Abstract
Therapeutic treatment with a non-neutralizing monoclonal antibody (mAb) (131-2G) specific to respiratory syncytial virus (RSV) G glycoprotein mediates virus clearance and decreases leukocyte trafficking and interferon gamma (IFN-γ) production in the lungs of RSV-infected mice. Its F(ab′)2 component only mediates decreased leukocyte trafficking and IFN-γ production without reducing virus replication. Thus, this mAb has two independent actions that could facilitate treatment and/or prevention of RSV infection by reducing both virus replication and virus-induced pulmonary inflammation.
Respiratory syncytial virus (RSV) is an important cause of severe respiratory disease in infants, young children and the elderly (Panitch, 2001; Shay et al., 1999; Hall et al., 1991; Falsey et al., 1995). Despite several decades of research, there is still no effective treatment for or licensed vaccine to prevent RSV infection. While prophylaxis with Palivizumab, a humanized IgG monoclonal antibody (mAb) directed against the F protein of RSV, has demonstrated a level of effectiveness in reducing hospitalization, neither it nor any other antiviral therapy has been effective in treating disease once infection is established. One possible explanation for the difficulty in treating RSV disease is that the host inflammatory response induced early in infection is an important contributor to disease pathogenesis and persists after virus replication has ended (Varga & Brachiale, 2002; Mejias et al., 2004).
It is clear from animal studies that RSV proteins modulate many aspects of the immune response to infection, particularly the RSV G protein (Tripp, 2004). A potential contributor to immune modulation and disease pathogenesis is CX3C chemokine mimicry mediated by the CX3C motif in the central conserved region of the G glycoprotein of RSV (Tripp et al., 2001). Through this motif, the RSV G protein binds to the fractalkine receptor, CX3CR1, and this interaction facilitates virus infection and modulates leukocyte chemotaxis (Tripp et al., 2001), including adversely affecting CX3CR1+ T cell responses (Harcourt et al., 2006). Studies have shown that antibodies that block G protein CX3C–CX3CR1 interaction protect against some of the G protein-associated enhanced inflammation observed after RSV infection (Haynes et al., 2003). In addition, anti-RSV G protein antibody responses after natural infection or vaccination with live-attenuated RSV vaccine in young children are associated with the inhibition of G protein-mediated human leukocyte migration and G protein CX3C–CX3CR1 interaction (Harcourt et al., 2004). A non-neutralizing anti-RSV G protein mAb, 131-2G, has been shown to inhibit RSV G protein binding to CX3CR1 and RSV G protein-induced chemotaxis (Tripp et al., 2001), so we hypothesized that this mAb might also decrease RSV G protein-mediated inflammation that might contribute to disease pathogenesis. In a murine model of RSV infection, we found that treatment with mAb 131-2G 3 days after infection not only reduced pulmonary inflammation and inflammatory cytokine production but also increased virus clearance (L. Haynes and others, unpublished results). The increase in viral clearance was not expected because mAb 131-2G does not neutralize RSV (Anderson et al., 1988). One possible mechanism used by non-neutralizing antibodies to mediate increased viral clearance is antibody-dependent cellular cytotoxicity (ADCC) (Hirsch, 1982). ADCC is triggered when antibody bound to the surface of a virus interacts with Fc receptors on leukocytes, whereby the virus is eliminated. To determine whether ADCC contributed to mAb 131-2G-associated viral clearance, we examined viral clearance and pulmonary inflammation following treatment with intact or F(ab′)2 forms of mAb 131-2G in an established BALB/c mouse model.
