Abstract
Like obesity, the prevalence of asthma has increased over the past several decades. Accelerated patters of infant growth have been associated with obesity and its co-morbidities. We aimed to determine if infant weight gain pattern is associated with asthma development later in childhood. Birth weight, growth, pulmonary function, and symptom data were collected in a trial of 2–3 year old children at-risk for asthma randomized to a two-year treatment with inhaled corticosteroids or placebo followed by a one year observation period off study medication. Patterns of infant weight gain between birth and study enrollment were categorized as accelerated, average, or decelerated. Regression analyses were used to test the effects of infant weight gain pattern prior to study enrolment on outcomes during the observation year and at study conclusion while adjusting for demographics, baseline symptom severity, study treatment, and atopic indicators. Among the 197 study participants, early life weight gain pattern was not associated with daily asthma symptoms or lung function at the study’s conclusion. However, both prednisone courses (P=.01) and urgent physician visits (P<.001) were significantly associated with weight gain pattern with fewer exacerbations occurring amongst those with a decelerated weight gain pattern. We conclude that early life patterns of weight change were associated with subsequent asthma exacerbations, but were not associated with asthma symptoms or pulmonary function during the preschool years for these children at-risk for asthma.
Keywords: Asthma, Weight Gain, Infant
INTRODUCTION
Accelerated or “rapid” weight gain during infancy has emerged as a strong risk factor for obesity in both childhood and adulthood.1–16 Some co-morbid chronic conditions related to obesity have also been independently linked to rate of weight gain during infancy such as hypertension,17–20 coronary heart disease,21, 22 and type 2 diabetes mellitus.23, 24 Investigations into these associations began with the fetal growth restriction, or “Barker” hypothesis.25 This hypothesis linked retarded growth in utero and catch-up growth to subsequent morbidities related to cardiovascular health and insulin resistance.26, 27 As studies continued to evaluate this hypothesis, recent data have revealed that the rate of growth in the months after birth may be more important in determining future outcomes.4, 28 It has been theorized that over-nutrition in infancy adversely “programs” the components of the metabolic syndrome by promoting growth acceleration.29
Like obesity, the prevalence of asthma has increased over the past several decades.30–33 While both low birth weight34–42 and high body mass index in childhood43–51 have been associated with an increased prevalence of asthma, a potential link between the two, rate of growth during childhood has received little attention. Therefore, we hypothesized that infant weight gain pattern would be associated with asthma development in a cohort of 2–3 year old children at high-risk for asthma enrolled in the Prevention of Early Asthma in Kids (PEAK) study.52 To test this hypothesis, asthma development was characterized by the two domains described in the new National Asthma Education and Prevention Program Guidelines: impairment and risk.53 The impairment domain is rooted in current burden of symptoms, quality of life, and functional capacity while the risk domain reflects adverse events, such as exacerbations of asthma and medication side effects.
PATIENTS AND METHODS
Subjects
Children 2 and 3 years of age with frequent intermittent wheezing at high risk of developing persistent asthma but currently without persistent symptoms were identified by meeting the criteria for a positive modified Asthma Predictive Index54 and enrolled in the PEAK trial. Major exclusion criteria included a history of prematurity of 35 weeks or less, growth less than the 10th percentile, chronic lung disease, or other major medical illness. The cohort meeting these inclusion and exclusion criteria was population based and identified by primary care physicians at the 5 clinical centers of the Childhood Asthma Research and Education Network, and the participants were screened for study eligibility based on inclusion and exclusion criteria that have been published previously in more detail.52, 55, 56
Study Design
Briefly, the PEAK trial was a multicenter, double-blind, randomized, placebo-controlled, parallel-group trial of inhaled fluticasone compared with placebo aimed to assess whether treatment of high-risk subjects with inhaled corticosteroids early in life could alter the natural history of asthma upon their cessation. For two treatment years, the participants received either inhaled corticosteroid (fluticasone propionate, 44 mcg/puff) or placebo, two puffs twice daily delivered through a valved spacer with a mask. Treatment was then stopped, and the subjects were then followed for an additional observation year when asthma symptoms and lung function were assessed. Institutional review boards at all participating centers approved the protocol and consent forms.
