopril |
| Clopidogrel | |
| Mercury | |
| Penicillamine | |
| Nonsteroidal anti-inflammatory | |
| Gold | |
| Autoimmune diseases | Systemic lupus erythematosus |
| Rheumatoid arthritis | |
| Autoimmune thyroiditis | |
| Sjögren’s syndrome | |
| Mixed connective tissue disease | |
| Neoplasms | Carcinomas of bladder, breast, pancreas, prostate |
| Hematological malignancies: lymphoma, chronic lymphocytic leukemia | |
| Others | Diabetes mellitus |
| Sarcoidosis | |
| Sickle-cell disease | |
| Hematopoietic stem-cell transplant | |
| Postrenal transplant |
It is important to differentiate between primary and secondary MN due to differences in management and outcome. Whereas there may be some clinical clues that point toward a possible etiology, histology may also show features suggestive of a secondary cause, especially that of an autoimmune disease. Histologic features in favor of MN associated with SLE include mesangial or endocapillary proliferation on light microscopy, a “full-house” pattern of Ig staining, including C1q and non-IgG4 on immunofluorescence microscopy, and tubuloreticular inclusions within the glomerular endothelial cells on electron microscopy (Fig. 3).
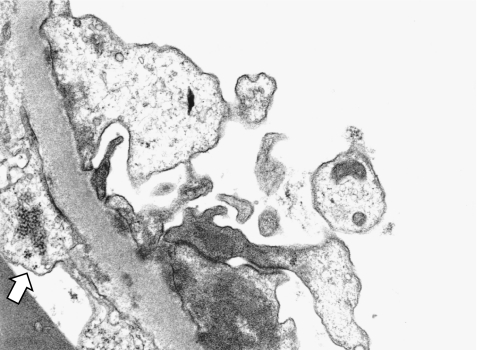
Fig. 3.
Secondary membranous nephropathy (MN) (lupus nephritis) (electron microscopy x10,000). Tubuloreticular inclusion in cytoplasm of endothelial cells (arrow)
Another potentially distinguishing histologic feature includes the anatomic location of the electron-dense deposits. In primary MN, these deposits are found exclusively in the subepithelial and intramembranous region. In contrast, some secondary forms of MN (those related to drugs such as gold or penicillamine) are characterized by deposits in the subendothelial region of the capillary wall with a lower likelihood of deposits in the subepithelial region. Membranous lupus nephritis, however, cannot be distinguished from idiopathic MN by the presence of subepithelial deposits, as they are frequent in both types. On the other hand, mesangial deposits have been reported to occur more commonly in membranous lupus nephritis (Fig. 4) [19].
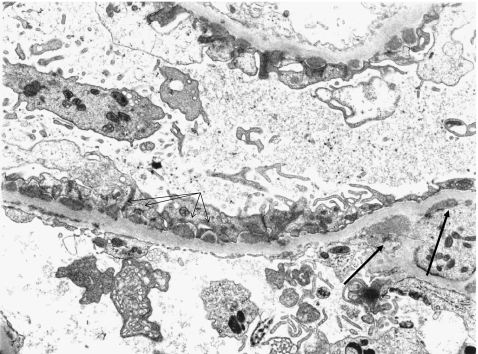
Fig. 4.
Secondary membranous nephropathy (MN) (lupus nephritis) (electron microscopy x5,000). Subepithelial deposits are juxtaposed with intervening glomerular basement membrane (thin arrows). Mesangial deposits characteristic of lupus nephritis (thick arrows)
Pathogenesis
Due to the characteristic subepithelial immune complex deposits seen on the electron microscopy, MN is believed to be an immune-complex-mediated disease. However, no circulating immune complexes have been identified in patients with idiopathic MN. Most data on the pathogenesis of MN comes from an animal model, the Heymann model of experimental MN in rats, which suggests that the podocyte is the target of injury. Studies show that there is in situ binding of a circulating antibody to antigen in the subepithelial space [7]. In the Heymann nephritis model, megalin was identified as the antigenic target. However, megalin, which is a member of the low-density lipoprotein receptor family and is expressed with clathrin at the base of podocyte foot processes (the site of immune complex formation) in rats, is an unlikely antigen for human MN [7, 20, 21]. Certain other podocyte membrane proteins, such as dipeptidyl peptidase IV and neutral endopeptidase, have been postulated to play an antigenic role in the pathogenesis of MN [21, 22]. The identification on neutral endopeptidase, as the target antigen in cases with neonatal MN strongly supports the in situ immune complex formation hypothesis [23].
The subepithelial immune complexes associated with MN have been identified as consisting of IgG (usually IgG4) and unidentified antigens [21]. T cells play a significant role in the pathogenesis. The presence of IgG4, which is a product of the type 2 response T helper cells (Th2) and an upregulation of cytokines, such as interleukins (IL) -4 and -10, suggest Th2 involvement [24, 25]. This CD4, T-cell-dependent, humoral response leads to subsequent Ig deposition and complement activation. These observations are further supported by experimental studies that show mycophenolate mofetil (MMF) prevents induction of Heymann nephritis by causing preferential suppression of Th2 cytokines; furthermore, this model of nephritis is modified by treatment with monoclonal anti-CD4 and anti-CD8 treatment [26].
