Abstract
Background
Female recipients of male kidneys have an inferior graft survival, and patients receiving larger kidneys relative to their body size may have a graft survival advantage. Thus, graft survival may be affected by both gender and kidney size mismatches. The objective of this study was to analyze the possible confounding effect of body mass mismatch (body mass as proxy for kidney size) between female recipients of male donor kidneys.
Study Design
A total of 668 kidney transplants between 1996 and 2005 at our center were studied retrospectively. Graft and patient survival were determined by Kaplan-Meier estimation. Multivariate Cox proportional analyses were performed to determine the hazards of graft loss.
Results
There were 146 female recipients of male kidneys. Compared to all gender combinations, this group had the lowest, unadjusted graft survival (86%, 79%, and 78% vs. 92%, 88%, and 86% at 1, 2, and 3 years, respectively, log-rank p=0.01). Donor body mass index (BMI) correlated with donor kidney size (p<0.001). Male kidneys were a risk factor of graft loss for female recipients (hazard ratio [HR] 3.45, 95% CI 1.40–8.51, p=0.01), but male donors with a larger BMI relative to female recipients’ significantly reduced the risk (HR 0.19, 95% CI 0.05–0.67, p=0.01).
Conclusions
The inferior graft survival for female recipients of male donor kidneys is mitigated by male donors with a larger BMI.
Introduction
Although current registry data from the United Network of Organ Sharing report similar graft survival rates for males and females,1 a 2005 systematic review on gender differences in kidney transplantation identified 14 studies with contradicting results.2 More recently, an analysis from the Collaborative Transplant Study3 demonstrated that female recipients of male donor kidneys had the worst graft survival after the first year and up to ten years post transplant.4 The authors hypothesized that an alloimmune response mediated by H-Y minor histocompatibility antigens could be responsible. H-Y antigens have been associated with acute rejection in smaller gender mismatch investigations of bone marrow5 and corneal and kidney transplants.6–8
Also, several investigations have suggested a graft survival advantage for recipients receiving larger kidneys relative to their body size.9–11 Compared to males, females usually have smaller kidneys.12–14 It has been theorized when the recipient’s metabolic demand exceeds the capacity of the smaller donor kidney, hyperfiltration from nephron under-dosing could occur.15, 16 Larger donor kidney mass in relation to smaller recipient mass diminishes hyperfiltration injury, and subsequently, immune-mediated rejection.9 Earlier studies of donor-recipient gender mismatch have not explored the possible confounding of body size mismatch between female recipients of male donor kidneys, which is the purpose of the current study.
Methods
Study participants
With Institutional Review Board approval a retrospective chart review of 863 consecutive kidney transplants performed between January 1996 and August 2005 at Tulane University Medical Center was conducted. Cases were excluded if either donor or recipient age, race, gender, height, and/or weight values were not documented. Six-hundred and sixty-eight subjects met the inclusion criteria. Missing information on graft status and/or death was obtained from the United Network for Organ Sharing and National Death Index, respectively. A database for data entry and cleaning was created using MS Access 2003. A quality check of the data entry was performed by randomly selecting 10% of the final sample for double entry. An individual was trained and assigned exclusively to perform the quality check. The discrepancy rate was less than 5%.
Immunosuppression therapy
Patients received standard triple immunosuppression—steroids, tacrolimus or cyclosporine, and mycophenolic acid. High risk patients, including those with a prior transplant, 6-antigen human leukocyte (HLA) mismatches, and/or a panel reactive antibody >20%, received induction therapy with the IL-2 receptor antagonist basiliximab. All patients received standard antifungal, antibacterial, and cytomegalovirus prophylaxis. Acute rejection was confirmed by kidney biopsy, and the severity was graded according to the Banff classification.17
Correlating donor body mass index and donor kidney size
Donor kidney surface area was calculated (kidney length × width) from measurements provided by Louisiana Organ Procurement Agency stored records. Univariate correlations between donor body mass index (BMI) and kidney size using Pearson’s correlation coefficient were calculated for available donors, 205 males and 155 female donors.
Donor-recipient body mass index match and mismatch
BMI (kg/m2) was calculated for donors and recipients. There is no consensus as to what constitutes a match of donor-recipient BMI. In the current study this concept was operationalized noting a match if the donor’s BMI fell within ± 2 units of the recipient’s BMI (Appendix 1). BMI values outside the designated parameters were categorized as mismatches.
Appendix 1.
Operationalizing BMI Match and Mismatch in Kidney Transplantation
| Example determinations of donor-recipient body mass index match* | |||||
|---|---|---|---|---|---|
| Case | Donor BMI (kg/m2) |
Recipient BMI (kg/m2) |
Donor- recipient BMI unit difference |
BMI match or mismatch |
Final determination |
| 1 | 26 | 27 | −1 | M | Match |
| 2 | 26 | 30 | −4 | MM | Mismatch-larger recipient |
| 3 | 35 | 25 | +10 | MM | Mismatch-larger donor |
BMI, body mass index; M, match; MM, mismatch.
