Abstract
Burkholderia mallei is a facultative intracellular pathogen that survives and replicates in phagocytic cell lines. The bacterial burden recovered from naïve BALB/c mice infected by intranasal delivery indicated that B. mallei persists in the lower respiratory system. To address whether B. mallei invades respiratory non-professional phagocytes, this study utilized A549 and LA-4 respiratory epithelial cells and demonstrated that B. mallei possesses the capacity to adhere poorly to, but not to invade, these cells. Furthermore, it was found that B. mallei was taken up by the murine alveolar macrophage cell line MH-S following serum coating, an attribute suggestive of complement- or Fc receptor-mediated uptake. Invasion/intracellular survival assays of B. mallei-infected MH-S cells demonstrated decreased intracellular survival, whilst a type III secretion system effector bopA mutant strain survived longer than the wild-type. Evaluation of the potential mechanism(s) responsible for efficient clearing of intracellular organisms demonstrated comparable levels of caspase-3 in both the wild-type and bopA mutant with characteristics consistent with apoptosis of infected MH-S cells. Furthermore, challenge of BALB/c mice with the bopA mutant by the intranasal route resulted in increased survival. Overall, these data suggest that B. mallei induces apoptotic cell death, whilst the BopA effector protein participates in intracellular survival.
INTRODUCTION
Burkholderia mallei, the aetiological agent of glanders disease, is a Gram-negative, capsulated and non-motile facultative intracellular bacterium. Naturally acquired human infection with B. mallei has not been recorded in the USA since 1945, although there have been reports of laboratory-acquired glanders cases (Srinivasan et al., 2001). Direct contact with the skin can result in a localized cutaneous infection, whilst inhalation of aerosol containing B. mallei may lead to acute septicaemia and pulmonary infections (Jennings, 1963). Furthermore, when left untreated, septicaemia caused by the disease results in a 95 % case fatality rate, which is reduced to only 50 % in antibiotic-treated individuals (Mandell et al., 1995).
Members of the genus Burkholderia include Burkholderia pseudomallei, the causative agent of melioidosis; Burkholderia cepacia, an important pulmonary pathogen in cystic fibrosis cases; and Burkholderia thailandensis, a bacterium of relatively low virulence (Brett et al., 1998; Coenye et al., 2001; White, 2003). A feature common to these three Burkholderia species is the localization of infection to the lungs and airways (Isles et al., 1984; Lever et al., 2003). Pulmonary involvement by B. mallei justifies the inclusion of this pathogen as a member of the CDC category B select agents (Rotz et al., 2002). Aerogenic B. mallei infection of BALB/c mice results in localization of the pathogen to the upper and lower sections of the respiratory tract and transportation of bacteria within alveolar macrophages to regional lymph nodes, suggestive of intracellular survival (Lever et al., 2003).
Experiments investigating the intracellular characteristics of Burkholderia species in phagocytic cell lines have historically used macrophage-like cell lines not derived from the lungs, but rather those with similarities to peritoneal macrophages. The macrophage populations obtained from different anatomical sites differ functionally and phenotypically (Caignard et al., 1985). In studies of B. cepacia using the human alveolar epithelial cell line A549 model, a correlation was reported between in vitro and in vivo invasiveness (Cieri et al., 2002). B. pseudomallei-infected mice, via intranasal (i.n.) delivery, demonstrated bacterial outgrowth from the lungs and significant lung inflammation (Wiersinga et al., 2008). In contrast, data are lacking regarding B. mallei intracellular survival, adhesion and/or colonization studies examining interactions with respiratory cells.
Identification of virulence determinants responsible for intracellular survival is currently limited to the presence of a functional type III secretion system (TTSS) (Ribot & Ulrich, 2006). A variety of Gram-negative bacteria utilize the TTSS for translocation of effector proteins into the host-cell cytoplasm, allowing the bacteria to evade host defences and survive the intracellular environment (Waterman & Holden, 2003; Zhou & Galan, 2001). Previous studies with the murine macrophage-like J774.2 cell line have shown the ability of B. mallei to escape into the host-cell cytoplasm, replicate intracellularly and induce directional actin polymerization, dependent on a functional TTSS (Ribot & Ulrich, 2006; Stevens et al., 2005). At least five genes have been identified as encoding potential TTSS effector proteins (Ulrich & DeShazer, 2004; Whitlock et al., 2007). One of these TTSS effector proteins, BopA, shares homology with the Shigella TTSS effector IcsB, which has been shown to play a role in intracellular replication (Ogawa et al., 2003). Interestingly, the B. pseudomallei bopA mutant showed a significant delay in time to death when challenged by the intraperitoneal route (Stevens et al., 2004).
Finally, studies involving B. mallei-induced cell death as it relates to a respiratory cell model are also lacking. Apoptosis, or programmed cell death, is a normal biochemical program of multicellular organisms manifested by the cleavage of chromosomal DNA, which occurs frequently during invasion of different Gram-negative pathogens (Monack & Falkow, 2000). Activation of the apoptotic pathway is characterized by a family of cysteine proteases (caspases) that cleave specific target proteins. This apoptotic activation plays an important role in the pathogenic process of various infectious diseases (Weinrauch & Zychlinsky, 1999). Bacterial-induced apoptosis has been reported to require the internalization and translocation of effector molecules through a functional TTSS (Hersh et al., 1999; Hilbi et al., 1998).
The present study examined the ability of B. mallei ATCC 23344 to adhere to and invade respiratory epithelial cells in vitro and survive within murine alveolar macrophages. In addition, we compared the wild-type strain with a B. mallei TTSS bopA mutant (known for its defective intracellular survival during infection of the J774A.1 macrophage cell line; Whitlock et al., 2008), using a mouse model of infection, for their ability to invade and survive in the intracellular environment and cause disease.
