Abstract
Endoscopic ultrasound (EUS) has become a well accepted test for the diagnosis of chronic pancreatitis. Advantages include its ability to detect subtle and severe changes of the pancreatic duct and parenchyma, and its relative safety compared with endoscopic retrograde cholangiopancreatography. Limitations include inter- and intra-observer variability, operator dependence, and an incomplete understanding of its true accuracy. The Rosemont classification has recently been proposed as a weighted, standardized method that may improve EUS chronic pancreatitis scoring. This paper reviews the published evidence regarding the accuracy of EUS in chronic pancreatitis diagnosis, and enumerates the emerging technologies that have been recently studied which may ultimately improve endosonographic imaging of the pancreas.
Keywords: Chronic pancreatitis, Contrast-enhanced endoscopic ultrasound, Diagnosis, Digital image analysis, Elastography, Endoscopic ultrasound, Pancreatic function testing, Rosemont classification
INTRODUCTION
Endoscopic ultrasound (EUS) has been used for the diagnosis of chronic pancreatitis (CP) for over two decades. Its primary attribute is its ability to detect mild parenchymal and ductal abnormalities not seen with computed tomography (CT) scans. EUS is of most use in patients with abdominal pain of suspected pancreatic origin and non-diagnostic cross-sectional imaging. In a report entitled “Minimal change chronic pancreatitis”, Walsh described 16 patients with typical pancreatic pain and negative or equivocal CT and endoscopic retrograde cholangiopancreatography (ERCP) images[1]. All patients underwent pancreatic resection due to a strong suspicion of CP. Fifteen of the 16 patients had definite histological features of CP, including periductal fibrosis, duct dilation, intralobular inflammation, and atrophy. The histological features were often subtle and mild, out of proportion to the severity of pain. Had EUS been widely available, it might have been used to verify the presence of parenchymal and ductal abnormalities of CP prior to resection. This review focuses on the role of EUS in the diagnosis of CP, including its accuracy, strengths, and limitations. An update on emerging techniques to augment endosonographic diagnosis is also presented.
THE NORMAL PANCREAS
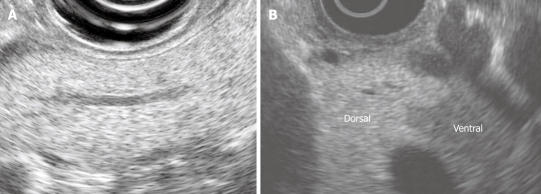
The normal endosonographic appearance of the pancreas has been characterized in three studies of healthy volunteers[2] and patients undergoing EUS for non-pancreatic indications[3,4]. The normal pancreatic parenchyma is homogenous and granular (“salt and pepper echotexture”), and echogenic relative to the liver parenchyma (Figure 1A). The border of the gland is smooth. The main pancreatic duct is also smooth in contour and tapers from the head to the tail. In one study, the upper limit of normal for duct diameter was 3.6 mm in the head, 3.0 mm in the body, and 2.0 mm in the tail[2]. The main pancreatic duct wall is not easily visible, and side-branches are difficult to visualize or narrow in caliber (< 1.0 mm). A ventral anlage is found in one-half of patients (Figure 1B). This is a relative echogenicity of the dorsal compared with the ventral pancreas.
Figure 1.
The normal endosonographic appearance of the pancreas. A: View of pancreatic body from gastric station. The parenchyma is homogeneous and granular (“salt and pepper”). The duct is neither dilated nor ectatic; B: Dorsal ventral anlage. The ventral pancreas is relatively echogenic compared with the dorsal pancreas. There is a distinct border between the dorsal and ventral pancreas.
CP CRITERIA
The endosonographic features that have been used for CP diagnosis have evolved since the inception of EUS. In the seminal studies of Dancygier et al[5] and Lees[6], a number of parenchymal and ductal features were characterized (Table 1). At that time, EUS diagnosis was qualitative and focused on the global separation of benign from malignant pancreatic diseases[7]. Subsequent studies codified these features into numbered scoring systems[2,5,8]. The most common scoring system includes 5 ductal and 4 parenchymal features (Figures 2 and 3)[8]. The criteria are counted and compared against a cutpoint. Typical cutpoints range from 3 (maximizing sensitivity) to 5 (maximizing specificity).
Table 1.
