Abstract
This study examined the sensitivity of diffusion tensor imaging (DTI) to microstructural white matter (WM) damage in mild and moderate pediatric traumatic brain injury (TBI). Fourteen children with TBI and 14 controls ages 10–18 had DTI scans and neurocognitive evaluations at 6–12 months post-injury. Groups did not differ in intelligence, but children with TBI showed slower processing speed, working memory and executive deficits, and greater behavioral dysregulation. The TBI group had lower fractional anisotropy (FA) in three WM regions: inferior frontal, superior frontal, and supracallosal. There were no group differences in corpus callosum. FA in the frontal and supracallosal regions was correlated with executive functioning. Supracallosal FA was also correlated with motor speed. Behavior ratings showed correlations with supracallosal FA. Parent-reported executive deficits were inversely correlated with FA. Results suggest that DTI measures are sensitive to long-term WM changes and associated with cognitive functioning following pediatric TBI.
Keywords: Traumatic brain injury (TBI), Diffusion tensor imaging (DTI), White matter, Pediatric
1. Introduction
1.1. Background and significance
The Centers for Disease Control and Prevention estimate that there are more than one million new pediatric traumatic brain injuries (TBIs) each year, resulting in 435,000 emergency room visits and making TBI the most common neurological condition in children (Centers for Disease Control and Prevention, 2006). Most likely, these data underestimate the actual number of injuries because many go unreported or are treated outside of the ER (Langlois, Rutland-Brown, & Wald, 2006). The majority of TBIs (75% or greater) are classified as mild (Langlois, Rutland-Brown, & Thomas, 2005; National Center for Injury Prevention and Control, 2003). Although the literature provides ample evidence of long-term cognitive and behavioral changes following moderate and severe TBI, the consequences of mild TBI are less clear (Asarnow, Satz, Light, Lewis, & Neumann, 1991; Ewing-Cobbs, Miner, Fletcher, & Levin, 1989; Farmer, Singer, Mellits, Hall, & Charney, 1987; Fletcher, Ewing-Cobbs, Miner, Levin, & Eisenberg, 1990; Yeates et al., 1999). Furthermore, across all levels of TBI, it has been challenging to establish clear links between the observable sequelae of TBI and underlying structural brain changes.
Many previous imaging studies of pediatric TBI have focused on either the location and volume of focal lesions or on gross volumetric changes in grey and white matter. Presently, there is a growing interest in newer imaging techniques, such as diffusion tensor imaging (DTI), which may prove to be more sensitive to the diffuse white matter damage that often occurs in TBI (Levin, 2003). DTI may have particular utility in the study of pediatric TBI because of its sensitivity to normal developmental brain changes (Ben Bashat et al., 2005; Eluvathingal, Hasan, Kramer, Fletcher, & Ewing-Cobbs, 2007; Hermoye et al., 2006; Mabbott, Noseworthy, Bouffet, Laughlin, & Rockel, 2006; Salat et al., 2005) and its potential to expose mechanisms of neuroplasticity involved in recovery from TBI. DTI indices reflect normal developmental changes in brain tissue including increased fiber organization with age, changes in tissue water content, and myelination of axons (Neil, Miller, Mukherjee, & Huppi, 2002). DTI’s sensitivity to this normal developmental “background” change in children’s brains may eventually allow for greater sensitivity in measuring the effects of neurodevelopmental insults, including TBI. Large-scale DTI studies of normally developing children will provide particularly useful “normative” data about brain development against which clinical samples can be compared. Such normative data will also be critical to future studies examining tissue recovery following TBI in young, developing brains.
The current study employed DTI in examining the relationship between microstructural white matter damage and neurocognitive and behavioral sequelae in a sample of children and adolescents with mild and moderate TBI.
1.2. Neurocognitive and neurobehavioral outcomes in pediatric TBI
The clinical outcome of pediatric TBI is highly varied, but often includes persistent cognitive problems such as attention deficit, memory impairment, slowed processing speed, word-finding difficulties, impaired executive function, behavioral disinhibition, and emotional liability (Taylor, 2004; Yeates & Taylor, 2005). One of the most common cognitive correlates of childhood TBI is impairment in attentional functioning. Although there are indications in the literature that some children with TBI have higher rates of premorbid attention deficits (Donders & Strom, 2000; Yeates et al., 2002), there is substantial evidence that TBI is associated with the onset of new attention problems in children (Anderson, Fenwick, Manly, & Robertson, 1998; Dennis, Guger, Roncadin, Barnes, & Schachar, 2001; Fenwick & Anderson, 1999; Yeates et al., 2002). Yeates et al. (2005) provided neurocognitive and behavioral evidence of long-term attention deficits in children following TBI and demonstrated a clear relationship between cognitive and behavioral aspects of attention in this group. Group differences were significant even after controlling for important subject variables including premorbid attention problems.
Working memory deficits occur frequently in pediatric TBI (Levin et al., 2002; Levin, Hanten, et al., 2004). In a longitudinal study, Levin and colleagues demonstrated that children with TBI showed persistent working memory deficits as far out as 2 years post-injury. Roncadin, Guger, Archibald, Barnes, and Dennis (2004) found working memory impairments in moderate and severe TBI groups, but not in a mild group.
Slowed processing speed is also common in pediatric TBI (Anderson, Catroppa, Morse, Haritou, & Rosenfeld, 2005; Bawden, Knights, & Winogron, 1985; Knights et al., 1991; Yeates, 2000), particularly in children with more severe injuries. In an examination of Wechsler Intelligence Scale for Children—Third Edition (WISC-III) index scores, Donders and Warschausky (1997) found a distinct subtype of children with TBI characterized by selective impairments on tasks comprising the processing speed and perceptual organization factor indices. These children tended to have more severe injuries and evidence of diffuse lesions on CT and MRI scans.