The formation of subepithelial immune deposits leads to complement activation and subsequent insertion of sublytic quantities of C5b-9 complex into the podocyte membrane [7, 20, 21]. Various studies have shown that complement activation and formation of C5b-9 is an important mediator of podocyte injury in experimental MN. Inhibition of complement deposition has been associated with the absence of proteinuria in the passive Heymann nephritis model of MN, despite not having any effect on antipodocyte antibody deposits [25].
Complement activation triggers an inflammatory cascade that includes upregulation of gene expression for production of prostanoids, proteases, reactive oxygen species, extracellular matrix, and cytokines. Reactive oxygen species initiate lipid peroxidation and degradation of GBM type IV collagen. There is increased production of laminin and type IV and type I collagen, which accumulates in the extracellular matrix. This matrix accumulation gives MN its characteristic morphological appearance with thickened basement membranes and spike-like extensions of matrix between podocytes. C5b-9 also leads to abnormal distribution of slit diaphragm protein with dissociation of nephrin from the actin cytoskeleton and causes detachment of podocytes that are then shed into Bowman’s space. Complement activation and C5b-9 decrease nephrin expression and reduce F-actin-bound nephrin, thus further interrupting slit diaphragm integrity [25]. Along with the disruption of GBM, there is also podocyte apoptosis mediated by reactive oxygen species [21]. The detachment of podocytes from the GBM, as a result of both apoptosis and cytoskeletal damage from C5b-9, contributes to an increase in permeability of the protein filtration barrier. This sequence of events disrupts the GBM and the protein filtration barrier formed by podocytes, thereby resulting in proteinuria. Thus, whereas the exact pathophysiologic mechanism is still unclear, there is sufficient evidence to suggest the role of an antigenic target in the podocyte membrane, with subsequent in situ subepithelial immune complex deposit formation and accompanying complement activation, resulting in glomerular injury and proteinuria.
Natural history
Due to the relatively low incidence of MN in children, most data on its natural history and progression have been extrapolated from studies in adults. In adults, the disease is often characterized by spontaneous remission and relapses. In general, adults with MN are believed to be equally divided into patients who show spontaneous remission, those who have persistent proteinuria with preserved renal function, and those who progress to ESRD [17, 20]. A pooled analysis of randomized and prospective studies in adults with MN showed a 10-year renal survival rate of 65−75% [27]. However, an important difference in the clinical course between children and adults is that the younger patients have been noted to have a relatively better outcome. The older series by Olbing and others reported rates of proteinuria remission of approximately 30% [6, 12]. Some of the more recent studies have reported higher overall remission rates of 75% [2, 13]. The percentage of patients with MN showing impaired renal function is also lower in children. According to the 2008 Annual Report of NAPRTCS, MN contributes to 0.4 % of children on dialysis and 0.5 % of children with CKD [15]. Lee et al. reported a 17.6% rate of progression to chronic renal insufficiency [13]. In another study, by Chen et al., one fourth of patients were in CKD stage 3 (mean estimated GFR of 37 ± 9 ml/min/1.73 m2) at the end of a mean follow-up of 42 months [4]. In a series on 12 patients reported from our center, only one patient had an eGFR < 75 ml/min per 1.73 m2 at a mean follow-up of 27 months [2] .
Predictive factors
A number of adult studies have allowed practitioners to characterize prognostic factors in adult patients with MN. Laluck et al. showed that low-grade, subnephrotic proteinuria and female gender were associated with spontaneous remission [28]. In a review on 350 patients with MN and nephrotic syndrome, Troyanov et al. reported that the 10-year renal survival rate was 100% in the complete remission group, 90% in the partial remission group, and 45% in the no remission group [29]. Male gender, age >50 years, persistent high-grade proteinuria, impaired renal function at onset, presence of segmental glomerular sclerosis, and tubulointerstitial damage on the kidney biopsy have been considered to be poor prognostic factors in adult idiopathic MN [7, 20]. The Toronto Glomerulonephritis Registry created a model for identifying patients at risk for progression of renal insufficiency, taking into account the initial creatinine clearance (CrCl), the slope of the CrCl, and the lowest amount of proteinuria during a 6-month period [30]. According to this model, patients who present with a normal CrCl, proteinuria <4 g/24 h, and stable renal function over 6 months are considered to be at low risk for progression. On the other hand, patients with persistent proteinuria (>8 g/24 h) have a 66−80% probability of progression to ESRD within 10 years, independent of the degree of renal dysfunction. This model or an analogous model has not been applied in children.