Evolution of endoscopic ultrasound criteria for chronic pancreatitis
| Lees[6] (1986) | Wiersema et al[2] (1993) | Sahai et al[8] (1998) | Catalano et al[9] (2009)1 | Histological correlation | |
| Number of criteria | Qualitative | 11 | 9 | 3 major; 6 minor | |
| Parenchymal | X | Reduced echogenic foci | X | X | |
| Heterogeneity | |||||
| Strongly echogenic foci | Echogenic foci (> 3 mm) | Hyperechoic foci | Hyperechoic foci without shadowing (minor). Echogenic structures ≥ 2 mm in length and width with no shadowing | Focal fibrosis | |
| Hyperechoic foci with shadowing (major A). Echogenic structures ≥ 2 mm in length and width that shadow | Calcifications in side-branches | ||||
| Echogenic bands | X | Hyperechoic strands | Stranding (minor). Hyperechoic lines of ≥ 3 mm in length in at least 2 different directions with respect to the imaged plane | Fibrotic bands | |
| Cavities | Cysts (> 3 mm) | Cysts | Cysts (minor). Anechoic, rounded/elliptical structures with or without septations | Pseudocysts | |
| Accentuation of lobular architecture | Accentuation of lobular pattern | Parenchymal lobularity | Lobularity with honeycombing (major B). Well-circumscribed, ≥ 5 mm structures with enhancing rim and relatively echo-poor center, with ≥ 3 contiguous lobules | Edema separated by fibrotic bands | |
| Lobularity without honeycombing (minor). Well-circumscribed, ≥ 5 mm structures with enhancing rim and relatively echo-poor center, with noncontiguous lobules | |||||
| Enlargement | X | X | X | ||
| Duct | X | Narrowing | X | X | |
| Increase in caliber | Dilation | MPD dilation | MPD dilation (minor). ≥ 3.5 mm in body or > 1.5 mm in tail | Obstructed duct | |
| Irregularity of the lumen | Irregular contour | Irregular MPD margins | Irregular MPD contour (minor). Uneven or irregular outline and ectatic course | Ductal fibrosis and strictures | |
| Increase echogenicity of duct wall | Duct wall echogenicity | Hyperechoic MPD margins | Hyperechoic MPD margin (minor). Echogenic, distinct structure greater than 50% of entire MPD in the body and tail | Periductal fibrosis | |
| Intraluminal echoes | Calculi | Shadowing calcifications | MPD calculi (major A). Echogenic structures within MPD with acoustic shadowing | Calcifications in the main duct | |
| Visualization of side branches | Side-branch dilation | Visible side-branches | Dilated side branches (minor). 3 or more tubular anechoic structures each measuring ≥ 1 mm in width, budding from the MPD | Side-branch ectasia | |
| Strictures with dilation | X | X | X | ||
| Main or branch duct disruptions with cyst | X | X | X |
Rosemont diagnostic stratification: Normal: < 3 minor features (excluding non-shadowing echogenic foci, visible side-branches, cysts, and MPD dilation) and no major features. Indeterminate: 3 or 4 minor features and no major features, or major B feature alone with < 3 minor features. Suggestive: 1 major A feature and < 3 minor features, or 1 major B feature and ≥ 3 minor features, or ≥ 5 minor features. Most consistent: 1 major A feature and ≥ 3 minor features, or 1 major A feature and major B feature, or 2 major A features. MPD: Main pancreatic duct.
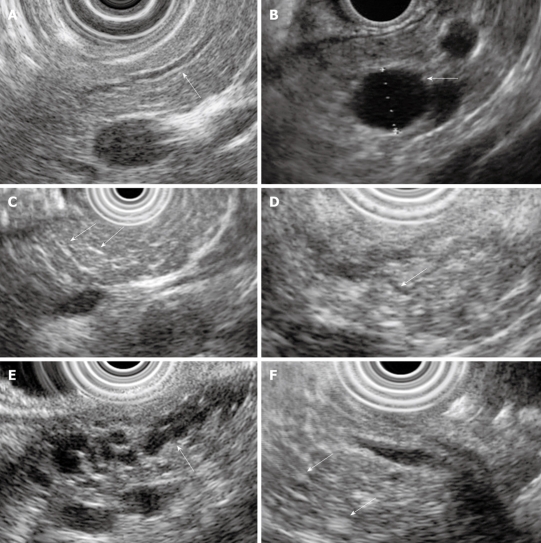
Figure 2.
Examples of endoscopic ultrasound (EUS) chronic pancreatitis (CP) criteria. A: Hyperechoic duct wall (arrow); B: Cyst (arrow); C: Hyperechoic strands (arrows); D: Visible side-branch (arrow); E: Dilated and irregular main pancreatic duct with visible side-branches (arrow); F: Hyperechoic foci (arrows).
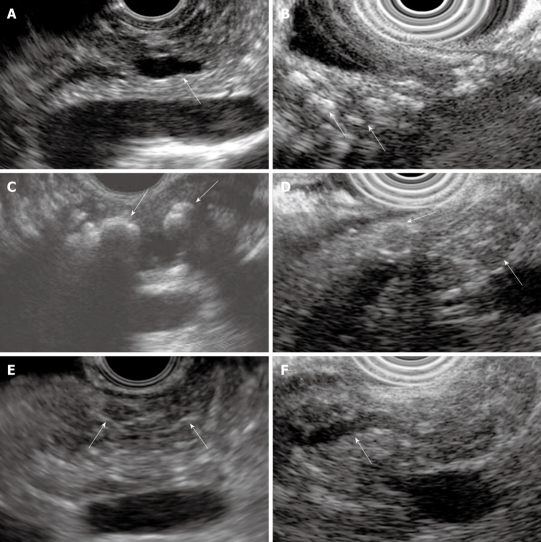
Figure 3.
Examples of EUS CP criteria. A: Dilated main pancreatic duct (arrow); B: Parenchymal calcifications (arrows); C: Main duct calcifications (arrows); D: Lobules (arrows); E: Stranding (arrows); F: Irregular main pancreatic duct (arrow).
A frequent criticism of standard scoring is that each criterion is counted equally, even though some criteria have more diagnostic importance than others. In April 2007, thirty-two international experts met in Rosemont Illinois, USA to create a consensus-based scoring system[9]. The Rosemont classification includes strictly defined major and minor criteria, and a four-level diagnostic stratification (Table 1). Although this new scoring system may help standardize the EUS diagnosis of CP, it requires further validation in multicenter studies.
TECHNICAL ASPECTS
A complete EUS examination to evaluate CP includes a careful inventory of all major and minor criteria. The body and tail is viewed from the gastric station, where the interpretation of CP criteria is consider most reliable. It is good practice to photograph, digitally annotate, count, and measure all identified features based on the Rosemont definitions. The main pancreatic duct should be carefully traced to ascertain the presence of ≥ 3 visible side-branches and echogenic wall. The duct diameter should be measured at the genu (in view of the portosplenic confluence) and in the pancreatic tail (in view of the splenic hilum). A 10-s video clip of the body and tail provides a useful frame of reference for future examinations. The scope should be advanced to the duodenum for a careful inspection of the head and uncinate process. Examination of the head detects cysts and main duct calculi (the only criteria properly detected in the head) and rules out biliary pathology, solid masses, and pancreas divisum.