METHODS
Bacterial strains and culture conditions.
The human isolates B. mallei strain ATCC 23344 (China 7), B. pseudomallei strain 576 and B. pseudomallei strain K96243 and the horse isolate B. mallei strain China 5 were cultured on Luria–Bertani (LB) agar plates supplemented with 4 % glycerol (LBG) for 48 h at 37 °C. Escherichia coli strains HB101 and DH5α were cultured on LB agar plates for 24 h at 37 °C. Isolated colonies were subcultured on LBG or in LB broth and cultures were incubated at 37 °C with shaking at 200 r.p.m. to obtain an exponential growth phase inoculum. Construction of the GCW005 strain (bopA mutant) has been described elsewhere (Whitlock et al., 2008). Briefly, a 300 bp PCR amplimer within the bopA gene was generated using primers bopAmut-F (5′-CCGAATTCTGTACGAGCACGTCAGTTGG-3′; EcoRI site underlined) and bopAmut-R (5′-CCGAATTCATCGCCGGAAAATAGACCTTG-3′), and cloned into the gentamicin-resistant suicide vector pGSV3. The resulting plasmid, pGCW02, was introduced into B. mallei by conjugation using the donor strain SM10 (λ pir). Homologous recombination of the plasmid into B. mallei ATCC 23344 was verified by PCR analysis. Polar effects of bopA disruption are unlikely, based on the Virtual Institute of Microbial Stress and Survival operon predictions server and computational algorithms, placing the gene at the end of an operon (Ermolaeva et al., 2001). All bacterial strains were verified as kanamycin-sensitive. The B. mallei strain GCW005 (bopA mutant) was generated in 2007. Authorization was granted by the Centers for Disease Control Select Agent Program (CDC-SAP) to utilize and publish data generated from the Gmr B. mallei bopA mutant. The studies performed herein were in accordance with CDC-SAP-mandated guidelines and procedures. Pursuant to the CDC-SAP guidelines, the strain has since been destroyed.
Animal experiments.
Female 6–8-week-old BALB/c mice were obtained from Harlan Laboratories. The in vivo effects of GCW005 on survival were determined by comparing BALB/c mice (n=8) challenged i.n. with 5×105 c.f.u. (2 LD50) or 1×106 c.f.u. (4 LD50) of GCW005 or 2 LD50 of wild-type B. mallei ATCC 23344. LBG was delivered i.n. (50 μl) as a vehicle control. Animals were monitored for 10 days post-infection (p.i.) and the percentage survival was calculated. At day 10, 2 LD50 survivors [wild-type (n=1) and GCW005 (n=4)] were sacrificed, and the lungs and spleen were harvested, weighed and homogenized in 5 ml PBS. Bacterial colonization of tissues was quantified by plating 10-fold serial dilutions of tissue homogenates on LBG agar plates. The stability of GCW005 following in vivo infection was confirmed by subculturing colonies recovered on polymyxin B (15 μg ml−1) agar plates on gentamicin (5 μg ml−1) plates.
Bacterial burden studies were performed on BALB/c mice (n=7) challenged i.n. with 1 LD50 B. mallei ATCC 23344. At days 2 and 21 p.i., two and five animals, respectively, were sacrificed, and the lungs and spleen were harvested, weighed and homogenized in 5 ml PBS. Bacterial colonization of tissues was quantified as described above.
Cell lines.
The human respiratory epithelial cell line A549 was a generous gift of Dr Kent Tseng (University of Texas Medical Branch) and was cultured in Dulbecco's modified Eagle's medium (DMEM)/F12 (Gibco) supplemented with l-glutamine, 15 mM HEPES and 10 % heat-inactivated fetal bovine serum (FBS) at 37 °C with 5 % CO2. The mouse respiratory epithelial cell line LA-4 and mouse alveolar macrophage cell line MH-S were kind gifts from Dr Istvan Boldogh (University of Texas Medical Branch) and were cultured in DMEM/F12 supplemented with l-glutamine, 15 mM HEPES and 10 % heat-inactivated FBS or in DMEM supplemented with l-glutamine and 10 % heat-inactivated FBS, respectively, at 37 °C with 5 % CO2.
Cell adherence/invasion assays.
Human A549 and murine LA-4 respiratory epithelial cells were incubated at 37 °C with 5 % CO2 in 24-well plates (Corning) at a concentration of 5×105 cells per well. Inocula of B. mallei, B. pseudomallei and E. coli were prepared as described above, and 100 μl was added to each well at an m.o.i. of 10, followed by centrifugation at 800 g for 2 min and incubation at 37 °C for 2 h.
In adherence assays, non-adherent bacteria were removed with five washes of sterile PBS. Cell monolayers were lysed with 0.1 % Triton X-100 in PBS, and serial dilutions were plated and incubated at 37 °C for 48 h. The percentage of adherence was calculated as: (adherent c.f.u./total inoculum c.f.u.)×100. E. coli was used as both a negative control for invasion assays and a positive control for adherence assays, whilst medium only (no cells) served as a negative control of non-specific binding to plastic wells.
For invasion assays, the wells were washed twice with sterile PBS and fresh culture medium containing 150 μg kanamycin ml−1 was added, followed by an additional 2 h incubation at 37 °C in 5 % CO2 to kill extracellular bacteria. Thereafter, cell monolayers were washed twice with sterile PBS and lysed with 0.1 % Triton X-100. Serial dilutions of the lysate were plated on LBG agar and the number of c.f.u. ml−1 was determined. The percentage invasion was calculated as described above. All assays were performed in triplicate and reported as means±sem.
Intracellular survival assay.