Donor-recipient BMI within +/− 2 units.
Statistical method and outcome analysis
Donor and recipient demographic and clinical parameters were stratified by gender. Significant differences between groups were ascertained by least squared means and maximum likelihood ratio for continuous and categorical variables, respectively, and subsequently for the male donor-female recipient group versus all others.
Kaplan-Meier estimates of graft and patient survival were calculated for donors and recipients by gender and for the male donor-female recipient group versus others. Sub-analyses on survival were performed for living and deceased donor recipients by donor gender, recipient gender, and donor-recipient gender combination. Log-rank p-value was used as a test of significance.
A multivariate Cox proportional hazards model ascertained independent associations of graft loss. Covariates for model adjustment included deceased donor and recipient age, deceased donor and recipient black race, hypertension, diabetes mellitus, number of HLA mismatches, cold and warm ischemia times, peak panel-reactive lymphocytotoxic antibody (PRA), previous kidney transplantation, donor type, 30-day acute rejection, donor BMI greater than 2 units compared to recipient, and male donor-female recipient vs. others. Estimates of risk were also calculated after censoring for death. A second regression model to assess the risk of graft loss was stratified by recipient gender and adjusted for previous covariates plus 1) male donor BMI 2 units larger and 2) male vs. female donor.
All statistical analyses were performed using SAS 9.1.3 (Cary, NC), and p-values less than 0.05 were considered statistically significant.
Results
Donor-recipient demographics and baseline clinical characteristics
There were 146 male donor to female recipient transplants (MDFR group) and 213, 179, and 130 male to male, female to male, and female to female combinations, respectively. In this study the latter three combinations were pooled for most analyses to form a single group (others) as no statistical differences in demographic characteristics or outcome were identified in the exploratory analyses. Donor and recipient age, race, and BMI did not differ significantly between the MDFR group and others (Table 1). Compared to recipients among the others, recipients in the MDFR group had a higher prevalence of diabetes (31% vs. 22%, p=0.03) but were less sensitized (70% vs. 65% for peak PRA <50 and 30% vs. 35%, for PRA ≥50, p<0.01. Table 1, bottom panel).
Table 1.
Demographics and Clinical Characteristics in a Single-Center Study of 668 Kidney Transplants by Donor-Recipient Gender Combination
| Donor-recipient gender | p Value | ||
|---|---|---|---|
| Male-female | Others | ||
| Characteristic | n=146 | n=522 | |
| Donor | |||
| Mean age, y (SD) | 28 (15) | 35 (16) | NS |
| Race, % | |||
| White | 62 | 71 | |
| African American | 30 | 21 | NS |
| Other | 8 | 8 | |
| Male,% | 100 | 41 | <0.01 |
| Mean BMI, kg/m2 (SD) | 25 (7) | 26 (7) | NS |
| Donor type, % | |||
| Living | 14 | 20 | NS |
| Deceased | 86 | 80 | |
| Recipient | |||
| Mean age, y (SD) | 43 (16) | 43 (17) | NS |
| Race, % | |||
| White | 38 | 40 | |
| African American | 59 | 56 | NS |
| Other | 3 | 4 | |
| Male,% | 0 | 75 | <0.01 |
| Mean BMI, kg/m2 (SD) | 27 (7) | 27 (7) | NS |
| Past medical history, % | |||
| Diabetes mellitus | 31 | 22 | 0.03 |
| Hypertension | 86 | 88 | NS |
| Polycystic kidney disease | 7 | 7 | NS |
| Coronary artery disease | 10 | 13 | NS |
| Peripheral vascular disease | 5 | 6 | NS |
| Previous kidney transplant, % | 10.6 | 11.6 | NS |
| Mean cold ischemia time, h (SD) | 9.4 (10.4) | 10.4 (10.9) | NS |
| Mean warm ischemia time, min (SD) | 13.8 (17.9) | 14.3 (19.0) | NS |
| Peak PRA, % | |||
| <50 | 70 | 65 | <0.01 |
| ≥50 | 30 | 35 | |
| HLA mismatches, % | |||
| 0–1 | 20 | 26 | |
| 2–4 | 47 | 42 | NS |
| 5–6 | 33 | 32 | |
SD, standard deviation; BMI, body mass index; PRA, panel-reactive lymphocytotoxic antibodies; HLA, human leukocyte antigen.
Pearson’s correlation of donor body mass index and donor kidney size
Regardless of calculation method, either by combined gender or single gender, donor BMI and kidney size directly correlated (combined male and female donors (n=360) r=0.40, p<0.0001; male donors only (n=205) r=0.40, p<0.0001; and female donors only (n=155) r=0.40, p<0.0001).