Most validation studies have used mechanical radial scopes for CP diagnosis. However, most current endosonographers prefer linear scopes to examine the pancreas due to higher resolution, color flow Doppler capability, and capability for tissue sampling and celiac plexus blockade. In a blinded study, we found radial EUS to be non-inferior to linear EUS for CP diagnosis[10]. Newer electronic radial scopes have similar resolution and the same Doppler capability as older generation linear scopes.
TEST PERFORMANCE
The ideal study of EUS accuracy might include 1000 consecutive patients with non-diagnostic CT scans from multiple centers undergoing total pancreatectomy for suspected CP. Each patient would undergo a preoperative EUS examination and blinded histological analysis of the resection specimen. Of course, this study is unlikely to ever be performed. Even if it were carried out, some would quibble with the significance of mild or patchy fibrosis on the histological specimen!
To estimate the test performance of EUS, we must interpret cross-sectional studies with flawed reference standards (e.g. ERCP or functional testing), small histological comparisons with significant selection, spectrum, or sampling biases, and post-EUS observational studies with insufficient follow-up time (Table 2). Sadly, the actual sensitivity and specificity of EUS for diagnosing CP may never be known.
Table 2.
Test performance of endoscopic ultrasound in comparative studies
| Study | Gold standard | Cut-point |
Overall test performance |
Minimal change CP test performance |
||
| Sensitivity | Specificity | Sensitivity | Specificity | |||
| Buscail et al[11] | Composite | None | 89% (39/44) | 100% (18/18) | NR | NR |
| Nattermann et al[4] | ERCP | None | 98% (50/51) | 63% (27/43) | 88% (7/8) | 63% (27/43) |
| Wiersema et al[2] | ERCP | ≥ 3 | 100% (19/19) | 79% (38/48) | 100% (18/18) | 79% (38/48) |
| Catalano et al[3] | ERCP | ≥ 3 | 86% (31/36) | 95% (42/44) | 82% (22/27) | 95% (42/44) |
| Sahai et al[8] | ERCP | ≥ 3 | 63% NR | 68% NR | NR | NR |
| Hastier et al[25] | ERCP | None | 93% (13/14) | NR | NR | NR |
| Hollerbach et al[36] | ERCP | Grade 0-3 | 97% (30/31) | 67% (4/6) | 95% (20/21) | 67% (4/6) |
| Wiersema et al[2] | Secretin ID-PFT | ≥ 3 | 67% (6/9) | 29% (2/7) | 67% (6/9) | 29% (2/7) |
| Catalano et al[3] | Secretin ID-PFT | ≥ 3 | 84% (21/25) | 78% (43/55) | NR | NR |
| Raimondo et al[13] | CCK PFT | ≥ 4 | 43% (3/7) | 50% (4/8) | NR | NR |
| Chowdhury et al[14] | Secretin PFT | ≥ 3 | 71% (5/7) | 35% (5/14) | 71% (5/7) | 35% (5/14) |
| Stevens et al[15] | Secretin PFT | ≥ 3 | 68% (34/50) | 79% (30/38) | NR | NR |
| Stevens et al[16] | Secretin PFT | ≥ 4 | 71% (17/24) | 92% (24/26) | NR | NR |
| Stevens et al[16] | CCK PFT | ≥ 4 | 63% (15/24) | 85% (22/26) | NR | NR |
| Chong et al[19] | Histology | ≥ 3 | NR | NR | 83% (30/36) | 80% (4/5) |
| Varadarajulu et al[18] | Histology | ≥ 4 | 91% (19/21) | 86% (18/21) | 91% (19/21) | 86% (18/21) |
| Gupta et al[20] | Histology | ≥ 3 | 71% (10/14) | 100% (1/1) | 71% (10/14) | 100% (1/1) |
| Albashir et al[21] | Histology | ≥ 4 | 75% (12/16) | NR | NR | NR |
| Bhutani et al[49] | Histology (Autopsy) | ≥ 3 | 100% (10/10) | 100% (1/1) | 100% (10/10) | 100% (1/1) |
| Kahl et al[22] | Baseline or repeat ERCP | ≥ 1 | 100% (114/114) | NR | NR | NR |
| Morris-Stiff et al[24] | Radiographic progression | None | 100% (13/13) | NR | 100% (13/13) | NR |
Calc: Calcification; CCK: Cholecystokinin; NR: Not reported or calculable; PFT: Pancreatic function test; ID-PFT: Intraductal pancreatic function test.
ERCP
The earlier studies compared EUS with ERCP, since ERCP was the defacto radiographic reference standard and considered ethical at the time for diagnostic purposes[2-4,8,11]. These studies included performance of both EUS and ERCP in succession, and sometimes blinded independent interpretation of the EUS and ERCP images. Combining 273 patients from the three ERCP studies that used a cutpoint of ≥ 3 EUS criteria, the sensitivity is 87% and the specificity is 75%[2,3,8]. Limitations of the ERCP reference standard include limited specificity (e.g. in advancing age or following acute pancreatitis), frequent inadequacy of the pancreatogram to interpret small duct changes, and inability to assess the parenchyma[12].
Pancreatic function testing
We might expect high accuracy for EUS in these ERCP comparison studies since one structural test is being compared to another. The comparison of EUS with a pancreatic function test (PFT) reference standard is based on the rationale that impaired exocrine function is a sensitive surrogate for early fibrosis. An unadjusted pooling of the 255 patients in the secretin PFT studies reveals a sensitivity of 72% and specificity of 74%[2,3,13-16], noticeably lower than ERCP studies. A shortcoming of these studies is substantial methodological variation in secretin- and CCK-based PFT protocols. Also, acinar-cell and duct-cell exocrine insufficiency do not perfectly correlate with histological fibrosis. A subset of patients even with advanced structural changes has preserved exocrine function[17].
