Abstract
This study compared the ability of mosquito and mammalian cell-derived dengue virus (DENV) to infect human dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN)-expressing cells and characterized the structure of envelope (E) protein N-linked glycans on DENV derived from the two cell types. DENVs derived from both cell types were equally effective at infecting DC-SIGN-expressing human monocytes and dendritic cells. The N-linked glycans on mosquito cell-derived virus were a mix of high-mannose and paucimannose glycans. In virus derived from mammalian cells, the N-linked glycans were a mix of high-mannose and complex glycans. These results indicate that N-linked glycans are incompletely processed during DENV egress from cells, resulting in high-mannose glycans on viruses derived from both cell types. Studies with full-length and truncated E protein demonstrated that incomplete processing was most likely a result of the poor accessibility of glycans on the membrane-anchored protein.
INTRODUCTION
Dengue viruses (DENVs) are enveloped, positive-sense RNA viruses of the genus Flavivirus that are transmitted via the bite of Aedes mosquitoes. Each year, over 2.5 billion people are at risk of contracting dengue, 100 million people develop symptomatic infections and up to 2.5 % of dengue haemorrhagic fever (DHF) patients die (Gubler, 2002; Gubler & Clark, 1995; WHO, 2009). Despite the public health importance of dengue, the cell biology of DENV is poorly understood. Vector-borne viruses such as DENV must productively infect cells of both arthropod and mammalian origin. As the post-translational protein-processing machinery is different in insect and mammalian cells, the structure of glycoproteins produced in the two hosts may be different. Recent work with a wide variety of viruses has shown that protein glycosylation can influence viral virulence (reviewed by Vigerust & Shepherd, 2007). Recent studies with alphaviruses, which are also transmitted by arthropod vectors, have demonstrated that membrane-protein N-linked oligosaccharides are differentially processed by enzymes in insect and mammalian cells (Shabman et al., 2008). Structural differences in the glycans derived from insect and mammalian cells influence the ability of the viruses to infect target cells (Klimstra et al., 2003; Shabman et al., 2007, 2008). In this study, we characterized the N-linked glycans on the envelope protein of DENVs grown in different cell types and assessed the functional consequences of these differences.
The DENV particle is made up of three structural proteins: envelope (E), membrane (M) and capsid (C) (Chambers et al., 1990b; Kuhn et al., 2002). The E protein is the major membrane glycoprotein on the surface of the virion, responsible for virus attachment and fusion (Chambers et al., 1990b). Human dendritic cells (DCs) are a target of DENV infection (Ho et al., 2001; Marovich et al., 2001; Wu et al., 2000). Infection of DCs is mediated by the binding of DENV to DC-specific ICAM3-grabbing non-integrin (DC-SIGN), a C-type lectin that preferentially binds to terminal mannose sugars on glycans (Cambi & Figdor, 2003; Cambi et al., 2003; Engering et al., 2002; Navarro-Sanchez et al., 2003; Tassaneetrithep et al., 2003). DENV E protein has two potential N-linked glycosylation sites at N67 and N153, whereas most other flaviviruses have only a single N-linked glycosylation site at N154 (Chambers et al., 1990a; Heinz & Allison, 2003). DC-SIGN binds to DENV via the glycan at N67 on the E protein (Lozach et al., 2005; Mondotte et al., 2007; Navarro-Sanchez et al., 2003; Pokidysheva et al., 2006; Tassaneetrithep et al., 2003).
Virions produced in the mosquito vector and human host may have structurally different N-linked glycans, as insect and mammalian cells process glycans differently during exocytosis (Jarvis, 2003). Both hosts transfer a lipid-linked oligosaccharide, Glc3Man9GlcNAc2, to asparagine residues co-translationally in the endoplasmic reticulum (ER) (Hubbard & Ivatt, 1981). Insects and mammals have mannosidases and glucosidases capable of trimming mannose and glucose residues during exocytosis. In mosquito cells, proteins can make it through the exocytic pathway maximally (paucimannose) or minimally (high-mannose sugars) trimmed (Hsieh & Robbins, 1984; Hubbard & Ivatt, 1981; Jarvis, 2003; Marchal et al., 2001). In either case, terminal mannose residues are present on N-linked oligosaccharides in insects. Mammalian cells have enzymes that can further process trimmed oligosaccharides by adding sialic acid, glucose, galactose and other sugars to produce complex N-linked oligosaccharides with no terminal mannose residues (Jarvis, 2003; Pfeffer & Rothman, 1987). As DC-SIGN preferentially binds to terminal mannose residues, one would predict that viruses grown in insect cells would bind to this receptor and infect DCs better than viruses grown in mammalian cells. In fact, DC-SIGN can distinguish between mosquito- and mammalian cell-derived alphavirus E2 glycans, resulting in more efficient infection by mosquito cell-derived virus (Klimstra et al., 2003; Shabman et al., 2007, 2008). Similarly, mosquito-derived West Nile virus (WNV), a flavivirus relative of DENV, also infects cells through DC-SIGN better than mammalian cell-derived virus. The ability of DC-SIGN to interact preferentially with mosquito-derived WNV maps to the lectin carbohydrate-recognition domains (Davis et al., 2006b).
The glycans on the DENV envelope have been roughly characterized for some serotypes. There is no consensus on the number and structure of the sugars added to the E protein in mosquito or mammalian cells. Smith & Wright (1985) first reported that the mouse-adapted DENV-2 (strain New Guinea C) has two N-linked glycans on the E glycoprotein. They also concluded that the sugars added were heterogeneous in structure and composition. Johnson et al. (1994) confirmed the addition of two glycans to the E protein of DENV-1 (strain Hawaii) grown in C6/36 cells, but found only a single glycan at position 67 in DENV-2 (strain Jamaica) grown in the same cells. They concluded that these sugars were high mannose based on their ability to bind concanavalin A (ConA). More recently, the structure of glycans has been characterized on the soluble E protein of DENV-1, DENV-2 and DENV-3. In these systems, both glycans were processed to paucimannose or complex glycans in insect and mammalian cells, respectively (Lozach et al., 2005; Miller et al., 2008; Modis et al., 2005). Two recent studies have examined the phenotype of E protein glycan-mutant DENVs and shown that the glycan at N153 is not necessary for virus production and spread in either mosquito or mammalian cells (Bryant et al., 2007; Miller et al., 2008). N67 was essential for virus spread in mammalian cells, which is consistent with a role for this glycan in binding to host-cell receptors. There is disagreement over whether the glycan at N67 is necessary for viral spread in mosquito cells (Bryant et al., 2007; Mondotte et al., 2007). Further studies are needed to characterize the structure and functional significance of N-linked glycans on the four serotypes of DENVs produced in mosquito and mammalian cells.