A range of verbal memory deficits has been reported in pediatric TBI (Anderson, Morse, Catroppa, Haritou, & Rosenfeld, 2004; Catroppa & Anderson, 2002; Ewing-Cobbs, Levin, & Fletcher, 1998; Hoffman, Donders, & Thompson, 2000; Salorio et al., 2005). Salorio et al. (2005) found significant memory impairment at 1 year post-injury and further observed that frontal and temporal lesion sizes (determined from MRI) were associated with the degree of impairment. Warschausky, Kay, Chi, and Donders (2005) showed impairment on a verbal memory task and demonstrated that children with severe TBI had slower rates of acquisition than those with less severe injuries.
Lastly, significant persistent behavioral changes are frequently noted following pediatric TBI. Yeates, Taylor, Drotar, Wade, and Stancin (2001) reported increased rates of emotional and behavioral symptoms, including mood changes and impulsivity, following TBI. The authors also noted that these symptoms increased with time elapsed since injury, whereas cognitive and somatic symptoms declined over time. Levin, Zhang, et al. (2004) reported maladaptive behavior and poor socialization skills in a pediatric TBI sample and found a relationship between level of impairment and lesion volume, particularly in frontal tissue.
1.3. Sensitivity of DTI in pediatric TBI
DTI is a relatively new MRI technique that measures the diffusion properties of water molecules in tissue (Basser & Jones, 2002; Beaulieu, 2002; Moseley et al., 1990). In brain tissue, water molecules diffuse more easily in the direction parallel to long axons compared to the directions perpendicular to axons. This differential diffusion can be measured in three dimensions, mapped, and reconstructed into images that closely correspond to underlying tissue anatomy. Furthermore, the technique also provides a quantitative assessment of the integrity of white matter tissue. Fractional anisotropy (FA) is the name of the measure commonly used to represent the ratio of the diffusion in the principal direction of axons to diffusion in the perpendicular directions. FA, which ranges from 0 to 1.0, is greatest in highly organized white matter because of the directionally restrictive structure of bundled axons. The shearing forces of TBI simultaneously result in the tearing of axons and in localized bleeding resulting from damaged capillaries. Over time, this damage to axons results in the development of gaps between previously tightly packed fibers. This change, along with degradation of myelin over time, allows water diffusion to occur more easily in the directions perpendicular to the axons, thus lowering FA (Arfanakis et al., 2002).
Clinically, white matter shearing injury is referred to as diffuse axonal injury (DAI). Typically, DAI is diagnosed based upon the appearance of small, scattered lesions or petechial hemorrhages from burst capillaries (as seen on a conventional anatomical MRI) along with specific clinical indications, such as loss of consciousness (Smith, Meaney, & Shull, 2003). The lesions are commonly seen in the cerebral (especially frontal) white matter, corpus callosum, upper brainstem, and internal capsule (Levin et al., 1997). Because conventional MRI is primarily sensitive to blood from nearby torn capillaries, but not to axonal damage itself, conventional MRI likely underestimates the presence of diffuse axonal injury, especially in milder cases. In contrast, DTI provides a more direct measure of the integrity of the white matter fibers themselves. As a result, DTI may be sensitive to milder forms of damage.
The earliest published use of DTI in a pediatric TBI sample was a series of case reports (Lee et al., 2003). Lee et al.’s investigation demonstrated that the use of DTI led to more accurate delineation of the extent of white matter injury than did clinical MRI. A second set of case studies that compared two severely injured children to their non-injured twins showed reduced corpus callosum FA at three years post-injury (Ewing-Cobbs, Hasan, Prasad, Kramer, & Bachevalier, 2006). The single controlled study of pediatric TBI using DTI published to date (Wilde et al., 2006) compared 16 children with severe TBI (1 year or more post-injury) to control subjects and found lower FA in the genu, body, and splenium of the corpus callosum in the TBI group. Across all subjects, FA was positively correlated with cognitive processing speed and performance on a behavioral inhibition task. For TBI subjects, FA was positively correlated with functional outcome based on the Glasgow Outcome Scale. The current investigation represents a controlled study of pediatric TBI using DTI to evaluate WM status in both cortical white matter and the corpus callosum. In contrast to the Wilde et al. study, which included children with moderate and severe TBI, the current study focuses on children with mild and moderate TBI and includes additional white matter regions of interest.
2. Methods
2.1. Participants
Male and female subjects between ages 10 and 18 were recruited from two local hospitals with pediatric rehabilitation units. Inclusion criteria for subjects in the TBI group included a documented traumatic brain injury within the previous 6–12 months, accompanied by one or more of the following: loss of consciousness, loss of memory for events around the incident, alteration in mental status, recurrent emesis or persistent headache, or transient focal neurological deficits. Severity was determined by the lowest reported score on the Glasgow Coma Scale (GCS; Teasdale & Jennett, 1974); mild TBI was defined by a GCS of 13–15 and moderate TBI defined by a GSC from 9 to 12. Children with Severe TBI, as indicated by GCS scores below 9 or post-traumatic amnesia greater than 24 h, were excluded. Potential TBI subjects also were excluded for a prior history of TBI, any pre-existing neurological condition, or documented pre-existing mental retardation, pervasive developmental disorder, learning disability, or serious psychopathology such as major depressive disorder or bipolar disorder. Subjects with substance abuse histories (prior to injury or post-injury) were also excluded. All of the subjects had closed head injuries and none required neurosurgical intervention. None of the children with TBI had significant intra-axial bleeds; four subjects had small punctate hemorrhages on initial CT scan and three subjects had evidence of cerebral contusion in frontal and temporal regions. None of the subjects required intervention for increased intracranial pressure. None of the subjects experienced post-injury seizures, nor were any subjects on anti-epileptic medications at the time of evaluation. Subjects with major mood disorders at the time of evaluation, including major depressive disorder, bipolar disorder, or anxiety disorders were excluded. Subjects with pre-existing attention-deficit/hyperactivity disorder (ADHD) were not excluded. One subject with TBI had a pre-existing diagnosis of ADHD. Nine of the TBI subjects had been injured in motor vehicle accidents and five were motor vehicle versus pedestrian or bicyclist injuries. Additional subject characteristics are included in Table 1.