Histology
Histological comparison may be the “Holy Grail” for studying diagnostic tests for CP. There have been four studies comparing EUS with histology, two of which are published in abstract form only[18-21]. Varadarajulu evaluated 42 patients who underwent preoperative EUS within 2 mo of a partial or complete pancreatic resection[18]. Based on an ROC curve analysis, ≥ 4 criteria optimized sensitivity (91%) and specificity (86%) of EUS. There was a very good correlation of the histological fibrosis score with the EUS score (r = 0.85, P < 0.0001). A strength of this study was the predominance of mild fibrosis which minimizes spectrum bias. Also, half the patients did not have histological fibrosis allowing a reasonable estimation of specificity. A limitation was that most patients underwent resection for pancreatic cancer, making it difficult to extrapolate the results to the typical patient with abdominal pain.
Chong et al[19] reported a larger study in 71 patients with abdominal pain and suspected CP who underwent resection or open biopsy. This study had a substantial proportion of patients with calcific CP. Sixteen of the patients had calcifications on CT; an additional 14 patients had small calcifications detected by EUS. In the 41 patients without calcifications (minimal change group), ≥ 3 EUS criteria had 83% sensitivity (95% CI: 67%-94%) and 80% (95% CI: 28%-99%) specificity for the diagnosis of CP. The wide confidence limits surrounding the specificity estimate are due to only 5 patients without fibrosis.
These studies are quite helpful in providing histological correlations to validate individual EUS criteria and EUS scoring. One potential limitation of these studies is selection bias since only patients undergoing surgery were included. The increasing performance of total pancreatectomy with autologus islet cell transplant (TP/AIT) for earlier stages of CP may allow future multicenter histological studies in patients with milder fibrosis[20].
Long-term follow-up
Long-term follow-up may be a useful reference standard. Patients with normal and abnormal EUS can be observed to see whether obvious disease progression develops on subsequent imaging tests. This approach may shed light on the predictive value of minimal or mild EUS features. Kahl et al[22] followed 32 alcoholic patients with normal baseline ERCP and abnormal EUS (≥ 1 criterion) for a median observation time of 18 mo (range 6 to 25 mo). During the follow-up period, ERCP was repeated in 22 patients, and demonstrated either Cambridge Class I (n = 12) or II (n = 10) ductal changes. The authors concluded that mild EUS changes had 100% sensitivity for CP, and predicted the development of CP at an earlier stage than ERCP. The conclusion of this study is based on subtle endpoints of questionable specificity (Cambridge I and II changes). It is not clear whether there were meaningful differences between the baseline and follow-up pancreatograms since interpretation was not blinded. Calcifications and atrophy may be more convincing endpoints, but would require longer follow-up. Natural history studies suggest that calcifications may develop after more than 10 years from symptom onset[23]. Also, Kahl et al[22] considered EUS positive if only 1 criterion was present. This cutpoint is likely to result in many false diagnoses. Finally, repeat ERCP was not performed in all patients, which produces verification bias and weakens the estimate of specificity.
Morris-Stiff et al[24] identified 16 patients who underwent EUS for evaluation of CP after normal conventional imaging (CT or MRCP). Thirteen of the patients had EUS changes of CP, and all 13 subsequently developed “progression of disease” on subsequent conventional imaging. Neither the follow-up time nor the specific imaging findings referenced as disease progression were reported in this publication.
Hastier et al[25] presented results that conflict with Kahl’s and Morris-Stiff’s findings. In this study, 18 alcoholic cirrhotic patients with isolated parenchymal features identified by EUS and normal ERCP were followed. After a mean duration of 22 mo, the EUS appearance was unchanged in all patients. The subsequent repeat pancreatograms were normal in all 10 patients who had a follow-up ERCP. This study raises doubts over the significance of the importance of minimal EUS features. Since follow-up time was short, firm conclusions cannot be drawn.
In summary, the test performance of EUS can only be estimated based on a variety of imperfect evidence. In interpreting these studies, it is useful to calculate the sensitivity and specificity estimates in the subset of patients with non-calcific (minimal change) CP, where EUS is of most clinical value (Table 2). At the present time, we must take a “nihilistic” view of the diagnostic performance of EUS accuracy and interpret the results with care in the context of the history and ancillary laboratory, imaging, and functional test results.
RELIABILITY
The major strengths of EUS are its ability to detect mild structural abnormalities of the entire pancreas and its safety relative to ERCP. Unfortunately, there are also several caveats.
Questionable specificity
Minimal or mild parenchymal and ductal EUS features may have poor specificity, resulting in the “overdiagnosis” of CP. A number of clinical variables can lead to EUS changes that mimic those in CP. Rajan performed a prospective study of 120 patients undergoing EGD/EUS for non-pancreatic indications[26]. At least 1 EUS CP criterion was found in 38% of these non-alcoholic and asymptomatic patients. Patients in the > 60 years age group had a statistically insignificant trend toward more frequent EUS abnormalities (39%) compared with those in the < 40 years (23%) and 40-60 years (25%) age groups (P = 0.13). Male gender was a statistically significant predictor of an abnormal EUS (OR 2.9, 95% CI: 1.2-6.8).
In another study, 189 asymptomatic patients undergoing EUS for non-pancreatic indications were administered an alcohol questionnaire[27]. EUS features were more frequent in alcoholic patients (70%) compared with non-alcoholic patients (31%) (P < 0.001), suggesting the EUS changes may be present in alcohol drinkers who lack symptoms of CP. In a much larger study (n = 1157), heavy ethanol intake (OR 5.1, 95% CI: 3.1-8.5), male sex (OR 1.8, 95% CI: 1.3-2.6), clinical suspicion of pancreatic disease (OR 1.7, 95% CI: 1.2-2.3), and heavy smoking (OR 1.7, 95% CI: 1.2-2.4) were each independent predictors of severe EUS changes[28].