Study Procedures
As previously described in detail,52 data collected during the PEAK study included allergy skin testing, demographic data, and family history obtained at enrollment, symptom recall interviews, asthma medication use, and growth parameters throughout the study, and pulmonary function tests at the end of the study observation year. Upon study completion, birth weight was obtained via phone call or at an office visit on a sub-group of PEAK participants. Birth weight was reported by parents from several sources including birth certificates, baby books, medical records, and memory. Documented birth weights were obtained on 35% of participants while 65% were from memory, but importantly, parental recall of birth weight has been shown to be a reliable and accurate source of this information.57–66 Enrollment weights were obtained at the study visit using clinic scales.
Episode-free days, exacerbations, and use of supplementary asthma medication were assessed by direct contact (by telephone or during a clinic visit) to evaluate asthma symptoms and medication use during the preceding 14 days and the use of healthcare resources since the last telephone call or clinic visit, as well as by review of the parents’ written records of all medication used by the children. Episode-free days were determined from the parents’ reported data, which were corrected according to the coordinators’ records in cases in which the family did not report previously prescribed supplementary controller medication that had been recorded and dispensed by the coordinators. The proportion of episode-free days for each participant was calculated as the number of episode-free days divided by the number of days of observation. Data from all participants were used in the analysis regardless of the number of days observed.
Data Analysis
After obtaining birth weight data, participants were categorized based upon their pattern of weight gain between birth and study enrollment at age 2 or 3 years. As has been done previously in numerous investigations, accelerated or “rapid” weight gain was defined as weight-for-age gains greater than 0.67 Z-scores between birth and study enrollment while decelerated or “slow” and average patterns of weight gain were defined as gains of less than −0.67 Z-scores and 0.67 to −0.67 Z-scores, respectively.2, 9, 10 These values were based upon the norms established from the 2000 Centers for Disease Control weight-for-age growth chart.67
Analysis of covariance was used to test for differences between patterns of weight gain with respect to the following continuous outcomes: episode free days, use of controller medicines, and pulmonary function testing. Pattern of weight gain was the factor of primary interest and covariates included demographics (age, sex, race/ethnicity, center), baseline symptom severity (number of episode free days during the study run-in period, duration of asthma-like symptoms prior to enrollment), treatment group, and indicators of atopy (aeroallergen skin test reactivity, percentage of blood eosinophils, and presence or absence of eczema). Unlike the episode free day analysis that used all three years of data, the outcome of use of controller medicines was based on data collected during the observation year only because the treatment group all received a controller medication during the treatment years of the study, Pulmonary function testing data were collected at the final study visit.