Table 1.
Subject characteristics for children with TBI and controls
| N (%) or mean ± S.D. | TBI (n = 14) | Control (n = 14) |
|---|---|---|
| Age at MRI scan | 15.1 ± 2.3 years | 15.8 ± 2.3 years |
| Gender | ||
| Male | 5 (35.7%) | 6 (42.9%) |
| Female | 9 (64.3%) | 8 (57.1%) |
| Injury type (TBI only) | ||
| Motor vehicle accident | 9 (64.3%) | |
| Bicycle versus motor vehicle | 3 (21.4%) | |
| Pedestrian versus motor vehicle | 2 (14.3%) | |
| Injury category | ||
| Mild | 6 (42.9%) | |
| Moderate | 8 (57.1%) | |
| Mean time from injury to testing and scan | 8.2 ± 2.2 months | |
| Glasgow coma scale score | 12.25 ± 1.9 |
A control group consisted of age-matched subjects who were recruited through demographically targeted advertising, hospital employee newsletters, and word-of-mouth. Control participants were excluded for a history of traumatic brain injury or concussion. Control participants also were excluded for the same neurological, neurodevelopmental, and psychiatric conditions as the patients. One control subject with a diagnosis of ADHD was included in the study as a match for the TBI subject with pre-existing ADHD.
2.2. Procedures
All procedures were reviewed and approved by a university Institutional Review Board. The informed consent process included a discussion of the study with the patient and caregiver, a consent form, and an assent form that was signed by the child or adolescent participant.
2.2.1. Neurocognitive assessment battery
All subjects completed a neurocognitive test battery designed to assess general intellectual functioning, working memory and long-term memory, executive function, and processing speed. The battery included the following measures: Wechsler Intelligence Scale for Children – Fourth Edition (Wechsler, 2003) or the Wechsler Adult Intelligence Scale – Third Edition (Wechsler, 1997); Controlled Oral Word Association Test (Benton, Hamsher, & Sivan, 1983); Wisconsin Card Sorting Test (Heaton, 1981); Trailmaking Test (Reitan & Wolfson, 1985); a computerized version of the Tower of London (Culbertson & Zillmer, 2000); and the Stroop Color and Word Test (Golden, Freshwater, & Golden, 1998). Parents completed the Behavior Rating Inventory of Executive Functioning (BRIEF) (Gioia, Isquith, Guy, & Kenworthy, 2000) and Behavioral Assessment System for Children – Second Edition – Parent Report Version (BASC-II) (Reynolds & Kamphaus, 2004).
2.2.2. MRI data acquisition
The MRI was performed on a 3 T Trio scanner (Siemens, Erlangen, Germany). A three-plane localizer sequence was acquired to position subsequent scans. Scans with T1 and proton density (PD) contrasts were collected for tissue segmentation. T1 images were acquired coronally, using a 3D MPRAGE sequence (TR = 2530 ms, TE = 3.65 ms, TI = 1100 ms, 224 slices, 1 mm × 1 mm × 1 mm voxel, flip angle = 7 degrees, FOV = 256 mm × 176 mm). PD weighted images were acquired axially using a hyper-echo turbo spin echo (TSE) sequence (TR = 8550 ms, TE = 14 ms, 80 slices, 1 mm × 1 mm × 2 mm voxel, flip angle = 120 degrees, FOV = 256 mm). DTI data were acquired axially, aligned with the TSE images, using a dual spin echo, single shot, pulsed gradient, echo planar imaging sequence (TR = 8300 ms, TE = 86 ms, 64 contiguous slices, 2 mm × 2 mm × 2 mm voxel, FOV = 256 mm, three averages, b value = 1000 s/mm2). Thirteen unique volumes were collected to compute the tensor: a, b = 0 s/mm2 image and 12 images with diffusion gradients applied in 12 non-collinear directions: (Gx, Gy, Gz) = [1.0, 0.0, 0.5], [0.0, 0.5, 1.0], [0.5, 1.0, 0.0], [1.0, 0.5, 0.0], [0.0, 1.0, 0.5], [0.5, 0.0, 1.0], [1.0, 0.0, −0.5], [0.0, −0.5, 1.0], [−0.5, 1.0, 0.0], [1.0, −0.5, 0.0], [0.0, 1.0, −0.5], [−0.5, 0.0, 1.0]. A dual echo flash field map sequence with voxel parameters common to the DTI was acquired and used to correct the DTI data for geometric distortion caused by magnetic field inhomogeneity (TR = 700 ms, TE = 4.62/7.08 ms, flip angle = 90 degrees, magnitude and phase difference contrasts).