A fatty pancreas causes hyperechogenicity of the parenchyma that can imitate the echogenic changes of CP. In a logistic regression analysis, obesity, fatty liver disease, and alcohol intake were independent predictors of fatty infiltration of the pancreas[29]. Another study showed a high rate of EUS abnormalities in patients with dyspepsia (and low suspicion of pancreatic disease) compared with controls[30]. This may indicate that mild CP is a more common etiology of dyspepsia than previously thought. Other plausible explanations include tertiary referral bias and information bias, since the endoscopist was aware of the clinical history during the EUS examination[31]. In summary, age, gender, smoking, alcohol use, and BMI may influence EUS results. Future scoring systems may include these clinical variables in addition to EUS features.
Interobserver variability
Interobserver variability pertains to differences in interpretation of the same exam by multiple observers. In a study by Wallace, 45 videotaped examinations were privately scored by 11 expert endosonographers[32]. The κ (agreement beyond chance) for global diagnosis was only moderate (κ = 0.45). Interobserver agreement was worse (κ < 0.40) for most individual criteria, except for lobularity (κ = 0.51) and main duct dilation (κ = 0.61). Similar sub-optimal interobserver agreement was observed in a study of CP criteria in familial pancreatic cancer kindreds (κ < 0.40 for all CP criteria except cysts)[33].
It was expected that the Rosemont classification might improve interobserver agreement because it incorporates more stringent definitions. In a recent study, 50 clips were interpreted by 14 experts on two occasions, first with standard scoring and then with Rosemont scoring[34]. The interobserver agreement was slightly higher for Rosemont scoring [κ = 0.65 (95% CI: 0.52-0.77)] compared with standard scoring [κ = 0.54 (95% CI: 0.44-0.66)]; however, the difference was not statistically significant (P = 0.12).
Intraobserver variability
Intraobserver variability relates to differences in scoring of the same images from multiple interpretations by the same observer. A single study has examined the intraobserver variability of EUS CP criteria[35]. Thirty still-frame images were scored by the same observers on repeat occasions over 6 wk. The intraobserver κ for overall diagnosis of CP was 0.77, and ranged from 0.61 to 0.88 for individual criteria. In other words, endosonographers have reasonable (but not perfect) agreement with themselves when interpreting the same image on multiple occasions.
Pretest probability
EUS is known as an operator dependent test. Even with Rosemont scoring, there is inherent subjectivity in measuring each criterion. Most endosonographers are aware of the patient’s history as they are performing the examination, and may have already formed a “likelihood of disease” estimate in their mind. This pre-test probability may influence the interpretation of EUS results. For example, in a non-smoker with dyspepsia and no history of heavy alcohol intake or acute pancreatitis, many endosonographers would ignore mild stranding and lobularity in fear of mislabeling the patient. There are no available studies characterizing the affect of pretest probability on EUS interpretation.
ANCILLARY TECHNIQUES
Several supplementary techniques have been studied which may complement standard EUS scoring in the diagnosis of CP.
Tissue sampling
Linear EUS allows tissue acquisition through fine needle aspiration (FNA). Hollerbach et al[36] reported 27 patients with suspicion of CP who underwent targeted fine-needle aspiration of lobular or inflamed appearing areas of the pancreas. An average of 2.3 passes was required to obtain satisfactory tissue in all patients. The cytological findings were graded 0 to 2 based on the severity of chronic inflammation. The performance of FNA minimally increased the specificity of EUS (67% with FNA, 60% without FNA). Two patients developed mild acute pancreatitis following the procedure. Based on this study, routine FNA does not seem very useful given its minimal impact on test performance, potential for acute pancreatitis, and the lack of a validated cytological grading system.
Obtaining a core biopsy may provide sufficient tissue for a histological diagnosis. Dewitt performed EUS-guided Trucut biopsy (TCB) of the pancreatic body in 16 patients with suspected non-focal CP[37]. Only 1 patient had CP based on the histological analysis; 8 had normal pancreas; 6 had non-diagnostic findings; 1 had device malfunction. Interestingly, all of the patients with normal pancreatic histology had 3-5 features on EUS imaging. Most of the patients with non-diagnostic histopathology had severe (≥ 5) EUS features, with only connective tissue apparent on the pathologic specimen. There were 2 hospitalizations for post-procedural abdominal pain and pancreatitis. The authors concluded that EUS-TCB was not recommended based on low yield and risk of complications. However, a more favorable interpretation is that TCB lowers the number of false positive EUS results.
Digital image analysis
Digital image analysis (DIA) limits operator dependence by trading human interpretation for computerized pattern recognition. The colors, distribution, and patterns of individual pixels are algorithmically quantified within a specified region of interest. Irisawa et al[38] digitally quantified the parenchymal echogenicity in CP patients and controls. The mean parenchymal echogenicity was determined as the amount of field area over a threshold level of echogenicity. The mean size above the threshold area was significantly higher in CP patients compared to controls. Also, echogenicity correlated with disease severity.
In another study, a sophisticated program was used to compare images from patients with CP, pancreatic cancer, and controls[39]. A total of 256 statistical parameters were extracted from each region of interest and filtered into a compact dataset of 11 textural parameters. These 11 variables were used to train and validate a neural network model for interpretation of each image. The end model was 100% accurate in classifying normal pancreas from CP. Further refinement of existing software is required to make DIA an easy and reliable method for real-time image interpretation.