MDFR graft survival and the risk of graft loss
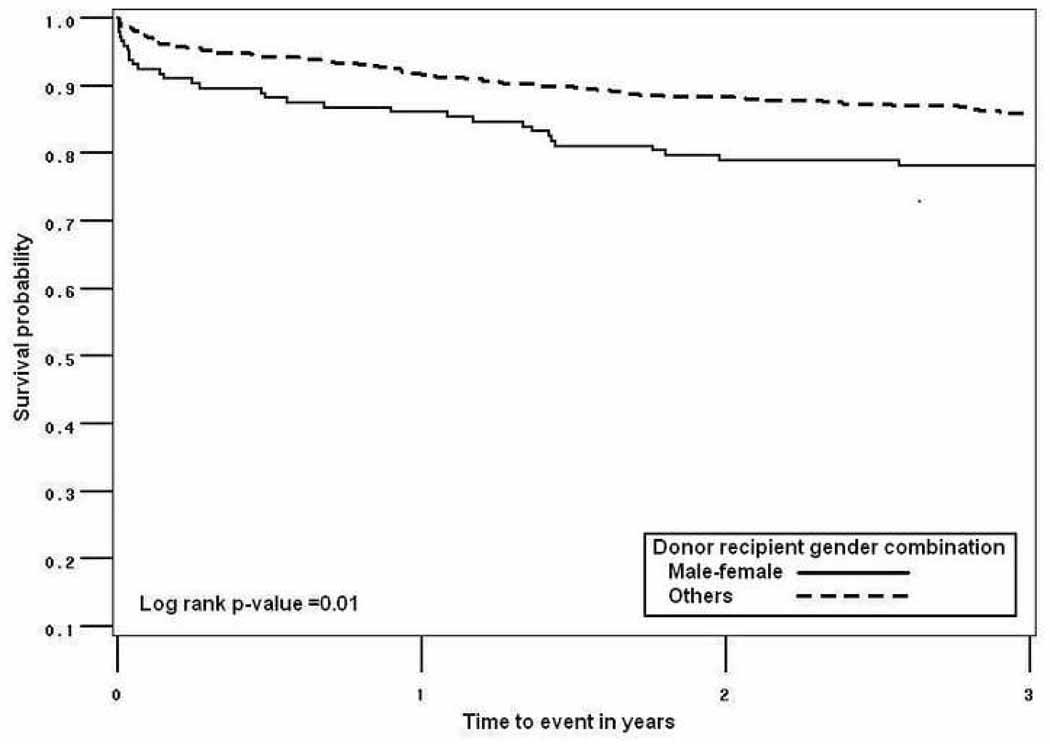
Kaplan Meier estimates of graft survival for the MDFR group were significantly lower than for the others. Unadjusted graft survival at 1, 2, and 3 years was 86%, 79%, and 78% for MDFR group, and 92%, 88%, and 86% for the others (log-rank-p=0.01, Figure 1).
Figure 1.
1-, 2-, and 3-year graft survival in a single-center study of 668 kidney. transplants by male donor-female recipient gender combination (86%, 79%, and 78%) versus others (92%, 88%, and 86%).
Sub-analyses of graft survival according to donor type were also performed. Overall, compared to deceased donors, living donors provided a significantly better rate of graft survival at 1, 2, and 3 years after transplant (92%, 92%, and 91% vs. 90%, 85%, and 83%, log-rank p=0.05). When living donors were stratified by the MDFR group vs. others, the rate of graft survival was consistently poorer for male donor to female recipient transplants though the difference was not statistically significant. For deceased donor transplants analyzed by donor-recipient gender combinations, compared to the others, the MDFR group had significantly lower graft survival rates (86%, 78%, and 77% at 1, 2, 3 years versus 91%, 87%, and 85%, log-rank p=0.03).
A multivariate adjusted Cox proportional analysis of graft loss demonstrated that the hazard ratio (HR) of graft loss for additional HLA mismatch among all study patients was 1.24 (95% CI 1.08–1.44, p<0.01) and was 1.29 (95% CI 1.10–1.52, p<0.01) after censoring for death (Table 2). When compared to others, a strong association was demonstrated between the MDFR group and graft loss (HR 1.78, 95% CI 1.12–2.85, p=0.02). The association increased after censorship for death (HR 1.97, 95% CI 1.18–3.30, p=0.01).
Table 2.