2.2.3. Anatomical image processing
Image data was processed using software (BET, FLIRT, FAST, FUGUE and FDT) from the FMRIB Software Library (http://www.fmrib.ox.ac.uk/). The brain was extracted from the T1 and PD acquisitions using BET. The PD brain was aligned to the T1 brain using FLIRT, allowing for translations and rotations but no scaling or shear (six degrees of freedom (DoF) fit). Dual channel segmentation was performed on T1 and aligned PD brains using FAST, producing four tissue classes (CSF, white, gray, and blood). The T1 brain was registered to the FSL template brain using a FLIRT (12 DoF). A cerebrum mask, which consisted of the whole brain excluding the cerebellum and brain stem, was generated for each T1 image by transforming the template mask created on the FSL MNI 152 brain onto the T1 brain.
2.2.4. DTI processing
FDT was first used to correct the diffusion-weighted images for distortion caused by the effects of eddy currents. FDT was then used to compute the diffusion tensor. Maps of mean diffusivity (MD—mean of the three eigenvalues) and fractional anisotropy (FA) were derived. FA, the anisotropic component of the tensor (Basser, 1995), ranges between 0 (perfectly isotropic) and 1 (diffusion occurring in only one direction). The geometric distortion caused by the magnetic field inhomogeneity was determined from the field map image, and FUGUE was used to dewarp the b = 0 diffusion image and the scalar maps (FA, MD) for this distortion.
Subject specific white matter masks for the dewarped DTI scalar maps were then determined. The partial volume estimate (PVE) map from the dual channel FAST segmentation of the T1 and aligned PD images was transformed onto the distortion corrected DTI image using the inverse of the previously determined transform. Voxels in the dewarped DTI images were classified as white matter for this analysis if the white matter PVE value was at least 50%.
2.2.5. Regions of interest definition
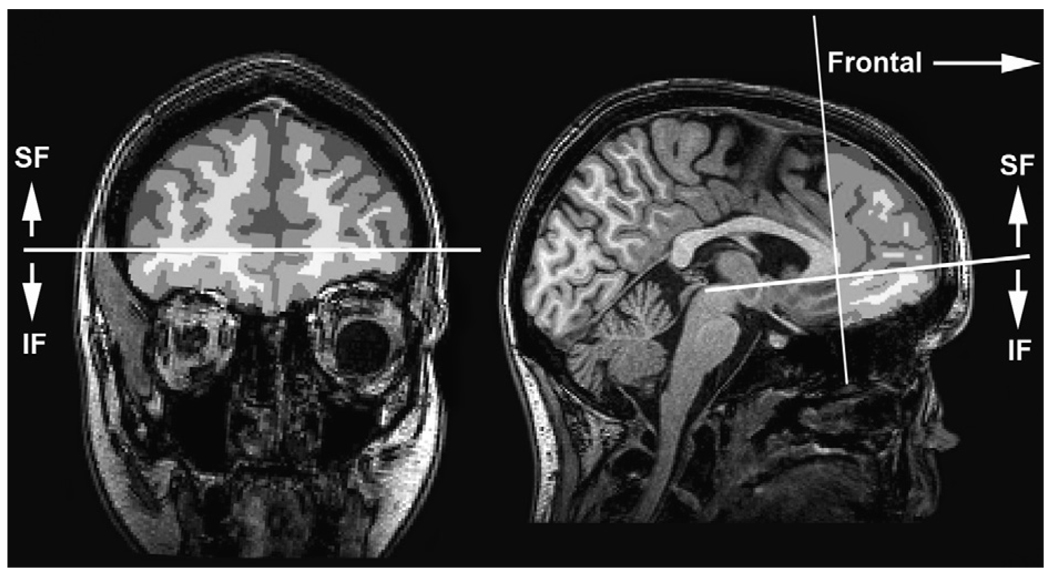
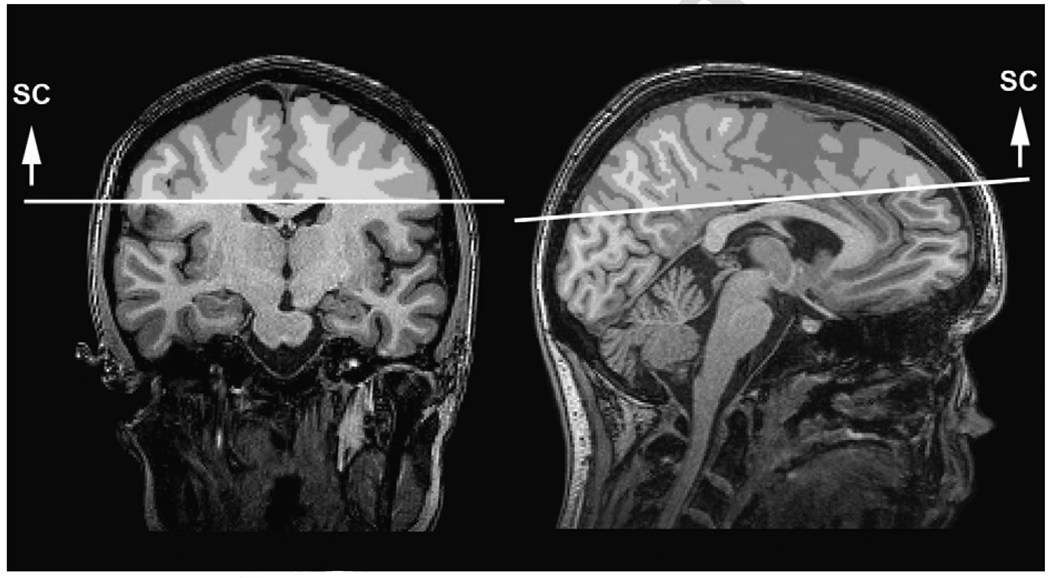
Semi-automated procedures were used to define regions of interest (ROIs) for analysis. A trained operator identified three slices on the MNI aligned T1 image from each subject: the axial slice containing the anterior and posterior commissure (AC–PC), the coronal slice just anterior to the mid-sagittal point of the genu of the corpus callosum, and the axial slice just superior to the corpus callosum at the longitudinal fissure. Based on these delineations, masks were defined for three ROIs: the inferior frontal (IF) mask was anterior to the genu and inferior to and including the AC–PC plane (Fig. 1); the superior frontal (SF) mask was anterior to the genu and superior to the AC–PC plane (Fig. 1); the mask above the corpus callosum (supracallosal, or SC) was superior to the corpus callosum (Fig. 2). The ROI masks were transformed from the MNI image to the dewarped DTI images, and then convolved with the white matter mask to yield the ROI specific the white matter mask. Mean FA and MD values were computed for the white matter within each ROI.