In this paper, we report that both mosquito- and mammalian-derived DENVs infect DC-SIGN-expressing cells with similar efficiency. We used lectin blots and enzymes that specifically cleave glycans of defined structure to characterize the N-linked glycans on mosquito and mammalian cell-derived DENV. All four serotypes of DENV grown in insect and mammalian cells had two N-linked glycans on the E protein. In both cell types, one of the glycans had a high-mannose structure indicating incomplete processing. The second sugar was either paucimannose or complex on virus produced in mosquito or mammalian cells, respectively. We propose that, unlike alphaviruses and other flaviviruses, DENVs derived from both hosts can efficiently infect DCs because of the presence of unprocessed, high-mannose glycans on the E protein.
METHODS
Cell lines and virus strains.
C6/36 Aedes albopictus cells (ATCC CRL-1660) were propagated in minimal essential media with Earle's salts (E-MEM) supplemented with 1 % l-glutamine, 1 % penicillin/streptomycin/Fungizone, 1 % non-essential amino acids and 10 % fetal bovine serum (FBS; Gibco/Invitrogen) at 28 °C in 5 % CO2. Vero (African green monkey) clone 81 cells were a gift from Robert Putnak at the Walter Reed Army Medical Center (Washington, DC, USA) and were propagated in Dulbecco's modified Eagle's medium/Nutrient Mixture F-12 (DMEM/F-12) supplemented with 1 % l-glutamine, 1 % penicillin/streptomycin/Fungizone, 1 % non-essential amino acids, 0.2 % sodium bicarbonate and 10 % FBS (Gibco/Invitrogen) at 37 °C in 5 % CO2. The DENV strains used in this study were DENV-1 West Pac 74, DENV-2 New Guinea C and 16681, DENV-3 H87 and CH53489, and DENV-4 TVP 360.
Virus propagation and purification.
DENV stocks were grown in C6/36 mosquito cells or Vero cells. To generate stocks, seed virus was added to 80 % confluent cells at an m.o.i. of 0.01 in reduced-serum medium (E-MEM supplemented with 1 % l-glutamine, 1 % penicillin/streptomycin/Fungizone, 1 % non-essential amino acids and 2 % FBS for C6/36 cells, and DMEM/F-12 supplemented with 1 % l-glutamine, 1 % penicillin/streptomycin/Fungizone, 1 % non-essential amino acids, 0.2 % sodium bicarbonate and 5 % FBS for Vero cells). After 7 days, the medium was harvested from the cells and clarified by centrifugation at 10 000 g for 30 min. The virus-containing supernatant was supplemented with 20 % FBS and stored at −80 °C. For studies requiring purified and concentrated virus, the supernatant was centrifuged at 76 221 g for 5 h to pellet the virus under a 20 % sucrose : PBS (w/v) cushion. Pelleted virus was resuspended in PBS, loaded onto a 15–60 % (v/v) continuous iodixanol gradient, and centrifuged at 29 331 g for 154 min. Fractions containing purified virus were diluted in PBS and centrifuged at 76 221 g for 5 h to pellet the virus and remove the iodixanol. Purified virus was stored at −80 °C.
Virus genome quantification and titration.
Viral genomes were quantified by real-time PCR as described previously using primers to amplify the 3′-untranslated region (Houng et al., 2001). To calculate the m.o.i. for infection assays, virus titres were determined using flow cytometry using a method similar to that described by Lambeth et al. (2005). Briefly, 2×103 Vero cells were plated in 96-well plates and infected with known amounts of viral genomes. At 24 h post-infection (p.i.), infected cells were stained and infection was determined by flow cytometry. Cells were fixed, permeabilized and stained with Alexa Fluor 488-conjugated anti-flavivirus E protein monoclonal antibody (mAb) 4G2 (ATCC HB-112). Virus released into the supernatant of infected cells was quantified in Vero cells in a 24-well-format immunofocus assay modified from that described in AP61 cells (Despres et al., 1993). Subconfluent Vero monolayers were infected with serial dilutions of infected cells. Overlay medium (Opti-MEM I containing 0.8 % methyl cellulose; Electron Microscopy Sciences) was then added to plates and the cells were incubated for 5 days at 37 °C in 5 % CO2. Monolayers were stained with 400 ng mAb 4G2 followed by a 1 : 500 dilution of horseradish peroxidase (HRP)-conjugated secondary antibody (Sigma-Aldrich) for 1 h, each at 37 °C. Foci were visualized with HRP substrate. Titres were expressed as focus forming units (f.f.u.) ml−1 and were calculated by multiplying the mean number of foci per well at a given dilution by the inverse dilution factor and dividing by the volume added to each well.
DENV infection of U937 cells expressing DC-SIGN.
A human monocytic cell line (U937) constitutively expressing DC-SIGN was obtained from Mark Heise at the University of North Carolina at Chapel Hill, NC, USA (Kraus et al., 2007). The parental and DC-SIGN transduced cells were maintained at 37 °C in 5 % CO2 in complete RPMI medium (RPMI 1640 supplemented with 1 % l-glutamine, 1 % penicillin/streptomycin/Fungizone, 1 % non-essential amino acids, 50 mM 2-mercaptoethanol and 10 % FBS). For infection assays, virus was added to cells at an m.o.i. of 0.001, 0.01 or 0.05. Cells and virus were incubated for 2 h in the presence of medium alone, isotype control mAb clone 20116 (100 μg ml−1; R&D Systems), anti-CD209 blocking mAb clone 120507 (100 μg ml−1; R&D Systems), 20 μg mannan ml−1 or 5 mM EDTA. After 2 h, the cells were washed and resuspended in complete RPMI medium. At 24 h p.i., cells were processed and stained for infection with mAb 4G2 as described above. For the 48 h DENV2 time-course experiment, cells and supernatants were harvested at 12 h intervals. Cells were processed for fluorescence-activated cell sorting and supernatants were titrated on Vero cells in an immunofocus assay as described above.
Isolation and infection of monocytes-derived DCs.
Human DCs were derived from peripheral blood monocytes as described by Moran et al. (2005). Briefly, buffy coats obtained from the American Red Cross were diluted 1 : 2 in PBS and peripheral blood monocytic cells were isolated by centrifugation over Ficoll-Hypaque (Sigma). Monocytes were enriched by adding 1×108 cells to a tissue culture flask for 2 h and removing non-adherent cells. Adherent cells were cultured in complete RPMI medium supplemented with 800 U granulocyte–macrophage colony-stimulating factor ml−1 and 500 U interleukin-4 (Peprotech) ml−1. Fresh cytokines were added on day 3 and immature DCs were harvested on day 6. Immature DCs were infected with DENV at an m.o.i. of 0.05 and processed for flow cytometry as described above for U937+DC-SIGN cells.