Poisson regression was used to test for differences between patterns of weight gain with respect to the following frequency outcomes: unscheduled physician visits and exacerbations requiring oral steroids over the three year study period. Exacerbations were defined as the need for a course of prednisolone to control asthma-like symptoms, These analyses were also adjusted for the covariates listed above.
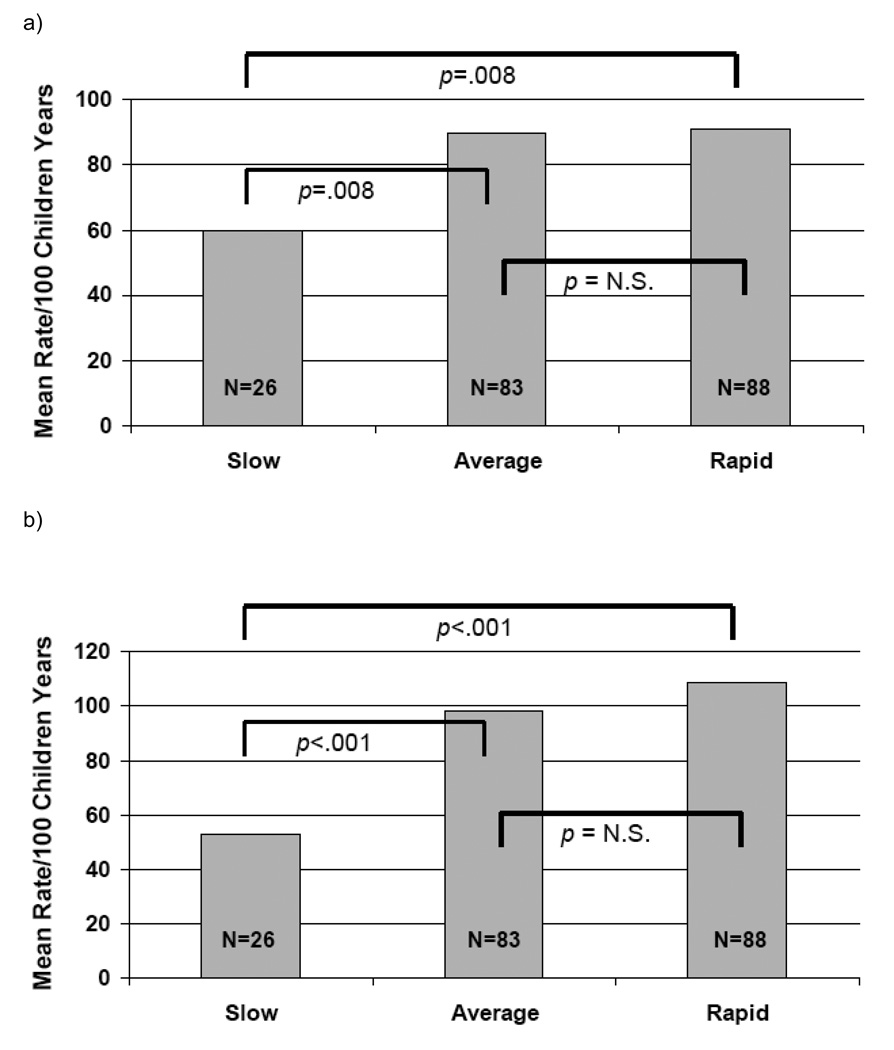
Overall p-values for the test of the null hypothesis of no differences between the 3 patterns of weight gain are reported in Table 2. P-values for the three pairwise comparisons: slow vs. average weight gain, slow vs. rapid, and average vs. rapid are reported in Figure 1. Bonferroni correction to the standard 0.05 significance level is commonly used in this type of multiple comparison setting. Therefore, the statistical significance of these p-values could be assessed in comparison to 0.017 instead of 0.05. All analyses were carried out using the SAS statistical software package, version 9.1 (SAS Inc., Cary, NC).
Table 2.
Relationship between Infant Weight Gain Group and Features of Asthma Impairment and Risk Domains at Ages 5–6 Years
| Variable | Decelerated N=26 |
Weight Gain Category Average N=83 |
Accelerated N=88 |
P |
|---|---|---|---|---|
| FEV0.5 – mean % predicted (95% CI) |
92 (84–100) | 89 (84–94) | 94 (90–98) | .26 |
| FEV0.5/FVC – mean % (95% CI) |
69.7 (65.0–74.3) | 67.5 (65.0–70.0) | 66.3 (64.1–68.6) | .41 |
| Episode Free Days – mean no of days/yr (95% CI)* | 330 (303–346) | 321 (305–334) | 316 (299–329) | .57 |
| Days Using Controller Medication – mean no days/yr (95% CI) † | 19.6 (−23.4–62.6) | 49.0 (25.6–72.5) | 44.3 (21.6–66.9) | .45 |
| Exacerbations requiring a course of systemic corticosteroid – no./100 child-yr (95% CI)* |
59.8 (44.7–80.1) | 89.8 (78.0–103.5) | 91.1 (79.6–104.4) | .01 |
| Unscheduled physician visits – no./100 child-yr (95% CI)* | 53.1 (38.8–72.8) | 98.3 (85.8–112.6) | 108.8 (95.9–123.4) | <.001 |
data from all three study years, but adjusted for treatment group
observation year only
Figure 1.
Relationship Between Infant Weight Gain Group and a) systemic corticosteroid courses (P=.01 overall group comparisons) and b) unscheduled physician visits (P<.001 overall group comparisons) during the three year study period
RESULTS
Demographics and Baseline Characteristics
From the previously described PEAK cohort, birth weight data were obtained on 197/285 (69.1%), and this subgroup is the subject of the results of this analysis. The subjects with birth weight data were not significantly different from those without these data for any baseline characteristic. Among the 197 participants with data used for this analysis, 126 (64%) were male, and the mean age at study enrollment was 3.0 ± 0.6 years (Table 1). 58% of the cohort were classified by their parents to be White, and 42% were from racial or ethnic minority groups. Atopic features at enrollment were present in many of the participants with 122 (62%) having a positive aeroallergen skin test and 105 (53%) having a history of eczema. The median IgE level was 48.0 IU/mL (interquartile range 13.7–115.0 IU/mL).
Table 1.