131-2G F(ab′)2 fragments were generated by pepsin digestion (Sigma). Briefly, purified mAb 131-2G was digested with porcine pepsin overnight at 37 °C, and digested mAb was passed through a protein G Sepharose column (GE Healthcare) to eliminate Fc fragments and undigested antibodies. Purified F(ab′)2 fragments were dialysed and concentrated using Centricon spin column (Millipore) with a 30 kDa cut-off. The purity of the F(ab′)2 fragments was determined by SDS-PAGE (Bio-Rad) under non-reducing conditions followed by Western blot analysis, using anti-Fab and anti-Fc antibodies (Bethyl Laboratories). The protein concentration was determined by micro BCA protein assay (Pierce) and the reactivity of the F(ab′)2 fragments was determined by RSV ELISA as described previously (Harcourt et al., 2003). Anaesthetized (Avertin), 6–8-week-old BALB/c mice (Charles Rivers) were intranasally inoculated with 106 p.f.u. RSV A2 strain (Centers for Disease Control and Prevention) in 50 μl serum-free Dulbecco's modified Eagle's medium (Invitrogen). On day 3 post-infection (p.i.), mice were treated intraperitoneally with 150–300 μg intact or F(ab′)2 forms of mAb 131-2G per mouse. Control mice were treated with the same isotype and concentration of intact or F(ab′)2 normal mouse IgG (nIg) (Pierce). Appropriate groups of mice were anaesthetized with Avertin and exsanguinated by severing the right caudal artery on days 3 (prior to treatment), 5 (the peak of virus replication) and 7 p.i. No fewer than three mice per treatment were examined per time point and the experiments were independently repeated three times. All studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Virus titration was performed on individual lungs that were homogenized in sterile PBS (Invitrogen) and virus titres were determined by immunostaining plaque assays as described previously (Haynes et al., 2002). Bronchoalveolar leukocytes (BAL) were harvested by lavaging the lungs of anaesthetized mice three times with 1 ml sterile PBS and the cell types were enumerated using Turk's staining. The procedure used for extracellular staining of BAL was modified for microculture staining as described previously (Tripp et al., 1999), using fluorescein isothiocyanate-, phycoerythrin- or allophycocyanin-conjugated antibodies anti-CD3 (145-2C11), anti-CD45R/B220 (RA3-6B2), anti-pan natural killer (NK) cell (DX5), anti-CD11b (M1/70), anti-neutrophil [polymorphonuclear leukocyte (PMN)] (RB6-8C5) and isotype control antibodies (BD Biosciences and eBiosciences). The concentrations of interferon gamma (IFN-γ) and interleukin (IL)-4 in the BAL cell-free supernatant were measured by ELISA (eBioscience) and the results were expressed as pg (ml BAL fluid)−1. Statistical significance was determined using Student's t test, where a P-value <0.05 was considered statistically significant.
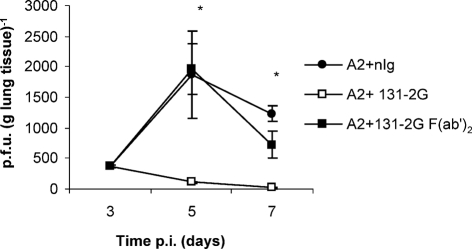
Treatment of BALB/c mice 1 day prior to or 3 days after RSV infection with mAb 131-2G dramatically inhibited RSV replication at day 5 p.i. [<200 p.f.u. (g lung tissue)−1] compared with normal isotype control mAb treatment [1400–5000 p.f.u. (g lung tissue)−1] (L. Haynes and others, unpublished results). To understand the mechanism of virus clearance linked to treatment with this mAb better, RSV-infected mice were treated with either intact or F(ab′)2 fragments of nIg or mAb 131-2G on day 3 p.i. and the virus titres were determined (Fig. 1). The viral load in lungs of mice treated with intact nIg increased from days 3 to 5 p.i. and, as expected, virus was cleared by day 7 p.i. Two days after mAb 131-2G treatment, the viral load in mice treated with intact mAb was significantly (P<0.048) reduced compared with lung titres in F(ab′)2-treated mice which showed no significant reduction compared with nIg-treated mice. The ability of this non-neutralizing antibody to prevent virus replication in vivo is consistent with earlier observations and reports using other non-neutralizing RSV G protein antibodies (Corbeil et al., 1996; Plotnicky-Gilquin et al., 1999; Mekseepralard et al., 2006). The results suggest that mAb 131-2G may mediate virus clearance through an ADCC mechanism. Failure of the mAb 131-2G F(ab′)2 fragments to control virus replication may also be related to differences in pharmacokinetic properties, serum half life, tissue distribution and accumulation of F(ab′)2 fragments compared with the intact immunoglobulin (Brown et al. 1987).
Fig. 1.
RSV titres in lungs following antibody treatment. The lungs from antibody-treated [nIg, anti-RSV G or anti-RSV G F(ab′)2] mice were harvested on days 3, 5 and 7 p.i. and virus titres were determined by immunostaining plaque assays. Results are representative of three independent experiments (mean±se). Asterisks indicate a significant difference (P<0.05) between nIg-treated and antibody-treated mice.