Multivariate Adjusted Cox Proportional Hazards of Graft Loss in a Single-Center Study of 668 Kidney Transplants
| All Patients | Death-Censored | |||
|---|---|---|---|---|
| Variable | Hazard Ratio | p Value | Hazard Ratio | p Value |
| Donor age (continuous) | 0.98 (0.97–1.00) | NS | 0.99 (0.97–1.01) | NS |
| Recipient age (continuous) | 1.00 (0.98–1.01) | NS | 0.98 (0.97–1.00) | NS |
| African-American race versus non-African- American race (donor) |
0.95 (0.59–1.69) | NS | 0.81 (0.45–1.47) | NS |
| African-American race versus non- African- American race (recipient) |
1.21 (0.73–2.02) | NS | 1.21 (0.69–2.13) | NS |
| Hypertension (yes versus no) | 1.03 (0.51–2.09) | NS | 1.22 (0.56–2.67) | NS |
| Diabetes mellitus (yes versus no) | 0.58 (0.32–1.06) | NS | 0.76 (0.40–1.44) | NS |
| Number of HLA mismatch (continuous) | 1.24 (1.08–1.44) | <0.01 | 1.29 (1.10–1.52) | <0.01 |
| Cold ischemia time (continuous) | 0.99 (0.97–1.02) | NS | 0.99 (0.96–1.02) | NS |
| Warm ischemia time (continuous) | 1.00 (0.99–1.02) | NS | 1.00 (0.98–1.01) | NS |
| Peak PRA (≥50 versus <50) | 1.13 (0.66–1.92) | NS | 0.84 (0.45–1.55) | NS |
| Previous kidney transplant (yes versus no) | 1.27 (0.64–2.52) | NS | 1.61 (0.79–3.28) | NS |
| Donor type (deceased versus living donor) | 1.10 (0.52–2.31) | NS | 1.26 (0.55–2.89) | NS |
| 30-day acute rejection (yes versus no) | 5.72 (2.16–15.12) | <0.01 | 5.10 (1.67–15.5) | <0.01 |
| Donor BMI >2 units compared to recipient versus others |
0.94 (0.58–1.52) | NS | 0.84 (0.49–1.44) | NS |
| Male donor-female recipient versus others | 1.78 (1.12–2.85) | 0.02 | 1.97 (1.18–3.30) | 0.01 |
HLA, human leukocyte antigen; PRA, panel-reactive lymphocytotoxic antibody.
BMI matches and mismatches, gender and size matches and mismatches and the risk of graft loss
Separate sets of analyses were performed to identify associations of graft loss between different donor-recipient BMI combination groups (data presented as text only). From a Kaplan Meier graft survival estimate, there was no survival difference among three categories of BMI combinations (similar, heavier donor, and heavier recipient). Three year graft survival was 84%, 86%, and 84% respectively, log rank p-value=0.86). To further assess the effect of donor-recipient BMI matching we performed a multivariate adjusted Cox regression hazards model within each donor-recipient gender combination subgroup. The donor-recipient gender combinations that composed the other group were not pooled for this analysis, and the donor-recipient similar BMI group acted as reference. The MDFR group displayed hazard ratios of graft loss among heavier donors and heavier recipients of 0.41 (95% CI 0.09–1.87) and 0.96 (95% CI 0.26–3.59), respectively. Though not statistically significant, except for male donor to male recipient subgroup, having a heavier donor was associated with a lower risk of graft loss compared to having a heavier recipient.
An additional multivariate model of graft loss stratified by recipient gender demonstrated that for female recipients the risk of graft loss was more than three times higher if the donor was male versus female (HR 3.45 95% CI 1.40–8.51, p=0.01). An analogous risk did not exist for male recipients (Table 3). After adjusting for all other risk factors and compared to others, female recipients of male donors whose BMI was 2 units larger than their own BMI had a statistically significant reduction in the risk of graft loss (HR 0.19, 95% CI 0.05–0.67, p=0.01). Again, an analogous risk did not exist for male recipients. In a separate model not presented in table form, female recipients whose donor BMI regardless of gender was 2 units larger than their own BMI had a statistically significant reduction in the risk of graft loss (HR 0.23, 95% CI 0.08–0.68, p=0.01).
Table 3.