To determine the intracellular survival of B. mallei in murine alveolar (MH-S) macrophages, we carried out an invasion assay at an m.o.i. of 1. After 2 h, wells were washed twice with sterile PBS and incubated for an additional 2 h at 37 °C in 5 % CO2 with fresh culture medium containing 250 μg kanamycin ml−1. Thereafter, cell monolayers were either washed twice with sterile PBS and lysed with 0.1 % Triton X-100 or replenished with fresh culture medium containing 100 μg kanamycin ml−1 and incubated for an additional 2 or 6 h. Serial dilutions of the lysates were plated and the number of c.f.u. ml−1 was determined. The percentage survival at 2, 4 and 8 h p.i. was calculated as described above. All assays were performed in triplicate and reported as means±sem.
Cell cytotoxicity assay.
Lactate dehydrogenase (LDH) assays of cultured supernatants were performed as described previously (Decker & Lohmann-Matthes, 1988). Prior to Triton X-100 lysis, 100 μl supernatant from infected cultures was added to 96-well plates and allowed to incubate at room temperature with 100 μl LDH detection reagent (BioVision). Control wells of tissue-cultured cells alone were representative of spontaneous LDH release, whilst maximum release was achieved by adding 200 μl 0.1 % Triton X-100. The percentage toxicity was calculated as follows: (A485 experimental−A485 spontaneous)/(A485 maximum−A485 spontaneous), and was performed in triplicate and reported as the mean±sem.
Caspase-3 activity.
Spectrophotometric (405 nm) caspase-3 activity by cleavage of DEVD-p-nitroanilide (Biovision Research Products) in cell lysates was performed following the manufacturer's guidelines. Briefly, MH-S cells were infected at an m.o.i. of 1 with B. mallei or its bopA mutant for 4 h, followed by two washes with PBS and lysing of cells in 50 μl lysis buffer (Biovision Research Products). Cell lysates were incubated for 1 h at 37 °C with 50 μl reaction buffer and substrate prior to colorimetric detection. Incubation with the phosphatidylinositol-3-kinase inhibitor 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride (10 μM; LY294002) or medium only for 14 h served as positive and negative controls, respectively. Results were reported as fold change compared with the uninduced negative control. Assays were performed in triplicate.
DNA fragmentation analysis.
MH-S cells (infected and uninfected) were incubated at 37 °C for 4 h prior to DNA extraction with a DNeasy extraction kit (Qiagen) and treated with proteinase K (100 μg ml−1). Extracted DNA was separated by electrophoresis on 1.3 % agarose gel (containing 10 μg ethidium bromide ml−1) for analysis of fragmentation. Cells treated with 10 μM LY294002 or medium alone for 14 h served as positive and negative controls, respectively.
Flow cytometry analysis of cell death.
Cytometric analysis of B. mallei-infected MH-S cells was performed by incubating the fluorescent-reactive dye ethidium monoazide bromide (emission peak 450 nm; Molecular Probes) with 4, 8 and 24 h-infected MH-S cells following the manufacturer's guidelines. Following cell staining, samples were immediately analysed with BD CellQuest Pro software on a FACSCaliber flow cytometer. More than 10 000 events were analysed per sample. Gating of uninfected and 70 % ethanol-treated cells served as negative and positive controls, respectively.
Statistical analysis.
Survival curves were calculated by Kaplan–Meier survival analysis with log-rank tests between groups using GraphPad Prism (version 4.03 for Windows). Statistical analysis was generally performed with a paired Student's t-test. A P value of ≤0.05 was considered significant.
RESULTS
Bacterial burden in tissues
Challenge of lungs from BALB/c mice with 1×105 c.f.u. B. mallei strain ATCC 23344 i.n. resulted in recoverable organisms from the lungs by day 2 p.i. with a mean c.f.u. (g tissue)−1 of 5.5×106 (data not shown). Bacterial outgrowth in the lung tissue decreased substantially by day 21 p.i. to 2.95×102 c.f.u. g−1, whilst bacterial burden in the spleen increased from 3×106 to 1.8×107 (P=0.0475). Similar work utilizing aerosol delivery of B. mallei in BALB/c mice demonstrated a course of infection comparable to that in our findings (Alekseev et al., 1994; Lever et al., 2003). Whilst respiratory involvement is evident, a cellular basis for infection is lacking. Therefore, we initiated in vitro cellular adhesion and invasion assays to identify a potential pulmonary host cell targeted during B. mallei infection.
Invasive and adherent properties of B. mallei and B. pseudomallei in murine and human respiratory epithelial cell lines
To begin investigating the invasive properties of B. mallei, we conducted standard invasion assays utilizing the human alveolar type II pneumocyte line A549 and the murine lower respiratory epithelial cell line LA-4. B. pseudomallei strain 576 and E. coli HB101 were used as positive and negative controls, respectively. B. mallei and a TTSS effector mutant bopA (GCW005) exhibited <0.5 % invasion in both cell lines, compared with the invasive B. pseudomallei control (Fig. 1a, b). To determine whether the non-invasive phenotype exhibited by B. mallei was a result of host-cell destruction, followed by exposure to extracellular kanamycin, we tested for LDH cytotoxicity on cultured cells (data not shown). Additionally, non-infected cells were examined for potential kanamycin-induced, compromised host-cell membrane integrity. All strains tested resulted in LDH values less than those of the kanamycin control, with the exception of the values found for B. pseudomallei on the LA-4 cell line, which were not statistically significant (P=0.202; data not shown). Furthermore, invasion assays performed at an alternative m.o.i. of 1 resulted in a similar non-invasive phenotype for B. mallei (data not shown).
Fig. 1.
Burkholderia invasion of respiratory epithelial cells in vitro. Monolayers of murine LA-4 (a) and human A549 (b) cells were infected with B. mallei (B.m), the bopA mutant (GCW005), B. pseudomallei (B.pm 576) or E. coli at an m.o.i. of 10 and the percentage invasion (from the initial inoculum) was determined at 4 h p.i. Experiments were performed in triplicate and data are expressed as means±sem. *, P <0.05.