Fig. 1.
Frontal regions of interest defined by a plane at the genu of the corpus callosum. Inferior frontal region (IF) is inferior to the plane formed by the anterior and posterior commisures (AC–PC); superior frontal region (SF) is superior to the AC–PC plane.
Fig. 2.
Supracallosal (SC) region of interest above the corpus callosum, defined by a plane at the superior portion of the corpus callosum.
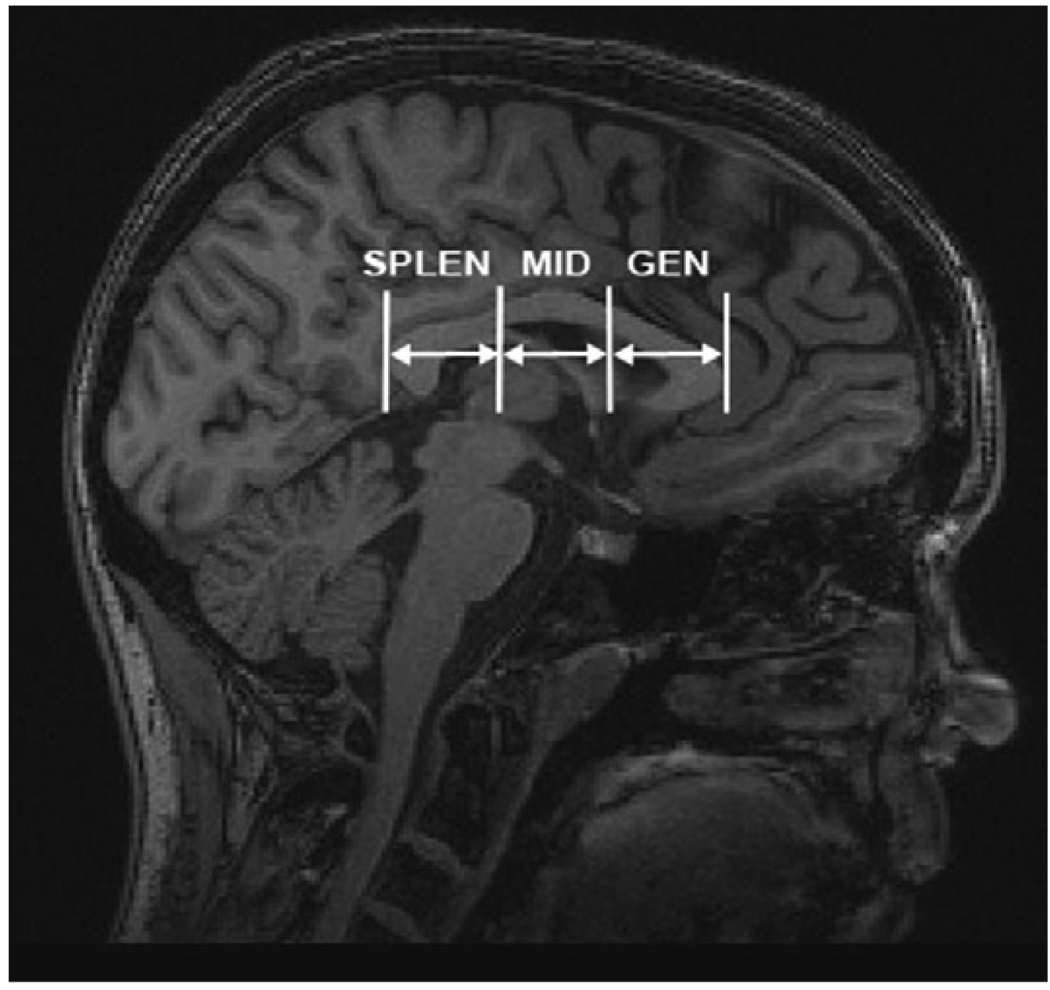
Three corpus callosum ROIs also were defined in a similar semi-automated fashion: genu region (GEN), midbody region (MID), and splenium region (SPLEN). A rectangular region was defined on three contiguous mid-sagittal slices of the MNI aligned T1 image, with edges defined by the anterior, posterior, inferior and superior extent of the corpus callosum. The rectangular volume was edited with a template fornix exclusion mask and then was divided into three equal regions based on the distance from the genu to the splenium. Each of the three regions was transformed onto the DTI images from the MNI template, and then was convolved with the DTI white matter mask, producing white matter masks for the GEN, MID and SPLEN regions of the corpus callosum (Fig. 3). Mean FA values were computed for the white matter within each ROI.