Multivariate Adjusted Cox Proportional Hazards of Death-Censored Graft Loss in a Single Center Study of 392 Male Recipients and 276 Female Recipients
| Male Recipients | Female Recipients | |||
|---|---|---|---|---|
| Variable | Hazard Ratio | p Value | Hazard Ratio | p Value |
| Donor age (continuous) | 0.99 (0.97–1.02) | NS | 0.99 (0.96–1.02) | NS |
| Recipient age (continuous) | 0.98 (0.96–1.01) | NS | 0.97 (0.94–1.00) | NS |
| African-American race versus non-African- American race (donor) |
0.44 (0.13–1.55) | NS | 0.63 (0.27–1.46) | NS |
| African-American race versus non- African-American race (recipient) |
1.75 (0.66–4.63) | NS | 1.20 (0.47–3.06) | NS |
| Hypertension (yes versus no) | 1.01 (0.31–3.24) | NS | 2.71 (0.64–11.40) | NS |
| Diabetes mellitus (yes versus no) | 0.52 (0.15–1.85) | NS | 1.34 (0.57–3.15) | NS |
| Number of HLA mismatch (continuous) | 1.31 (0.99–1.75) | 0.03 | 1.44 (1.10–1.87) | 0.01 |
| Cold ischemia time (continuous) | 1.03 (0.99–1.07) | NS | 0.95 (0.90–0.99) | 0.02 |
| Warm ischemia time (continuous) | 0.98 (0.95–1.00) | NS | 1.01 (0.99–1.04) | NS |
| Peak PRA (≥50 versus <50) | 1.68 (0.48–5.82) | NS | 0.42 (0.17–1.04) | NS |
| Previous kidney transplant (yes versus no) | 0.66 (0.18–2.48) | NS | 4.46 (1.43–13.8) | 0.01 |
| Donor type (deceased versus living donor) | 0.60 (0.14–2.64) | NS | 2.67 (0.55–12.9) | NS |
| 30-day acute rejection (yes versus no) | 13.5 (2.44–74.5) | <0.01 | 1.49 (0.24–9.29) | NS |
| Male donor BMI >2 units compared to recipient versus others |
1.86 (0.72–4.81) | NS | 0.19 (0.05–0.67) | 0.01 |
| Male donor versus female donor | 0.91 (0.35–2.33) | NS | 3.45 (1.40–8.51) | 0.01 |
HLA, human leukocyte antigen; PRA, panel-reactive lymphocytotoxic antibody.
Increasing HLA mismatches augmented the risk of graft loss for both male (HR 1.31, 95% CI 0.99–1.75, p=0.03) and female (HR 1.44, 95% CI 1.10–1.87, p=0.01) recipients. For the covariates cold ischemia time and previous kidney transplantation the hazards of graft loss differed by gender. Compared to males, female recipients appeared less susceptible to cold ischemia time (HR 0.95 95% CI 0.90–0.99, p=0.02). Receiving a previous kidney transplant did not significantly impact graft loss for male recipients, but female recipients had over four times the risk of graft failure (HR 4.46, 95% CI 1.43–13.8, p=0.01).
Gender and acute rejection
The prevalence of 30-day acute rejection (AR) did not differ by male donor-female recipients versus others. Acute rejection was, however, associated with a significant risk of graft loss for the whole sample (HR 5.72, 95% CI 2.16–15.12, p<0.01), as well as the death censored population (HR 5.10, 95% CI 1.67–15.5, p<0.01) (Table 2). When stratified by recipient gender, 30-day AR was a statistically significant risk for males (HR 13.5, 95% CI 2.44–74.5, p<0.01) but not for females (Table 3).
Discussion
Our data support previous works reporting that the male donor to female recipient transplant combination is an independent risk for an inferior graft survival. More importantly, our study showed a confounding effect when body mass mismatch (kidney size mismatch) was considered for male donor-female recipient kidney transplants. We demonstrated a significantly decreased risk of graft loss for female recipients of male kidneys when donor BMI was 2 or more units larger than recipient BMI.
A seminal paper by Gratwohl and colleagues retrospectively studied almost 160,000 deceased donor kidney transplant recipients and demonstrated in a multivariate regression model that female recipients of male donor kidneys had an increased risk of poor graft survival.4 Investigators in the study speculated that the H-Y antigens/the H-Y effect was responsible. H-Y antigens are ubiquitously expressed18, 19 and despite a “minor” classification have been implicated in clinically relevant immunologic events such as graft versus host disease after bone marrow transplantation5 and acute rejection in solid organ transplantation (corneal transplants6 and kidney transplants7, 8). Non-HLA antigens such has H-Y have been poorly characterized in the current literature. Most recently, however, Tan and colleagues measured H-Y antibody expression in kidney transplant recipients.8 The investigators found that compared to all other donor-recipient gender groups, the male donor-female recipient population demonstrated significantly higher percentage of H-Y antibody production (54% vs. 8%) 8
It is not known whether the H-Y effect is a short or a long-term phenomenon. Several investigations have suggested that the H-Y effect can be clinically apparent as early as two weeks post-transplantation.7, 20 Thus, to assess the H-Y effect in our population we analyzed the frequency of 30-day AR episodes by donor gender and donor-recipient gender combination. In our study the prevalence of 30-day AR was statistically similar for recipients of male and female grafts. Also, 30-day AR events by donor-recipient gender combination failed to differ significantly between the MDFR group and the others. Therefore, our data does not support an early H-Y effect after kidney transplantation, but we recognize that our null result does not definitively exclude a late effect, especially the potential long-term influence. Though time to rejection was not reported in the Tan et al. study, 26 demographically similar male donor to female recipient transplants were examined. The investigators found that compared to recipients not experiencing AR, recipients experiencing rejection developed de novo anti-HY antibodies significantly more often, 79% vs. 8% (p=0.00048).8 Because the H-Y antigens measured in the study were intracellular cytosolic and nuclear, authors hypothesized that de novo antibodies, not preformed, are responsible for the strong, H-Y initiated alloimmune response associated with AR.8 Further, characterization of the H-Y minor histocompatibility antigen is needed to fully understand its impact in kidney transplant outcomes.