Demographic Variables and Baseline Characteristics
| Characteristic | Entire Cohort | Weight Gain Category Subgroup | |||
|---|---|---|---|---|---|
| N=197 | Decelerated N=26 |
Average N=83 |
Accelerated N=88 |
P | |
| Age – years | 3.0 ± 0.6 | 3.3 ± 0.6 | 3.1 ± 0.6 | 3.0 ± 0.6 | .03 |
| Sex – no. (%) | .24 | ||||
| Male | 126 (64) | 20 (77) | 49 (59) | 57 (65) | |
| Female | 71 (36) | 6 (23) | 34 (41) | 31 (35) | |
| Race or Ethnic Group – no. (%) | .76 | ||||
| Non-Hispanic White | 114 (58) | 18 (69) | 51 (61) | 46 (52) | |
| Non-Hispanic Black | 22 (11) | 2 (8) | 7 (8) | 12 (14) | |
| Hispanic | 33 (17) | 3 (12) | 13 (16) | 18 (20) | |
| Other | 28 (14) | 3 (12) | 12 (14) | 12 (14) | |
| Birth Weight, Mean ± SD – kg | 3.4 ± 0.6 | 4.1 ± 0.4 | 3.5 ± 0.4 | 3.1 (0.5) | <.001 |
| Enrollment Weight, Mean ± SD – kg |
15.4 ± 2.4 | 14.8 ± 1.6 | 14.4 ± 1.8 | 16.5 ± 2.7 | <.001 |
| Positive Aeroallergen Skin Tests – no (%) |
122 (62) | 21 (81) | 54 (65) | 47 (53) | .03 |
| Eczema – no (%) | 105 (53) | 13 (50) | 48 (58) | 44 (50) | .56 |
| IgE, median (interquartile range) – IU/mL |
48.0 (13.7–115.0) | 97.4 (42.6–196.5) | 41.8 (13.1–97.1) | 40.2 (8.5–117.0) | .02 |
| Study Treatment Group | |||||
| Fluticasone – N (%) | 103 (52) | 17 (65) | 49 (59) | 37 (42) | 0.03 |
| Placebo – N (%) | 94 (48) | 9 (35) | 34 (41) | 51 (58) | |
The mean birth weight for this subgroup was 3.4 ± 0.6 kg, equivalent to the 46th percentile on the 2000 Centers for Disease Control weight-for-age growth chart.67 Birth weight was not significantly correlated with any study outcomes with the exception of FEV0.5 (P<.001). At enrollment, the mean weight was 15.4 ± 2.4 kg, equivalent to the 62nd percentile of the growth chart. Between birth and study enrollment, 88/197 (45%) demonstrated an accelerated pattern of weight gain while 83 (42%) and 26 (13%) had average and decelerated patterns of weight change, respectively. At study enrollment, weight gain pattern was significantly associated with aeroallergen skin test positivity (P=.03) and IgE level (P=.02), with the decelerated group demonstrating the highest degree of atopy. No significant relationship was found between weight gain and eczema at enrollment.
Relationship between Infant Weight Gain Pattern and Later Asthma Outcomes
Impairment domain
The relationship between weight gain pattern and pulmonary function testing, episode free days, and days using controller medication were evaluated (Table 2). Because pulmonary function testing of the participants was done at a young age (mean age 5.8 ± 0.6 years), FEV0.5 was used instead of FEV1.68, 69 No significant relationship was found between weight change pattern during infancy and either FEV0.5 or FEV0.5/FVC when performed at age 5–6 years. For both episode free days and days using controller medication, data were analyzed only for the observation year. Again, no significant relationship was discovered between weight gain groups and these outcomes.
Risk domain
Significant differences were detected between weight gain patterns for the outcomes of exacerbations requiring systemic corticosteroids and unscheduled physician visits (Figure 1). For exacerbations requiring systemic corticosteroids, those with the slowest pattern of weight gain had a mean of 59.8 (95% CI 44.7–80.1) courses per 100 child years compared with 89.8 (78.0–103.5) for the average weight change group and 91.1 (79.6–104.4) for the children with accelerated weight gain (P=.01). Similarly, for unscheduled physician visits, significant differences again were detected between the decelerated group that had a mean of 53.1 (38.8–72.8) visits per 100 child years and the average and accelerated groups that had 98.3 (85.5–112.6) and 108.8 (95.9–123.4) visits per 100 child years, respectively (P<.001). Data regarding hospitalizations over the 3 year study period and weight change pattern were difficult to analyze because such events were rare; there were only 4 for the entire cohort over the 3 year study period. Analyses describing all outcomes were adjusted for demographics, baseline symptom severity, study treatment group, and atopic status.