Fig. 3.
Corpus callosum regions of interest (ROIs): splenium (SPLEN), midbody (MID), and genu (GEN).
3. Results
3.1. Group differences in cognitive functioning
The groups were not expected to differ in general cognitive functioning. A MANOVA tested for group differences (TBI versus controls) in full-scale IQ, verbal comprehension index score, and perceptual reasoning score. The MANOVA was non-significant, Wilks’ lambda = 0.923, F(3, 24) = 0.664, p = 0.582.
Four separate statistical tests were used to examine group differences in the following cognitive domains: processing speed, working memory, motor speed, and executive functioning. Cognitive measures were grouped into these domains based on common neuropsychological categorization (Lezak, Howieson, & Loring, 2004) for purposes of clarity and in order to reduce the overall number of statistical tests. For processing speed, an ANOVA examining group differences (TBI versus controls) in Wechsler processing speed index scores revealed significantly lower scores for the TBI group, F(1, 26) = 4.521, p = 0.043. For working memory, an ANOVA examining group differences in Wechsler working memory index (WMI) standard scores was not significant, F(1, 26) = 0.134, p = 0.717. For motor speed, a MANOVA examining dominant-hand and non-dominant-hand performance time z-scores on the Grooved Pegboard task, as well as performance on the Trails-A measure (z-score), was significant, Wilks’ Lambda = 0.713, F(3, 23) = 3.079, p = 0.048. The univariate tests were examined, revealing significantly slower non-dominant-hand motor speed in the TBI group (p = 0.030) but no significant difference for the dominant hand. On the Trails-A task, the control group performed significantly better (p = 0.030) than the TBI group. Lastly, for executive functioning, a MANOVA examining group differences in Stroop interference score (t-score), Wisconsin card sorting test total errors (standard score), COWAT total score (z-score), Trails-B (z-score), and Tower of London total excess moves (z-score) was significant, Wilks’ Lambda = 0.501, F(5, 17) = 3.386, p = 0.026. Univariate tests were then examined on individual measures, and only the Tower of London excess moves score was significantly different between the two groups [F(1, 24) = 10.399, p = 0.004], with the control group making fewer errors than the TBI group. There was a trend-level difference between the groups on the Stroop interference score [F(1, 26) = 4.083, p = 0.055], with the control group performing better than the TBI group, as shown in Table 2.
Table 2.
Neurocognitive data for children with TBI and controls
| Neurocognitive domain | TBI (n = 14) mean (S.D.) | Control (n = 14) mean (S.D.) | p-Value |
|---|---|---|---|
| Intelligence: Wechsler Intelligence Scale for Children or Wechsler Adult Intelligence Scale | |||
| Full scale IQ (standard score) | 109.93 (15.74) | 113.29 (9.14) | 0.496 |
| Verbal comprehension index (standard score) | 108.79 (20.02) | 111.43 (15.36) | 0.698 |
| Perceptual reasoning index (standard score) | 113.00 (18.09) | 112.50 (10.63) | 0.930 |
| Processing speed | |||
| Processing speed index (standard score) | 100.36 (12.47) | 109.00 (8.71) | 0.043 |
| Executive functioning | |||
| Wisconsin card sort test (% errors) | 97.77 (18.40) | 104.15 (16.54) | 0.361 |
| COWAT total (z-score) | −0.701 (0.750) | −0.575 (0.755) | 0.662 |
| Stroop interference score (t-score) | 51.50 (5.79) | 55.79 (5.49) | 0.055 |
| Trails-B (time) | 61.69 (24.06) | 50.94 (16.10) | 0.181 |
| Tower of London—excess moves (z-score) | −0.120 (0.922) | 0.740 (0.360) | 0.004 |
| Working memory | |||
| Working memory index (standard score) | 104.93 (15.33) | 106.93 (13.47) | 0.717 |
| Motor speed | |||
| Trails-A (time) | 25.53 (8.14) | 19.96 (3.89) | 0.030 |
| Grooved Pegboard—dominant hand (z-score) | −0.203 (0.841) | 0.166 (0.630) | 0.218 |
| Grooved Pegboard—non-dominant hand (z-score) | −0.578 (0.967) | 0.238 (0.835) | 0.030 |
3.2. Group behavioral differences
MANOVA was used to test for a group difference (TBI versus controls) on the eight executive functioning subscales from the BRIEF. The MANOVA was significant, Wilks’ Lambda = 0.207, F(8, 17) = 8.152, p = <0.001. Univariate tests revealed that the organization of materials subtest was not significant, but all other subtests showed significant elevations for the TBI group. An additional MANOVA was used to test for group differences in three specific behavioral domains of interest from the BASC-II. The MANOVA was significant, Wilks’ Lambda = 0.523, F(3, 22) = 6.682, p = 0.002. Univariate tests revealed significant group differences for hyperactivity, aggression, and attention problems, as shown in Table 3.
Table 3.