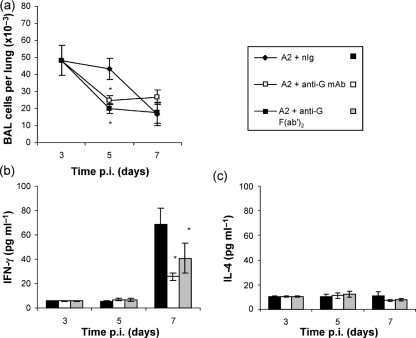
The levels of pulmonary cell infiltration and cytokine expression were compared to determine the effect of intact and F(ab′)2 mAb 131-2G treatment on aspects of the inflammatory response associated with RSV infection. By day 5 p.i., which is the period of maximal virus replication (Fig. 1), treatment with mAb 131-2G was associated with a significant (P<0.05) decrease in the total BAL cell infiltration compared with nIg antibody-treated mice (Fig. 2a). Mice treated with intact mAb 131-2G exhibited a 65–78 % reduction in cell types expressing PMN, B220+ and CD11b+, and a modest decrease in CD3+ and DX5+ cells (Table 1), suggesting that mAb 131-2G treatment reduces the parameters of RSV-associated pulmonary inflammation. Interestingly, mAb 131-2G F(ab′)2-treated mice also displayed a similar reduction in total pulmonary infiltration, particularly in B220+, CD11b+, CD3+ and PMN cells. Furthermore, mAb 131-2G F(ab′)2 treatment was associated with a significant (P<0.032) decrease in DX5+ NK cell infiltration (56 % reduction) (Table 1). It is of note that BAL cell infiltration and levels of cytokine production after treatment with nIg F(ab′)2 fragments or IgG1 isotype-matched control mAbs were comparable to nIg controls (data not shown). Additionally, IFN-γ production in BAL fluids at day 7 p.i. was dramatically and significantly (P<0.05) decreased following intact or F(ab′)2 mAb 131-2G treatment (Fig. 2b), but neither antibody treatment affected the already low levels of IL-4 (Fig. 2c). These results suggest that, while the Fc portion of 131-2G mAb is necessary for mediating clearance of RSV, it is not required for modulating the pulmonary cell infiltration and pro-inflammatory cytokine production. Taken together, these results show that mAb 131-2G can both decrease replication of RSV and alter the virus-induced host immune response to infection. Interestingly, viral clearance mediated by mAb 131-2G requires the Fc portion, while the ability to modify pulmonary inflammation is independent of both reduction in virus replication and the presence of Fc receptor.
Fig. 2.
Pulmonary infiltration and cytokine production following antibody treatment. (a) BAL cell numbers in the lungs of mice treated at day 3 p.i. with nIg, anti-RSV G mAb or anti-RSV G F(ab′)2 mAb were determined at days 3 (prior to treatment), 5 and 7 p.i. Data shown are the means±se. (b, c) ELISA was used to determine the IFN-γ (b) and IL-4 (c) cytokine levels in cell-free BAL lavage fluid from antibody-treated mice. The data are expressed as the mean (±se) cytokine concentration. Asterisks indicate a significant difference (P<0.05) between nIg-treated and antibody-treated mice. Results are representative of three independent experiments.
Table 1.
Pulmonary leukocyte trafficking in nIg-, anti-G- or anti-G F(ab′)2-treated RSV strain A2-infected mice
RSV-infected mice were treated with nIg, anti-G mAb or anti-G F(ab′)2 mAb 3 days p.i. BAL were recovered from three mice per group on days 3, 5 and 7 p.i. The results from day 5 p.i. from a representative experiment are shown. Data represent the mean (±se) total BAL cells of each phenotype per lung (representative of two separate experiments). The total number of BAL cells expressing a particular phenotype was determined from the total number of BAL cells. Values in bold type are significantly different (P<0.05).
| Phenotype | nIg | Anti-G | Reduction* | Anti-G F(ab′)2 | Reduction |
|---|---|---|---|---|---|
| CD3 | 14733±2043 | 12650±1462 | 14 | 9280±1341 | 37 |
| B220 | 2513±349 | 550±63 | 78 | 1220±176 | 51 |
| DX5 | 11786±1634 | 8750±1011 | 26 | 5200±751 | 56 |
| CD11b | 7713±1069 | 2675±309 | 65 | 2000±289 | 74 |
| PMN | 5720±793 | 1525±176 | 73 | 2200±319 | 62 |
*The reduction is the change in total cell type after antibody treatment relative to total cell type after nIg treatment (%).
