Abstract
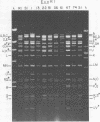
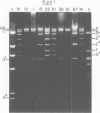
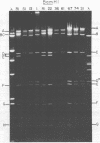
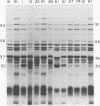
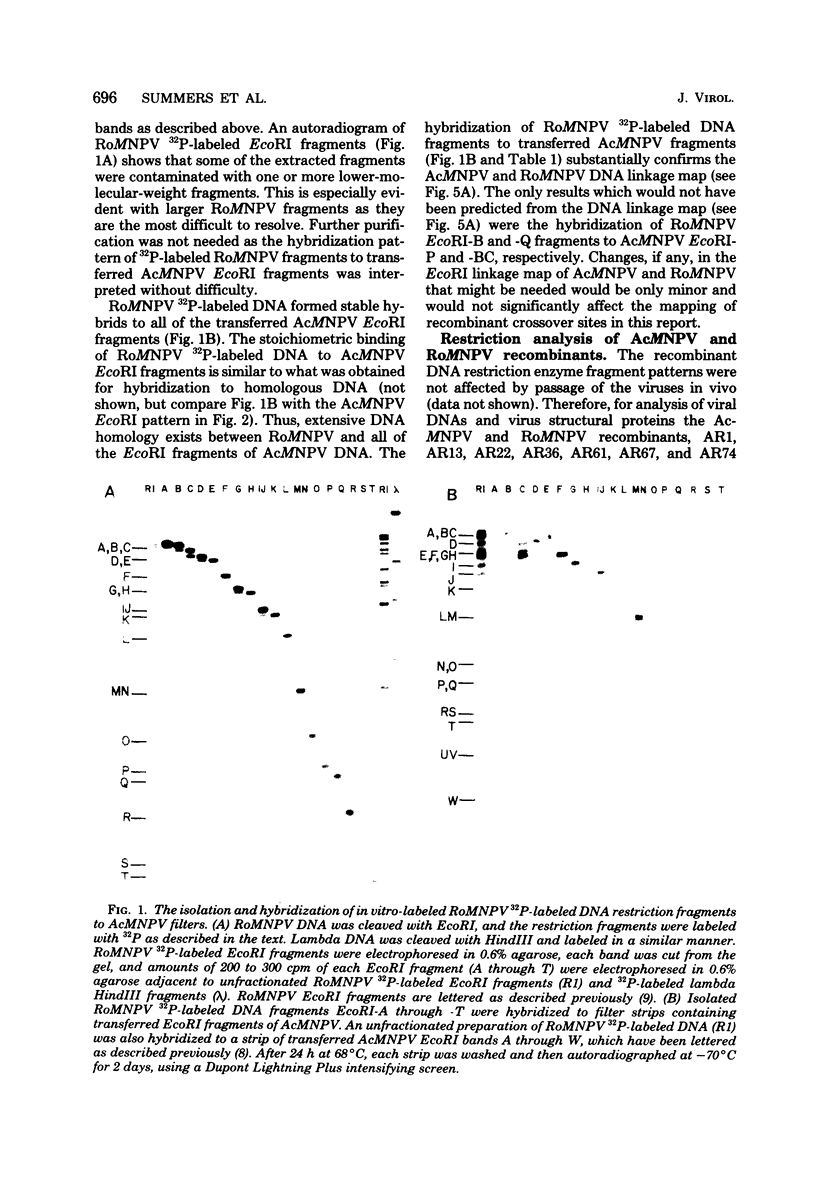
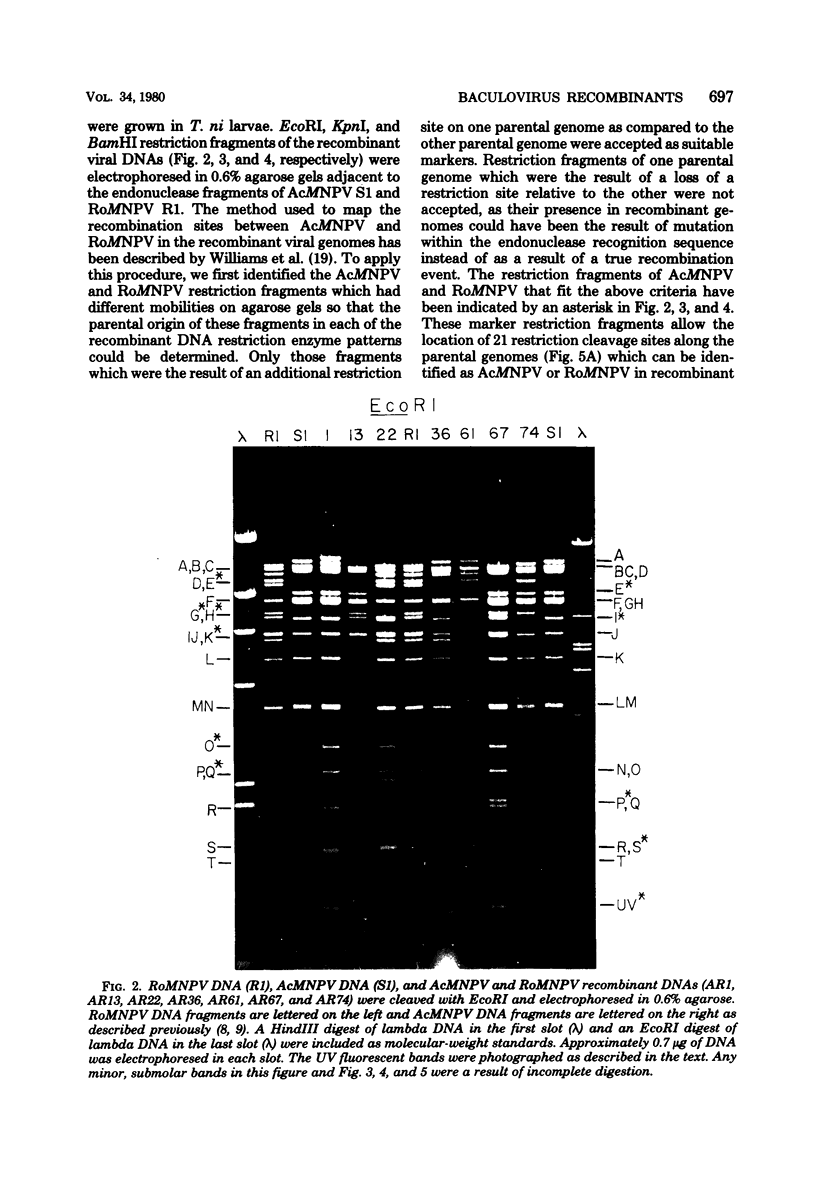
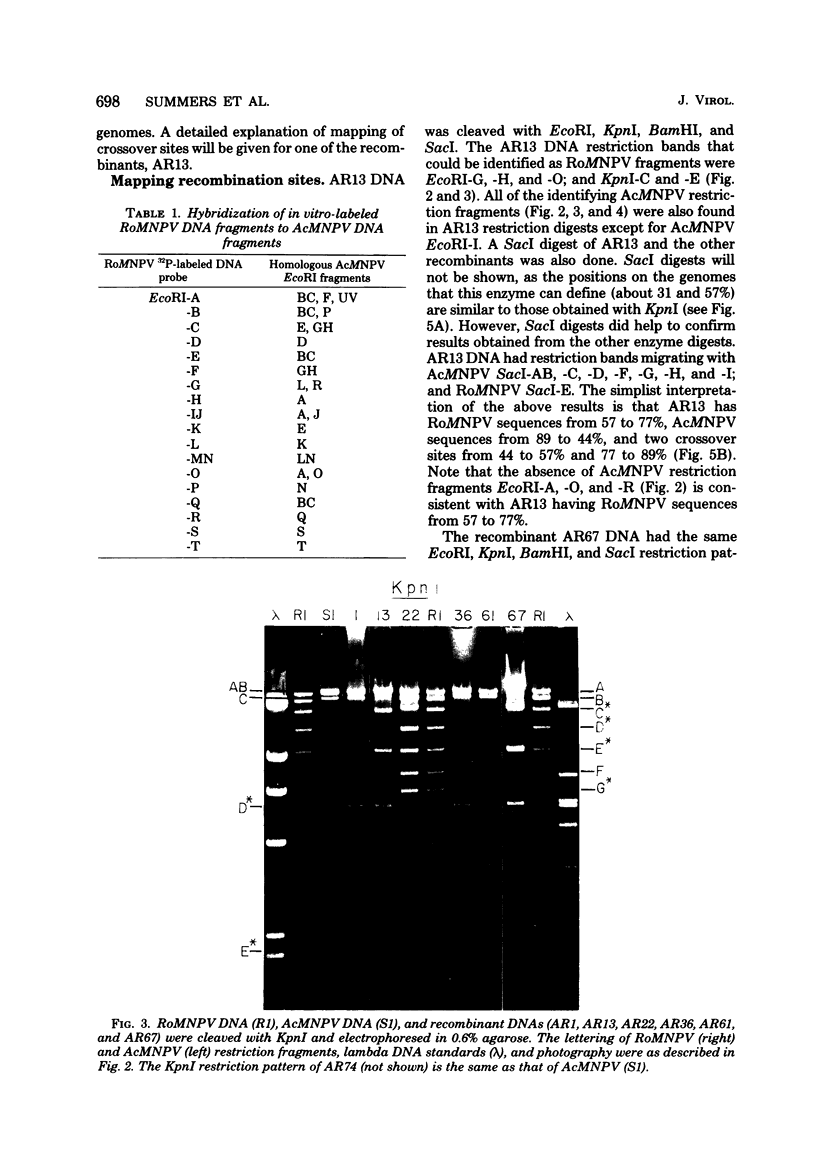
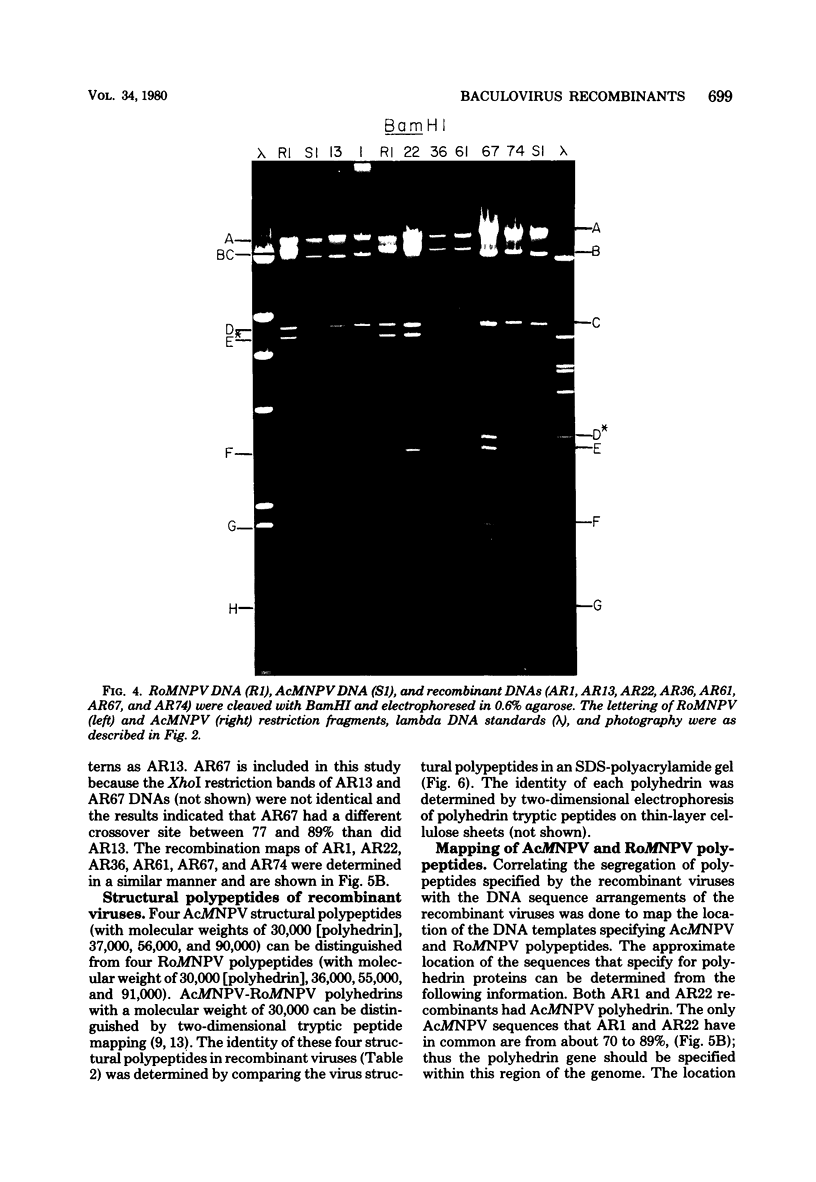
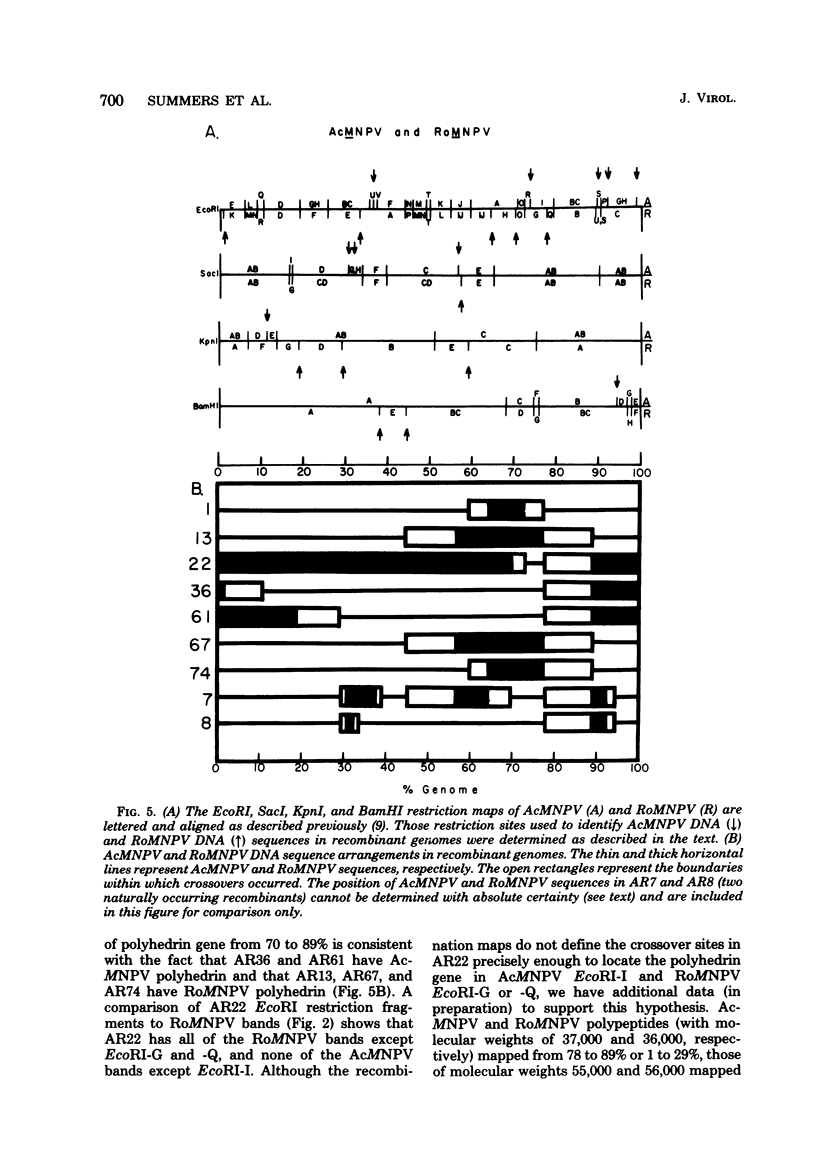
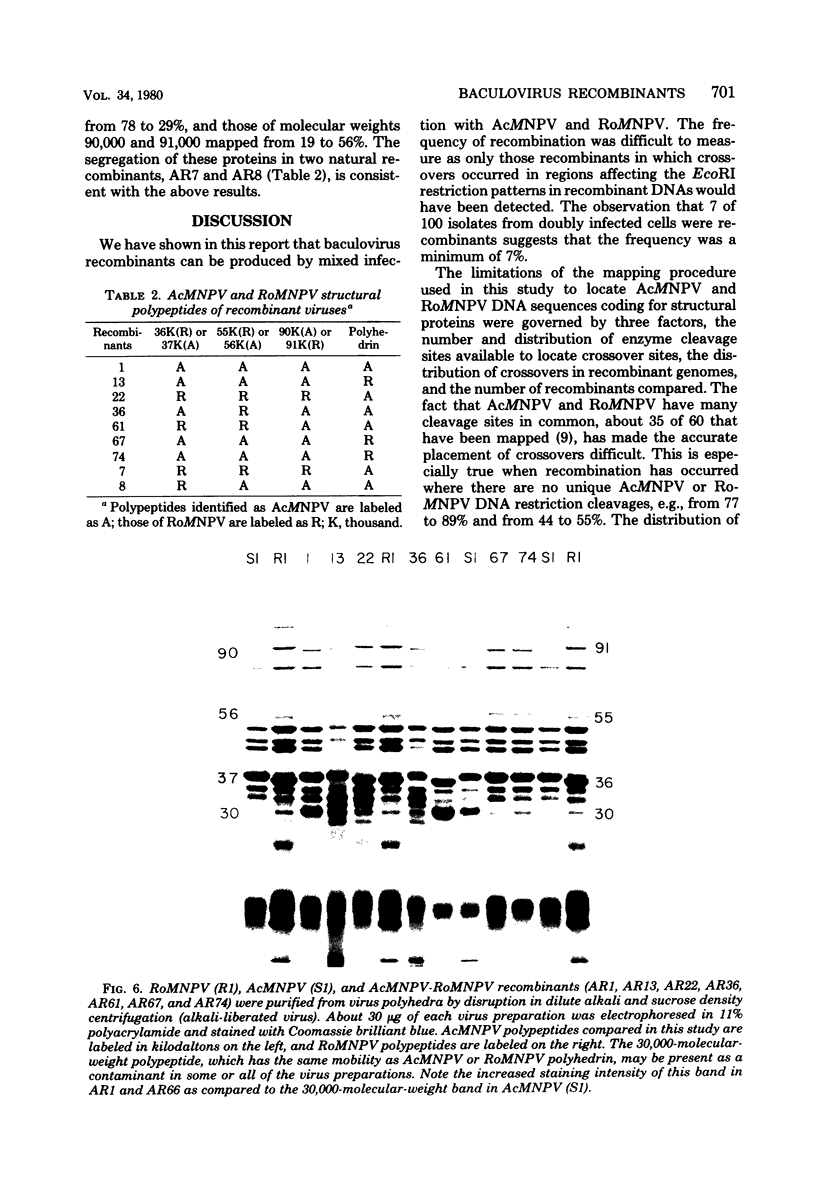
TN-368 cells were infected simultaneously with the closely related Autographa california (AcMNPV) and Rachiplusia ou (RoMNPV) nuclear polyhedrosis viruses. Progeny viral isolates were plaque purified, and their DNAs were analyzed with restriction endonucleases. Of 100 randomly cloned plaques, 7 were AcMNPV and RoMNPV recombinants, 5 were RoMNPV, and 88 were AcMNPV. The recombinants contained DNA sequences derived from both