Various pediatric studies have tried to characterize the prognostic factors in children with idiopathic MN. In their series of nine patients, Olbing et al. noted that age <9 years and normal blood pressure at the time of onset were associated with a good prognosis [12]. They did not observe any correlation between initial edema, proteinuria, hypoalbuminemia, and hypercholesterolemia and the subsequent course of disease. Other pediatric studies failed to demonstrate any advantage of younger age and normal blood pressure at presentation on final outcome.
There is some evidence from adult series that suggests that histopathological staging of MN may be useful in predicting prognosis and response to therapy [31]. In children, however, there is no consistent data on the use of this histopathological staging to predict response. Whereas Ramirez et al. reported an increased rate of progression to renal insufficiency with stage 3 and 4 lesions on initial biopsy compared with stage 1 or 2 lesions, this observation was not noted in a study of Canadian children with MN [32, 33]. A few studies have noted a correlation between chronic features such as glomerular sclerosis or tubulointerstitial changes on biopsy and disease severity at onset or its response to treatment [20, 34].
Management
The treatment algorithm for managing MN in adults relies on assessing the prognostic factors at illness onset and trying to achieve a balance between the probability of renal failure versus the risks of immunosuppression [17, 20]. According to the risk of renal disease progression, patients are assigned to receive either conservative, nonimmunosuppressive therapy versus immunosuppressive therapy. However, due to the rarity of the disease in the pediatric population and the paucity of natural history studies, there is no standardized approach to therapy in children. Most knowledge in the pediatric literature pertaining to natural history of MN, treatment options, and long-term outcome is derived from small, uncontrolled case series (Table 2) [2, 4, 6, 13, 32]. None of the studies have shown consistency in their therapeutic approaches, with differences in dosage and duration of corticosteroids as well as use of other immunosuppressive and nonimmunosuppressive agents [angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB)].
Table 2.
Pediatric membranous nephropathy studies
| Author | Number of patients | NS | Steroids | Other immunosuppression | Remission | Persistent disease | CRI | ESRD |
|---|---|---|---|---|---|---|---|---|
| Habib et al. [55] | 50 | 72% | 54% | 44% (mechlorethamine and chlorambucil) | 52% | 38% | ? | 10% |
| Olbing et al. [12] | 9 | 78% | 89% | 22% CYP, 11% AZA | 33% | 33% | 33% | 0% |
| Chan and Tsao [14] | 10 | 80% | 100% | None | 50% | 40% | 0% | 10% |
| Trainin et al. [56] | 14 | 79% | 79% | 57% “cytotoxics” | 43% | 29% | 7% | 21% |
| Latham et al. [33] | 14 | 100% | ≤ 93% | ≤93%: CYP | 29% | 50% | 7% | 14% |
| Ramirez et al. [32] | 22 | 82% | 50% | 5% AZA+CYP, 5% chlorambucil | 27% | 45% | 23% | 5% |
| Tsukahara et al. [57] | 12 | 25% | 42% | 17% CYP | 67% | 33% | 0% | 0% |
| Lee et al. [13] | 19 | 58% | 84% | 16% CsA | 68% | 16% | 5% | 11% |
| Chen et al. [4] | 13 | 38% | 77% | 38% CNI, 23% AZA or MMF | ? | 61% | 23% | 0% |
| Valentini et al. [2] | 12 | 75% | 83% | 58% CYP | 75% | 17% | 8% | 0% |
CYP cyclophosphamide, AZA azathioprine, CsA cyclosporine, CNI calcineurin inhibitors, MMF mycophenolate mofetil, CRI chronic renal insufficiency, ESRD end-stage renal disease
There is evidence that not all children with MN merit aggressive immunosuppressive treatment. Similar to adults, it has been noted that children with asymptomatic, nonnephrotic proteinuria have a better prognosis than those with nephrotic syndrome. Hence, the primary aim of management is to avoid aggressive therapeutic measures for patients who are not likely to develop progressive disease. Children with MN can be divided into two groups based on their clinical presentation:
Children with asymptomatic, nonnephrotic proteinuria. These children typically present with no edema, normal serum albumin, and a urine protein to creatinine ratio between 0.2 and 2 [35]. As it has been shown that this group is at low risk of progressive renal disease, this group of patients should be managed conservatively with ACEi or ARB, with the goals of treatment being to reduce proteinuria and optimize blood pressure control. Whereas the effect of angiotensin blockade has not been evaluated with controlled trials in children, there is some inferential evidence from adults on the benefits of ACEi or ARB. Gansevoort et al. reported a 30% decrease in proteinuria in patients with MN, especially in those who had lower levels of proteinuria [36]. In another review of 348 patients, Troyanov et al. reported a modest renoprotective effect with the use of ACEi or ARB (hazard ratio for renal survival on univariate analysis 0.40, p = 0.009); however, this advantage was not seen on multivariate analysis [29]. There may be a potential synergistic antiproteinuric effect of lipid-lowering agents when used with angiotensin blockade [17, 37]. Irrespective of the antiproteinuric effect, lipid-lowering agents, particularly statins, play an important role in the management of lipid abnormalities with the aim of decreasing this cardiovascular risk factor.