Characterization of N-linked glycans on DENV.
Purified DENVs were digested with endoglycosidase H (EndoH) or peptide: N-glycosidase F (PNGaseF; New England BioLabs) according to the manufacturer's protocol with a minor modification. Instead of denaturation in the provided glycoprotein denaturation buffer containing dithiothreitol, viruses were denatured in 0.5 % SDS. Treatment with these enzymes can determine whether glycans are high mannose or complex (Maley et al., 1989). Digested viral proteins were separated by SDS-PAGE under non-reducing conditions and analysed by Western or lectin blots. In Western blots, E protein was detected using mAb 4G2 as a primary antibody and HRP-conjugated goat anti-mouse IgG as a secondary antibody. Glycosylated envelope was detected with the lectins Galanthus nivalis agglutinin (GNA) or Datura stramonium agglutinin (DSA) according to the manufacturer's protocol (DIG Glycan Differentiation kit; Roche). Western and lectin blot films were scanned and images were processed using Adobe Photoshop.
Determination of the role of prM in E protein N-linked glycan processing.
To study whether E packaging in viral or subviral particles affected the processing of N-linked glycans in the E protein, we created constructs of DENV-2 truncated ectodomain E and full-length E protein for expression using Venezuelan equine encephalitis virus (VEEV) replicons as described previously (White et al., 2007). RT-PCR was used to create full-length E protein with prM (prM/E) and the soluble ectodomain of E with prM (prM/E85). The amplified DNAs were cloned into the multicloning sites of VEEV replicon vectors provided by Nancy Davis of the Carolina Vaccine Institute (NC, USA). Full-length T7 transcripts were generated as described previously and electroporated into Vero 81 cells (Davis et al., 2000). Cells were plated into six-well plates. At 4 h post-electroporation, the cells were starved for 1 h in cysteine/methionine-free medium (MP Biomedicals). At 5 h post-electroporation, cells were metabolically labelled with [35S]methionine/cysteine [100 μCi (3.7 MBq) per well, Promix amino acid mixture; GE Healthcare] for 7 h. At the end of the labelling period, the cells and medium were harvested to examine cell-associated and secreted E protein. The medium was clarified by centrifugation at 10 000 g for 10 min. Labelled cells were lysed in TNE buffer [10 mM Tris/HCl (pH 7.4), 200 mM NaCl, 1 mM EDTA] containing 1 % (v/v) NP-40 and protease inhibitors. Cell-associated and secreted E proteins were immunoprecipitated with 2 μg mAb 4G2 and protein A–Sepharose beads (Sigma). Purified proteins were digested with the endoglycosidases described above, separated by SDS-PAGE under reducing conditions and visualized using a PhosphorImager or X-ray film.
RESULTS
Infection of DC-SIGN-expressing monocytes and DCs by mosquito and mammalian cell-derived DENV
Studies with some mosquito-borne viruses have demonstrated that virions produced in insect cells infect DCs better than virions produced in mammalian cells (Davis et al., 2006b; Klimstra et al., 2003; Shabman et al., 2007). In these studies, the superior infectivity of mosquito-derived virus was linked to high-mannose N-linked glycans present in mosquito but not mammalian cell-derived virus (Davis et al., 2006a, b; Klimstra et al., 2003; Shabman et al., 2007, 2008). We performed experiments to determine whether mosquito cell-derived DENV also infected monocytes and DCs better than mammalian cell-derived virus.
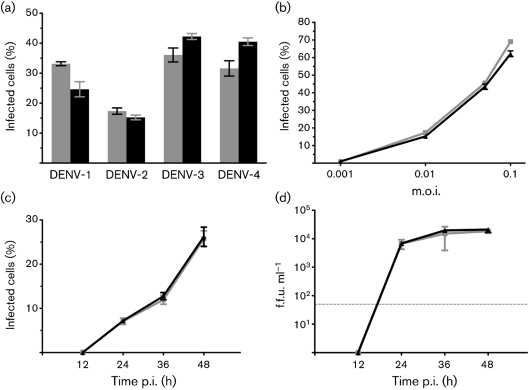
Initial experiments were conducted with a human monocytic cell line (U937) engineered to stably express human DC-SIGN, a DENV attachment factor (Kraus et al., 2007). Mosquito and mammalian cell-derived DENVs were used to infect DC-SIGN-expressing U937 cells (Fig. 1a). Cells were harvested at 24 h p.i. following a single cycle of replication. We determined the efficiency of infection by measuring the percentage of cells expressing DENV E protein. Both mosquito and mammalian cell-derived viruses of all four DENV serotypes infected similar numbers of cells (Fig. 1a). To determine whether this phenotype was dependent on the amount of virus used to infect cells or the time of testing, we performed dose–response experiments and growth curves with DENV-2. At all m.o.i. tested, mosquito and mammalian cell-derived DENV-2 infected similar percentages of cells (Fig. 1b). Additionally, similar percentages of DC-SIGN-expressing U937 cells were infected at all time points tested (Fig. 1c) and similar amounts of virus were released into the supernatant (Fig. 1d).
Fig. 1.
Infection of human U937 cells expressing DC-SIGN by mosquito and mammalian cell-derived DENV. (a) DC-SIGN-expressing U937 cells were infected with all four serotypes of DENV derived from insect cells (shaded bars) or mammalian cells (filled bars). DENV-1 and DENV-4 were added at an m.o.i. of 0.001, whilst DENV-2 and DENV-3 were added at an m.o.i. of 0.01. (b) Mosquito-derived (grey squares) and mammalian-derived (black triangles) DENV-2 was added to U937 cells expressing DC-SIGN at an m.o.i. of 0.001, 0.01, 0.05 or 0.1. (c, d) DC-SIGN-expressing U937 cells were infected with mosquito-derived (grey squares) or mammalian-derived (black triangles) DENV-2 at an m.o.i. of 0.01. At 12 h intervals for 48 h, cells were harvested and stained for intracellular antigen (c) and supernatants were titrated to determine virus release (d). All values represent the means±sd of experiments performed in triplicate and are representative of one of two experiments. The dotted horizontal line in (d) indicates the level of detection of our immunofocus titration assay.