In our adjusted analyses looking at the influence of 30-day AR on graft survival, the entire population was at increased risk for graft loss when AR occurred. Interestingly, when stratified by recipient gender there was a significantly increased risk for males, but not females. Of the 392 male recipients in the study almost half had female donors. This is relevant with respect to early gender studies in kidney transplantation as female donors were frequently implicated in inferior outcomes. Vereerstraeten and colleagues reviewed 741 deceased donor transplants and found after multivariate analysis that female donation to male recipients increased the risk of graft failure by 60%.21 Authors observed a greater number of technical failures for the female donor-male recipient transplant group, and they proposed greater immunogenicity among female donors.21 Similarly, a registry study of over 100,000 first deceased donor kidney transplants concluded that compared to female recipients of female donors, the risk of graft loss for male recipients of a female donors was significantly greater (RR 1.22, 95% CI 1.16–1.29, p<0.0001 vs. RR 1.15, 95% CI 1.07–1.23, p<0.0001).22 These investigators speculated that nephron under-dosing and donor immunologic factors were responsible for the poor graft outcomes of the female donor to male recipient group.22 To completely discern the relationship between the female donor-male recipient group and graft outcomes, we would need to include examination of associated donor-recipient size mismatch.
Another group investigating the role of gender in kidney transplantation studied 30,000 living donor kidney transplants, and found in an adjusted analysis, as we found for our deceased donors, that male donor to female recipient transplants had significantly increased risk of graft loss (HR 1.19, 95% CI 1.09–1.30).23 Interestingly, investigators speculated that in the context of nephron under-dosing the male to female transplant combination should have improved graft survival as male kidneys are characteristically larger.23 While our living donor analysis did not reach statistical significance, compared to the others, the living donor MDFR group demonstrated a trend toward a worse survival rate at all 3 years reviewed post transplant. Failure of significance was most likely due to a smaller living donor sample size for the MDFR group (n=21) compared to all others (n=102).
Previous studies examining donor-recipient size mismatch suggested a graft survival advantage for kidney recipients who receive larger organs in relation to their own body size.9–11 An early study described the magnitude of advantage comparable to the benefits for grafts implanted within 48 hours of procurement as well as HLA matched grafts.15 Investigators have also described the advantage as beginning at five years after transplant with an almost 20% difference in graft survival between recipients of larger donors compared to donors of similar or smaller size.24 The pathophysiology has been explained by larger kidneys having more glomeruli,14, 25 and more glomeruli translates to less susceptibility to progressive renal failure.13 As a consequence, in the setting of a larger donor compared to recipient there is less metabolic demand on the donor graft, thus less hyperfiltration induced injury.15, 16, 26 Hyperfiltration from nephron under-dosing has been hypothesized to initiate pathologic, structural kidney damage to the transplanted kidney ultimately resulting in graft dysfunction and failure.15, 16
After confirming the correlation between donor BMI and kidney size in our population, donor BMI was accepted as a proxy for kidney size in concordance with earlier studies.12, 14, 25, 27 We then tested the relationship between donor-recipient gender, donor-recipient size, and kidney transplant outcomes. Multivariate analyses found that a larger donor BMI compared to the recipient, regardless of donor gender, was protective against the risk of graft loss. This relationship was particularly strong for female recipients of male donor kidneys. To our knowledge this is the first report investigating the combined effects of donor-recipient gender and size on kidney graft outcomes. Our results suggest that perhaps the donor-recipient gender and size relationship should be considered in deceased donor kidney allocation schemas and that female recipients of male kidneys may benefit from protocol driven, tailored immunosuppression to improve their outcomes. However, prior to implementing changes in clinical practice much more knowledge is needed on the impact of the interaction between donor-recipient gender and size.
As expected, regardless of gender our data describing the relationship between HLA mismatches and adverse graft events are consistent with established conclusions affirming the risk of poor graft outcomes as the number of HLA mismatches increases.28 Somewhat unexpectedly, gender differences were identified for the cold ischemia variable. It is difficult to explain why female recipients in our cohort may be less susceptible to longer cold ischemia times. The majority of our female patients received male donor kidneys. Although controversial, male kidney grafts have been shown to be less susceptible to cold ischemia.22 We speculate, however, that the result in our study has limited statistical and/or clinical relevance because the upper limit of the 95% confidence interval approaches unity. Our data support existing reports that 1) compared to male recipients, female recipients may have increased immunoreactivity because of sensitizing events such as pregnancy and higher prevalence of autoimmune diseases29 and 2) compared to first transplants, repeat kidney transplant recipients have worse graft survival.30, 31 Taken together, it was not surprising that compared to male recipients, female recipients in our study were at increased risk of graft loss if they had been a previous kidney transplant recipient.
