Abstract
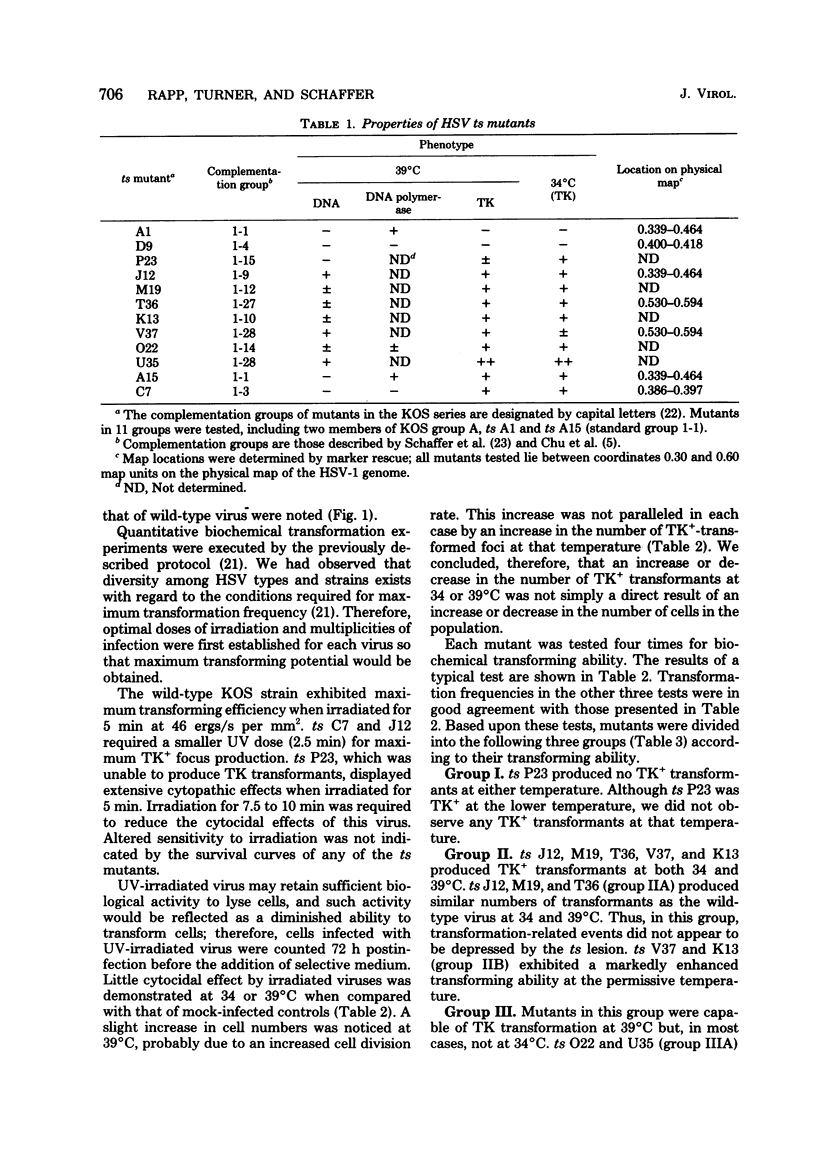
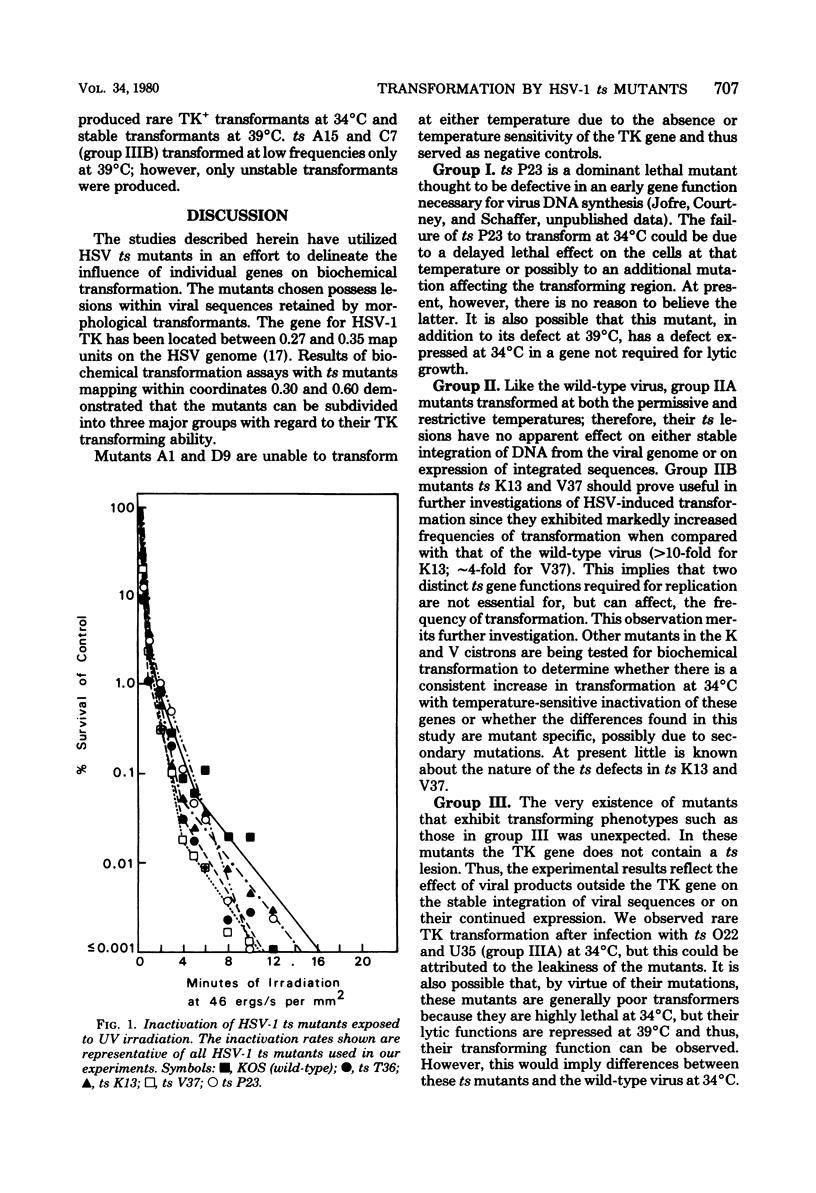
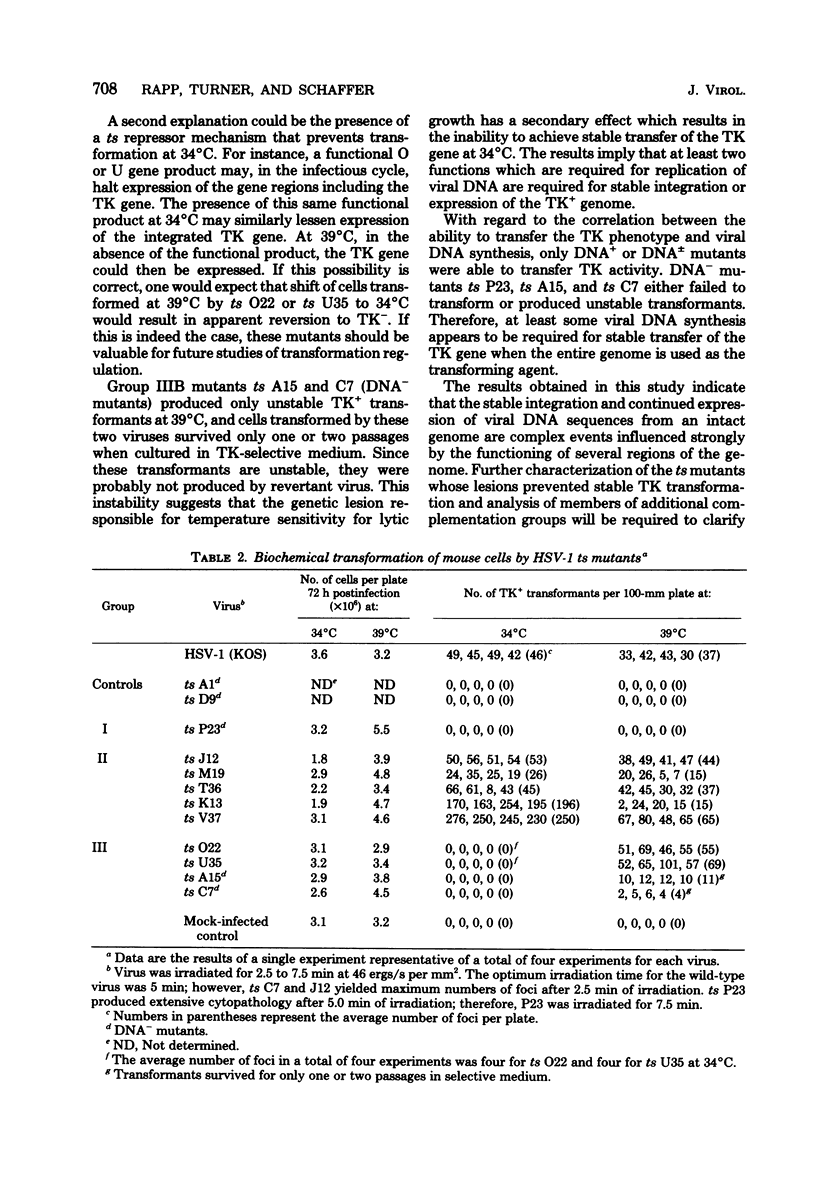
Biochemical transformation assays of herpes simplex virus type 1 temperature-sensitive (ts) mutants distinguished three groups of mutants with regard to their thymidine kinase (TK) transforming ability: those incapable of transferring the TK gene at either the permissive or restrictive temperatures (group I); those resembling the wild-type virus, and therefore able to transform at both the permissive and nonpermissive temperatures (group II); and those that failed to transform or exhibited very low transformation frequencies at the permissive temperature but were able to transform at the nonpermissive temperature (group III). Two mutants in group II exhibited greatly enhanced transformation efficiency at the permissive temperature. The ts lesions in the majority of the mutants tested map between 0.30 and 0.60 units on the viral genome. Mutants with TK-positive (TK+), but DNA-negative, phenotypes at the nonpermissive temperature produced no TK+ transformants at the permissive temperature and only unstable transformants at the nonpermissive temperature. This suggests that a function which is required for viral DNA synthesis is also required to obtain stable expression or to transfer the TK+ gene or both when transfer is mediated by the entire viral genome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A. L., Orme T. W. Transformation of mouse cells after infection with ultraviolet irradiation-inactivated herpes simplex virus type 2. Int J Cancer. 1975 Oct 15;16(4):526–538. doi: 10.1002/ijc.2910160403. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Spear P. G. Regulation of the herpes simplex virus gene for thymidine kinase in clonal derivatives of transformed mouse L-cells. IARC Sci Publ. 1978;(24 Pt 1):517–522. [PubMed] [Google Scholar]
