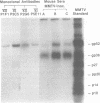
Abstract
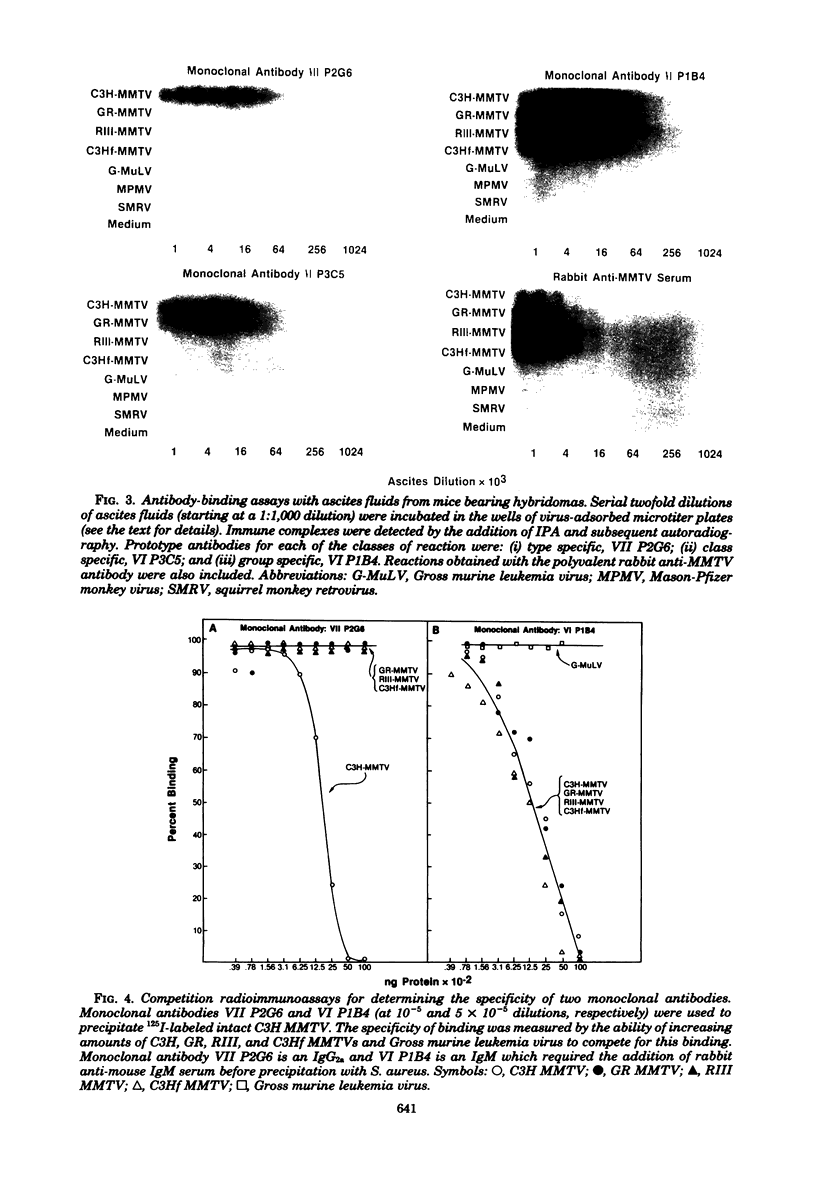
Hybrid cell lines producing monoclonal antibodies against the C3H strain of mouse mammary tumor virus (C3H MMTV) were prepared by the fusion of mouse myeloma cells with the lymphocytes of BALB/c mice that were immunized with C3H MMTV. Approximately 10% of the hybrid cells initially plated after cell fusion produced immunoglobulins that reacted in antibody-binding assays with C3H MMTV; 40 of these cells were cloned, and 6 eventually yielded stable cell lines. High concentrations of monoclonal antibodies (5 to 20 mg/ml) were obtained from serum and ascites fluid of syngeneic mice inoculated with the hybrid cells. All of the monoclonal antibodies were directed against the envelope glycoprotein gp52. Three of the hybrid cell lines produced immunoglobulins of the immunoglobulin M subclass and three produced immunoglobulin G2a. The monoclonal antibodies showed limited charge heterogeneity in light and heavy chains when analyzed by high-resolution, two-dimensional gel electrophoresis. Three serologically distinct specificities were observed when these ascites fluids were tested against different strains of MMTV. The antigenic determinants detected were the following: (i) a type-specific determinant unique to the C3H strain of MMTV; (ii) class-specific determinants shared between C3H and GR MMTVs; and (iii) a group-specific determinant found on C3H, GR, RIII, and the endogenous C3H (C3Hf) MMTVs. Because monoclonal antibodies recognize single antigenic determinants, these results demonstrate for the first time that the three patterns of antigenic reactivity for MMTV are related to individual determinants on the gp52 molecule and also clearly show that one strain of MMTV can be distinguished from other strains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Bauer R. F., Orme L. S., Fine D. L. Coexistence of the mouse mammary tumor virus (MMTV) major glycoprotein and natural antibodies to MMTV in sera of mammary tumor-bearing mice. Virology. 1978 Jun 15;87(2):266–275. doi: 10.1016/0042-6822(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Arthur L. O., Fine D. L. Immunological characterization of mouse mammary tumor virus p10 and its presence in mammary tumors and sera of tumor-bearing mice. J Virol. 1979 Apr;30(1):148–156. doi: 10.1128/jvi.30.1.148-156.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur L. O., Long C. W., Smith G. H., Fine D. L. Immunological characterization of the low-molecular-weight DNA binding protein of mouse mammary tumor virus. Int J Cancer. 1978 Oct 15;22(4):433–440. doi: 10.1002/ijc.2910220411. [DOI] [PubMed] [Google Scholar]
- Arthur L. O., Lovinger G. G., Schochetman G. Establishment of a C3Hf mammary tumor cell line expressing endogenous mouse mammary tumor virus: antigenic and genetic relationships of this virus with highly oncogenic mouse mammary tumor viruses. J Virol. 1979 Dec;32(3):852–859. doi: 10.1128/jvi.32.3.852-859.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiocchi M., Nowinski R. C. Polymorphism in the major core protein (p30) of murine leukemia viruses as identified by mouse antisera. Virology. 1978 Feb;84(2):530–535. doi: 10.1016/0042-6822(78)90269-6. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Colombatti A., Hilgers J. A radioimmunoassay for virus antibody using binding of 125I-labelled protein A. J Gen Virol. 1979 May;43(2):395–401. doi: 10.1099/0022-1317-43-2-395. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Arthur L. O., PLOWMAN J. K., Hillman E. A., Klein F. In vitro system for production of mouse mammary tumor virus. Appl Microbiol. 1974 Dec;28(6):1040–1046. doi: 10.1128/am.28.6.1040-1046.