Interactions between DC-SIGN and its mannose ligand can be inhibited by anti-DC-SIGN antibody, excess mannan or EDTA treatment (Navarro-Sanchez et al., 2003; Tassaneetrithep et al., 2003). To confirm that mosquito and mammalian cell-derived DENV infection was DC-SIGN dependent, we infected parental U937 cells and DC-SIGN-expressing U937 cells in the presence of inhibitors (Fig. 2a). As expected, the expression of DC-SIGN enhanced DENV infection (Navarro-Sanchez et al., 2003; Tassaneetrithep et al., 2003). Pre-incubation of cells with DC-SIGN blocking antibody (100 μg ml−1), mannan (20 μg ml−1) or EDTA (5 mM) for 30 min reduced infection of both mosquito and mammalian cell-derived DENV (Fig. 2a). These studies indicated that mosquito and mammalian cell-derived DENVs use DC-SIGN and infect cells with similar efficiency.
Fig. 2.
Infection of primary human myeloid DCs and DC-SIGN-expressing monocytes by mosquito and mammalian cell-derived DENV is dependent of DC-SIGN. DC-SIGN-expressing U937 cells (a) and DCs from three different donors (b) were infected with mosquito-derived (shaded bars) or mammalian-derived (filled bars) DENV-2 at an m.o.i. of 0.05. Infections were carried out in the presence or absence of anti-DC-SIGN antibody, mannan or EDTA. Cells were harvested at 24 h p.i. and infected cells were detected by staining with an anti-E protein antibody followed by flow cytometry. Values represent the means±sd of experiments performed in triplicate and are representative of one of three experiments.
Primary human myeloid DCs are susceptible to DENV infection and are likely to be an important target during natural infection (Ho et al., 2001; Marovich et al., 2001). Experiments were carried out to compare infection of these primary cells with mosquito and mammalian cell-derived DENV. Myeloid DCs were obtained from three different donors and infected with DENV at an m.o.i. of 0.05. Both mosquito and mammalian cell-derived viruses infected similar proportions of primary DCs (Fig. 2b). Moreover, DENV-2 infection of primary DCs was reduced by DC-SIGN antibody, mannan and EDTA, indicating that infection was dependent on DC-SIGN (Fig. 2b). We concluded that, unlike some other mosquito-borne viruses, insect and mammalian cell-derived DENVs infect DC-SIGN-expressing cells with similar efficiency (Davis et al., 2006b; Klimstra et al., 2003; Shabman et al., 2007).
Composition of N-linked glycans on DENV
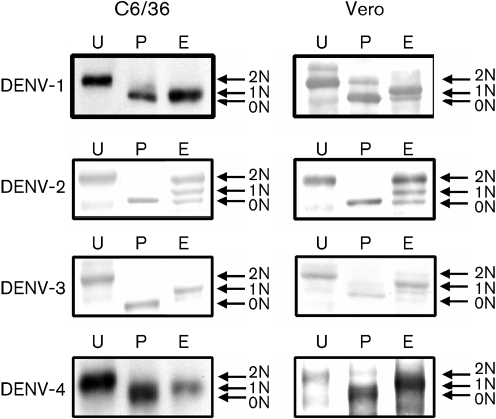
The DENV E protein possesses two conserved, potential N-linked glycosylation sites at N67 and N153 (Chambers et al., 1990b; Smith & Wright, 1985). To determine why mosquito cell-derived DENV did not have an advantage infecting via DC-SIGN compared with mammalian cell-derived virus, we compared the structures of the glycans added to the E protein of DENV produced in the two cell types. Purified DENV-2 and DENV-3 were treated with PNGaseF or EndoH. PNGaseF removes all N-linked glycans, whereas EndoH removes only N-linked sugars containing more than three terminal mannose residues, typically found in immature N-linked glycans (Maley et al., 1989). Following treatment with PNGaseF, DENV grown in Vero and C6/36 cells had an electrophoretic shift of approximately 4 kDa, corresponding to the loss of two glycans (Fig. 3, labelled 0N) (Smith & Wright, 1985). When the viruses were treated with EndoH, DENV-2 displayed differing digestion patterns compared with DENV-1, DENV-3 and DENV-4. The E protein produced in both mosquito and mammalian cell-derived DENV-1, DENV-2 and DENV-4 exhibited an electrophoretic shift corresponding to 2 kDa, indicating the presence of one high-mannose, EndoH-sensitive glycan and another more processed glycan that was EndoH-resistant (Fig. 3, labelled 1N). DENV-2 did not exhibit one distinct band when digested with EndoH. Instead, there were three bands corresponding to the E protein: one migrated with undigested E protein (2N), one migrated with PNGaseF-digested E protein (0N) and one migrated between the undigested and deglycosylated E protein (1N) (Fig. 3). These results indicated that the glycans on DENV-2 E protein are heterogeneous. A significant portion of the E protein had at least one EndoH-sensitive or high-mannose glycan. Importantly, the digestion patterns exhibited by mosquito and mammalian cell-derived DENV did not differ. Thus, both cell types produced virus in which at least one N-linked glycan on DENV was not fully processed during virus egress from cells. The resulting high-mannose glycan is probably responsible for the ability of both mosquito and mammalian cell-derived virus to infect DC-SIGN-expressing cells efficiently.
Fig. 3.
DENVs grown in both insect and mammalian cells have high-mannose N-linked glycans. DENVs were grown in C6/36 (mosquito) and Vero 81 (mammalian) cells. Purified virus was digested with PNGaseF (P), EndoH (E) or mock digested (U), and the relative gel mobility of the E protein was determined by Western blotting. Bands are labelled 0N, 1N or 2N, corresponding to the number of N-linked glycans on the E protein.
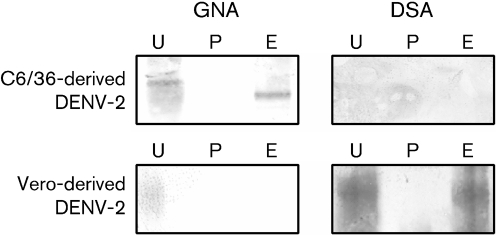
To characterize further the EndoH-resistant and -sensitive N-linked glycans on DENV, we used two lectins with defined specificity for terminal sugar residues in lectin blotting assays – GNA and DSA (Fig. 4). GNA specifically recognizes terminal mannose linked to mannose, whereas DSA recognizes Gal(1–4)GlcNAc present in complex N-linked glycans (Crowley et al., 1984; Shibuya et al., 1988). In insect cells, both unprocessed (EndoH-sensitive) and fully processed (EndoH-resistant) N-linked glycans should contain terminal mannose residues, as insects do not have Golgi enzymes for the addition of terminal sugars to produce complex sugars (Jarvis, 2003). As expected, when insect-derived DENV-2 was analysed by lectin blotting, the E protein bound to GNA but not to DSA. GNA binding was lost following treatment with PNGase, which removes all N-linked glycans. The EndoH-resistant glycans bound to GNA but not to DSA, confirming that they have a paucimannose structure.
