Abstract
Phosphorylation of H2AX functions to recruit DNA repair complexes to sites of DNA damage. Here, we report that H2AX is constitutively acetylated on lysine 36 (H2AXK36Ac) by the CBP/p300 acetyltransferases. H2AXK36Ac is required for cells to survive exposure to ionizing radiation; however, H2AXK36Ac levels are not increased by DNA damage. Further, acetylation of H2AX did not affect phosphorylation of H2AX or the formation of DNA damage foci. Finally, cells with a double mutation in both the H2AX acetylation and phosphorylation sites were more radiosensitive than cells containing individual mutations. H2AXK36Ac is therefore a novel, constitutive histone modification located within the histone core region which regulates radiation sensitivity independently of H2AX phosphorylation.
Keywords: H2AX, acetylation, chromatin, DNA-double strand breaks, Histone acetyltransferase, IR
1. Introduction
In response to DSBs, serine 139 of H2AX is rapidly phosphorylated by ATM and related kinases [1]. Formation of γH2AX provides binding sites to recruit the mdc1 protein [2], which then functions as a platform to concentrate DNA repair proteins at DSBs [3,4]. Consequently, loss of H2AX is associated with increased genomic instability and sensitivity to ionizing radiation [5,6]. Histones are also modified on lysine by methylation, ubiquitination and acetylation [7]. Histone acetylation is significantly increased in response to DNA damage, and it is now clear that chromatin acetylation is important for DSB repair. The acetylation of H2A and H2AX on lysine 5 by Tip60 plays a key role in the DNA damage response [8-11]. In drosophila, acetylation of H2Av on lysine 5 is required for removal of H2Av from the chromatin and for its subsequent dephosphorylation [10]. Acetylation of histone H4 [8,9,12] and H2AX [10,11] is required for the formation of open chromatin structures at DSBs, regulating the extent of γH2AX formation and is critical for facilitating access of the DNA repair machinery to DSBs [13]. Increased acetylation of histones at DSBs therefore makes a key contribution to DSB repair by regulating histone exchange, remodeling chromatin structure and facilitating the recruitment of DNA repair proteins to suites of DNA damage. However, whether H2AX contains other sites for lysine acetylation which are important for DNA damage response is not known. Here, we demonstrate that acetylation of lysine 36 of H2AX is essential for cells to survive exposure to DNA damage, and show that this function of lysine 36 is independent of the phosphorylation of serine 139.
2. Materials and methods
Cell culture
Flag-HA-H2AX cDNA was inserted into the pIRESpuro3 (Clontech, CA) expression vector. Mutations were created using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, CA). 293T cells, and H2AX−/− MEF cells (provided by A. Nussenzweig [6]) were maintained in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum. Transfections were carried out using FuGene6 (Roche, IN) or Lipofectamine 2000 (Invitrogen, CA) and stable cell lines established using puromycin. Cell survival assays, flow cytometry and immunofluorescent staining are described in [14,15]. For cell synchronization, exponentially growing 293T cells were incubated twice in the presence of 100mM thymidine for 18h, with 10h in thymidine free medium between exposures. G1/S phase and S phase cells were obtained immediately upon or 2h after the release from thymidine block. G2/M arrest was achieved with nocodazole (40ng/ml for 18h).
Western blotting
Rabbit polyclonal antibody AbK36Ac was raised using the synthetic peptide 29-RVHRLLR(AcK)GHYAER-42. AbK36Ac was purified by sequential affinity chromatography against unacetylated peptide and acetylated peptide. Preparation of cell lysates, antibodies, acid extraction of histones and immunoprecipitation are described in [15,16].
3. Results
To determine the contribution of conserved lysines on H2AX to the cells DNA damage response, lysine residues were individually mutated and expressed in H2AX−/− MEFs. H2AX was cloned into the inducible pIRES vector to allow controlled expression of H2AX. Figure 1a demonstrates that the expression levels and phosphorylation of exogenous H2AX (H2AXwt) were similar to that of endogenous H2AX in H2AX+/+ MEFs. Each of the H2AX constructs were efficiently incorporated into the chromatin at similar levels to H2AXwt and were phosphorylated in response to DNA damage (figure 1b and 1c). Further, mutation of lysines 118 and 119 eliminated the ubiquitinated form of H2AX, confirming that this is the site of H2AX ubiquitination (figure 1b).