DISCUSSION
Through study of the developmental origins of adult diseases, early life weight gain has been explored for its relationship with numerous diseases later in life.70 Associations have previously been found with obesity,1–14 hypertension,17–20 coronary heart disease,21, 22 and type 2 diabetes mellitus.23, 24 The results of this study suggest that pattern of infant weight gain is associated with the risk domain of asthma morbidity for young children at high-risk for chronic asthma. Specifically, slower weight gain during the infant years relative to growth chart norms was protective for asthma exacerbations during the preschool years. Though no association was found between weight gain pattern and components of the impairment domain of asthma morbidity, the data describing an association between early life weight gain and asthma exacerbations does provide evidence of a link between early life growth and subsequent asthma for children.
Given the small sample size, the findings should be viewed as preliminary, but the results of our study are consistent with the single other report examining the relationship between early life weight gain and asthma outcomes by Rona et al.71 In that study, a cohort of 1232 Chilean children born between 1974 and 1978 were followed until a final study visit conducted between 2001 and 2003. Despite the collection of data on numerous variables related to birth and the infant period of life, only weight gain in the first year of life was significantly associated with wheezing illnesses during the 12 months prior to the final study assessment during adulthood.
Several previous studies have shown that increasing birth weight is inversely associated with subsequent asthma development.36, 37, 39, 40 Relating this fact to the current findings, children born heavier and near the upper percentile lines on the growth chart are less likely to upwardly cross major percentile lines due to the regression to the mean concept. Such children therefore are unlikely to have accelerated patterns of weight gain and more likely to have decelerated patterns of gain. The opposite is true for those born at lower weights who are unlikely to show decelerated patterns of growth and more likely to have accelerated weight gain during infancy. Though this study was underpowered to describe associations between asthma and birth weight, the current findings are related and consistent with an overall conceptual framework relating early life weight status to subsequent asthma outcomes.
Outside of infancy, the prevalence of asthma is associated with increased body mass index during childhood.43–51 Further, overweight children were recently shown to have a higher hospital admission rate when presenting to the emergency department when compared with normal weight children with asthma.72 In addition to the studies examining single point weight measurements, weight change over time has also been associated with asthma symptoms for children. The Tucson Children’s Respiratory Study demonstrated that females who became overweight or obese between ages 6 and 11 years had an increased risk of developing new asthma symptoms and increased bronchial reactivity during the early adolescent period.73, 74 Similarly, the body mass index slope increases for those with unremitting asthma and unremitting wheezing were steeper than those with remitting asthma, remitting wheezing, or no wheezing.74 The investigators remarked that the prevention of accelerated weight gain during the school years could prevent asthma related morbidities.
Several mechanisms have been proposed to explain the relationship between obesity and asthma symptoms, and many of them can also be applied to weight gain pattern and accelerated weight gain during infancy, a time period where many confounders relevant to older subjects like sedentary lifestyle are not relevant. As recently reviewed by Shore,75 obesity is associated with mechanical factors, chronic systemic inflammation, and adipose tissue and energy-related hormones that all may be related to an individual’s asthma. Notably, accelerated weight gain during infancy has been associated with acquisition of adipose tissue as opposed to lean body mass.6, 9, 15, 76 The adipokines produced by adipose tissue may have a disease modifying effect.77 Further, co-morbidities of obesity such as gastroesophageal reflux disease can also contribute to asthma symptoms, and for infants, gastroesophageal reflux in particular is often clinically associated with overfeeding, which can lead to accelerated weight gain.
It is notable that the weight gain groups differed at baseline with respect to atopic status. Those in the decelerated group had higher IgE levels and were more likely to have aeroallergen skin test positivity. The fewer asthma exacerbations for this group is most likely explained by the fact that for toddlers, most exacerbations are induced by viral upper respiratory infections.