Fig. 4.
Mosquito-derived DENV has two terminal mannose sugars whilst mammalian-derived DENV has only one. Purified DENV-2 produced in C6/36 and Vero cells was digested with PNGaseF (P) or EndoH (E) or mock digested (U) and lectin blots were performed. GNA is a lectin that recognizes terminal mannose residues. Both mosquito and mammalian cell-derived DENV bound to this lectin, indicating the presence of terminal mannose residues. Following digestion with EndoH, only the mosquito-derived virus bound to the lectin. These results are consistent with the mammalian-derived virus having one EndoH-sensitive high-mannose glycan and one EndoH-resistant complex glycan with no terminal mannose residues. The results are also consistent with the insect-derived virus having one EndoH-sensitive high-mannose glycan and one EndoH-resistant paucimannose glycan with terminal mannose residues. DSA is a lectin that recognizes Gal(1-4)GlcNAc, which is found only in complex sugars. Only the virus grown in Vero cells bound to this lectin.
When DENV-2 E protein from mammalian-derived virus was tested for lectin binding, both lectins bound to the E protein, indicating that a high-mannose and a complex glycan were present on the protein (Fig. 4). Following EndoH treatment, GNA binding was lost whilst DSA binding remained, confirming that the virus had a heterogeneous population of both high-mannose and complex glycans. The lectin-binding studies also confirmed that, irrespective of host, DENV contains one high-mannose N-linked glycan. The second glycan was a complex sugar or a paucimannose sugar in virus derived from mammals or mosquitoes, respectively.
Role of the E protein membrane anchor in N-linked glycan processing
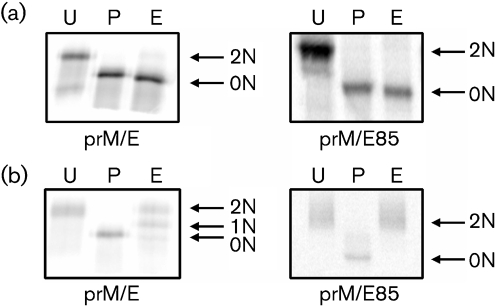
DENVs assemble on the ER membrane. Virions containing a lipid envelope with prM and E proteins and a capsid with the RNA genome bud into the lumen of the ER and the virus particles are secreted out of the cell. E and prM expressed without other viral proteins are secreted out of cells as subviral particles that bud into the ER (Ferlenghi et al., 2001). To determine whether membrane anchoring and/or viral particle formation was responsible for incomplete processing of DENV N-linked glycans, a VEEV replicon particle protein expression system was used to express prM with full-length E, and prM with just the ectodomain of E (prM/E85) (Davis et al., 2000). Full-length E protein is secreted out of cells as a subviral particle, whereas E85 is secreted as a soluble protein (White et al., 2007). The constructs were expressed in Vero cells, metabolically labelled with 35S, and the glycans on intra- and extracellular E protein were characterized by glycosidase digestion (Fig. 5). Full-length E and E85 had two glycans added, as indicated by an electrophoretic shift of 4 kDa following PNGaseF digestion (Fig. 5). All intracellular forms of E were sensitive to EndoH, indicating that the majority of this protein was in the ER and had not progressed through the Golgi (Fig. 5a). Full-length E protein expressed with prM and secreted into the medium as subviral particles exhibited an EndoH digestion pattern identical to whole virus (Fig. 3) and consisted of a mixed population of EndoH-resistant and -sensitive glycans. In contrast, E85 expressed with prM was completely EndoH-resistant following secretion from the cells, indicating that both glycans were fully processed into complex sugars (Fig. 5b). These findings indicated that incomplete N-linked glycan processing of DENV is a result of the membrane anchor and/or secretion as a subviral particle.
Fig. 5.
Role of the E protein membrane anchor in glycan processing. DENV-2 full-length (prM/E) and soluble (prM/E85) E protein were expressed in Vero cells using a VEEV replicon particle expression vector. Vero cells were electroporated with the replicon particle RNA and radiolabelled with [35S]methionine/cysteine. E protein was immunoprecipitated from cell lysates (a) and supernatants (b), digested with PNGaseF (P) or EndoH (E) or mock digested (U), and separated by SDS-PAGE. Intracellular forms of the E protein (a) were completely sensitive to EndoH, indicating that most of the protein had not proceeded beyond the ER. The secreted full-length E protein (b, prM/E) was incompletely processed and consisted of a mix of EndoH-resistant and -sensitive glycans. In contrast, soluble E (b, prM/E85) was fully processed and contained two glycans that were EndoH-resistant.
DISCUSSION
In this study, we compared the ability of DENVs derived from mosquito and mammalian cells to utilize DC-SIGN as an attachment factor for infecting monocytic cells. Our results demonstrated that viruses derived from both cell types were equally effective at infecting DC-SIGN-expressing human monocytes and DCs. We also characterized the structure of N-linked glycans on DENVs grown in insect and mammalian cells. Two N-linked glycans were added to the E protein in both cell types. In virus derived from mammalian cells, the N-linked glycans were a mix of high-mannose sugars and complex sugars. The N-linked glycans on mosquito-derived virus were a mix of high-mannose sugars and paucimannose sugars. The carbohydrate recognition domains of human DC-SIGN preferentially interact with high-mannose glycans when compared with single mannoses and complex carbohydrates (Feinberg et al., 2001; Mitchell et al., 2001). We propose that the presence of unprocessed, high-mannose sugars in both mosquito and mammalian cell-derived virus is responsible for the ability of these viruses to infect DC-SIGN-expressing cells with similar efficiency. We chose Vero cells as a representative cell line to produce DENV as it is commonly used in vaccine studies to grow virus (reviewed by Whitehead et al., 2007). DENV produced in other mammalian cell types can also infect DC-SIGN-expressing U937 cells, including virus produced in human monocytic cells (data not shown).