Behavior ratings for children with TBI and control subjects
| Behavior measure | TBI (n = 14) mean (S.D.) | Control (n = 14) mean (S.D.) | p-Value |
|---|---|---|---|
| Behavior Rating Inventory of Executive Function (t-scores) | |||
| Emotional control | 61.85 (10.07) | 46.92 (8.03) | <0.001 |
| Inhibit | 59.69 (8.57) | 50.85 (9.93) | 0.023 |
| Shift | 58.69 (7.65) | 49.77 (9.04) | 0.012 |
| Initiate | 60.77 (9.58) | 49.23 (9.51) | 0.005 |
| Monitor | 63.46 (10.57) | 47.31 (7.77) | <0.001 |
| Plan/organize | 65.92 (11.51) | 48.23 (10.18) | <0.001 |
| Organization of materials | 56.38 (13.00) | 52.31 (10.58) | 0.389 |
| Working memory | 67.23 (8.96) | 46.62 (7.90) | <0.001 |
| BASC-II (t-scores) | |||
| Hyperactivity | 61.77 (14.53) | 47.62 (8.20) | 0.005 |
| Aggression | 58.23 (12.32) | 47.92 (5.94) | 0.012 |
| Attention problems | 60.31 (8.57) | 44.62 (8.58) | <0.001 |
3.3. Group DTI differences
A MANOVA testing for group differences in fractional anisotropy (FA) in the three cortical white matter regions was significant, Wilks’ Lambda = 0.639, F(3, 24) = 4.527, p = 0.012. Univariate tests showed significantly decreased FA in all three regions in children with TBI compared to controls: IF, p = 0.025; SF, p = 0.039, and SC, p = 0.003. An additional MANOVA tested for group differences in FA in the three corpus callosum regions: GEN, MID, and SPLEN. This analysis was non-significant, Wilks’ Lambda = 0.910, F(3, 24) = 0.795, p = 0.509.
3.4. Exploratory analyses
Although group sizes were small, a set of exploratory analyses was conducted in order to examine potential differences between children with mild (n = 6) and moderate (n = 8) injuries. A one-way ANOVA showed significant differences between the three groups (control, mild TBI, moderate TBI) in the IF region, F(2, 25) = 5.626, p = 0.010, with post-hoc tests (Tukey correction for family-wise error) showing significantly lower FA for the moderate TBI group compared to the control group (p = 0.008), but no difference between the mild TBI group and the controls. Similar results were seen in the SF region, with a significant effect for group, F(2, 25) = 4.265, p = 0.025, and post-hoc tests revealing a significant difference between the moderate TBI group compared to the controls (p = 0.020), but no difference between the mild TBI group and the controls. In contrast, both mild and moderate TBI groups had lower FA than controls in the SCC region. The overall ANOVA was significant, F(2, 25) = 5.302, p = 0.012, with post-hoc tests showing significant differences between the mild TBI group and controls (p = 0.049) and between the moderate group and controls (p = 0.026).
Additional exploratory analyses were performed to examine the possibility that the two subjects with ADHD (one TBI and one control) could have disproportionately affected their respective groups. FA values in the IF, SF, and SC regions of the two ADHD subjects were compared with their appropriate group means (TBI or control); neither subject was an outlier for his/her group. For the child with ADHD in the TBI group, FA values in the three ROIs ranged from −0.096 to 0.83 standard deviations from the group mean. For the control subject with ADHD, FA values in the three ROIs ranged from 0.10 to 1.3 standard deviations from the mean. Because neither subject was considered an outlier, they were retained in order to maximize the power of the statistical analyses.
3.5. DTI associations with cognition and behavior
Relationships between cortical white matter integrity and neurocognitive performance were examined with a set of correlations. In order to reduce the number of correlations, the two frontal ROIs were combined into a single ROI by averaging. Pearson correlations were computed between only those cognitive measures that showed group differences (TBI versus controls) and FA in both the SC and the combined-frontal regions. Frontal FA was significantly correlated with the Tower of London Test excess moves score, r = 0.40, p = 0.038 and Trails-A completion time, r =−0.578, p = 0.002, but not with the Wechsler processing speed index, r = 0.238, p = 0.223, nor with Grooved Pegboard non-dominant hand performance, r = 0.107, p = 0.587. FA in the SC region was significantly correlated with Tower of London excess moves score, r = 0.524, p = 0.006, Trails-A completion time, r = −0.601, p − 0.001, and processing speed, r = 0.407, p = 0.031, but not Grooved Pegboard non-dominant hand performance, r = 0.108, p = 0.585. After applying a conservative Bonferroni correction, the following correlations remained significant: frontal FA and Tower of London, SC FA and Tower of London, and SC FA and Trails-A.
A set of correlations was used to examine associations between cortical FA and behavioral ratings from the BASC-II subtests of interest. FA in the SC region was significantly inversely correlated with the BASC-II aggression scale, r = −0.427, p = 0.029, and the attention problems scale, r = −0.417, p = 0.034. There was a trend-level association with the hyperactivity scale, r = −0.373, p = 0.060. Frontal FA was not significantly correlated with any of the three BASC-II scales. None of these correlations survived Bonferroni error correction, however.
An additional set of correlations examined associations between cortical FA and the four BRIEF executive functioning scales that showed the largest group effects. BRIEF emotional control problems were significantly inversely correlated with both frontal FA, r = −0.454, p = 0.020, and FA in the SC region, r = −0.534, p = 0.005. This significant correlation survived the Bonferroni error correction. None of the correlations with the other scales (monitor, plan/organize, or working memory) reached a significant level.
4. Discussion
The current study investigated the relationship between white matter microstructure, as measured by DTI, and cognitive and behavioral outcome measures in a pediatric sample with mild and moderate TBI. The results revealed decreased cortical WM FA, which is consistent with a previous study demonstrating decreased WM FA in a severely injured pediatric TBI group (Wilde et al., 2006). The findings are also in line with a number of adult DTI studies showing long-term white matter changes following TBI (Arfanakis et al., 2002; Huisman et al., 2004; Nakayama et al., 2006; Salmond et al., 2006). As expected, based on previous research, the current investigation provided evidence of associations between FA and performance on selected neurocognitive measures, as well as on behavior ratings. Specifically, low cortical WM FA was associated with slow motor speed, poor executive functioning on neurocognitive testing, and poor executive functioning as defined by parent-reports of behavior. Although the statistical power of the correlations was limited, the data suggested that lower FA may also be related to decreased processing speed and increased behavioral problems including aggression, attention, and emotional dysregulation. Larger sample sizes in future studies may allow for more definitive conclusions about these potential associations.