Due to the relatively low invasive phenotype of B. mallei, and given that the interaction of bacteria with host-cell membranes is a prerequisite for the infectious process, we investigated whether the defective invasion phenotype was due to the inability of B. mallei to bind in our tissue culture model. Murine respiratory epithelial LA-4 adherence percentages for B. mallei and GCW005 were significantly less than for B. pseudomallei (P=0.024 and P=0.012, respectively; Fig. 2a), whilst the percentage adherence to A549 cells was similar for wild-type B. mallei and B. pseudomallei (Fig. 2b). Overall, B. mallei adhered relatively poorly to both cell lines. Control wells of infected medium alone confirmed the lack of adherence to plastic wells, and the positive-control E. coli demonstrated higher percentages of adherence, further validating our model. Adherence of B. pseudomallei to A549 cells (∼2 %) at 37 °C has been reported and is in agreement with the present findings (Brown et al., 2002). To our knowledge, this is the first report of adherence and invasion assays performed on murine LA-4 cells with Burkholderia species.
Fig. 2.
Burkholderia adherence to respiratory epithelial cells in vitro. Monolayers of murine LA-4 (a) and human A549 (b) cells were infected with B. mallei (B.m), the bopA mutant (GCW005), B. pseudomallei (B.pm 576) or E. coli at an m.o.i. of 10 and the percentage adherence (from the initial inoculum) was determined at 2 h p.i. Experiments were performed in triplicate and data are expressed as means±sem. *, P <0.05.
Giemsa staining and transmission electron microscopy substantiated the adherent but non-invasive phenotype of B. mallei (data not shown). Thus, under the conditions tested, B. mallei appeared to lack (or failed to express) the necessary factors required for respiratory epithelial invasion. In contrast, B. pseudomallei adhered and invaded LA-4 epithelial cells. In the murine model of disease, the i.n. doses required for infection by B. mallei and B. pseudomallei are very different. Thus this divergence may explain the reduced ability of B. mallei to interact with respiratory epithelial cells.
Murine alveolar macrophage uptake and intracellular survival of B. mallei and B. pseudomallei
To evaluate the phagocytic capability of the murine alveolar macrophage cell line MH-S, we performed complement opsonization studies. The coating of organisms with naïve mouse serum enhanced opsonization, whilst heat-killed treated serum reduced uptake to the baseline level, comparable to that of the organism alone (Fig. 3a). Additionally, coating of B. mallei with heat-killed immune serum obtained from infected BALB/c mice demonstrated enhanced opsonization results comparable to those found with naïve serum (Fig. 3a), suggestive of Fc receptor-mediated uptake.
Fig. 3.
Phagocytosis and survival of Burkholderia in alveolar macrophages in in vitro experiments. (a) Monolayers of MH-S cells were infected with wild-type B. mallei (B.m) at an m.o.i. of 10 pre-incubated with naïve serum (NS), 56 °C heat-inactivated naïve serum (HK NS) or heat-inactivated immune serum (HK IS) and the percentage uptake/survival was determined after a 2 h infection. (b) MH-S monolayers were infected with B. mallei (B.m) or the bopA mutant (GCW005) at an m.o.i. of 1 and the percentage intracellular survival (from the initial inoculum) was determined at 2 h (filled columns), 4 h (open columns) and 8 h (hatched columns) p.i. Experiments were performed in triplicate and data are expressed as means±sem. *, P <0.05; **, P <0.01.
To complete our investigation into bacteria–respiratory cell interactions, we infected MH-S cells with B. mallei ATCC 23344 and GCW005 at an initial m.o.i. of 10. At 2, 4 and 8 h post-inoculation, we failed to recover intracellular B. mallei from the exposed cells. Additional experiments performed at 30 min and 1 h post-inoculation failed to produce recoverable organisms. LDH assays indicated no cytotoxicity to the host cells (data not shown). Alternative strains of B. mallei (China 5) and B. pseudomallei (K96243) were tested and resulted in a similar pattern of intracellular recovery (data not shown). Similar studies of B. mallei uptake and survival in RAW 264.7 macrophages demonstrated the inability to survive intracellularly at an m.o.i. of 10, whilst an m.o.i. of 1 was conducive to survival (Brett et al., 2008). Therefore, the potential for MH-S uptake of B. mallei was investigated at a lower m.o.i. of 1 and the percentage survival indicated an initial uptake of both the wild-type and GCW005 mutant strain (Fig. 3b). Interestingly, recovery of wild-type B. mallei from intracellular compartments at 4 and 8 h p.i. was not significant, whilst the mutant GCW005 was recovered at 10 % of the initial uptake at these time points (Fig. 3b), which suggested to us that the bopA mutant may remain for a longer period of time in the intracellular compartments, leading to decreased cellular escape. No significant LDH release was detected at an m.o.i. of 1 (data not shown).
B. mallei-induced cell death of MH-S alveolar macrophages
Based on our previous data, we rationalized that the TTSS effector BopA may contribute to bacterial survival and/or escape from the MH-S host cell, thereby exposing B. mallei to extracellular kanamycin with subsequent killing of the organism. Shigella, Salmonella and Pseudomonas are capable of evading the killing effects of macrophages by cellular escape and host-cell death induction (Hauser & Engel, 1999; Hersh et al., 1999; Zychlinsky et al., 1992), and our in vivo studies performed on i.n.-infected BALB/c mice suggested that B. mallei was capable of dissemination, although how this dissemination is accomplished remains unclear.