This analysis is limited by several factors. First, the data were generated from a prospective clinical trial, and the sample size and statistical power are therefore relatively small to describe associations between weight change and asthma outcomes. This fact may contribute to our inability to show any differences between the average weight gain group and those that had accelerated patterns of weight gain. Similarly, the lack of significant findings with asthma outcomes in the impairment domain should be viewed cautiously. Next, interval weight measurements between birth and study enrollment were not collected or available for analysis. It is possible that there is a critical early life period of growth that may have a stronger association with asthma outcomes than we were able to detect. A cohort followed from birth throughout childhood would be ideal to further evaluate the questions posed by this study. Further, data regarding other relevant perinatal covariates with relationships to infant weight status such as maternal body mass index and newborn gestational age were either unavailable or imprecise. Finally, this cohort was not representative of the general population or the overall population of asthmatic children, but rather a very specific cohort of young children with frequent intermittent wheezing at high risk for asthma development. Therefore, this report cannot be generalized to other populations, but instead can be used to prompt further studies.
In summary, the results of this analysis describe a relationship between weight gain pattern during infancy and components of asthma later in childhood, specifically those related to exacerbations. While the overall contribution of weight change to asthma morbidity may be relatively minor compared with genetics, atopic status, and environmental exposures,78 weight status and weight change over time are modifiable. If future studies confirm that weight change is associated with asthma exacerbations, interventions could be developed with asthma prevention as a goal.
ACKNOWLEDGEMENTS
This study was supported by Grants 5U10HL064287, 5U10HL064288, 5U10HL064295, 5U10HL064307, 5U10HL064305, 5U10HL064313 from the National Heart, Lung, and Blood Institute; General Clinical Research Centers at Washington University School of Medicine (M01 RR00036) and National Jewish Medical and Research Center (M01 RR00051)
References
- 1.Eid EE. Follow-up study of physical growth of children who had excessive weight gain in first six months of life. Br Med J. 1970;2:74–76. doi: 10.1136/bmj.2.5701.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Bmj. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. Bmj. 2005;330:1358–1360. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 5.von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep--a cross-sectional study. Int J Obes Relat Metab Disord. 2002;26:710–716. doi: 10.1038/sj.ijo.0801980. [DOI] [PubMed] [Google Scholar]
- 6.Cameron N, Pettifor J, De Wet T, Norris S. The relationship of rapid weight gain in infancy to obesity and skeletal maturity in childhood. Obes Res. 2003;11:457–460. doi: 10.1038/oby.2003.62. [DOI] [PubMed] [Google Scholar]
- 7.Mellbin T, Vuille JC. Physical development at 7 years of age in relation to velocity of weight gain in infancy with special reference to incidence of overweight. Br J Prev Soc Med. 1973;27:225–235. doi: 10.1136/jech.27.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77:1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 9.Ekelund U, Ong K, Linne Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro PO, Victora CG, Barros FC, Monteiro LM. Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27:1274–1282. doi: 10.1038/sj.ijo.0802409. [DOI] [PubMed] [Google Scholar]
- 11.Wells JC, Hallal PC, Wright A, Singhal A, Victora CG. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes (Lond) 2005;29:1192–1198. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- 12.Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 13.Blair NJ, Thompson JM, Black PN, et al. Risk factors for obesity in 7-year-old European children: the Auckland Birthweight Collaborative Study. Arch Dis Child. 2007;92:866–871. doi: 10.1136/adc.2007.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006;14:491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 15.Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 16.Gillman MW. The first months of life: a critical period for development of obesity. Am J Clin Nutr. 2008;87:1587–1589. doi: 10.1093/ajcn/87.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Is slower early growth beneficial for long-term cardiovascular health? Circulation. 2004;109:1108–1113. doi: 10.1161/01.CIR.0000118500.23649.DF. [DOI] [PubMed] [Google Scholar]