Previous studies with alphaviruses and WNV have demonstrated that mosquito cell-derived virus infects DC-SIGN-expressing cells better than mammalian cell-derived virus (Davis et al., 2006b; Klimstra et al., 2003; Shabman et al., 2007). The superior infectivity of mosquito-derived virus was attributed to the presence of terminal high-mannose glycans in mosquito cell- but not in mammalian cell-derived virus. Our results demonstrated that this phenomenon cannot be generalized to DENV as both mosquito and mammalian cell-derived viruses had incompletely processed, high-mannose glycans and were able to infect DC-SIGN-bearing cells efficiently. In studies with alphaviruses and WNV, the superior infectivity of mosquito cell-derived virus has also been attributed to the ability of mosquito cell- but not mammalian cell-grown virus to suppress type I interferon responses (Shabman et al., 2007; Silva et al., 2007). The mechanism by which mosquito cell-derived virus suppresses this innate immune response is currently unknown. Confirming previous reports, we were unable to detect type I interferon by bioassay in either our mosquito or our mammalian DENV-infected cultures at 24 h p.i. (data not shown) (Chen & Wang, 2002; Palmer et al., 2005; Sun et al., 2009). Further studies are needed to explore whether mosquito and mammalian cell-derived DENVs differ in their ability to suppress host innate antiviral responses.
Previous studies have come to different conclusions about the number and structure of N-linked glycans on the DENV E protein. Smith & Wright (1985) reported that DENV-2 E protein had two N-linked glycans and that the glycans were heterogeneous in structure. Johnson et al. (1994) confirmed the addition of two glycans in DENV-1, but found only a single glycan in DENV-2. Moreover, they concluded that the sugars were high mannose due to the binding of ConA. More recently, the structure of glycans has been characterized for recombinant soluble E protein expressed in insect, human and rodent cell lines: both glycans were EndoH-resistant, indicating heavy glycan processing (Lozach et al., 2005; Miller et al., 2008; Modis et al., 2003, 2005). Here, we compared the number of glycans in multiple isolates of DENV belonging to all four serotypes and observed the presence of two glycans in all but one case (Fig. 3 and data not shown). Thus, we propose that two N-linked glycans is the norm for the DENV E protein. We also observed a mix of fully processed (paucimannose or complex) and unprocessed (high-mannose) N-linked glycans for all four serotypes grown in both mosquito and mammalian cells. The presence of high-mannose and complex glycans on mammalian cell-derived virus is not unique to DENV (Leonard et al., 1990). We conclude that incomplete glycan processing is a general feature of DENVs, resulting in terminal high-mannose N-linked glycans being a part of the virion, irrespective of the host cells used to propagate virus.
Two recent papers have examined the phenotype of mutant DENVs and demonstrated that the glycan at N153 is not necessary for virus production and spread in mosquito or mammalian cells (Bryant et al., 2007; Mondotte et al., 2007). These studies demonstrated that N67 is essential for virus spread in mammalian cells, which is consistent with a role for this glycan in binding to host-cell receptors and in other steps of the viral life cycle. N67 was not required for the production of infectious virus from mosquitoes or mosquito cells, indicating a non-essential role for N67 in the vector (Bryant et al., 2007; Mondotte et al., 2007). In the current study, we did not determine the location of the unprocessed and processed N-linked glycans. We predict that the processed complex (mammalian-derived virus) or paucimannose (insect-derived virus) glycan is at position N153, as the WNV glycan at the corresponding position is processed to an EndoH-resistant form (Hanna et al., 2005). Consequently, the second potential glycosylation site at N67 is likely to contain the unprocessed, high-mannose glycan we observed in DENV virions. This location is consistent with published data indicating that this glycan binds to DC-SIGN (Mondotte et al., 2007; Pokidysheva et al., 2006). A glycan at N67 is present in DENV but not in other flaviviruses. However, when an N-linked glycan was artificially added to this position of WNV reporter particles, the glycan was unprocessed (high mannose) and mediated infection of DC-SIGN-expressing cells (Davis et al., 2006a).
When expressing recombinant DENV E proteins, we observed incomplete processing of N-linked glycans only when the protein was membrane anchored. In E protein secreted from cells expressing DENV E ectodomain, both glycans were in a processed, EndoH-resistant form. In contrast, when full-length E protein, which is membrane anchored, was expressed, the secreted protein had an EndoH digestion pattern identical to virus, with some E proteins containing EndoH-sensitive, high-mannose glycans. Membrane-anchored E protein and prM bud into the ER to form subviral particles that are secreted out of cells (Ferlenghi et al., 2001). We speculate that the membrane anchoring of these proteins may alter glycosidase processing of N67 by placing structural constraints on cellular glycosidases, thereby limiting processing. When E protein is produced as a soluble form, as in the prM/E85 VEEV constructs, the protein passes through the host ER and Golgi as monomers or dimers that may be associated with prM (Lorenz et al., 2002). In this context, the glycans are probably readily accessible by host glycosidases. Previous work with Sindbis virus has shown that the ability of host glycosidases to access specific locations determines the structure of E protein glycans (Hubbard, 1988). Our results have implications for the interpretation of DENV E protein crystal structures. The structures that have been solved for the E protein to date are based on recombinant, soluble E protein secreted from Drosophila cells (Modis et al., 2003, 2005). We demonstrated that glycans on the virion are processed differently by ER and Golgi enzymes compared with glycans on soluble recombinant proteins. If glycan structure is important for dimer formation or protein folding, the E protein structures based on the secreted E ectodomain may be misleading.
Our results demonstrated that the heavily processed N-linked glycan (most probably at N153) has a different structure in mosquito (paucimannose, with terminal mannose) and mammalian (complex, with no terminal mannose) cells. The differences between these structures may influence receptor interactions. Davis et al. (2006a, b) showed that E protein N154 of WNV, which corresponds to N153 of DENV, can mediate infection via the related receptor DC-SIGNR. DC-SIGNR recognizes both high-mannose and complex glycans. The recognition of complex glycans is mediated by binding to terminal N-acetylglucosamine (Davis et al., 2006a). DENV infection of myeloid cells can also be mediated by mannose receptor, which specifically recognizes glycans terminating in mannose, fucose and N-acetylglucosamine (Miller et al., 2008). LSECtin (liver and lymph node sinusoidal endothelial cell C-type lectin) can act as an attachment factor for viruses such as filoviruses and severe acute respiratory syndrome coronavirus (Gramberg et al., 2005). Although the specificity of LSECtin is not known, mannan cannot inhibit binding to filovirus glycoproteins, suggesting that it does not recognize high- or terminal-mannose glycans (Gramberg et al., 2005). As mammalian and mosquito cell-derived DENVs contain different glycans at N153, and because DC-SIGNR, LSECtin and the mannose receptor differentially recognize these glycans, there may be cell types expressing these lectins that are differentially infected by mosquito and mammalian cell-derived DENVs.