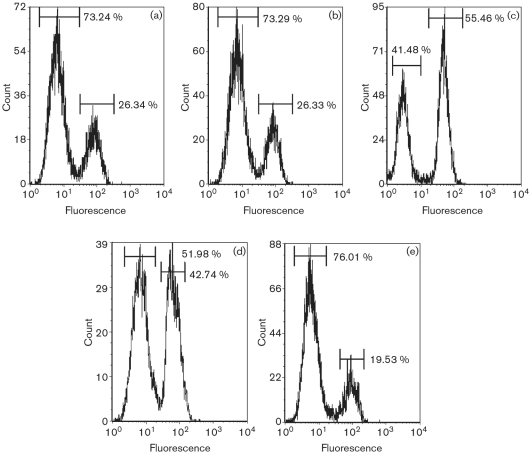
In order to investigate the possible mechanisms involved in the intracellular recovery of the GCW005 strain and the contribution of BopA towards host-cell escape, we evaluated the relationship of B. mallei infection and apoptosis. The MH-S cells demonstrated features consistent with apoptosis when we analysed DNA fragmentation at 4 h p.i. (m.o.i. of 1; data not shown). Further investigation into a possible mechanism for this cell death led us to analyse caspase-3 activity, which was found to be increased (∼1.6-fold at 4 h treatment) for both wild-type B. mallei- and GCW005-infected cells when compared with medium-only control cells. To determine the cytotoxic effect associated with apoptosis, we analysed B. mallei-infected MH-S cells by flow cytometry at 4, 8 and 24 h p.i. for compromised cell-wall permeability (as assessed by access of a reactive dye to the bacterial cytoplasm). MH-S cells infected at an m.o.i. of 1 demonstrated low levels of altered membrane integrity at 4 and 8 h p.i., with approximately 73 % of the population remaining viable (Fig. 4a, b). These findings were validated by our positive and negative controls (Fig. 4d, e). At 24 h p.i., cells demonstrating altered membrane permeability increased 100 % in MH-S cells treated with B. mallei (Fig. 4c) compared with levels recorded at early time points (Fig. 4a, b). GCW005-infected cells showed levels of altered cell-membrane integrity similar to those in wild-type B. mallei (data not shown). These results imply that an apoptotic secondary necrosis may occur.
Fig. 4.
Secondary necrosis of B. mallei-infected MH-S cells. MH-S cells were infected at an m.o.i. of 1 with wild-type B. mallei. After 4 (a), 8 (b) and 24 (c) h p.i., cells were washed and incubated with fluorescent reactive dye for cell viability assessment by flow cytometry. Ethanol treatment (70 %) of 24 h-incubated MH-S cells (d) and medium only (e) served as positive and negative controls, respectively. Data are representative of two independent experiments.
Contribution of the TTSS effector BopA to virulence in vivo
To substantiate the involvement and contribution of the BopA effector protein to the pathogenicity of B. mallei, we infected BALB/c mice by the i.n. route with 2 and 4 LD50 GCW005 and compared the percentage survival with that in wild-type infected animals. The mean survival time for animals infected with 2 LD50 GCW005 was 7.5 days, with 50 % overall survival (P=0.022), whilst wild-type B. mallei-infected animals had a 5 day mean survival time and 0 % survived by day 10 p.i. (Fig. 5a). No bacteria were recovered from the lung tissue taken at day 10 p.i. from GCW005-infected BALB/c mice challenged at the 2 LD50 level (Fig. 5b). Although there was no correlation between GCW005 in vitro cytotoxicity and attenuated in vivo virulence, the potential for BopA contributing to intracellular survival could explain the attenuation in virulence observed in our mouse model. These studies further support our in vitro data, which suggest that BopA plays a role during infection and may be required for intracellular survival within alveolar macrophages by mechanisms that remain to be investigated.
Fig. 5.
Attenuated virulence of the B. mallei bopA mutant. BALB/c mice (n=8) were challenged by i.n. delivery with B. mallei or the bopA mutant (GCW005) and monitored for survival. (a) GCW005 animals challenged with 2 LD50 resulted in a 50 % survival rate (P=0.022) compared with that of the wild-type. Control animals were dosed with vehicle only.  , Control; ▴, B. mallei 2 LD50; ▵, GCW005 2 LD50; ○, GCW005 4 LD50. (b) At day 10 p.i., lungs and spleen were harvested from surviving animals infected with wild-type (filled columns, n=1) or GCW005 (shaded columns, n=4) and plated for bacterial burden. No organisms were detected in GCW005-infected lungs. Data are expressed as means±sem for GCW005.
, Control; ▴, B. mallei 2 LD50; ▵, GCW005 2 LD50; ○, GCW005 4 LD50. (b) At day 10 p.i., lungs and spleen were harvested from surviving animals infected with wild-type (filled columns, n=1) or GCW005 (shaded columns, n=4) and plated for bacterial burden. No organisms were detected in GCW005-infected lungs. Data are expressed as means±sem for GCW005.
DISCUSSION
The inhalational exposure of mice to B. mallei initially results in localization of the bacteria within the upper and lower respiratory tract, followed by general dissemination, presumably through bacteraemia (Alekseev et al., 1994). Here, we described the ability of B. mallei ATCC 23344 to adhere to, but not invade, human and murine respiratory epithelial cells during in vitro experimentation. An overall lower adherence to human A549 cells was observed in all species evaluated. This finding was in agreement with previous studies utilizing the B. pseudomallei strains 08 and K96243 (Ahmed et al., 1999; Brown et al., 2002), which showed a low adherence to human epithelial cells in vitro. We found that, despite the low level of attachment of B. mallei to human respiratory epithelia, an increased attachment occurred in the murine respiratory cell line LA-4, although this adherence was still relatively poor compared with that of B. pseudomallei. These findings confirm the observations of the bacterial burden of B. mallei in infected murine lungs and substantiate previous in vivo findings of the ability of B. pseudomallei to adhere to the murine respiratory tract (Ahmed et al., 1999).