Spectrum analysis
B-mode ultrasound gray-scale images are derived from the amplitude of reflected radiofrequency waves. Additional information can be gained from the spectrum of hidden back-scattered radiofrequency signals. This frequency information is typically ignored and not used to generate the image. However, backscattering is related to the density and sound speed of the tissue, and may represent a useful parameter to quantify pancreatic fibrosis. In a pilot study, statistically significant differences in spectral parameters computed from the backscatter were observed between normal pancreas and CP[40].
Elastography
A method to measure the “hardness” of tissue may be very applicable for early CP diagnosis. Tissue elastography assesses tissue stiffness by measuring the response of the tissue to mechanical compression. As the scope gently compresses the tissue, less tissue displacement occurs with hard relative to soft tissue. This displacement (or “strain”) can be quantified, color coded, and superimposed over the B-mode image.
There have been two initial reports of EUS elastography for CP. Janssen compared the elastography images in controls, patients with CP, and patients with pancreatic masses[41]. A “honeycombed” elastography pattern was apparent in most of the CP patients and patients with pancreatic masses. Most of the controls lacked the honeycombed appearance. These results suggest that elastography is useful in separating normal pancreas from CP, and less helpful in differentiating benign from malignant masses.
Săftoiu et al[42] applied sophisticated image analysis to the interpretation of EUS elastography. Mean hue histograms were calculated for regions of interest from controls, inflammatory masses, and malignant masses. Receiver operating characteristics (ROC) analysis revealed that a mean hue histogram cutpoint of 175 had 91.4% sensitivity and 89.9% specificity for diagnosis of benign from malignant masses. Neural network analysis of the images was employed to refine the diagnostic performance. After training the neural network, accuracy was as high as 97%.
Although initial results with elastography and other image analysis approaches are encouraging, many questions remain. Do these new techniques improve upon standard EUS scoring? Are they easily applied in real time? How much does operator dependence affect the results? It is worth noting that many of these pilot studies have not incorporated blinded interpretation of the images.
Contrast-enhanced EUS
Venous contrast agents may improve EUS resolution of pancreatic tissue and help differentiate benign from malignant masses. In one study, injection of the contrast agent Albumex produced enhancement in 3 of 4 patients with inflammatory masses vs 0 of 11 patients with pancreatic cancer[43]. In a study of 86 patients with benign and malignant masses, contrast enhancement improved sensitivity from 73.2% to 91.1%, and specificity from 83.3% to 93.3%[44]. There are no published studies of contrast-enhanced EUS for the diagnosis of early CP.
Endoscopic pancreatic function testing
Some experts suggest that both structural and functional testing is needed to optimally diagnose early CP[45]. Mild exocrine insufficiency detected by direct pancreatic function tests is a surrogate marker for early fibrosis. Duodenal aspirates can be collected through the endoscope following secretin stimulation, and analyzed for bicarbonate as a measure of duct-cell exocrine function. This endoscopic pancreatic function test method (ePFT) has shown equivalent bicarbonate concentration results as the traditional “Dreiling tube” secretin pancreatic function test in a crossover study[46]. We have recently combined EUS and ePFT in a single 50-min endoscopic session to allow a simultaneous assessment of pancreatic structure and function[16,47]. Concordant normal results (< 4 EUS criteria and peak bicarbonate concentration ≥ 80 mmol/L) rule out CP; concordant abnormal results (≥ 4 EUS criteria and peak bicarbonate concentration < 80 mmol/L) rule in CP. The greater diagnostic challenge arises when the structural and function results are discordant. Long-term observational studies are underway to clarify the relative importance of early structural and functional changes in predicting early fibrosis. In the meantime, the EUS and ePFT results should be interpreted in the context of the patient’s symptoms and risk factors.
Secretin enhanced EUS
Secretin may also be used to “dynamically” image the pancreatic duct in patients with suspected CP. Catalano et al[48] serially measured the pancreatic duct following secretin injection in 40 patients evaluated for suspected CP. Secretin-induced duct dilation was 80% sensitive and 96% specific for detecting ductal obstruction (stones and strictures, and also predicted response to minor papillotomy in patients with pancreas divisum.
CONCLUSION
Endoscopic ultrasound is a useful and safe technique for detecting pancreatic parenchymal and ductal abnormalities suggestive of chronic pancreatitis. Even after many comparative studies, the accuracy of EUS for diagnosing early chronic pancreatitis is poorly understood. In spite of this and other limitations, many gastroenterologists consider EUS the best available structural reference standard. It is hoped that emerging ancillary technologies will further refine the EUS diagnosis of chronic pancreatitis in coming years.