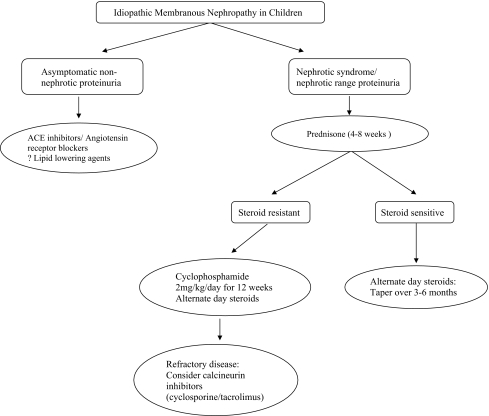
Children with nephrotic syndrome. The standard therapy for adults with MN and nephrotic proteinuria, as defined as a urine protein to creatinine ratio >2, is usually a combination of oral corticosteroids and alkylating agents (chlorambucil or cyclophosphamide) or cyclosporine [35]. The effects of these immunosuppressive agents have not yet been proven in children. A few uncontrolled studies have reported the lack of effectiveness of exclusively using oral corticosteroids in children with MN [38]. On the other hand, there have been some reports about the efficacy of combined immunosuppressive therapy. Children with nephrotic syndrome also benefit from concomitant treatment with ACEi or ARB, and lipid-lowering agents for optimal management of blood pressure and lipid abnormalities (Fig. 5).
Fig. 5.
Management of children with idiopathic membranous nephropathy
Medications (adults and children)
Corticosteroids A small number of prospective controlled trials with corticosteroids have been reported in adults with MN. These studies used different steroid regimens ranging from low dose (45 mg/m2 on alternate days for 6 months) to high dose (100–150 mg on alternate days for 8 weeks). The US Collaborative Study of Adult Idiopathic Nephrotic Syndrome reported a significant reduction in the rate of progression to renal failure [39]. However, a double-blind study by the British Medical Research Council, which used the same steroid dose in a larger patient population and with a longer duration of follow-up, did not show any beneficial effect of corticosteroids on renal function or urinary protein excretion [40]. Cattran et al. and the Toronto Study Group conducted a prospective randomized study in which patients with idiopathic MN were assigned to receive either alternate-day steroids for 6 months or no specific treatment [41]. After a mean follow-up of 48 months, they reported that there was no difference in the proportion of patients achieving complete remission or those progressing to renal failure.To summarize, these controlled adult trials do not show a definite advantage of using corticosteroids over placebo. This was also confirmed by the Cochrane Review on immunosuppressive treatment for idiopathic MN in adults with nephrotic syndrome, which reported no beneficial effect on total mortality or ESRD in patients treated with glucocorticoids [relative risk (RR) 0.88, 95% confidence interval (CI) 0.39−1.97, p = 0.75] [42]. A similar analysis has not been done in pediatrics.
Alkylating agents (cyclophosphamide or chlorambucil)
Alkylating agents such as cyclophosphamide and chlorambucil emerged as a second-line therapy for MN as a result of the uncertain efficacy of corticosteroids alone. Ponticelli et al. noted that a 6-month course of intravenous methylprednisolone and oral steroids alternating monthly with chlorambucil was superior to conservative treatment in inducing remission. Complete remission was achieved in 50% of cases compared with 7% of controls [43]. At 10 years, only 8% of treated cases had reached ESRD compared with 40% of controls. Another randomized controlled trial comparing a cyclic combination of steroids and chlorambucil with cyclic steroids and oral cyclophosphamide observed that both treatment regimens were equally effective in achieving remission and preserving renal function [44]. However, cyclophosphamide was associated with fewer side effects. According to the Cochrane Review, alkylating agents showed a significant effect on complete remission when compared with placebo (RR 2.37, 95% CI 1.32−4.25, p = 0.004) or with corticosteroids (RR 1.89, 95% CI 1.34−2.67, p = 0.0003) [42]. Another meta-analysis of controlled trials of treatment with cyclophosphamide or chlorambucil in patients with idiopathic MN and nephrotic-range proteinuria showed better chances of achieving complete remission with cytotoxic agents (RR 4.6, 95% CI 2.2 to 9.3) [45]. Our center reported favorable outcomes with a 12-week course of oral cyclophosphamide (2 mg/kg per day) in children with idiopathic MN [2]. Six of seven patients (five steroid resistant, one each partial responder and steroid dependent) achieved complete remission with no significant adverse effects.