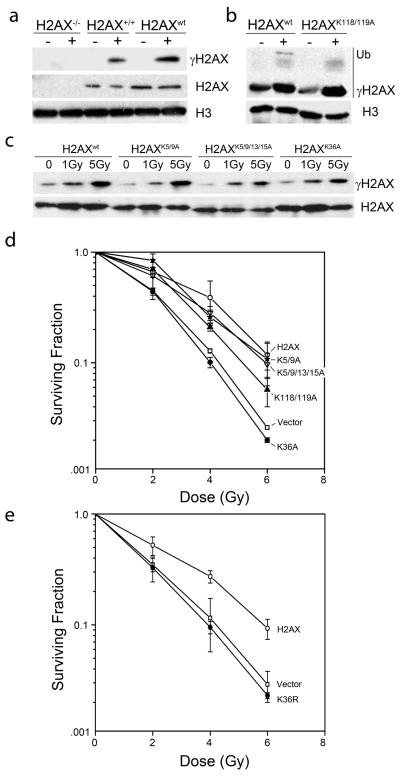
Figure 1. Mutation of lysine 36 of H2AX confers sensitivity to IR.
(a) H2AX−/− MEFs, wild type H2AX MEFs (H2AX+/+) or H2AX−/− MEFs reconstituted with H2AX (H2AXwt) were irradiated (5Gy). H2AX, γH2AX and H3 were measured by western blot. (b) H2AX−/− MEFs reconstituted with H2AXwt or H2AX with alanine mutations at lysines 118 and 119 (H2AXK118/119A) were irradiated (IR: 5Gy). H2AX and γH2AX were measured by western blot analysis. Ubiquitinated γH2AX is shown. (c) H2AX−/− MEF cells reconstituted with H2AXwt or H2AX with alanine mutations at lysines 5 and 9 (H2AXK5/9A), lysines 5, 9, 13 and 15 (H2AXK5/9/13/15A) or lysine 36 (H2AXK36A) were irradiated. H2AX and γH2AX were measured by western blot. (d) H2AX−/− MEFs expressing vector, wild type H2AX or H2AX with mutations at K5/9A, K5/9/13/15A, K118/119A or K36A were irradiated as indicated. 12 days later, clonogenic cell survival assays were carried out. Results ± SE (n = 3). (e) H2AX−/− MEFs expressing H2AX, Vector or H2AX with the mutation K36R were irradiated as indicated. 12 days later, clonogenic cell survival assays were carried out. Results ± SE (n = 3).
H2AX−/− MEF cells exhibit increased sensitivity to IR [6]. MEFs expressing the various H2AX proteins were irradiated and clonogenic cell survival measured. Complementation of H2AX−/− MEFs with H2AX increased the radioresistance of the cells (figure 1d, H2AXwt). Mutations targeted to the n-terminal regulatory domain of H2AX (H2AXK5/9A and H2AXK5/9/13/15A) had similar radiosensitivity to cells expressing wild type H2AX (figure 1d). Therefore, although lysine 5 of H2AX is acetylated after DNA damage [10], neither lysine 5 nor other n-terminal lysines contribute to the level of γH2AX (figure 1c) or the ability of cells to survive exposure to IR (figure 1d). Therefore, while acetylation of lysine 5 of H2AX may play a key role in regulating the turnover of γH2AX [10], loss of this acetylation sites does not significantly impact the ability of cells to repair and survive exposure to IR. A double mutation of lysines 118 and 119, which abolishes ubiquitinated H2AX (figure 1b), only partially complemented the radiosensitivity of the H2AX−/− MEFs (figure 1d), even though it demonstrated normal levels of γH2AX (figure 1b). Previous reports indicate that H2AX ubiquitination by RNF8 is required to recruit the rap80-brca1 complex and 53BP1 to sites of DNA damage [17,18]. Ubiquitination of H2AX may therefore function to recruit brca1 to DSBs and be important for cells to survive IR-induced DNA damage.