Activation of mitogen-activated protein kinase pathways has been reported to contribute to bacterial invasion (Kohler et al., 2002). Bacterial components, including LPS and, potentially, TTSS effector proteins in the case of B. pseudomallei, have been linked to a mitogen-activated protein kinase-dependent invasive phenotype (Utaisincharoen et al., 2000). Sequence alignments within the O-antigen regions of B. mallei and B. pseudomallei have revealed 99 % identity at the nucleotide level (Burtnick et al., 2002). Of the strains tested in the current study, only B. pseudomallei strain 576 possesses an atypical LPS profile (Anuntagool et al., 2000). However, results with the alternative B. pseudomallei strain K96243 (with a typical LPS profile) were similar to those with B. pseudomallei strain 576. Therefore, differences in LPS could not account for the lack of invasion observed with B. mallei. Furthermore, minimal toxicity was recorded following infection of respiratory epithelial cell lines with B. mallei and B. pseudomallei. Therefore, our results supported the idea that lack of recoverable B. mallei is not due to a lack of host-cell viability.
The identification of B. mallei within alveolar macrophages during aerosol infection of mice provides evidence for bacteria–host cell interactions (Lever et al., 2003). An initial m.o.i. of 10 resulted in no recoverable B. mallei from MH-S cells. However, MH-S cells effectively phagocytosed B. mallei, as demonstrated by serum coating of organisms. Additionally, we evaluated the potential of Fc receptor-mediated uptake by MH-S cells. By coating organisms with heat-treated immune serum from infected BALB/c mice, we enhanced organism uptake compared with the uptake in untreated MH-S cells. These data suggest that MH-S cells are capable of passive B. mallei phagocytosis.
The MH-S macrophages were permissive to B. mallei uptake and exhibited reduced numbers of intracellular organisms when compared with those found with B. pseudomallei uptake. We confirmed the contribution of the TTSS effector BopA protein to the intracellular life cycle of B. mallei. Furthermore, we recently described the effects of BopA on intracellular survival within the peritoneal macrophage cell line J774A.1 (Whitlock et al., 2008). Moreover, Cullinane et al. (2008) reported that B. pseudomallei BopA plays a role in mediating bacterial evasion of autophagy, allowing for increased intracellular survival. Interestingly, it appears that both B. mallei and GCW005 (bopA mutant) exhibit distinct intracellular survival characteristics in alveolar MH-S macrophages compared with the J774A.1 cell line, which displayed increased intracellular survival of GCW005 compared with wild-type B. mallei. This finding was unexpected based on previous intracellular survival data of B. mallei in murine macrophage-like cell lines (Ribot & Ulrich, 2006). Lowering the m.o.i. to 1 enabled us to recover intracellular B. mallei (presumably through the reduced activation of macrophages) from infected MH-S cells at numbers equivalent to those seen in Legionella pneumophila (Yan & Cirillo, 2004), as well as in B. mallei-infected RAW 264.7 macrophages (Brett et al., 2008). Therefore, it is plausible to suggest that B. mallei possesses the ability to escape killing by MH-S cells by an uncharacterized mechanism. The delay of the bopA mutant in escaping from the intracellular compartment may explain the recovery of organisms after 4 h of infection. If BopA contributes to escape from intracellular compartments of MH-S cells, this may result in the escape of wild-type B. mallei into antibiotic-containing medium, whereas GCW005 remains within the intracellular compartment. The increased efficiency of B. pseudomallei to survive within MH-S cells correlated with its ability to induce cellular damage as represented by cellular LDH release at 8 h p.i. Conversely, there was insignificant LDH release from B. mallei-infected MH-S cells (data not shown).
Several bacteria, including Shigella, Salmonella and Legionella, can escape macrophages by an apoptotic pathway (Gao & Kwaik, 2000). B. pseudomallei induces apoptotic cell death in macrophages (Kespichayawattana et al., 2000), and this induction appears to involve the B. pseudomallei TTSS protein BipB, although its role as a translocator or effector remains unknown (Suparak et al., 2005). The lack of an elevated LDH level in our B. mallei-infected MH-S cell supernatants indicated the absence of cell-membrane permeability. Thus we investigated the possibility of apoptosis-induced cell cytotoxicity. DNA fragmentation and caspase-3 studies showed evidence consistent with caspase-induced apoptosis in B. mallei-infected MH-S cells; therefore, evaluation of cell death was carried out via flow cytometry analysis. B. mallei did not alter cell-membrane permeability at 4 and 8 h p.i.; in contrast, a sharp increase was seen at 24 h after infection of MH-S cells (Fig. 4c). B. pseudomallei has been shown to induce caspase-1-dependent cell death, which correlates with bacterial entry (Sun et al., 2005). In contrast, we demonstrated that B. mallei appears to induce cell death without correlation with invasion of alveolar macrophages, perhaps through an apoptotic pathway that rapidly leads to secondary necrosis.
Our in vivo data, with increased survival of GCW005-challenged mice, demonstrate the contribution of BopA to virulence. This reduction in virulence may contribute to the inability of GCW005 to escape the intracellular compartment of alveolar macrophages effectively. Bacterial burdens of lung tissues determined at 10 days p.i. resulted in no recoverable organisms, substantiating our theory that GCW005 has a reduced ability to survive within host cells. In conclusion, this is the first report of adherence and invasion assays developed by utilizing a respiratory cell model for B. mallei. A possible mechanism of dissemination may involve cellular escape from alveolar macrophages, allowing the entry of B. mallei into capillary beds within the lung interstitium. Cellular escape may also occur at lymph nodes, given that alveolar macrophages are capable of trafficking to tracheobronchial lymph nodes (Harmsen et al., 1985). Intracellular survival and replication within peritoneal macrophages may allow maintenance of the organism within the spleen following dissemination.