Footnotes
Peer reviewer: Claudio Bassi, MD, Professor, Department of Surgery and Gastroenterology, Hospital GB Rossi, University of Verona, Piazza LA Scuro 37134 Verona, Italy
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
References
- 1.Walsh TN, Rode J, Theis BA, Russell RC. Minimal change chronic pancreatitis. Gut. 1992;33:1566–1571. doi: 10.1136/gut.33.11.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555–564. doi: 10.1055/s-2007-1010405. [DOI] [PubMed] [Google Scholar]
- 3.Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11–17. doi: 10.1016/s0016-5107(98)70122-1. [DOI] [PubMed] [Google Scholar]
- 4.Nattermann C, Goldschmidt AJ, Dancygier H. Endosonography in chronic pancreatitis--a comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy. 1993;25:565–570. doi: 10.1055/s-2007-1010406. [DOI] [PubMed] [Google Scholar]
- 5.Dancygier H, Classen M. Endosonographic diagnosis of benign pancreatic and biliary lesions. Scand J Gastroenterol Suppl. 1986;123:119–122. doi: 10.3109/00365528609091872. [DOI] [PubMed] [Google Scholar]
- 6.Lees WR. Endoscopic ultrasonography of chronic pancreatitis and pancreatic pseudocysts. Scand J Gastroenterol Suppl. 1986;123:123–129. doi: 10.3109/00365528609091873. [DOI] [PubMed] [Google Scholar]
- 7.Sivak MV, Kaufman A. Endoscopic ultrasonography in the differential diagnosis of pancreatic disease. A preliminary report. Scand J Gastroenterol Suppl. 1986;123:130–134. doi: 10.3109/00365528609091874. [DOI] [PubMed] [Google Scholar]
- 8.Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, Hawes RH, Hoffman BJ. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 9.Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251–1261. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Stevens T, Zuccaro G Jr, Dumot JA, Vargo JJ, Parsi MA, Lopez R, Kirchner HL, Purich E, Conwell DL. Prospective comparison of radial and linear endoscopic ultrasound for diagnosis of chronic pancreatitis. Endoscopy. 2009;41:836–841. doi: 10.1055/s-0029-1215061. [DOI] [PubMed] [Google Scholar]
- 11.Buscail L, Escourrou J, Moreau J, Delvaux M, Louvel D, Lapeyre F, Tregant P, Frexinos J. Endoscopic ultrasonography in chronic pancreatitis: a comparative prospective study with conventional ultrasonography, computed tomography, and ERCP. Pancreas. 1995;10:251–257. [PubMed] [Google Scholar]
- 12.Forsmark CE, Toskes PP. What does an abnormal pancreatogram mean? Gastrointest Endosc Clin N Am. 1995;5:105–123. [PubMed] [Google Scholar]
- 13.Raimondo M, Wiersema MJ, Vazquez-Sequeiros E, DiMagno EP. Endoscopic ultrasound (EUS) may not be as sensitive as previously thought to diagnose chronic pancreatitis (CP). A preliminary correlation study with CCK pancreatic function test. Gastrointest Endosc. 2001;53:AB69. [Google Scholar]
- 14.Chowdhury R, Bhutani MS, Mishra G, Toskes PP, Forsmark CE. Comparative analysis of direct pancreatic function testing versus morphological assessment by endoscopic ultrasonography for the evaluation of chronic unexplained abdominal pain of presumed pancreatic origin. Pancreas. 2005;31:63–68. doi: 10.1097/01.mpa.0000164451.69265.80. [DOI] [PubMed] [Google Scholar]
- 15.Stevens T, Conwell DL, Zuccaro G Jr, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci. 2008;53:1146–1151. doi: 10.1007/s10620-007-9975-1. [DOI] [PubMed] [Google Scholar]
- 16.Stevens T, Dumot JA, Zuccaro G Jr, Vargo JJ, Parsi MA, Lopez R, Kirchner HL, Purich E, Conwell DL. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol. 2009;7:114–119. doi: 10.1016/j.cgh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Malfertheiner P, Büchler M, Stanescu A, Ditschuneit H. Exocrine pancreatic function in correlation to ductal and parenchymal morphology in chronic pancreatitis. Hepatogastroenterology. 1986;33:110–114. [PubMed] [Google Scholar]
- 18.Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc. 2007;66:501–509. doi: 10.1016/j.gie.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 19.Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc. 2007;65:808–814. doi: 10.1016/j.gie.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Gupta K, Carlson A, Kobayashi T, Manivel C, Lai I, Mallerg S, Southeslond Freeman ML. Endoscopic ultrasound in minimal change chronic pancreatitis: Is it an ideal diagnostic tool? Correlation of EUS criteria with histopathology in patients undergoing total pancreatectomy with autologous islet cell transplantation. Pancreas. 2007;35:AB405. [Google Scholar]
- 21.Albashir S, Bronner M, Parsi M, Walsh M, Stevens T. Endoscopic ultrasound and secretin endoscopic pancreatic function test: histopathological correlation in chronic pancreatitis. Am J Gastroenterol. 2009;104:A230. doi: 10.1038/ajg.2010.274. [DOI] [PubMed] [Google Scholar]
- 22.Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc. 2002;55:507–511. doi: 10.1067/mge.2002.122610. [DOI] [PubMed] [Google Scholar]
- 23.Ammann RW. The natural history of alcoholic chronic pancreatitis. Intern Med. 2001;40:368–375. doi: 10.2169/internalmedicine.40.368. [DOI] [PubMed] [Google Scholar]
- 24.Morris-Stiff G, Webster P, Frost B, Lewis WG, Puntis MC, Roberts SA. Endoscopic ultrasound reliably identifies chronic pancreatitis when other imaging modalities have been non-diagnostic. JOP. 2009;10:280–283. [PubMed] [Google Scholar]
- 25.Hastier P, Buckley MJ, Francois E, Peten EP, Dumas R, Caroli-Bosc FX, Delmont JP. A prospective study of pancreatic disease in patients with alcoholic cirrhosis: comparative diagnostic value of ERCP and EUS and long-term significance of isolated parenchymal abnormalities. Gastrointest Endosc. 1999;49:705–709. doi: 10.1016/s0016-5107(99)70286-5. [DOI] [PubMed] [Google Scholar]