Calcineurin inhibitors
Cattran et al. conducted a randomized trial in patients with idiopathic MN with nephrotic-range proteinuria comparing 26 weeks of cyclosporine (CsA) and low-dose prednisone to placebo and prednisone [46]. They noted complete or partial remission in 75% patients from the treatment group versus 22% from the control group (p < 0.001). Although the rate of relapse after stopping CsA was high, at the end of a 78-week follow-up, 39% of patients continued to be in remission in the CsA group compared with 13% in the placebo group. Other studies have shown that CsA can induce partial or complete remission in 50−60% of patients [17]. Due to a high relapse rate after stopping cyclosporine, prolonged low-dose CsA (approximately 1.5 mg/kg per day) could be considered for patients who relapsed after discontinuing CsA [17]. Due to undesirable cosmetic side effects of CsA, namely, hirsutism and gingival hyperplasia, tacrolimus is emerging as a useful alternative. In a prospective randomized trial, Praga et al. evaluated monotherapy with tacrolimus in adult patients with MN [47]. Twenty-five patients were treated with tacrolimus (0.05 mg/kg per day) over 12 months with a 6-month taper and compared with 23 controls. At the end of 18 months, the probability of remission in the treatment group was 94% versus 35% in the control group. There was relapse of nephrotic syndrome in almost half of the patients after tacrolimus withdrawal. Despite these adult studies on CsA and tacrolimus, there is very limited published literature on the use of calcineurin inhibitors in pediatric MN [4, 13].
Mycophenolate mofetil (MMF)
MMF has been used in uncontrolled trials in adults with idiopathic MN. Miller et al. reported a 50% reduction in proteinuria in 6/16 patients who received treatment with MMF for a mean period of 8 months [48]. Another retrospective study in 17 patients reported a decrease in the median urine protein:creatinine ratio from 7.3 to 1.5 (p = 0.001) [7]. There are no published pediatric reports of MMF use in children with MN.
Rituximab
Due to the possible role of B-cell activation in the pathogenesis of MN, targeted B-cell therapy is being tried to stop the production of pathogenic antibodies and prevent further glomerular damage. In a pilot study of rituximab (four weekly infusions of 375 mg/m2) in eight patients with idiopathic MN with persistent nephrotic syndrome, Remuzzi et al. noted a 70% decline in albuminuria at week 20 [49]. In another prospective, open-label trial, 60% of patients achieved complete or partial remission, and there was a 48% reduction in proteinuria [17]. Bomback et al. recently conducted a systematic review on the use of rituximab for MN. They noted that in primary MN, rituximab achieved complete remission in 13–20% of patients, and partial remission was seen in 36–50%. Fourteen of the 17 studies in their review, which reported their rituximab protocols, used rituximab at 375 mg/m2 once weekly for 4 weeks. They noted that more prospective studies and randomized controlled trials were needed before rituximab could be considered an alternative to alkylating agents and calcineurin inhibitors [50]. Whereas the early adult outcomes are compelling, unknown long-term side effects of rituximab makes its use in the pediatric patient with MN likely restricted to children with nephrotic syndrome refractory to traditional immunosuppressive medication.
Posttransplantation membranous glomerulopathy
Idiopathic MN recurs in 10–30% of patients after kidney transplantation [51]. De novo MN, which is the most common de novo glomerulopathy in renal allografts, affects 2–9% of renal allografts [52]. De novo MN typically occurs 24–36 months posttransplant and recurrent MN after 10–24 months [53]. The exact pathogenesis of de novo MN is not clear. Recently, El Kossi et al. described a case of de novo MN in which the onset of nephrotic-range proteinuria was associated with a donor-specific antibody directed against a class II human leukocyte antigen (HLA) DQ7 [53]. The patient was treated successfully with MMF and an ARB. Remission of proteinuria was associated with a fall in the anti-DQ7 titer. The authors postulated that de novo membranous glomerulopathy could be considered an atypical manifestation of acute antibody-mediated damage. There are limited data on the treatment of recurrent or de novo MN. Some studies have reported the lack of any benefit from the use of steroids and other immunosuppressive drugs [51, 54]. However, others have reported some success with the standard drugs used for idiopathic MN [53].
Summary
MN in the pediatric patient poses many challenges to the pediatric nephrologist due to its infrequent occurrence, varying means of presentation, and inconsistent treatment response. Pediatric studies discourage the use of immunosuppressive medication in asymptomatic patients with minimal (subnephrotic) proteinuria. However, several uncontrolled case series support the use of corticosteroids coupled with alkylating agents for pediatric patients with MN and nephrotic syndrome. Fewer studies exist as to the role of calcineurin inhibitors in this setting. Other agents such as MMF and rituximab have not been studied in children to date. The prognosis of a child with MN has to be considered guarded, with the greatest attention being given to those with refractory proteinuria and/or altered renal function.
Acknowledgments
The authors thank Xu Zeng, Department of Laboratory Medicine and Pathology, Wayne State University, Detroit, MI, USA, for his assistance with the renal pathology slides.
Questions
(Answers appear following the reference list)
Which of the following would be the LEAST expected clinical presentation of a child diagnosed with idiopathic MN?
Asymptomatic proteinuria
Azotemia
Hypertension
Microscopic hematuria
Nephrotic syndrome
All of the following would be an expected association of secondary MN EXCEPT?