This study was limited by its retrospective nature. However, the single-center design minimized center-specific effects such as differences in immunosuppression protocols and peri-and post-operative care. The percentage of patients in this population receiving induction therapy or tacrolimus vs. cyclosporine did not significantly differ between the MDFR group and others, but because of limited sample size we were unable to analyze further by immunosuppression regimens. Though unlikely based on prevalence data, differing regimens might alter outcomes.
Conclusion
This study corroborated that the male donor to female recipient kidney transplant combination is an independent risk factor for poor graft survival. However, when an implanted kidney is from a larger donor this effect appears to be mitigated. As consideration is given to redefining the current kidney allocation system, perhaps the influence of donor size and gender effect should be taken into consideration.
Acknowledgment
The authors would like to thank Paul Muntner, PhD for his early support in the project’s design; Drs Anil Paramesh, Mary Killackey, and Eddie Hoover for critically reviewing the manuscript; Tiffany Haydel, Debbie Jeffreys, and the staff at the Louisiana Organ Procurement Agency for organizing the kidney size data; Joel Wells for abstracting the kidney size data; and the many students dedicated to data collection.
Funding/Support: The first author is partially supported by award number K12HD043451 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development.
Role of the Sponsor: Manuscript content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at Southern Surgical Association 121st Annual Meeting, Hot Springs, VA, December 2009.
Author contributions: Dr. McGee had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: McGee, Magnus, Jaffe
Acquisition of data: McGee, Mruthinti, Sullivan
Analysis and interpretation of data: McGee, Magnus, Islam
Drafting of manuscript: McGee, Magnus
Critical revision of the manuscript for important intellectual content: McGee, Magnus, Zhang, Jaffe, Florman, Hamm, Slakey
Statistical analysis: Islam
Administrative, technical, or material support: McGee, Mruthinti
Study supervision: McGee
Contributor Information
Jennifer McGee, Department of Surgery, Tulane University School of Medicine, New Orleans, LA.
Jeanette H Magnus, Department of Community Health Sciences, Tulane University School of Public Health and Tropical Medicine, New Orleans, LA.
Tareq M Islam, Department of Surgery, Tulane University School of Medicine, New Orleans, LA.
Bernard M Jaffe, Department of Surgery, Tulane University School of Medicine, New Orleans, LA.
Rubin Zhang, Tulane Abdominal Transplant Institute, Tulane University School of Medicine, New Orleans, LA.
Sander S Florman, Tulane Abdominal Transplant Institute, Tulane University School of Medicine, New Orleans, LA.
L Lee Hamm, Department of Medicine, Tulane University School of Medicine, New Orleans, LA.
Navyata Mruthinti, Department of Surgery, Tulane University School of Medicine, New Orleans, LA.
Karen Sullivan, Department of Medicine, Tulane University School of Medicine, New Orleans, LA.
Douglas P Slakey, Department of Surgery, Tulane University School of Medicine, New Orleans, LA.
References
- 1.Based on OPTN data. 2009 February 13; as of www.optn.org. [Google Scholar]
- 2.Jindal RM, Ryan JJ, Sajjad I, et al. Kidney transplantation and gender disparity. Am J Nephrol. 2005;25:474–483. doi: 10.1159/000087920. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Transplant Study. http://www.ctstransplant.org/ [Google Scholar]
- 4.Gratwohl A, Dohler B, Stern M, Opelz G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet. 2008;372:49–53. doi: 10.1016/S0140-6736(08)60992-7. [DOI] [PubMed] [Google Scholar]
- 5.Voogt PJ, Goulmy E, Fibbe WE, et al. Minor histocompatibility antigen H-Y is expressed on human hematopoietic progenitor cells. J Clin Invest. 1988;82:906–912. doi: 10.1172/JCI113697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohringer D, Spierings E, Enczmann J, et al. Matching of the minor histocompatibility antigen HLA-A1/H-Y may improve prognosis in corneal transplantation. Transplantation. 2006;82:1037–1041. doi: 10.1097/01.tp.0000235908.54766.44. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer PF, Thorsby E. HLA-restricted cytotoxicity against male-specific (H-Y) antigen after acute rejection of an HLA-identical sibling kidney: clonal distribution of the cytotoxic cells. Transplantation. 1982;33:52–56. doi: 10.1097/00007890-198201000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Tan JC, Wadia PP, Coram M, et al. H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation. 2008;86:75–81. doi: 10.1097/TP.0b013e31817352b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Fructuoso AI, Prats D, Marques M, et al. Does renal mass exert an independent effect on the determinants of antigen-dependent injury? Transplantation. 2001;71:381–386. doi: 10.1097/00007890-200102150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Poggio ED, Hila S, Stephany B, et al. Donor kidney volume and outcomes following live donor kidney transplantation. Am J Transplant. 2006;6:616–624. doi: 10.1111/j.1600-6143.2005.01225.x. [DOI] [PubMed] [Google Scholar]