Mutation of lysine 36 had a profound effect on H2AX function (figure 1d). Expression of H2AXK36A in H2AX−/− MEFs failed to complement the increased radiosensitivity of these cells, even though the H2AXK36A protein was efficiently phosphorylated after DNA damage (figure 1c). H2AXK36 is in the histone core domain, and may be important for histone-histone or histone DNA interactions. Because mutation of lysine 36 to alanine will eliminate the charge on this residue, lysine 36 was also mutated to arginine to retain the positive charge. H2AXK36R also failed to complement the increased sensitivity of H2AX−/− cells to IR (figure 1e). Further, the kinetics of H2AX phosphorylation and dephosphorylation were unaffected by mutation of lysine 36 of H2AX (figure 2a). Overall, these results indicate that H2AXK36 plays a critical role in the function of H2AX during DNA damage.
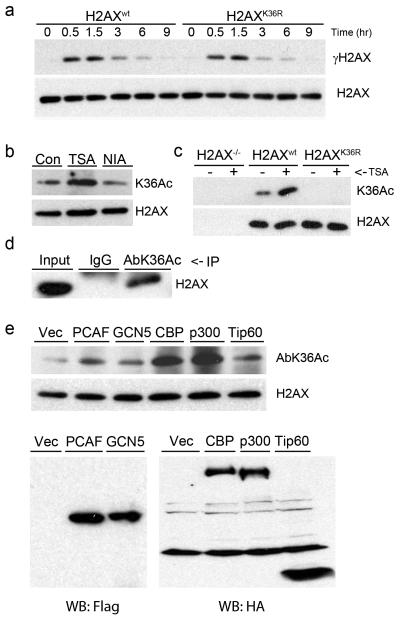
Figure 2. Lysine 36 of H2AX is acetylated.
(a) H2AX−/− MEFs expressing H2AXwt or H2AXK36R were irradiated (5Gy) and allowed to recover. γH2AX and H2AX were monitored by western blot. (b) 293T cells expressing Flag-H2AX were incubated with TSA (0.4 μM) or nicotinamide (NIA: 10mM) for 8hr. H2AX and H2AXK36Ac were monitored using antibody AbK36Ac. (c) 293T cells expressing Vector, Flag-H2AXwt or Flag-H2AXK36R were untreated or exposed to TSA (0.4μM for 8hr) and immunoprecipitated with Flag antibody. H2AXK36Ac was monitored by western blot using AbK36Ac. (d) Histones from H2AX+/+ MEF cells were immunoprecipitated with IgG or AbK36Ac. H2AX was detected by western blotting. (e) 293T cells were transiently transfected with Flag-H2AX and either vector, Flag-PCAF, Flag-GCN5, HA-CBP, HA-p300 or HA-Tip60. 72hr later, chromatin associated histones (upper panel) and total proteins (lower panel) were extracted. Acetylation of lysine 36 was detected with AbK36Ac antibody, and expression of HATs was monitored with anti-Flag or anti-HA antibody.
An acetylation specific antibody, AbK36Ac, was raised to examine H2AXK36 acetylation. 293T cells expressing Flag-H2AX were incubated with TSA (to inhibit class I and II HDACs) or nicotinamide (to inhibit SIRT deacetylases) [19]. AbK36Ac recognized an H2AX band whose intensity increased after incubation with TSA, but not nicotinamide (figure 2b), implicating class I and II HDACs in the regulation of H2AXK36Ac. The AbK36Ac antibody recognized a TSA stimulated H2AX band from H2AXwt cells but not from H2AXK36R cells (figure 2c), demonstrating that lysine 36 is required for acetylation. To determine if endogenous H2AX was acetylated at lysine 36, histones were isolated from wild type MEF cells. H2AX was immunoprecipitated with AbK36Ac antibody but not with IgG (figure 2d). These results demonstrate that H2AX is acetylated at lysine 36 in vivo, and that class I/II deacetylases regulate acetylation at this site.
To identify the HAT responsible for acetylation of H2AX, 293T cells were cotransfected with Flag-H2AX and the indicated HAT (figure 2e). Co-expression of CBP or p300 dramatically enhanced H2AX acetylation of lysine 36, whereas PCAF, GCN5 and Tip60 had minimal impact. CBP and p300 are therefore responsible for acetylation of H2AX on lysine 36, and provide additional evidence that lysine 36 of H2AX is a site for acetylation. Next, we examined if acetylation of lysine 36 was increased by DNA damage. However, although IR increased the phosphorylation of H2AX (figure 3a), no significant change in the levels of H2AXK36Ac were detected. Further, figure 3b demonstrates that the H2AXS139A phosphorylation mutant was still acetylated on lysine 36, and that the H2AXK36R mutant was still phosphorylated on serine 139, demonstrating that these 2 post-translational modifications are independent of each other. Finally, we examined the recruitment of 53BP1, a sensitive marker of DNA damage [18], to sites of DNA damage using immunofluorescent staining. 53BP1 was efficiently located to DSB in both H2AXwt and H2AXK36R cells (figure 3c), and there was no significant difference in the number of 53BP1 foci between the H2AXwt and H2AXK36R cells up to 5 hours after irradiation (figure 3d). This indicates that retention of 53BP1 does not require H2AXK36Ac. H2AXK36Ac does not, therefore, impact radiosensitivity by altering functions of H2AX which are processed through phosphorylation of serine 139.