Acknowledgments
This research was supported in part by the UTMB John Sealy Memorial Endowment Fund for Biomedical Research (A. G. T.), by NIH grant U54 AI057156 (D. M. E.) and by a contract from the National Institute of Allergy and Infectious Diseases NO1-AI-30065 (D. M. E. and A. G. T.). G. C. W. received a fellowship from the UTMB Sealy Center for Vaccine Development. We thank Yan Xia (University of Texas Medical Branch) for LY294002 and assistance with caspase studies and Mardelle Susman for critical reading of the manuscript.
Abbreviations
i.n., intranasal
LDH, lactate dehydrogenase
p.i., post-infection
TTSS, type III secretion system
References
- Ahmed, K., Enciso, H. D., Masaki, H., Tao, M., Omori, A., Tharavichikul, P. & Nagatake, T. (1999). Attachment of Burkholderia pseudomallei to pharyngeal epithelial cells: a highly pathogenic bacteria with low attachment ability. Am J Trop Med Hyg 60, 90–93. [DOI] [PubMed] [Google Scholar]
- Alekseev, V. V., Savchenko, S. T., Iakovlev, A. T., Rybkin, V. S., Kovalenko, A. A., Bykova, O. I. & Metlin, V. N. (1994). The early laboratory diagnosis of the pulmonary form of glanders and melioidosis by using rapid methods of immunochemical analysis. Zh Mikrobiol Epidemiol Immunobiol 59–63. [PubMed]
- Anuntagool, N., Aramsri, P., Panichakul, T., Wuthiekanun, V. R., Kinoshita, R., White, N. J. & Sirisinha, S. (2000). Antigenic heterogeneity of lipopolysaccharide among Burkholderia pseudomallei clinical isolates. Southeast Asian J Trop Med Public Health 31 (Suppl. 1), 146–152. [PubMed] [Google Scholar]
- Brett, P. J., DeShazer, D. & Woods, D. E. (1998). Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol 48, 317–320. [DOI] [PubMed] [Google Scholar]
- Brett, P. J., Burtnick, M. N., Su, H., Nair, V. & Gherardini, F. C. (2008). iNOS activity is critical for the clearance of Burkholderia mallei from infected RAW 264.7 murine macrophages. Cell Microbiol 10, 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N. F., Boddey, J. A., Flegg, C. P. & Beacham, I. R. (2002). Adherence of Burkholderia pseudomallei cells to cultured human epithelial cell lines is regulated by growth temperature. Infect Immun 70, 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick, M. N., Brett, P. J. & Woods, D. E. (2002). Molecular and physical characterization of Burkholderia mallei O antigens. J Bacteriol 184, 849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caignard, A., Martin, M. S., Hammann, A. & Martin, F. (1985). Heterogeneity of the rat macrophage antigenic specificity of resident peritoneal and pleural macrophages. Cell Mol Biol 31, 41–47. [PubMed] [Google Scholar]
- Cieri, M. V., Mayer-Hamblett, N., Griffith, A. & Burns, J. L. (2002). Correlation between an in vitro invasion assay and a murine model of Burkholderia cepacia lung infection. Infect Immun 70, 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenye, T., Vandamme, P., Govan, J. R. & LiPuma, J. J. (2001). Taxonomy and identification of the Burkholderia cepacia complex. J Clin Microbiol 39, 3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane, M., Gong, L., Li, X., Lazar-Adler, N., Tra, T., Wolvetang, E., Prescott, M., Boyce, J. D., Devenish, R. J. & Adler, B. (2008). Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy 4, 744–753. [DOI] [PubMed] [Google Scholar]
- Decker, T. & Lohmann-Matthes, M. L. (1988). A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods 115, 61–69. [DOI] [PubMed] [Google Scholar]
- Ermolaeva, M. D., White, O. & Salzberg, S. L. (2001). Prediction of operons in microbial genomes. Nucleic Acids Res 29, 1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, L. Y. & Kwaik, Y. A. (2000). The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol 8, 306–313. [DOI] [PubMed] [Google Scholar]
- Harmsen, A. G., Muggenburg, B. A., Snipes, M. B. & Bice, D. E. (1985). The role of macrophages in particle translocation from lungs to lymph nodes. Science 230, 1277–1280. [DOI] [PubMed] [Google Scholar]
- Hauser, A. R. & Engel, J. N. (1999). Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun 67, 5530–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh, D., Monack, D. M., Smith, M. R., Ghori, N., Falkow, S. & Zychlinsky, A. (1999). The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A 96, 2396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbi, H., Moss, J. E., Hersh, D., Chen, Y., Arondel, J., Banerjee, S., Flavell, R. A., Yuan, J., Sansonetti, P. J. & Zychlinsky, A. (1998). Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem 273, 32895–32900. [DOI] [PubMed] [Google Scholar]
- Isles, A., Maclusky, I., Corey, M., Gold, R., Prober, C., Fleming, P. & Levison, H. (1984). Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr 104, 206–210. [DOI] [PubMed] [Google Scholar]
- Jennings, W. E. (1963). Diseases transmitted from animals to man. In Glanders, pp. 264–292. Edited by T. G. Hull. Springfield, IL: Charles C. Thomas.