Celiac disease
Hepatitis B infection
Nonsteroidal anti-inflammatory drugs
Sarcoidosis
Systemic lupus erythematosus
A key histologic feature distinguishing MN associated with systemic lupus erythematosus from that of idiopathic MN is
C3 staining on immunofluorescence
Granular staining for IgG
Subepithelial electron-dense deposits
Thickened glomerular capillary walls
Tubuloreticular inclusions
Which of the following treatment strategies best characterizes the approach to a child with MN?
Alkylating agents with corticosteroids should be considered for children with MN and nephrotic syndrome and steroid-resistant disease
ACEi should be reserved for patients with refractory, nephrotic-range proteinuria
Calcineurin inhibitors appear to be effective in MN, with a low relapse rate upon discontinuation of medication
Oral corticosteroids should be used in all patients irrespective of clinical presentation
Rituximab appears to be a suitable second-line therapy for children with steroid-resistant MN based on published reports
Which of the following factors are associated with a favorable prognosis in the pediatric patient with MN?
Age <14 years
Azotemia
Male gender
Tubulointerstitial fibrosis
Subnephrotic proteinuria
Footnotes
Answers
1. b
2. a
3. e
4. a
5. e
References
- 1.Cameron JS. Membranous nephropathy in childhood and its treatment. Pediatr Nephrol. 1990;4:193–198. doi: 10.1007/BF00858840. [DOI] [PubMed] [Google Scholar]
- 2.Valentini RP, Mattoo TK, Kapur G, Imam A. Membranous glomerulonephritis: treatment response and outcome in children. Pediatr Nephrol. 2009;24:301–308. doi: 10.1007/s00467-008-1005-9. [DOI] [PubMed] [Google Scholar]
- 3.Filler G, Young E, Geier P, Carpenter B, Drukker A, Feber J. Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis. 2003;42:1107–1113. doi: 10.1053/j.ajkd.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Chen A, Frank R, Vento S, Crosby V, Chandra M, Gauthier B, Valderrama E, Trachtman H. Idiopathic membranous nephropathy in pediatric patients: presentation, response to therapy, and long-term outcome. BMC Nephrol. 2007;8:11. doi: 10.1186/1471-2369-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Study of Kidney Disease in Children (1978) Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13:159–165 [DOI] [PubMed]
- 6.Kleinknecht C, Levy M, Gagnadoux MF, Habib R. Membranous glomerulonephritis with extra-renal disorders in children. Medicine (Baltimore) 1979;58:219–228. doi: 10.1097/00005792-197905000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ponticelli C. Membranous nephropathy. J Nephrol. 2007;20:268–287. [PubMed] [Google Scholar]
- 8.Kawasaki Y, Suzuki J, Onishi N, Takahashi A, Isome M, Suzuki H. IgA deficiency and membranous glomerulonephritis presenting as nephrotic syndrome. Pediatr Nephrol. 2005;20:662–664. doi: 10.1007/s00467-004-1720-9. [DOI] [PubMed] [Google Scholar]
- 9.Ivanyi B, Haszon I, Endreffy E, Szenohradszky P, Petri IB, Kalmar T, Butkowski RJ, Charonis AS, Turi S. Childhood membranous nephropathy, circulating antibodies to the 58-kD TIN antigen, and anti-tubular basement membrane nephritis: an 11-year follow-up. Am J Kidney Dis. 1998;32:1068–1074. doi: 10.1016/S0272-6386(98)70085-X. [DOI] [PubMed] [Google Scholar]
- 10.Glassock RJ. Secondary membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7(Suppl 1):64–71. [PubMed] [Google Scholar]
- 11.Moxey-Mims MM, Stapleton FB, Feld LG. Applying decision analysis to management of adolescent idiopathic nephrotic syndrome. Pediatr Nephrol. 1994;8:660–664. doi: 10.1007/BF00869080. [DOI] [PubMed] [Google Scholar]
- 12.Olbing H, Greifer I, Bennett BP, Bernstein J, Spitzer A. Idiopathic membranous nephropathy in children. Kidney Int. 1973;3:381–390. doi: 10.1038/ki.1973.60. [DOI] [PubMed] [Google Scholar]
- 13.Lee BH, Cho HY, Kang HG, Ha IS, Cheong HI, Moon KC, Lim IS, Choy Y. Idiopathic membranous nephropathy in children. Pediatr Nephrol. 2006;21:1707–1715. doi: 10.1007/s00467-006-0246-8. [DOI] [PubMed] [Google Scholar]
- 14.Chan WC, Tsao YC. Diffuse membranous glomerulonephritis in children. J Clin Pathol. 1966;19:464–469. doi: 10.1136/jcp.19.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) (2007) Annual Report. Available at www.naprtcs.org. Accessed July 12, 2008