- 18.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 19.Law CM, Shiell AW, Newsome CA, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 20.Parker L, Lamont DW, Unwin N, et al. A lifecourse study of risk for hyperinsulinaemia, dyslipidaemia and obesity (the central metabolic syndrome) at age 49–51 years. Diabet Med. 2003;20:406–415. doi: 10.1046/j.1464-5491.2003.00949.x. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. Bmj. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 23.Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133:176–182. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 26.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. Bmj. 1993;306:422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 28.Hulman S, Kushner H, Katz S, Falkner B. Can cardiovascular risk be predicted by newborn, childhood, and adolescent body size? An examination of longitudinal data in urban African Americans. J Pediatr. 1998;132:90–97. doi: 10.1016/s0022-3476(98)70491-3. [DOI] [PubMed] [Google Scholar]
- 29.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet. 2004;363:1642–1645. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 30.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 31.Tantisira KG, Weiss ST. Complex interactions in complex traits: obesity and asthma. Thorax. 2001;56 Suppl 2:ii64–ii73. [PMC free article] [PubMed] [Google Scholar]
- 32.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 33.Mai XM, Chen Y, Krewski D. Does leptin play a role in obesity-asthma relationship? Pediatr Allergy Immunol. 2009 doi: 10.1111/j.1399-3038.2008.00812.x. Accessed on-line Epub, April 18, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. Am Rev Respir Dis. 1990;142:555–562. doi: 10.1164/ajrccm/142.3.555. [DOI] [PubMed] [Google Scholar]
- 35.Weitzman M, Gortmaker S, Sobol A. Racial, social, and environmental risks for childhood asthma. Am J Dis Child. 1990;144:1189–1194. doi: 10.1001/archpedi.1990.02150350021016. [DOI] [PubMed] [Google Scholar]
- 36.Seidman DS, Laor A, Gale R, Stevenson DK, Danon YL. Is low birth weight a risk factor for asthma during adolescence? Arch Dis Child. 1991;66:584–587. doi: 10.1136/adc.66.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svanes C, Omenaas E, Heuch JM, Irgens LM, Gulsvik A. Birth characteristics and asthma symptoms in young adults: results from a population-based cohort study in Norway. Eur Respir J. 1998;12:1366–1370. doi: 10.1183/09031936.98.12061366. [DOI] [PubMed] [Google Scholar]
- 38.Braback L, Hedberg A. Perinatal risk factors for atopic disease in conscripts. Clin Exp Allergy. 1998;28:936–942. doi: 10.1046/j.1365-2222.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 39.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 40.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54:396–402. doi: 10.1136/thx.54.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu B, Pekkanen J, Laitinen J, Jarvelin MR. Body build from birth to adulthood and risk of asthma. Eur J Public Health. 2002;12:166–170. doi: 10.1093/eurpub/12.3.166. [DOI] [PubMed] [Google Scholar]
- 42.Wjst M, Popescu M, Trepka MJ, Heinrich J, Wichmann HE. Pulmonary function in children with initial low birth weight. Pediatr Allergy Immunol. 1998;9:80–90. doi: 10.1111/j.1399-3038.1998.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 43.von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56:835–838. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthma-like symptoms in girls who become overweight or obese during the school years. American Journal of Respiratory and Critical Care Medicine. 2001;163:1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 45.Chinn S, Rona RJ. Can the increase in body mass index explain the rising trend in asthma in children? Thorax. 2001;56:845–850. doi: 10.1136/thorax.56.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epstein LH, Wu YW, Paluch RA, Cerny FJ, Dorn JP. Asthma and maternal body mass index are related to pediatric body mass index and obesity: results from the Third National Health and Nutrition Examination Survey. Obes Res. 2000;8:575–581. doi: 10.1038/oby.2000.74. [DOI] [PubMed] [Google Scholar]
- 47.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender BG, Fuhlbrigge A, Walders N, Zhang L. Overweight, race, and psychological distress in children in the Childhood Asthma Management Program. Pediatrics. 2007;120:805–813. doi: 10.1542/peds.2007-0500. [DOI] [PubMed] [Google Scholar]
- 49.Figueroa-Munoz JI, Chinn S, Rona RJ. Association between obesity and asthma in 4–11 year old children in the UK. Thorax. 2001;56:133–137. doi: 10.1136/thorax.56.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon HL, Ortiz B, Swaner R, et al. Childhood asthma and extreme values of body mass index: the Harlem Children's Zone Asthma Initiative. J Urban Health. 2006;83:421–433. doi: 10.1007/s11524-006-9050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oddy WH, Sherriff JL, de Klerk NH, et al. The relation of breastfeeding and body mass index to asthma and atopy in children: a prospective cohort study to age 6 years. Am J Public Health. 2004;94:1531–1537. doi: 10.2105/ajph.94.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 53.Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program: Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007
