Abstract
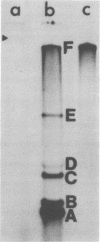
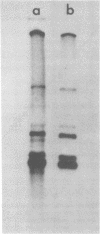
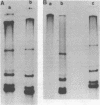
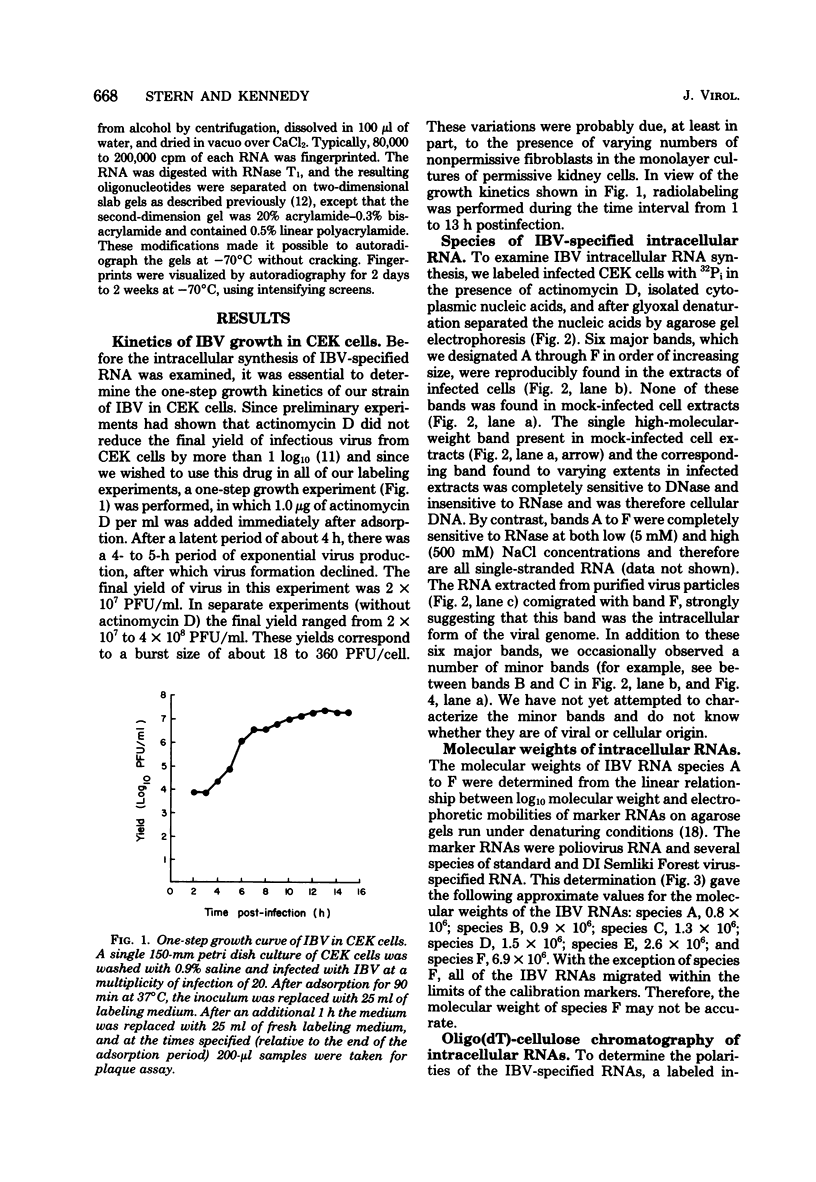
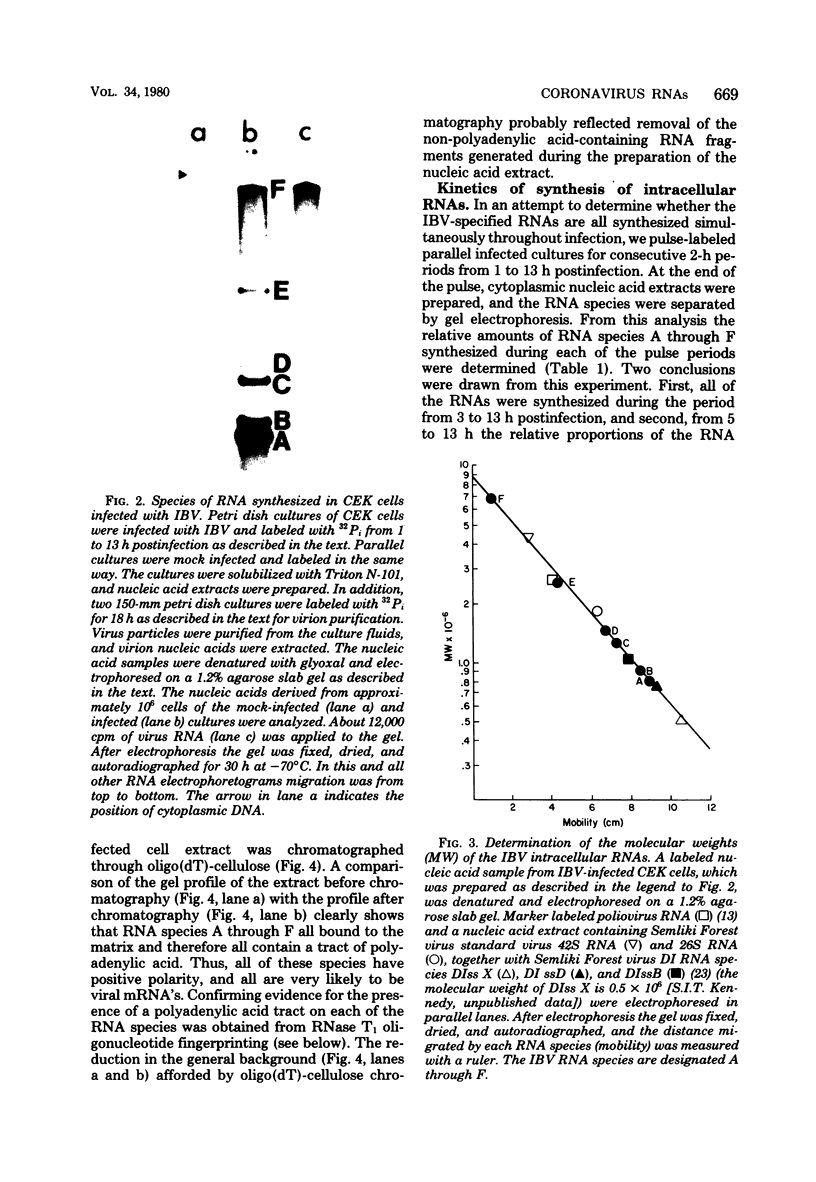
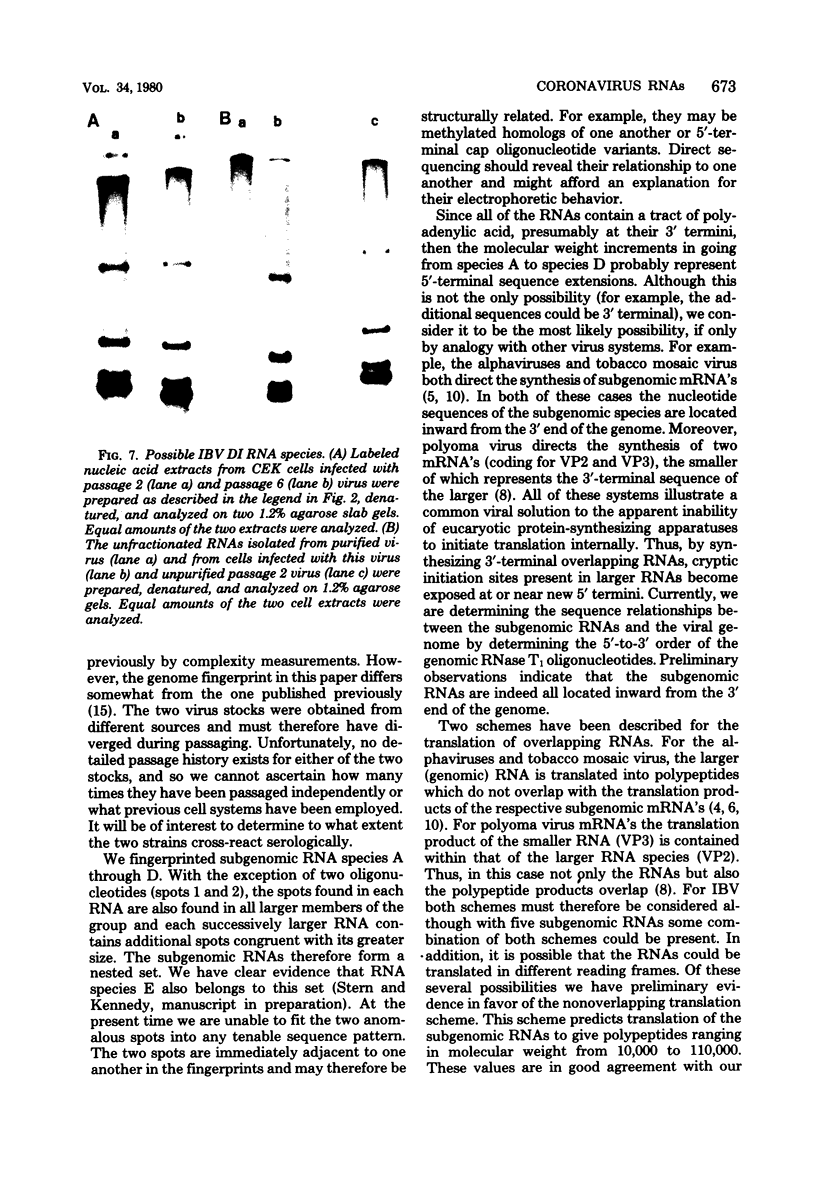
We examined the synthesis of intracellular RNA in primary chicken embryo kidney cells infected with the avian coronavirus infectious bronchitis virus. Infected cells were labeled with 32Pi in the presence of actinomycin D for the duration of the viral multiplication cycle, and nucleic acids were extracted, denatured, and analyzed on agarose slab gels. Six major RNA species were found. None of these RNAs was found in extracts of mock-infected cells. All six of the virus-specified RNAs (designated species A through F) were single stranded, and RNA species F had the same electrophoretic mobility as purified viral genome RNA. The molecular weights of the five subgenomic RNAs were estimated to be 0.8 × 106, 0.9 × 106, 1.3 × 106, 1.5 × 106, and 2.6 × 106 for species A through E, respectively. All of the RNAs were polyadenylated and are therefore likely to be viral mRNA's. The RNAs were synthesized in approximately constant proportions throughout the viral multiplication cycle. Intracellular RNA species A, B, C, D, and F and the purified viral genome were analyzed by RNase T1 fingerprinting. The results confirmed the identification of RNA species F as the intracellular genome and the derivation of the four smaller RNAs from the genome. Fingerprinting also showed that the intracellular RNAs constitute a nested set such that the nucleotide sequence of each RNA is contained within all