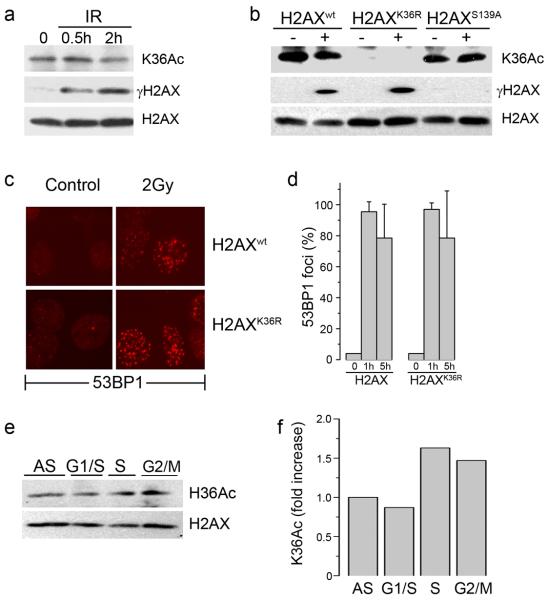
Figure 3. Acetylation of lysine 36 of H2AX is not altered by DNA damage.
(a) 293T cells expressing Flag-H2AX were irradiated (5 Gy) and chromatin associated histones isolated. H2AX, γH2AX and H2AXK36Ac were detected by western blot. (b) 293T cells expressing Flag-H2AX, Flag-H2AXK36R or Flag-H2AXS139A were irradiated (5Gy). H2AX, γH2AX and H2AXK36Ac were measured by western blot. (c) H2AX−/− MEFs expressing H2AXwt or H2AXK36R were irradiated (2Gy) and allowed to recover for 1hr. Cells were fixed and immunofluorescent staining carried out using anti-53BP1 antibody. (d) Quantitation of 53BP1 foci in cells expressing H2AX or H2AXK36R mutations. Cells were either untreated (0) or irradiated (2Gy) and the number of cells containing >5 foci counted 1h or 5h post-irradiation. (e) 293T cells stably expressing Flag-H2AX were either growing asynchronously (AS) or synchronized at the G1/S boundary, in S phase or G2/M as described in methods. H2AXK36Ac was monitored using AbK36Ac. (f) Western blots in (e) were scanned, and the ratio of K36 acetylation signal to total H2AX signal calculated.
H2AXK36Ac is a constitutive modification located in the core domain of H2AX, where it is likely to be inaccessible. Histone modifications within core domains are often deposited onto the DNA during replication; consequently, their levels increase during cell cycle progression. 293T cells were synchronized in G1/S, S phase or G2-M and H2AX acetylation compared to asynchronous cells. Cells in S-phase and G2 displayed significant increases in H2AXK36Ac compared to either asynchronous or G1 phase cells (figure 3e and 3f), implying that acetylated H2AX is deposited onto the chromatin during DNA replication.
The lack of functional interaction between H2AXK36Ac and phosphorylation of H2AX implies that phosphorylation and acetylation of H2AX regulate radiosensitivity through different pathways. To test this, H2AXwt, H2AXK36R, H2AXS139A and H2AXK36R/S139A were stably expressed in H2AX−/− (figure 4a). Clonogenic cell survival assays demonstrated that H2AXwt complemented the increased sensitivity of H2AX−/− cells to IR, whereas H2AXK36R and H2AXS139A were inactive, as expected (figure 4b). Significantly, the H2AXK36R/S139A double mutant cells were more sensitive to IR than either H2AXK36R or H2AXS139A alone. Therefore, phosphorylation of serine 139 and acetylation of lysine 36 function in 2 distinct pathways to regulate the ability of cells to repair and survive exposure to IR.