Acknowledgments
The authors would like to thank Mark Heise and Thomas Morrison for providing the U937 cells expressing DC-SIGN. The authors would also like to thank Nancy Davis and Martha Collier for scientific advice and technical assistance in expressing DENV E protein from VEEV replicon particles. Finally, the authors would like to thank Reed Shabman for advice regarding viral glycoproteins. K. H. was supported by T32 GM008719 and T32 AI007419.
References
- Bryant, J. E., Calvert, A. E., Mesesan, K., Crabtree, M. B., Volpe, K. E., Silengo, S., Kinney, R. M., Huang, C. Y., Miller, B. R. & Roehrig, J. T. (2007). Glycosylation of the dengue 2 virus E protein at N67 is critical for virus growth in vitro but not for growth in intrathoracically inoculated Aedes aegypti mosquitoes. Virology 366, 415–423. [DOI] [PubMed] [Google Scholar]
- Cambi, A. & Figdor, C. G. (2003). Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol 15, 539–546. [DOI] [PubMed] [Google Scholar]
- Cambi, A., Gijzen, K., de Vries, J. M., Torensma, R., Joosten, B., Adema, G. J., Netea, M. G., Kullberg, B. J., Romani, L. & Figdor, C. G. (2003). The C-type lectin DC-SIGN (CD209) is an antigen-uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 33, 532–538. [DOI] [PubMed] [Google Scholar]
- Chambers, T. J., McCourt, D. W. & Rice, C. M. (1990a). Production of yellow fever virus proteins in infected cells: identification of discrete polyprotein species and analysis of cleavage kinetics using region-specific polyclonal antisera. Virology 177, 159–174. [DOI] [PubMed] [Google Scholar]
- Chambers, T. J., Hahn, C. S., Galler, R. & Rice, C. M. (1990b). Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44, 649–688. [DOI] [PubMed] [Google Scholar]
- Chen, Y. C. & Wang, S. Y. (2002). Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J Virol 76, 9877–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley, J. F., Goldstein, I. J., Arnarp, J. & Lonngren, J. (1984). Carbohydrate binding studies on the lectin from Datura stramonium seeds. Arch Biochem Biophys 231, 524–533. [DOI] [PubMed] [Google Scholar]
- Davis, N. L., Caley, I. J., Brown, K. W., Betts, M. R., Irlbeck, D. M., McGrath, K. M., Connell, M. J., Montefiori, D. C., Frelinger, J. A. & other authors (2000). Vaccination of macaques against pathogenic simian immunodeficiency virus with Venezuelan equine encephalitis virus replicon particles. J Virol 74, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, C. W., Mattei, L. M., Nguyen, H. Y., Ansarah-Sobrinho, C., Doms, R. W. & Pierson, T. C. (2006a). The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin). J Biol Chem 281, 37183–37194. [DOI] [PubMed] [Google Scholar]
- Davis, C. W., Nguyen, H. Y., Hanna, S. L., Sanchez, M. D., Doms, R. W. & Pierson, T. C. (2006b). West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol 80, 1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres, P., Frenkiel, M. P. & Deubel, V. (1993). Differences between cell membrane fusion activities of two dengue type-1 isolates reflect modifications of viral structure. Virology 196, 209–219. [DOI] [PubMed] [Google Scholar]
- Engering, A., Geijtenbeek, T. B., van Vliet, S. J., Wijers, M., van Liempt, E., Demaurex, N., Lanzavecchia, A., Fransen, J., Figdor, C. G. & other authors (2002). The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol 168, 2118–2126. [DOI] [PubMed] [Google Scholar]
- Feinberg, H., Mitchell, D. A., Drickamer, K. & Weis, W. I. (2001). Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166. [DOI] [PubMed] [Google Scholar]
- Ferlenghi, I., Clarke, M., Ruttan, T., Allison, S. L., Schalich, J., Heinz, F. X., Harrison, S. C., Rey, F. A. & Fuller, S. D. (2001). Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol Cell 7, 593–602. [DOI] [PubMed] [Google Scholar]
- Gramberg, T., Hofmann, H., Moller, P., Lalor, P. F., Marzi, A., Geier, M., Krumbiegel, M., Winkler, T., Kirchhoff, F. & other authors (2005). LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology 340, 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler, D. J. (2002). Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 10, 100–103. [DOI] [PubMed] [Google Scholar]
- Gubler, D. J. & Clark, G. G. (1995). Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg Infect Dis 1, 55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna, S. L., Pierson, T. C., Sanchez, M. D., Ahmed, A. A., Murtadha, M. M. & Doms, R. W. (2005). N-linked glycosylation of West Nile virus envelope proteins influences particle assembly and infectivity. J Virol 79, 13262–13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz, F. X. & Allison, S. L. (2003). Flavivirus structure and membrane fusion. Adv Virus Res 59, 63–97. [DOI] [PubMed] [Google Scholar]
- Ho, L. J., Wang, J. J., Shaio, M. F., Kao, C. L., Chang, D. M., Han, S. W. & Lai, J. H. (2001). Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J Immunol 166, 1499–1506. [DOI] [PubMed] [Google Scholar]
- Houng, H. S., Chen, R. C.-M., Vaughn, D. W. & Kanesa-thasan, N. (2001). Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1–4 using conserved and serotype-specific 3′ noncoding sequences. J Virol Methods 95, 19–32. [DOI] [PubMed] [Google Scholar]
- Hsieh, P. & Robbins, P. W. (1984). Regulation of asparagine-linked oligosaccharide processing. oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem 259, 2375–2382. [PubMed] [Google Scholar]
- Hubbard, S. C. (1988). Regulation of glycosylation. the influence of protein structure on N-linked oligosaccharide processing. J Biol Chem 263, 19303–19317. [PubMed] [Google Scholar]
- Hubbard, S. C. & Ivatt, R. J. (1981). Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem 50, 555–583. [DOI] [PubMed] [Google Scholar]
- Jarvis, D. L. (2003). Developing baculovirus–insect cell expression systems for humanized recombinant glycoprotein production. Virology 310, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A. J., Guirakhoo, F. & Roehrig, J. T. (1994). The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology 203, 241–249. [DOI] [PubMed] [Google Scholar]