larger RNAs and each larger RNA contains an additional sequence congruent with its greater size. Finally, the possible modes of transcription and translation of the infectious bronchitis virus RNAs are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Expression of animal virus genomes. Bacteriol Rev. 1971 Sep;35(3):235–241. doi: 10.1128/br.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C. W., Leibowitz J. L., Robb J. A. Pathogenic murine coronaviruses. II. Characterization of virus-specific proteins of murine coronaviruses JHMV and A59V. Virology. 1979 Apr 30;94(2):371–384. doi: 10.1016/0042-6822(79)90468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Kennedy S. I. Defective-interfering particles of Semliki Forest Virus: structural differences between standard virus and defective-interfering particles. J Gen Virol. 1976 Jun;31(3):383–395. doi: 10.1099/0022-1317-31-3-383. [DOI] [PubMed] [Google Scholar]
- Brzeski H., Kennedy S. I. Synthesis of Sindbis virus nonstructural polypeptides in chicken embryo fibroblasts. J Virol. 1977 May;22(2):420–429. doi: 10.1128/jvi.22.2.420-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzeski H., Kennedy S. I. Synthesis of alphavirus-specified RNA. J Virol. 1978 Feb;25(2):630–640. doi: 10.1128/jvi.25.2.630-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Polyadenylic acid sequences in the virus RNA species of cells infected with Semliki Forest Virus. J Gen Virol. 1974 Mar;22(3):331–345. doi: 10.1099/0022-1317-22-3-331. [DOI] [PubMed] [Google Scholar]