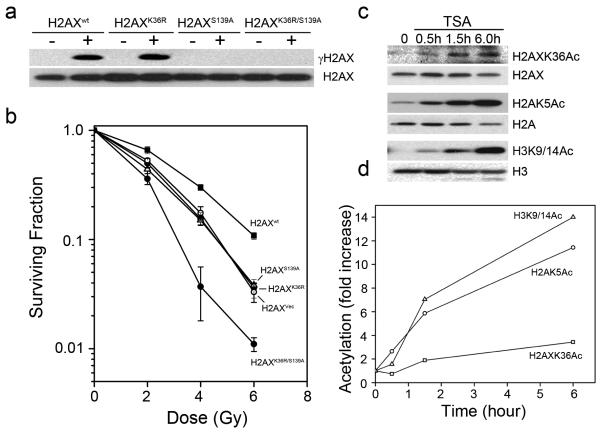
Figure 4. Acetylation of lysine 36 of H2AX is independent of H2AX phosphorylation.
(a) H2AXwt, H2AXK36R, H2AXS139A or H2AXK36R/S139A were irradiated (5Gy). H2AX and γH2AX measured by western blot. (b) H2AX−/− MEFs expressing vector (H2AXVec), H2AXwt, H2AXK36R, H2AXS139A or H2AXK36R/S139A were irradiated. 12 days later, clonogenic cell survival assays were carried. Results ± SD (n = 3). (c, d). 293T cells expressing Flag-H2AX were incubated with TSA (0.4μM). H2AX, H2A and H3 acetylation were measured using antibodies specific for H2AXK36Ac (AbK36Ac), H2AK5Ac or H3K9/14Ac. Western blots were scanned, and the ratio of acetylation signal to total histone signal calculated.
Because H2AXK36Ac is predicted to be buried inside the chromatin structure, we next examined the dynamics of lysine 36 acetylation. Cells were incubated with TSA to prevent deacetylation and the kinetics of histone acetylation monitored. TSA rapidly increased acetylation of lysine 5 of H2A and lysines 9 and 14 of H3, with an approximate 4-fold increase within 60 minutes and a greater than 10-fold increase within 6hr (figure 4c and 4d). This rapid acetylation of H2A and H3 reflects the fact that the n-terminals of these histones protrude from the nucleosome and are dynamically acetylated in the cell. In contrast, H2AXK36Ac exhibited a slow increase in acetylation after TSA addition, with only a 3-fold increase 6hr after treatment. The relatively slow dynamics of TSA induced acetylation indicates that H2AXK36Ac represents a stable modification which is buried within the nucleosome structure.
4. Discussion
Using immunological and genetic approaches, we demonstrated that lysine 36 of H2AX was acetylated, and that H2AXK36Ac was required for cells to survive exposure to IR. However, H2AXK36Ac was not altered by IR and did not impact either H2AX phosphorylation or the formation of mdc1 or 53BP1 foci. H2AXK36AC is therefore functionally distinct from DNA damage pathways which operate through phosphorylation of the c-terminal of H2AX. Further, H2AXK36Ac was not subject to dynamic regulation, since the turnover of H2AXK36Ac was much slower than the turnover of lysine acetylation on the n-terminal of H2A and H3 (figure 4). These results are consistent with H2AXK36Ac being a constitutive modification located within the core domain of H2AX, and therefore buried within the nucleosome structure.
The CBP and p300 acetyltransferases [20] were implicated in the acetylation of lysine 36 of H2AX. CBP and p300 also acetylate histone H3 on lysine 56 [21], and defects in the acetylation of H3K56 results in increased sensitivity to genotoxic agents [21,22]. Further, H3K56Ac, like H2AXK36, is located in the histone core domain. The acetylation of both H3K56 [22] and H2AXK36 (figure 3) are increased during S-phase, implying that the acetylated forms of both these histones are incorporated into the chromatin during DNA replication. Functionally, acetylation of H3K56 and H2AXK36 will neutralize the positive charge on these lysines, reducing the strength of the histone-DNA interaction within the nucleosomes. The loss of acetylation of H2AXK36 may therefore increase nucleosome stability, preventing the DNA repair machinery from processing the adjacent DSBs, and therefore leading to increased radiosensitivity.