- Klimstra, W. B., Nangle, E. M., Smith, M. S., Yurochko, A. D. & Ryman, K. D. (2003). DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol 77, 12022–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus, A. A., Messer, W., Haymore, L. B. & de Silva, A. M. (2007). Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J Clin Microbiol 45, 3777–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, R. J., Zhang, W., Rossmann, M. G., Pletnev, S. V., Corver, J., Lenches, E., Jones, C. T., Mukhopadhyay, S., Chipman, P. R. & other authors (2002). Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth, C. R., White, L. J., Johnston, R. E. & de Silva, A. M. (2005). Flow cytometry-based assay for titrating dengue virus. J Clin Microbiol 43, 3267–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, C. K., Spellman, M. W., Riddle, L., Harris, R. J., Thomas, J. N. & Gregory, T. J. (1990). Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem 265, 10373–10382. [PubMed] [Google Scholar]
- Lorenz, I. C., Allison, S. L., Heinz, F. X. & Helenius, A. (2002). Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J Virol 76, 5480–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozach, P. Y., Burleigh, L., Staropoli, I., Navarro-Sanchez, E., Harriague, J., Virelizier, J. L., Rey, F. A., Despres, P., Arenzana-Seisdedos, F. & Amara, A. (2005). Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem 280, 23698–23708. [DOI] [PubMed] [Google Scholar]
- Maley, F., Trimble, R. B., Tarentino, A. L. & Plummer, T. H., Jr (1989). Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem 180, 195–204. [DOI] [PubMed] [Google Scholar]
- Marchal, I., Jarvis, D. L., Cacan, R. & Verbert, A. (2001). Glycoproteins from insect cells: sialylated or not? Biol Chem 382, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marovich, M., Grouard-Vogel, G., Louder, M., Eller, M., Sun, W., Wu, S. J., Putvatana, R., Murphy, G., Tassaneetrithep, B. & other authors (2001). Human dendritic cells as targets of dengue virus infection. J Investig Dermatol Symp Proc 6, 219–224. [DOI] [PubMed] [Google Scholar]
- Miller, J. L., deWet, B. J., Martinez-Pomares, L., Radcliffe, C. M., Dwek, R. A., Rudd, P. M. & Gordon, S. (2008). The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog 4, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, D. A., Fadden, A. J. & Drickamer, K. (2001). A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem 276, 28939–28945. [DOI] [PubMed] [Google Scholar]
- Modis, Y., Ogata, S., Clements, D. & Harrison, S. C. (2003). A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A 100, 6986–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modis, Y., Ogata, S., Clements, D. & Harrison, S. C. (2005). Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol 79, 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondotte, J. A., Lozach, P. Y., Amara, A. & Gamarnik, A. V. (2007). Essential role of dengue virus envelope protein N glycosylation at asparagine-67 during viral propagation. J Virol 81, 7136–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, T. P., Collier, M., McKinnon, K. P., Davis, N. L., Johnston, R. E. & Serody, J. S. (2005). A novel viral system for generating antigen-specific T cells. J Immunol 175, 3431–3438. [DOI] [PubMed] [Google Scholar]
- Navarro-Sanchez, E., Altmeyer, R., Amara, A., Schwartz, O., Fieschi, F., Virelizier, J. L., Arenzana-Seisdedos, F. & Despres, P. (2003). Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 4, 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, D. R., Sun, P., Celluzzi, C., Bisbing, J., Pang, S., Sun, W., Marovich, M. A. & Burgess, T. (2005). Differential effects of dengue virus on infected and bystander dendritic cells. J Virol 79, 2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, S. R. & Rothman, J. E. (1987). Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem 56, 829–852. [DOI] [PubMed] [Google Scholar]
- Pokidysheva, E., Zhang, Y., Battisti, A. J., Bator-Kelly, C. M., Chipman, P. R., Xiao, C., Gregorio, G. G., Hendrickson, W. A., Kuhn, R. J. & Rossmann, M. G. (2006). Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell 124, 485–493. [DOI] [PubMed] [Google Scholar]
- Shabman, R. S., Morrison, T. E., Moore, C., White, L., Suthar, M. S., Hueston, L., Rulli, N., Lidbury, B., Ting, J. P. & other authors (2007). Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses. J Virol 81, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabman, R. S., Rogers, K. M. & Heise, M. T. (2008). Ross River virus envelope glycans contribute to type I interferon production in myeloid dendritic cells. J Virol 82, 12374–12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya, N., Goldstein, I. J., Van Damme, E. J. & Peumans, W. J. (1988). Binding properties of a mannose-specific lectin from the snowdrop (Galanthus nivalis) bulb. J Biol Chem 263, 728–734. [PubMed] [Google Scholar]
- Silva, M. C., Guerrero-Plata, A., Gilfoy, F. D., Garofalo, R. P. & Mason, P. W. (2007). Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. J Virol 81, 13640–13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G. W. & Wright, P. J. (1985). Synthesis of proteins and glycoproteins in dengue type 2 virus-infected Vero and Aedes albopictus cells. J Gen Virol 66, 559–571. [DOI] [PubMed] [Google Scholar]
- Sun, P., Fernandez, S., Marovich, M. A., Palmer, D. R., Celluzzi, C. M., Boonnak, K., Liang, Z., Subramanian, H., Porter, K. R. & other authors (2009). Functional characterization of ex vivo blood myeloid and plasmacytoid dendritic cells after infection with dengue virus. Virology 383, 207–215. [DOI] [PubMed] [Google Scholar]
- Tassaneetrithep, B., Burgess, T. H., Granelli-Piperno, A., Trumpfheller, C., Finke, J., Sun, W., Eller, M. A., Pattanapanyasat, K., Sarasombath, S. & other authors (2003). DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 197, 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigerust, D. J. & Shepherd, V. L. (2007). Virus glycosylation: role in virulence and immune interactions. Trends Microbiol 15, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, L. J., Parsons, M. M., Whitmore, A. C., Williams, B. M., de Silva, A. & Johnston, R. E. (2007). An immunogenic and protective alphavirus replicon particle-based dengue vaccine overcomes maternal antibody interference in weanling mice. J Virol 81, 10329–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, S. S., Blaney, J. E., Durbin, A. P. & Murphy, B. R. (2007). Prospects for a dengue virus vaccine. Nat Rev Microbiol 5, 518–528. [DOI] [PubMed] [Google Scholar]
- WHO (2009). Dengue and dengue hemorrhagic fever. Fact sheet no. 117. http://www.who.int/mediacentre/factsheets/fs117/en/
- Wu, S. J., Grouard-Vogel, G., Sun, W., Mascola, J. R., Brachtel, E., Putvatana, R., Louder, M. K., Filgueira, L., Marovich, M. A. & other authors (2000). Human skin Langerhans cells are targets of dengue virus infection. Nat Med 6, 816–820. [DOI] [PubMed] [Google Scholar]