- Deininger P., Esty A., LaPorte P., Friedmann T. Nucleotide sequence and genetic organization of the polyoma late region: features common to the polyoma early region and SV40. Cell. 1979 Nov;18(3):771–779. doi: 10.1016/0092-8674(79)90130-2. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. Improved method for staining cell monolayers for virus plaque counts. J Bacteriol. 1959 Oct;78:596–597. doi: 10.1128/jb.78.4.596-597.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Kennedy D. A., Johnson-Lussenburg C. M. Inhibition of coronavirus 229E replication by actinomycin D. J Virol. 1979 Jan;29(1):401–404. doi: 10.1128/jvi.29.1.401-404.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Kitamura N., Nomoto A., Wimmer E. Sequence studies of poliovirus RNA. IV. Nucleotide sequence complexities of poliovirus type 1, type 2 and two type 1 defective interfering particles RNAs, and fingerprint of the poliovirus type 3 genome. J Gen Virol. 1979 Aug;44(2):311–322. doi: 10.1099/0022-1317-44-2-311. [DOI] [PubMed] [Google Scholar]
- Lomniczi B. Biological properties of avian coronavirus RNA. J Gen Virol. 1977 Sep;36(3):531–533. doi: 10.1099/0022-1317-36-3-531. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Kennedy I. Genome of infectious bronchitis virus. J Virol. 1977 Oct;24(1):99–107. doi: 10.1128/jvi.24.1.99-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The characterisation of the virion RNA of avian infectious bronchitis virus. FEBS Lett. 1977 May 15;77(2):311–313. doi: 10.1016/0014-5793(77)80258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnaughton M. R., Madge M. H. The polypeptide composition of avain infectious bronchitis virus particles. Arch Virol. 1977;55(1-2):47–54. doi: 10.1007/BF01314478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Kennedy S. I. Semliki forest virus persistence in mouse L929 cells. Virology. 