- Camacho A., Spear G. Transformation of hamster embryo fibroblasts by a specific fragment of the herpes simplex virus genome. Cell. 1978 Nov;15(3):993–1002. doi: 10.1016/0092-8674(78)90283-0. [DOI] [PubMed] [Google Scholar]
- Chu C. T., Parris D. S., Dixon R. A., Farber F. E., Schaffer P. A. Hydroxylamine mutagenesis of HSV DNA and DNA fragments: introduction of mutations into selected regions of the viral genome. Virology. 1979 Oct 15;98(1):168–181. doi: 10.1016/0042-6822(79)90535-x. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K. J., Summers W. P., Summers W. C. The thymidine kinase gene of herpes simplex virus type 1: cell-free protein synthesis and substrate specificity studies. IARC Sci Publ. 1978;(24 Pt 1):337–345. [PubMed] [Google Scholar]
- Davidson R. L., Adelstein S. J., Oxman M. N. Herpes simplex virus as a source of thymidine kinase for thymidine kinase-deficient mouse cells: suppression and reactivation of the viral enzyme. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1912–1916. doi: 10.1073/pnas.70.7.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Kingsbury D. T. Quantitation of the viral DNA present in cells transformed by UV-irradiated herpes simplex virus. J Virol. 1976 Mar;17(3):788–793. doi: 10.1128/jvi.17.3.788-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiselburd E., Gage L. P., Weissbach A. Presence of a herpes simplex virus DNA fragment in an L cell clone obtained after infection with irradiated herpes simplex virus I. J Mol Biol. 1975 Oct 5;97(4):533–542. doi: 10.1016/s0022-2836(75)80057-x. [DOI] [PubMed] [Google Scholar]