parental genomes. By comparing the restriction cleavage patterns of parental and recombinant DNAs, the crossover sites were mapped. A single double crossover was detected in each of the seven recombinant genomes. In addition, six of the seven recombinants revealed a crossover site mapping between 78 and 89% of the genome. The structural polypeptides of the seven recombinants and two parental viruses were analyzed by polyacrylamide gel electrophoresis, and their polyhedrins were identified by tryptic peptide mapping. An analysis of the segregation of three enveloped nucleocapsid proteins and of the polyhedrins among the recombinants located the DNA sequences coding for AcMNPV structural polypeptides with molecular weights of 37,000 (a capsid polypeptide), 56,000, and 90,000 and the RoMNPV structural polypeptides with molecular weights of 36,000 (a capsid polypeptide), 56,000, and 91,000. The AcMNPV and RoMNPV polypeptides of molecular weights 37,000 and 36,000, respectively, mapped within 78 to 89% or 1 to 29%, the polypeptides of molecular weights 55,000 and 56,000 mapped within 78 to 29%, and the polypeptides of molecular weights 90,000 and 91,000 mapped within 19 to 56% of the genome. The region of the parental DNAs that codes for polyhedrin was located within 70 to 89% of the genome.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chartrand P., Stow N. D., Timbury M. C., Wilkie N. M. Physical mapping of paar mutations of herpes simplex virus type 1 and type 2 by intertypic marker rescue. J Virol. 1979 Aug;31(2):265–276. doi: 10.1128/jvi.31.2.265-276.