In conclusion, H2AXK36Ac is a stable, constitutive histone modification located within the histone core region. Importantly, H2AXK36Ac regulates radiosensitivity through a novel pathway which is independent of the well described γH2AX signaling pathway. A small number post-translational modifications have been identified within histone core domains, including methylation of H3K20 and acetylation of H3K56, both of which have been implicated in the DNA damage response [22,23]. The identification of H2AXK36Ac adds a new member to this category of functionally important constitutive core histone modifications which are critical for the repair of DSBs.
Acknowledgments
We thank members of the Price laboratory, Anyong Xie and Ralph Scully for valuable discussions and sharing unpublished data; X. Yang and Q. Liu for providing HAT constructs; J. Chen for MDC1 antibody and A. Nussenzweig for H2AX−/− MEF cells. Supported by grants from the NCI (CA64585 and CA93602) and the DOD Breast Cancer Program to BDP.
Abbreviations
- DSB
DNA double-strand break
- IR
ionizing radiation
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- H2AXK36Ac
H2AX acetylated on lysine 36
- TSA
Trichostatin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–67. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lou Z, Minter-Dykhouse K, Franco S, Gostissa M, Rivera MA, Celeste A, Manis JP, van Deursen J, Nussenzweig A, Paull TT, Alt FW, Chen J. MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell. 2006;21:187–200. doi: 10.1016/j.molcel.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Melander F, Bekker-Jensen S, Falck J, Bartek J, Mailand N, Lukas J. Phosphorylation of SDT repeats in the MDC1 N terminus triggers retention of NBS1 at the DNA damage-modified chromatin. J Cell Biol. 2008;181:213–26. doi: 10.1083/jcb.200708210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spycher C, Miller ES, Townsend K, Pavic L, Morrice NA, Janscak P, Stewart GS, Stucki M. Constitutive phosphorylation of MDC1 physically links the MRE11-RAD50-NBS1 complex to damaged chromatin. J Cell Biol. 2008;181:227–40. doi: 10.1083/jcb.200709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celeste A, Difilippantonio S, Difilippantonio MJ, Fernandez-Capetillo O, Pilch DR, Sedelnikova OA, Eckhaus M, Ried T, Bonner WM, Nussenzweig A. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–83. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–7. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ, Herceg Z. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8:91–9. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 9.Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC, Grant PA, Smith MM, Christman MF. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature. 2002;419:411–5. doi: 10.1038/nature01035. [DOI] [PubMed] [Google Scholar]
- 10.Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR, 3rd, Abmayr SM, Washburn MP, Workman JL. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science. 2004;306:2084–7. doi: 10.1126/science.1103455. [DOI] [PubMed] [Google Scholar]
- 11.Jha S, Shibata E, Dutta A. Human Rvb1/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the downregulation of phosphorylation on H2AX after DNA damage. Mol Cell Biol. 2008;28:2690–700. doi: 10.1128/MCB.01983-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Cote J. Binding of Chromatin-Modifying Activities to Phosphorylated Histone H2A at DNA Damage Sites. Mol Cell. 2004;16:979–90. doi: 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Choi JK, Howe LJ. Histone acetylation: truth of consequences? Biochem Cell Biol. 2009;87:139–50. doi: 10.1139/O08-112. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes ND, Sun Y, Price BD. Activation of the kinase activity of ATM by retinoic acid is required for CREB-dependent differentiation of neuroblastoma cells. J Biol Chem. 2007;282:16577–84. doi: 10.1074/jbc.M609628200. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Jiang X, Xu Y, Ayrapetov MK, Moreau LA, Whetstine JR, Price BD. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat Cell Biol. 2009;11:1376–82. doi: 10.1038/ncb1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 2005;102:13182–7. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huen MS, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–14. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolas NK, Chapman JR, Nakada S, Ylanko J, Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson TM, Pelletier L, Jackson SP, Durocher D. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science. 2007;318:1637–40. doi: 10.1126/science.1150034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–35. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Tang Y, Cole PA, Marmorstein R. Structure and chemistry of the p300/CBP and Rtt109 histone acetyltransferases: implications for histone acetyltransferase evolution and function. Curr Opin Struct Biol. 2008;18:741–7. doi: 10.1016/j.sbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–7. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature. 2005;436:294–8. doi: 10.1038/nature03714. [DOI] [PubMed] [Google Scholar]
- 23.Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127:1361–73. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]