- Kespichayawattana, W., Rattanachetkul, S., Wanun, T., Utaisincharoen, P. & Sirisinha, S. (2000). Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect Immun 68, 5377–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler, H., Rodrigues, S. P. & McCormick, B. A. (2002). Shigella flexneri interactions with the basolateral membrane domain of polarized model intestinal epithelium: role of lipopolysaccharide in cell invasion and in activation of the mitogen-activated protein kinase ERK. Infect Immun 70, 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever, M. S., Nelson, M., Ireland, P. I., Stagg, A. J., Beedham, R. J., Hall, G. A., Knight, G. & Titball, R. W. (2003). Experimental aerogenic Burkholderia mallei (glanders) infection in the BALB/c mouse. J Med Microbiol 52, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Mandell, G., Bennett, J. & Dolin, R. (1995). Pseudomonas species (including melioidosis and glanders). In Principles and Practices of Infectious Diseases, pp. 2006–2007. Edited by G. Mandell, J. Bennett & R. Dolin. New York: Churchill Livingstone.
- Monack, D. & Falkow, S. (2000). Apoptosis as a common bacterial virulence strategy. Int J Med Microbiol 290, 7–13. [DOI] [PubMed] [Google Scholar]
- Ogawa, M., Suzuki, T., Tatsuno, I., Abe, H. & Sasakawa, C. (2003). IcsB, secreted via the type III secretion system, is chaperoned by IpgA and required at the post-invasion stage of Shigella pathogenicity. Mol Microbiol 48, 913–931. [DOI] [PubMed] [Google Scholar]
- Ribot, W. J. & Ulrich, R. L. (2006). The animal pathogen-like type III secretion system is required for the intracellular survival of Burkholderia mallei within J774.2 macrophages. Infect Immun 74, 4349–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotz, L. D., Khan, A. S., Lillibridge, S. R., Ostroff, S. M. & Hughes, J. M. (2002). Public health assessment of potential biological terrorism agents. Emerg Infect Dis 8, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, A., Kraus, C. N., DeShazer, D., Becker, P. M., Dick, J. D., Spacek, L., Bartlett, J. G., Byrne, W. R. & Thomas, D. L. (2001). Glanders in a military research microbiologist. N Engl J Med 345, 256–258. [DOI] [PubMed] [Google Scholar]
- Stevens, M. P., Haque, A., Atkins, T., Hill, J., Wood, M. W., Easton, A., Nelson, M., Underwood-Fowler, C., Titball, R. W. & other authors (2004). Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 150, 2669–2676. [DOI] [PubMed] [Google Scholar]
- Stevens, J. M., Ulrich, R. L., Taylor, L. A., Wood, M. W., Deshazer, D., Stevens, M. P. & Galyov, E. E. (2005). Actin-binding proteins from Burkholderia mallei and Burkholderia thailandensis can functionally compensate for the actin-based motility defect of a Burkholderia pseudomallei bimA mutant. J Bacteriol 187, 7857–7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, G. W., Lu, J., Pervaiz, S., Cao, W. P. & Gan, Y. H. (2005). Caspase-1 dependent macrophage death induced by Burkholderia pseudomallei. Cell Microbiol 7, 1447–1458. [DOI] [PubMed] [Google Scholar]
- Suparak, S., Kespichayawattana, W., Haque, A., Easton, A., Damnin, S., Lertmemongkolchai, G., Bancroft, G. J. & Korbsrisate, S. (2005). Multinucleated giant cell formation and apoptosis in infected host cells is mediated by Burkholderia pseudomallei type III secretion protein BipB. J Bacteriol 187, 6556–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich, R. L. & DeShazer, D. (2004). Type III secretion: a virulence factor delivery system essential for the pathogenicity of Burkholderia mallei. Infect Immun 72, 1150–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utaisincharoen, P., Tangthawornchaikul, N., Kespichayawattana, W., Anuntagool, N., Chaisuriya, P. & Sirisinha, S. (2000). Kinetic studies of the production of nitric oxide (NO) and tumour necrosis factor-alpha (TNF-α) in macrophages stimulated with Burkholderia pseudomallei endotoxin. Clin Exp Immunol 122, 324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman, S. R. & Holden, D. W. (2003). Functions and effectors of the Salmonella pathogenicity island 2 type III secretion system. Cell Microbiol 5, 501–511. [DOI] [PubMed] [Google Scholar]
- Weinrauch, Y. & Zychlinsky, A. (1999). The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol 53, 155–187. [DOI] [PubMed] [Google Scholar]
- White, N. J. (2003). Melioidosis. Lancet 361, 1715–1722. [DOI] [PubMed] [Google Scholar]
- Whitlock, G. C., Estes, D. M. & Torres, A. G. (2007). Glanders: off to the races with Burkholderia mallei. FEMS Microbiol Lett 277, 115–122. [DOI] [PubMed] [Google Scholar]
- Whitlock, G. C., Estes, D. M., Young, G., Young, B. & Torres, A. G. (2008). Construction of a reporter system to study Burkholderia mallei type III secretion and identification of the BopA effector protein function in intracellular survival. Trans R Soc Trop Med Hyg 102 (Suppl. 1), S127–S133. [DOI] [PubMed] [Google Scholar]
- Wiersinga, W. J., de Vos, A. F., de Beer, R., Wieland, C. W., Roelofs, J. J., Woods, D. E. & van der Poll, T. (2008). Inflammation patterns induced by different Burkholderia species in mice. Cell Microbiol 10, 81–87. [DOI] [PubMed] [Google Scholar]
- Yan, L. & Cirillo, J. D. (2004). Infection of murine macrophage cell lines by Legionella pneumophila. FEMS Microbiol Lett 230, 147–152. [DOI] [PubMed] [Google Scholar]
- Zhou, D. & Galan, J. (2001). Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect 3, 1293–1298. [DOI] [PubMed] [Google Scholar]
- Zychlinsky, A., Prevost, M. C. & Sansonetti, P. J. (1992). Shigella flexneri induces apoptosis in infected macrophages. Nature 358, 167–169. [DOI] [PubMed] [Google Scholar]