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maruniak J. E., Summers M. D., Falcon L. A., Smith G. E. Autographa californica nuclear polyhedrosis virus structural proteins compared from in vivo and in vitro sources. Intervirology. 1979;11(2):82–88. doi: 10.1159/000149017. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. Restriction Map of Rachiplusia ou and Rachiplusia ou-Autographa californica Baculovirus Recombinants. J Virol. 1980 Jan;33(1):311–319. doi: 10.1128/jvi.33.1.311-319.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. Restriction Maps of Five Autographa californica MNPV Variants, Trichoplusia ni MNPV, and Galleria mellonella MNPV DNAs with Endonucleases SmaI, KpnI, BamHI, SacI, XhoI, and EcoRI. J Virol. 1979 Jun;30(3):828–838. doi: 10.1128/jvi.30.3.828-838.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. Physical mapping of temperature-sensitive mutations of herpes simplex virus type 1 by intertypic marker rescue. Virology. 1978 Oct 1;90(1):1–11. doi: 10.1016/0042-6822(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Egawa K. Physical and chemical properties of Trichoplusia ni granulosis virus granulin. J Virol. 1973 Nov;12(5):1092–1103. doi: 10.1128/jvi.12.5.1092-1103.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Baculovirus structural polypeptides. Virology. 1978 Feb;84(2):390–402. doi: 10.1016/0042-6822(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Comparative studies of baculovirus granulins and polyhedrins. Intervirology. 1975;6(3):168–180. doi: 10.1159/000149469. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Berg P. Hybridization in situ of SV40 plaques: detection of recombinant SV40 virus carrying specific sequences of nonviral DNA. Science. 1977 Apr 8;196(4286):183–185. doi: 10.1126/science.191907. [DOI] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D., Hsieh C. H. Occluded and nonoccluded nuclear polyhedrosis virus grown in Trichoplusia ni: comparative neutralization comparative infectivity, and in vitro growth studies. J Virol. 1976 Sep;19(3):820–832. doi: 10.1128/jvi.19.3.820-832.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman L. E., Summers M. D. Nuclear polyhedrosis virus detection: relative capabilities of clones developed from Trichoplusia ni ovarian cell line TN-368 to serve as indicator cells in a plaque assay. J Virol. 1975 Dec;16(6):1630–1637. doi: 10.1128/jvi.16.6.1630-1637.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Grodzicker T., Sharp P., Sambrook J. Adenovirus recombination: physical mapping of crossover events. Cell. 1975 Feb;4(2):113–119. doi: 10.1016/0092-8674(75)90117-8. [DOI] [PubMed] [Google Scholar]