- Kucera L. S., Gusdon J. P. Transformation of human embryonic fibroblasts by photodynamically inactivated herpes simplex virus, type 2 at supra-optimal temperature. J Gen Virol. 1976 Feb;30(2):257–261. doi: 10.1099/0022-1317-30-2-257. [DOI] [PubMed] [Google Scholar]
- Leiden J. M., Frenkel N., Polacek D., Rapp F. Mapping of the herpes simplex virus DNA sequences in three herpes simplex virus thymidine kinase-transformed cell lines. IARC Sci Publ. 1978;(24 Pt 1):473–488. [PubMed] [Google Scholar]
- Li J. L., Jerkofsky M. A., Rapp F. Demonstration of oncogenic potential of mammalian cells transformed by DNA-containing viruses following photodynamic inactivation. Int J Cancer. 1975 Feb 15;15(2):190–202. doi: 10.1002/ijc.2910150204. [DOI] [PubMed] [Google Scholar]
- Lin S. S., Munyon W. Expression of the viral thymidine kinase gene in herpes simplex virus-transformed L cells. J Virol. 1974 Nov;14(5):1199–1208. doi: 10.1128/jvi.14.5.1199-1208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Minson A. C., Wildy P., Buchan A., Darby G. Introduction of the herpes simplex virus thymidine kinase gene into mouse cells using virus DNA or transformed cell DNA. Cell. 1978 Mar;13(3):581–587. doi: 10.1016/0092-8674(78)90331-8. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Dixon R. A., Schaffer P. A. Physical mapping of herpes simplex virus type 1 ts mutants by marker rescue: correlation of the physical and genetic maps. Virology. 1980 Jan 30;100(2):275–287. doi: 10.1016/0042-6822(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Rapp F., Turner N. Biochemical Transformation of mouse cells by herpes simplex virus types 1 and 2: comparison of different methods for inactivation of viruses. Arch Virol. 1978;56(1-2):77–87. doi: 10.1007/BF01317284. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]