1980 Jan 15;100(1):141–155. doi: 10.1016/0042-6822(80)90560-7. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Bond C. W. Pathogenic murine coronaviruses. I. Characterization of biological behavior in vitro and virus-specific intracellular RNA of strongly neurotropic JHMV and weakly neurotropic A59V viruses. Virology. 1979 Apr 30;94(2):352–370. doi: 10.1016/0042-6822(79)90467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Stevens R. H., Simpson R. W. Presence of infectious polyadenylated RNA in coronavirus avian bronchitis virus. Virology. 1977 Apr;77(2):772–782. doi: 10.1016/0042-6822(77)90498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark C., Kennedy S. I. The generation and propagation of defective-interfering particles of Semliki Forest virus in different cell types. Virology. 1978 Aug;89(1):285–299. doi: 10.1016/0042-6822(78)90060-0. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. A. The nucleic acid of infectious bronchitis virus. Arch Gesamte Virusforsch. 1973;43(3):259–271. doi: 10.1007/BF01250421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Alexander D. J., Almeida J. D., Cunningham C. H., Easterday B. C., Garwes D. J., Hierholzer J. C., Kapikian A., Macnaughton M. R., McIntosh K. Coronaviridae: second report. Intervirology. 1978;10(6):321–328. doi: 10.1159/000148996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H., Reeve P., Alexander D. J. The ribonucleic acid of infectious bronchitis virus. Arch Virol. 1975;47(3):279–286. doi: 10.1007/BF01317815. [DOI] [PMC free article] [PubMed] [Google Scholar]