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering G., Old L. J., Boyse E. A. Antigens of leukemias induced by naturally occurring murine leukemia virus: their relation to the antigens of gross virus and other murine leukemia viruses. J Exp Med. 1966 Oct 1;124(4):753–772. doi: 10.1084/jem.124.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Arthur L. O., Fine D. L. Autogenous immunity to mouse mammary tumor virus in mouse strains of high and low mammary tumor incidence. Cancer Res. 1976 Aug;36(8):2840–2844. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Kopelman R., Sarkar N. H. Simultaneous purification of murine mammary tumor virus structural proteins: analysis of antigenic reactivities of native gp34 by radioimmunocompetition assays. J Virol. 1979 Aug;31(2):341–349. doi: 10.1128/jvi.31.2.341-349.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey R., Arthur L. O., Long C. W., Schochetman G. C3H/HeN mammary tumor-bearing mice develop type-specific neutralizing antibodies and group-specific precipitating antibodies for the mouse mammary tumor virus. J Virol. 1980 Jan;33(1):123–128. doi: 10.1128/jvi.33.1.123-128.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Wagenaar E., Nusse R. Autogenous antibodies against the murine mammary tumor virus in strains of mice with low incidences of mammary tumors. J Natl Cancer Inst. 1979 Apr;62(4):935–941. [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. V., Stockert E. Induction of GIX antigen and gross cell surface antigen after infection by ecotropic and xenotropic murine leukemia viruses in vitro. J Virol. 1976 Dec;20(3):545–554. doi: 10.1128/jvi.20.3.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A., STOCKERT E. TYPING OF MOUSE LEUKAEMIAS BY SEROLOGICAL METHODS. Nature. 1964 Feb 22;201:777–779. doi: 10.1038/201777a0. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Schochetman G., Arthur L. O., Long C. W., Massey R. J. Mice with spontaneous mammary tumors develop type-specific neutralizing and cytotoxic antibodies against the mouse mammary tumor virus envelope protein gp52. J Virol. 1979 Oct;32(1):131–139. doi: 10.1128/jvi.32.1.131-139.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Long C. W., Oroszlan S., Arthur L., Fine D. L. Isolation of separate precursor polypeptides for the mouse mammary tumor virus glycoproteins and nonglycoproteins. Virology. 1978 Mar;85(1):168–174. doi: 10.1016/0042-6822(78)90421-x. [DOI] [PubMed] [Google Scholar]
- Stone M. R., Lostrom M. E., Tam M. R., Nowinski R. C. Monoclonal mouse antibodies as probes for antigenic polymorphism in murine leukemia viruses. Virology. 1979 Jul 15;96(1):286–290. doi: 10.1016/0042-6822(79)90195-8. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of mammalian oncogenic RNA viruses: multiple antigenic determinants of the major internal protein and envelope glycoprotein. J Virol. 1974 Jan;13(1):171–180. doi: 10.1128/jvi.13.1.171-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Kufe D., Schlom J. Type-specific antigenic determinants on the major external glycoprotein of high- and low-oncogenic murine mammary tumor viruses. J Virol. 1977 Nov;24(2):525–533. doi: 10.1128/jvi.24.2.525-533.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Schlom J. Radioimmunoassays for the 36,000-dalton glycoprotein of murine mammary tumor viruses demonstrate type, group, and interspecies determinants. J Virol. 1979 Aug;31(2):334–340. doi: 10.1128/jvi.31.2.334-340.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Schlom J. Radioimmunoassays that demonstrate type-specific and group-specific antigenic reactivities for the major internal structural protein of murine mammary tumor viruses. Cancer Res. 1978 Jul;38(7):1990–1995. doi: 10.1203/00006450-199501000-00002. [DOI] [PubMed] [Google Scholar]