- 54.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 55.Guilbert TW, Morgan WJ, Krawiec M, et al. The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25:286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114:1282–1287. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 57.Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers' recall of birthweight and gestational age. Br J Obstet Gynaecol. 1987;94:731–735. doi: 10.1111/j.1471-0528.1987.tb03717.x. [DOI] [PubMed] [Google Scholar]
- 58.Burns TL, Moll PP, Rost CA, Lauer RM. Mothers remember birthweights of adolescent children: the Muscatine Ponderosity Family Study. Int J Epidemiol. 1987;16:550–555. doi: 10.1093/ije/16.4.550. [DOI] [PubMed] [Google Scholar]
- 59.Seidman DS, Gale R. Accuracy of maternal recall of birthweights of adolescent children. Int J Epidemiol. 1988;17:688–689. doi: 10.1093/ije/17.3.688. [DOI] [PubMed] [Google Scholar]
- 60.Gayle HD, Yip R, Frank MJ, Nieburg P, Binkin NJ. Validation of maternally reported birth weights among 46,637 Tennessee WIC program participants. Public Health Rep. 1988;103:143–147. [PMC free article] [PubMed] [Google Scholar]
- 61.Wilcox WD, Gold BD, Tuboku-Metzger AJ. Maternal recall of infant birth weight. Clin Pediatr (Phila) 1991;30:509–510. doi: 10.1177/000992289103000811. [DOI] [PubMed] [Google Scholar]
- 62.Lumey LH, Stein AD, Ravelli AC. Maternal recall of birthweights of adult children: validation by hospital and well baby clinic records. Int J Epidemiol. 1994;23:1006–1012. doi: 10.1093/ije/23.5.1006. [DOI] [PubMed] [Google Scholar]
- 63.Pless CE, Pless IB. How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med. 1995;149:553–558. doi: 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]
- 64.Gaskin P, Walker SP, Forrester TE, Grantham-McGregor SM. The validity of recalled birthweight in developing countries. Am J Public Health. 1997;87:114. doi: 10.2105/ajph.87.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Sullivan JJ, Pearce MS, Parker L. Parental recall of birth weight: how accurate is it? Arch Dis Child. 2000;82:202–203. doi: 10.1136/adc.82.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gofin R, Neumark YD, Adler B. Birthweight recall by mothers of Israeli children. Public Health. 2000;114:161–163. [PubMed] [Google Scholar]
- 67. http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm.
- 68.Vilozni D, Barker M, Jellouschek H, Heimann G, Blau H. An interactive computer-animated system (SpiroGame) facilitates spirometry in preschool children. Am J Respir Crit Care Med. 2001;164:2200–2205. doi: 10.1164/ajrccm.164.12.2101002. [DOI] [PubMed] [Google Scholar]
- 69.Sly PD, Morgan WJ. Respiratory function testing in infants and preschool children. In: Taussig LM, Landau LI, editors. Pediatric respiratory medicine. Second Edition. Philadelphia, PA: Mosby, Inc; 2008. [Google Scholar]
- 70.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353:1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rona RJ, Smeeton NC, Bustos P, Amigo H, Diaz PV. The early origins hypothesis with an emphasis on growth rate in the first year of life and asthma: a prospective study in Chile. Thorax. 2005;60:549–554. doi: 10.1136/thx.2004.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carroll CL, Stoltz P, Raykov N, Smith SR, Zucker AR. Childhood overweight increases hospital admission rates for asthma. Pediatrics. 2007;120:734–740. doi: 10.1542/peds.2007-0409. [DOI] [PubMed] [Google Scholar]
- 73.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163:1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 74.Guerra S, Wright AL, Morgan WJ, Sherrill DL, Holberg CJ, Martinez FD. Persistence of asthma symptoms during adolescence: role of obesity and age at the onset of puberty. Am J Respir Crit Care Med. 2004;170:78–85. doi: 10.1164/rccm.200309-1224OC. [DOI] [PubMed] [Google Scholar]
- 75.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. quiz 94-5. [DOI] [PubMed] [Google Scholar]
- 76.Karaolis-Danckert N, Buyken AE, Bolzenius K, Perim de Faria C, Lentze MJ, Kroke A. Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr. 2006;84:1449–1455. doi: 10.1093/ajcn/84.6.1449. [DOI] [PubMed] [Google Scholar]
- 77.Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol. 2008;19:535–540. doi: 10.1111/j.1399-3038.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 78.Matricardi PM, Illi S, Gruber C, et al. Wheezing in childhood: incidence, longitudinal patterns and factors predicting persistence. Eur Respir J. 2008;32:585–592. doi: 10.1183/09031936.00066307. [DOI] [PubMed] [Google Scholar]