- 11.Amante AJ, Pinon-Barretto SC. The correlation of renal allograft weight to metabolic index ratios and glomerular filtration rate among living-unrelated kidney transplant patients: a cross-sectional study. Transplant Proc. 2008;40:2313–2318. doi: 10.1016/j.transproceed.2008.07.071. [DOI] [PubMed] [Google Scholar]
- 12.Giral M, Nguyen JM, Karam G, et al. Impact of graft mass on the clinical outcome of kidney transplants. J Am Soc Nephrol. 2005;16:261–268. doi: 10.1681/ASN.2004030209. [DOI] [PubMed] [Google Scholar]
- 13.Hoy WE, Douglas-Denton RN, Hughson MD, et al. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003:S31–S37. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 14.Neugarten J, Kasiske B, Silbiger SR, Nyengaard JR. Effects of sex on renal structure. Nephron. 2002;90:139–144. doi: 10.1159/000049033. [DOI] [PubMed] [Google Scholar]
- 15.Brenner BM, Milford EL. Nephron underdosing: a programmed cause of chronic renal allograft failure. Am J Kidney Dis. 1993;21:66–72. doi: 10.1016/0272-6386(93)70097-i. [DOI] [PubMed] [Google Scholar]
- 16.Terasaki PI, Koyama H, Cecka JM, Gjertson DW. The hyperfiltration hypothesis in human renal transplantation. Transplantation. 1994;57:1450–1454. [PubMed] [Google Scholar]
- 17.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 18.Billingham RE, Silvers WK. Studies on tolerance of the Y chromosome antigen in mice. J Immunol. 1960;85:14–26. [PubMed] [Google Scholar]
- 19.Goulmy E. Human minor histocompatibility antigens. Curr Opin Immunol. 1996;8:75–81. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- 20.Spierings E, Vermeulen CJ, Vogt MH, et al. Identification of HLA class II-restricted H-Y-specific T-helper epitope evoking CD4+ T-helper cells in H-Y-mismatched transplantation. Lancet. 2003;362:610–615. doi: 10.1016/S0140-6736(03)14191-8. [DOI] [PubMed] [Google Scholar]
- 21.Vereerstraeten P, Wissing M, De Pauw L, et al. Male recipients of kidneys from female donors are at increased risk of graft loss from both rejection and technical failure. Clin Transplant. 1999;13:181–186. doi: 10.1034/j.1399-0012.1999.130205.x. [DOI] [PubMed] [Google Scholar]
- 22.Zeier M, Dohler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. 2002;13:2570–2576. doi: 10.1097/01.asn.0000030078.74889.69. [DOI] [PubMed] [Google Scholar]
- 23.Kayler LK, Rasmussen CS, Dykstra DM, et al. Gender imbalance and outcomes in living donor renal transplantation in the United States. Am J Transplant. 2003;3:452–458. doi: 10.1034/j.1600-6143.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 24.el-Agroudy AE, Hassan NA, Bakr MA, et al. Effect of donor/recipient body weight mismatch on patient and graft outcome in living-donor kidney transplantation. Am J Nephrol. 2003;23:294–299. doi: 10.1159/000072819. [DOI] [PubMed] [Google Scholar]
- 25.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 26.Oh CK, Lee BM, Jeon KO, et al. Gender-related differences of renal mass supply and metabolic demand after living donor kidney transplantation. Clin Transplant. 2006;20:163–170. doi: 10.1111/j.1399-0012.2005.00459.x. [DOI] [PubMed] [Google Scholar]
- 27.Kasiske BL, Umen AJ. The influence of age, sex, race, and body habitus on kidney weight in humans. Arch Pathol Lab Med. 1986;110:55–60. [PubMed] [Google Scholar]
- 28.Opelz G. New immunosuppressants and HLA matching. Transplant Proc. 2001;33:467–468. doi: 10.1016/s0041-1345(00)02095-9. [DOI] [PubMed] [Google Scholar]
- 29.Meier-Kriesche HU, Ojo AO, Leavey SF, et al. Gender differences in the risk for chronic renal allograft failure. Transplantation. 2001;71:429–432. doi: 10.1097/00007890-200102150-00016. [DOI] [PubMed] [Google Scholar]
- 30.Pour-Reza-Gholi F, Nafar M, Saeedinia A, et al. Kidney retransplantation in comparison with first kidney transplantation. Transplant Proc. 2005;37:2962–2964. doi: 10.1016/j.transproceed.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Magee JC, Barr ML, Basadonna GP, et al. Repeat organ transplantation in the United States, 1996–2005. Am J Transplant. 2007;7:1424–1433. doi: 10.1111/j.1600-6143.2007.01786.x. [DOI] [PubMed] [Google Scholar]