There are several possible mechanisms by which non-neutralizing antibodies can facilitate virus clearance, including ADCC, complement-dependent lysis of infected cells and complement-independent, cell-independent virus neutralization (Hirsch, 1982). Others have demonstrated that non-neutralizing anti-RSV G protein mAbs are sufficient to protect against RSV infection in mouse models (Corbeil et al., 1996; Plotnicky-Gilquin et al., 1999; Mekseepralard et al., 2006). For example, it was reported that passive administration of a non-neutralizing mAb (18A2B2) against the central conserved region of RSV G protein subgroup A protected mice from RSV infection (Corbeil et al. 1996). In these previous studies, treatment with the same mAb in complement-deficient mice or treatment with mAb F(ab′)2 fragments did not confer complete protection, suggesting that protection by the intact mAb involved both Fc-dependent pathways and the complement system (Corbeil et al., 1996). Other studies investigating a different subgroup A-specific anti-RSV G mAb (1C2) that recognized a similar region on the G protein, and a related mouse–human chimeric mAb (γ1 1C2), showed a level of protection against RSV infection when these antibodies were administered prophylactically to BALB/c mice (Mekseepralard et al. 2006). In these studies, virus replication was minimally inhibited in mice treated with an aglycosyl chimeric mAb defective in complement activation, FcγR binding and, therefore, ADCC activation, leading to the conclusion that protection from RSV infection was mediated by both Fc-dependent and Fc-independent mechanisms (Mekseepralard et al., 2006). In the studies cited here, F(ab′)2 fragments of mAb 131-2G did not facilitate virus clearance, suggesting that ADCC was involved in viral clearance from the lungs of RSV-infected mice. Previous in vitro neutralization assays demonstrated that mAb 131-2G does not neutralize RSV in the presence or absence of complement (data not shown), but these studies do not rule out the possibility that complement-mediated mechanisms are involved in mAb 131-2G-mediated viral clearance in vivo. We have not yet evaluated the role of the complement system in mAb 131-2G-mediated viral clearance.
The other role that mAb 131-2G plays in RSV infection in vivo, i.e. modifying the inflammatory response to RSV infection, is independent of the Fc portion of the antibody and not linked to viral clearance (Fig. 1). This finding is consistent with mAb 131-2G blocking immunomodulatory functions of the RSV G protein (Tripp, 2004). We suspect that this effect may be linked to blocking G protein CX3C–CX3CR1 interaction (Tripp et al., 2001). Our previous work has shown that mAb 131-2G can block RSV G protein CX3C–CX3CR1 interaction and associated downstream events that include altered leukocyte migration and activation that are mediated during RSV infection and that are associated with RSV G protein expression (Tripp et al., 2001).
The ability of mAb 131-2G to react against both A and B strains of RSV (Anderson et al., 1988) suggests that it binds within the central conserved region of G protein, from aa 164 to 176. The other regions of the G protein are hypervariable among A and B strains of RSV (Fodha et al., 2008). mAb 131-2G has been shown to bind to G protein from A and B strains of RSV between aa 1 and 173 (Sullender, 1995), which supports this hypothesis. Given its proximity to the CX3C chemokine motif, aa 182–186, it is not surprising that mAb 131-2G blocks G protein binding to CX3CR1.
In summary, the results show that 131-2G has two independent effects that could facilitate treatment and/or prevention of RSV disease, i.e. mediating virus clearance and decreasing virus-induced host pulmonary inflammation, a feature that is independent of the Fc portion of the antibody. Thus, mAb 131-2G or anti-G protein antibodies that react at the same region on the G protein could improve treatment and prevention of RSV disease, possibly in combination with a neutralizing F protein mAb or antiviral drugs, by increasing virus clearance or, possibly more importantly, by decreasing the RSV G protein-induced inflammatory response.