- 26.Rajan E, Clain JE, Levy MJ, Norton ID, Wang KK, Wiersema MJ, Vazquez-Sequeiros E, Nelson BJ, Jondal ML, Kendall RK, et al. Age-related changes in the pancreas identified by EUS: a prospective evaluation. Gastrointest Endosc. 2005;61:401–406. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 27.Thuler FP, Costa PP, Paulo GA, Nakao FS, Ardengh JC, Ferrari AP. Endoscopic ultrasonography and alcoholic patients: can one predict early pancreatic tissue abnormalities? JOP. 2005;6:568–574. [PubMed] [Google Scholar]
- 28.Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol. 2004;2:405–409. doi: 10.1016/s1542-3565(04)00126-0. [DOI] [PubMed] [Google Scholar]
- 29.Al-Haddad M, Khashab M, Zyromski N, Pungpapong S, Wallace MB, Scolapio J, Woodward T, Noh K, Raimondo M. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: a case-control study. Pancreas. 2009;38:672–675. doi: 10.1097/MPA.0b013e3181a9d5af. [DOI] [PubMed] [Google Scholar]
- 30.Sahai AV, Mishra G, Penman ID, Williams D, Wallace MB, Hadzijahic N, Pearson A, Vanvelse A, Hoffman BJ, Hawes RH. EUS to detect evidence of pancreatic disease in patients with persistent or nonspecific dyspepsia. Gastrointest Endosc. 2000;52:153–159. doi: 10.1067/mge.2000.107910. [DOI] [PubMed] [Google Scholar]
- 31.Forsmark CE. The diagnosis of chronic pancreatitis. Gastrointest Endosc. 2000;52:293–298. doi: 10.1067/mge.2000.106889. [DOI] [PubMed] [Google Scholar]
- 32.Wallace MB, Hawes RH, Durkalski V, Chak A, Mallery S, Catalano MF, Wiersema MJ, Bhutani MS, Ciaccia D, Kochman ML, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc. 2001;53:294–299. doi: 10.1016/s0016-5107(01)70401-4. [DOI] [PubMed] [Google Scholar]
- 33.Topazian M, Enders F, Kimmey M, Brand R, Chak A, Clain J, Cunningham J, Eloubeidi M, Gerdes H, Gress F, et al. Interobserver agreement for EUS findings in familial pancreatic-cancer kindreds. Gastrointest Endosc. 2007;66:62–67. doi: 10.1016/j.gie.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Stevens T, Lopez R, Adler DG, Al-Haddad MA, Conway J, Dewitt JM, Forsmark CE, Kahaleh M, Lee LS, Levy MJ, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:519–526. doi: 10.1016/j.gie.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 35.Lieb J, Palma D, Leblanc J, Romagnuolo J, Farrell J, Savides T, Eloubeidi M, Draganov P, Forsmark C, Wagh M. Intra-Observer Agreement Among Endosonographers for EUS Features of Chronic Pancreatitis. Gastrointest Endosc. 2009:69; AB239. [Google Scholar]
- 36.Hollerbach S, Klamann A, Topalidis T, Schmiegel WH. Endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33:824–831. doi: 10.1055/s-2001-17337. [DOI] [PubMed] [Google Scholar]
- 37.DeWitt J, McGreevy K, LeBlanc J, McHenry L, Cummings O, Sherman S. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointest Endosc. 2005;62:76–84. doi: 10.1016/s0016-5107(05)00504-3. [DOI] [PubMed] [Google Scholar]
- 38.Irisawa A, Mishra G, Hernandez LV, Bhutani MS. Quantitative analysis of endosonographic parenchymal echogenicity in patients with chronic pancreatitis. J Gastroenterol Hepatol. 2004;19:1199–1205. doi: 10.1111/j.1440-1746.2004.03468.x. [DOI] [PubMed] [Google Scholar]
- 39.Das A, Nguyen CC, Li F, Li B. Digital image analysis of EUS images accurately differentiates pancreatic cancer from chronic pancreatitis and normal tissue. Gastrointest Endosc. 2008;67:861–867. doi: 10.1016/j.gie.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 40.Kumon RE, Olowe K, Faulx AL, Farooq FT, Chen VK, Zhou Y, Wong RC, Isenberg GA, Sivak MV, Chak A, et al. EUS spectrum analysis for in vivo characterization of pancreatic and lymph node tissue: a pilot study. Gastrointest Endosc. 2007;66:1096–1106. doi: 10.1016/j.gie.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 41.Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–978. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 42.Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–1094. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 43.Hirooka Y, Goto H, Ito A, Hayakawa S, Watanabe Y, Ishiguro Y, Kojima S, Hayakawa T, Naitoh Y. Contrast-enhanced endoscopic ultrasonography in pancreatic diseases: a preliminary study. Am J Gastroenterol. 1998;93:632–635. doi: 10.1111/j.1572-0241.1998.179_b.x. [DOI] [PubMed] [Google Scholar]
- 44.Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–250. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lankisch PG, Seidensticker F, Otto J, Lubbers H, Mahlke R, Stockmann F, Folsch UR, Creutzfeldt W. Secretin-pancreozymin test (SPT) and endoscopic retrograde cholangiopancreatography (ERCP): both are necessary for diagnosing or excluding chronic pancreatitis. Pancreas. 1996;12:149–152. doi: 10.1097/00006676-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Stevens T, Conwell DL, Zuccaro G Jr, Van Lente F, Lopez R, Purich E, Fein S. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc. 2008;67:458–466. doi: 10.1016/j.gie.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 47.Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined Endoscopic Ultrasound and Secretin Endoscopic Pancreatic Function Test in Patients Evaluated for Chronic Pancreatitis. Dig Dis Sci. 2010:Epbub ahead of print. doi: 10.1007/s10620-009-1084-x. [DOI] [PubMed] [Google Scholar]
- 48.Catalano MF, Lahoti S, Alcocer E, Geenen JE, Hogan WJ. Dynamic imaging of the pancreas using real-time endoscopic ultrasonography with secretin stimulation. Gastrointest Endosc. 1998;48:580–587. doi: 10.1016/s0016-5107(98)70039-2. [DOI] [PubMed] [Google Scholar]
- 49.Bhutani MS, Arantes VN, Verma D, Moezzi J, Suryaprasad S, Kapadia AS, Gopalswamy N. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas. 2009;38:820–824. doi: 10.1097/MPA.0b013e3181b2bc1a. [DOI] [PubMed] [Google Scholar]