- 16.U.S. Renal Data System (2008) Atlas of chronic kidney disease and end-stage renal disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. In: USRDS 2008 Annual Data Report, Bethesda MD, 2008
- 17.Fervenza FC, Sethi S, Specks U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin J Am Soc Nephrol. 2008;3:905–919. doi: 10.2215/CJN.04321007. [DOI] [PubMed] [Google Scholar]
- 18.Ehrenreich T, Churg J. Pathology of membranous nephropathy. Pathology Annals. 1963;3:145. [Google Scholar]
- 19.Jennette JC, Iskandar SS, Dalldorf FG. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int. 1983;24:377–385. doi: 10.1038/ki.1983.170. [DOI] [PubMed] [Google Scholar]
- 20.Cattran DC. Idiopathic membranous glomerulonephritis. Kidney Int. 2001;59:1983–1994. doi: 10.1046/j.1523-1755.2001.0590051983.x. [DOI] [PubMed] [Google Scholar]
- 21.Couser WG, Nangaku M. Cellular and molecular biology of membranous nephropathy. J Nephrol. 2006;19:699–705. [PubMed] [Google Scholar]
- 22.Ronco P, Debiec H. Molecular dissection of target antigens and nephritogenic antibodies in membranous nephropathy: towards epitope-driven therapies. J Am Soc Nephrol. 2006;17:1772–1774. doi: 10.1681/ASN.2006050497. [DOI] [PubMed] [Google Scholar]
- 23.Ronco P, Debiec H. Molecular pathomechanisms of membranous nephropathy: from Heymann nephritis to alloimmunization. J Am Soc Nephrol. 2005;16:1205–1213. doi: 10.1681/ASN.2004121080. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama K, Ebihara I, Yamamoto S, Kai H, Muro K, Yamagata K, Kobayashi M, Koyama A. Predominance of type-2 immune response in idiopathic membranous nephropathy. Cytoplasmic cytokine analysis. Nephron. 2002;91:255–261. doi: 10.1159/000058401. [DOI] [PubMed] [Google Scholar]
- 25.Nangaku M, Shankland SJ, Couser WG. Cellular response to injury in membranous nephropathy. J Am Soc Nephrol. 2005;16:1195–1204. doi: 10.1681/ASN.2004121098. [DOI] [PubMed] [Google Scholar]
- 26.Penny MJ, Boyd RA, Hall BM. Mycophenolate mofetil prevents the induction of active Heymann nephritis: association with Th2 cytokine inhibition. J Am Soc Nephrol. 1998;9:2272–2282. doi: 10.1681/ASN.V9122272. [DOI] [PubMed] [Google Scholar]
- 27.Hogan SL, Muller KE, Jennette JC, Falk RJ. A review of therapeutic studies of idiopathic membranous glomerulopathy. Am J Kidney Dis. 1995;25:862–875. doi: 10.1016/0272-6386(95)90568-5. [DOI] [PubMed] [Google Scholar]
- 28.Laluck BJ, Jr, Cattran DC. Prognosis after a complete remission in adult patients with idiopathic membranous nephropathy. Am J Kidney Dis. 1999;33:1026–1032. doi: 10.1016/S0272-6386(99)70138-1. [DOI] [PubMed] [Google Scholar]
- 29.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66:1199–1205. doi: 10.1111/j.1523-1755.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 30.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int. 1997;51:901–907. doi: 10.1038/ki.1997.127. [DOI] [PubMed] [Google Scholar]
- 31.Marx BE, Marx M. Prediction in idiopathic membranous nephropathy. Kidney Int. 1999;56:666–673. doi: 10.1046/j.1523-1755.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez F, Brouhard BH, Travis LB, Ellis EN. Idiopathic membranous nephropathy in children. J Pediatr. 1982;101:677–681. doi: 10.1016/S0022-3476(82)80289-8. [DOI] [PubMed] [Google Scholar]
- 33.Latham P, Poucell S, Koresaar A, Arbus G, Baumal R. Idiopathic membranous glomerulopathy in Canadian children: a clinicopathologic study. J Pediatr. 1982;101:682–685. doi: 10.1016/S0022-3476(82)80290-4. [DOI] [PubMed] [Google Scholar]
- 34.Reichert LJ, Koene RA, Wetzels JF. Prognostic factors in idiopathic membranous nephropathy. Am J Kidney Dis. 1998;31:1–11. doi: 10.1053/ajkd.1998.v31.pm9428445. [DOI] [PubMed] [Google Scholar]
- 35.Abitbol CL, Chandar J, Onder AM, Nwobi O, Montane B, Zilleruelo G. Profiling proteinuria in pediatric patients. Pediatr Nephrol. 2006;21:995–1002. doi: 10.1007/s00467-006-0103-9. [DOI] [PubMed] [Google Scholar]
- 36.Gansevoort RT, Heeg JE, Vriesendorp R, Zeeuw D, Jong PE. Antiproteinuric drugs in patients with idiopathic membranous glomerulopathy. Nephrol Dial Transplant. 1992;7(Suppl 1):91–96. [PubMed] [Google Scholar]
- 37.Sandhu S, Wiebe N, Fried LF, Tonelli M. Statins for improving renal outcomes: a meta-analysis. J Am Soc Nephrol. 2006;17:2006–2016. doi: 10.1681/ASN.2006010012. [DOI] [PubMed] [Google Scholar]