Acknowledgments
This research was supported in part by the National Institutes of Health (5RO1AI06275-03) and through the Georgia Research Alliance to R. A. T. This research was also supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education (ORISE) through an inter-agency agreement between the US Department of Energy and the CDC. H. C. and G. U. R. are ORISE fellows. R. T. and L. J. A. have an invention related to the study. No conflicts of interest exist among the authors based on federal regulations. The authors thank Jenna Dare for technical assistance and Jennifer Harcourt for technical assistance and manuscript review. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- Anderson, L. J., Bingham, P. & Hierholzer, J. C. (1988). Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol 62, 4232–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B. A., Comeau, R. D., Jones, P. L., Liberatore, F. A., Neacy, W. P., Sands, H. & Gallagher, B. M. (1987). Pharmokinetics of the monoclonal antibody B72.3 and its fragments labeled with either 125I or 111In. Cancer Res 47, 1149–1154. [PubMed] [Google Scholar]
- Corbeil, S., Seguin, C. & Trudel, M. (1996). Involvement of the complement system in the protection of mice from challenge with respiratory syncytial virus Long strain following passive immunization with monoclonal antibody 18A2B2. Vaccine 14, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey, A. R., Cunningham, C. K., Barker, W. H., Kouides, R. W., Yuen, J. B., Menegus, M., Weiner, L. B., Bonville, C. A. & Betts, R. F. (1995). Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis 172, 389–394. [DOI] [PubMed] [Google Scholar]
- Fodha, I., Vabret, A., Bouslama, L., Leroux, M., Legrand, L., Dina, J., Gouarin, S., Petitjean, J., Dewar, J. & other authors (2008). Molecular diversity of the aminoterminal region of the G protein gene of human respiratory syncytial virus subgroup B. Pathol Biol (Paris) 56, 50–57. [DOI] [PubMed] [Google Scholar]
- Hall, C. B., Walsh, E. E., Long, C. E. & Schnabel, K. C. (1991). Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163, 693–698. [DOI] [PubMed] [Google Scholar]
- Harcourt, J. L., Brown, M. P., Anderson, L. J. & Tripp, R. A. (2003). CD40 ligand (CD154) improves the durability of respiratory syncytial virus DNA vaccination in BALB/c mice. Vaccine 21, 2964–2979. [DOI] [PubMed] [Google Scholar]
- Harcourt, J. L., Karron, R. A. & Tripp, R. A. (2004). Anti-G protein antibody responses to respiratory syncytial virus infection or vaccination are associated with inhibition of G protein CX3C–CX3CR1 binding and leukocyte chemotaxis. J Infect Dis 190, 1936–1940. [DOI] [PubMed] [Google Scholar]
- Harcourt, J. L., Alvarex, R., Jones, L. P., Henderson, C., Anderson, L. J. & Tripp, R. A. (2006). Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 176, 1600–1608. [DOI] [PubMed] [Google Scholar]
- Haynes, L. M., Tonkin, J., Anderson, L. J. & Tripp, R. A. (2002). Neutralizing anti-F glycoprotein and anti-substance P antibody treatment effectively reduces infection and inflammation associated with respiratory syncytial virus infection. J Virol 76, 6873–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, L. M., Jones, L. P., Barskey, A., Anderson, L. J. & Tripp, R. A. (2003). Enhanced disease and pulmonary eosinophilia associated with formalin-inactivated respiratory syncytial virus vaccination are linked to G glycoprotein CX3C–CX3CR1 interaction and expression of substance P. J Virol 77, 9831–9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, R. L. (1982). The complement system: its importance in the host response to viral infection. Microbiol Rev 46, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejías, A., Chávez-Beuno, S., Ríos, A. M., Saavedra-Lozano, J., Fonseca Aten, M., Hatfield, J., Kapur, P., Gómez, A. M., Jafri, H. S. & Ramilo, O. (2004). Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrob Agents Chemother 48, 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekseepralard, C., Toms, G. L. & Routledge, E. G. (2006). Protection of mice against human respiratory syncytial virus by wild-type and aglycosyl mouse–human chimeric IgG antibodies to subgroup-conserved epitopes on the G glycoprotein. J Gen Virol 87, 1267–1273. [DOI] [PubMed] [Google Scholar]
- Panitch, H. B. (2001). Bronchiolitis in infants. Curr Opin Pediatr 13, 256–260. [DOI] [PubMed] [Google Scholar]
- Plotnicky-Gilquin, H., Goetsch, L., Huss, T., Champion, T., Beck, A., Haeuw, J., Nguyen, T. N., Bonnefoy, J., Corvaïa, N. & Power, U. F. (1999). Identification of multiple protective epitopes (protectopes) in the central conserved domain of a prototype human respiratory syncytial virus G protein. J Virol 73, 5637–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay, D. K., Holman, R. C., Newman, R. D., Liu, L. L., Stout, J. W. & Anderson, L. J. (1999). Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282, 1440–1446. [DOI] [PubMed] [Google Scholar]
- Sullender, W. (1995). Antigenic analysis of chimeric and truncated G proteins of respiratory syncytial virus. Virology 209, 70–79. [DOI] [PubMed] [Google Scholar]
- Tripp, R. A. (2004). Pathogenesis of respiratory syncytial virus infection. Viral Immunol 17, 165–181. [DOI] [PubMed] [Google Scholar]
- Tripp, R. A., Moore, D., Jones, L., Sullender, W., Winter, J. & Anderson, L. J. (1999). Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol 73, 7099–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp, R. A., Jones, L. P., Haynes, L. M., Zheng, H., Murphy, P. M. & Anderson, L. J. (2001). CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2, 732–738. [DOI] [PubMed] [Google Scholar]
- Varga, S. M. & Brachiale, T. J. (2002). RSV-induced immunopathology: dynamic interplay between the virus and host immune response. Virology 295, 203–207. [DOI] [PubMed] [Google Scholar]