- 38.Makker SP. Treatment of membranous nephropathy in children. Semin Nephrol. 2003;23:379–385. doi: 10.1016/S0270-9295(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 39.Collaborative Study of the Adult Idiopathic Nephrotic Syndrome (1979) A controlled study of short-term prednisone treatment in adults with membranous nephropathy. N Engl J Med 301:1301–1306 [DOI] [PubMed]
- 40.Cameron JS, Healy MJ, Adu D. The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. The MRC Glomerulonephritis Working Party. Q J Med. 1990;74:133–156. [PubMed] [Google Scholar]
- 41.Cattran DC, Delmore T, Roscoe J, Cole E, Cardella C, Charron R, Ritchie S. A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med. 1989;320:210–215. doi: 10.1056/NEJM198901263200403. [DOI] [PubMed] [Google Scholar]
- 42.Schieppati A, Perna A, Zamora J, Giuliano GA, Braun N, Remuzzi G (2004) Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev (4):CD004293 [DOI] [PMC free article] [PubMed]
- 43.Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, Pasquali S, Imbasciati E, Grassi C, Redaelli B, Sasdelli M, Locatelli F. A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med. 1989;320:8–13. doi: 10.1056/NEJM198901053200102. [DOI] [PubMed] [Google Scholar]
- 44.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, Melis P, Valzorio B, Sasdelli M, Pasquali S, Pozzi C, Piccoli G, Lupo A, Segagni S, Antonucci F, Dugo M, MinariM SA, Pedrini L, Pisano G, Grassi C, Farina M, Bellazzi R. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. doi: 10.1681/ASN.V93444. [DOI] [PubMed] [Google Scholar]
- 45.Imperiale TF, Goldfarb S, Berns JS. Are cytotoxic agents beneficial in idiopathic membranous nephropathy? A meta-analysis of the controlled trials. J Am Soc Nephrol. 1995;5:1553–1558. doi: 10.1681/ASN.V581553. [DOI] [PubMed] [Google Scholar]
- 46.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL, North American Nephroltic Syndrome Study Group Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 47.Praga M, Barrio V, Juarez GF, Luno J, Grupo Espanol de Estudio de la Nefropatia Membranosa Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71:924–930. doi: 10.1038/sj.ki.5002215. [DOI] [PubMed] [Google Scholar]
- 48.Miller G, Zimmerman R, 3rd, Radhakrishnan J, Appel G. Use of mycophenolate mofetil in resistant membranous nephropathy. Am J Kidney Dis. 2000;36:250–256. doi: 10.1053/ajkd.2000.8968. [DOI] [PubMed] [Google Scholar]
- 49.Remuzzi G, Chiurchiu C, Abbate M, Brusegan V, Bontempelli M, Ruggenenti P. Rituximab for idiopathic membranous nephropathy. Lancet. 2002;360:923–924. doi: 10.1016/S0140-6736(02)11042-7. [DOI] [PubMed] [Google Scholar]
- 50.Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH. Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol. 2009;4:734–74. doi: 10.2215/CJN.05231008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant. 2006;6:2535–2542. doi: 10.1111/j.1600-6143.2006.01502.x. [DOI] [PubMed] [Google Scholar]
- 52.Kotanko P, Pusey CD, Levy JB. Recurrent glomerulonephritis following renal transplantation. Transplantation. 1997;63:1045–1052. doi: 10.1097/00007890-199704270-00001. [DOI] [PubMed] [Google Scholar]
- 53.El Kossi M, Harmer A, Goodwin J, Wagner B, Shortland J, Angel C, McKane W. De novo membranous nephropathy associated with donor-specific alloantibody. Clin Transplant. 2008;22:124–127. doi: 10.1111/j.1399-0012.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 54.Schwarz A, Krause PH, Offermann G, Keller F. Impact of de novo membranous glomerulonephritis on the clinical course after kidney transplantation. Transplantation. 1994;58:650–654. [PubMed] [Google Scholar]
- 55.Habib R, Kleinknecht C, Gubler MC. Extramembranous glomerulonephritis in children: report of 50 cases. J Pediatr. 1973;82:754–766. doi: 10.1016/S0022-3476(73)80063-0. [DOI] [PubMed] [Google Scholar]
- 56.Trainin EB, Boichis H, Spitzer A, Greifer I. Idiopathic membranous nephropathy. Clinical course in children. N Y State J Med. 1976;76:357–360. [PubMed] [Google Scholar]
- 57.Tsukahara H, Takahashi Y, Yoshimoto M, Hayashi S, Fujisawa S, Suehiro F, Akaishi K, Nomura Y, Morikawa K, Sudo M. Clinical course and outcome of idiopathic membranous nephropathy in Japanese children. Pediatr Nephrol. 1993;7:387–391. doi: 10.1007/BF00857546. [DOI] [PubMed] [Google Scholar]