It is noteworthy that greater disruption in frontal WM was associated with executive functioning deficits (planning, as measured by the Tower of London) and reports of “real-world” executive dysfunction on the BRIEF. Our finding that frontal white matter microstructural integrity is related to executive functioning in the TBI group is consistent with previous DTI research showing associations between frontal white matter integrity and executive functions in healthy, non-injured children and adolescents (Liston et al., 2006). It is generally recognized that the study of executive function deficits in pediatric TBI samples is complicated by significant changes in the underlying systems that occur in normal development (Levin & Hanten, 2005). In young children, frontal lobe injury may not manifest in executive deficits until later ages, when those systems are expected to fully develop and come “on line.” Because DTI and other imaging paradigms may be sensitive to subtle damage to these underlying systems at the time of injury and during the intervening span, they have the potential to shed new light on the effect of these disruptions on critical executive skills. The data also revealed associations between SC WM integrity and aspects of executive function and behavior, suggesting that the diffuse damage common in this region is also important in the clinical sequelae of TBI in children. It was particularly noteworthy that the integrity of WM in the SC region seemed to be related to overt behavioral symptoms including aggression, attention problems, and hyperactivity. Future investigations of more specific sub-regions, and possibly specific WM tracts, will shed more light on these relationships.
In contrast to the current findings, previous imaging studies have demonstrated that the corpus callosum is a frequent site of injury in TBI, perhaps because of its vulnerability to shearing and tearing forces (Graham et al., 1989).Volumetric studies of pediatric subjects have shown long-term changes, including atrophy, in the corpus callosum following TBI (Levin et al., 1990, 2000), particularly in the posterior regions of the callosum (Levin et al., 1997). Callosal damage generally has been shown to be related to neurocognitive outcome in children with TBI (Verger et al., 2001). However, the current study did not reveal evidence of corpus callosum abnormality in the TBI group. It is worth noting that most of the studies that identified corpus callosum abnormalities included children with severe TBI, whereas the current study examined only mild and moderate TBI cases. It is also possible that small differences in corpus callosum may have been “washed out” by the relatively coarse analysis technique used in the current study. Future studies with more subjects will be able to include more ROIs in the corpus callosum or to utilize voxel-based analyses which allow for a much more detailed analysis.
Although the neuropathological mechanisms underlying these observed changes in WM FA are not completely understood, changes of this type generally are thought to be related to the following processes: disruption to the organizational structure of the tissue, axonal degeneration, and demyelination (Arfanakis et al., 2002; Wozniak & Lim, 2006). In the non-injured developing brain, diffusion anisotropy in white matter, as measured by DTI, is a function of a number of factors including axon structure, axon packing, tissue water content, and myelin, among others (Neil et al., 2002). It is becoming increasingly clear that axonal damage from TBI cannot be conceptualized simply as a static event (Hurley, McGowan, Arfanakis, & Taber, 2004). The initial tearing, shearing, and misalignment of the axons initiates a series of events that leads to further WM damage, including Wallerian degeneration (dying back of the neurons following axonal damage) and loss of myelin. There is evidence that this subsequent damage continues over the next several days as disruption of axonal transport contributes to further axonal loss via axonal swelling and disconnection (Povlishock, 2000). Further structural changes continue for the next several months as illustrated by studies showing progressive loss of tissue volume over time (Bigler, 1999; Blatter et al., 1997). Additionally, myelin degeneration is thought to continue for one to two years post-injury (Meythaler, Peduzzi, Eleftheriou, & Novack, 2001).
The changes in FA observed in the current study at 6–12 months post-injury likely reflect axonal death, increased inter-axonal water where axons and myelin have been lost, and disruptions in the longitudinal structure of the axons themselves. Together, over the long term, these microstructural changes have the net effect of increasing the diffusivity of water molecules in the directions perpendicular to the principle orientation of the axons, thus lowering FA.
Clinically, WM injury in TBI is most commonly identified as diffuse axonal injury (DAI) and is typically identified on an early CT scan by the presence of small bleeds, or later on MRI (often on fluid attenuated inversion recovery—FLAIR images) as areas of hyperintense signal (Ashikaga, Araki, & Ishida, 1997; Takaoka et al., 2002). DAI is thought to be very common in moderate and severe TBI, and it is almost always present in high-speed motor vehicle accidents involving loss of consciousness (Meythaler et al., 2001). DAI is nearly universal in fatal cases of TBI (Gentleman et al., 1995). The axonal damage that may occur in moderate and, perhaps, even mild cases of TBI is much more likely to be identified using techniques such as diffusion-weighted imaging and DTI, which are sensitive to microstructural change (Ezaki, Tsutsumi, Morikawa, & Nagata, 2006; Hurley et al., 2004). At least one DTI study of mild TBI in adults has shown abnormalities in expected WM regions including the corpus callosum and internal capsule (Inglese et al., 2005). Although the exact pathology underlying the nature of the observed FA differences in this investigation of pediatric TBI requires further clarification, the fact that none of the children with TBI in the current study had evidence of DAI on clinical MRI (yet the group showed significant WM abnormalities on DTI measures) suggests that DTI and other newer imaging techniques ultimately will increase our ability to identify subtle WM damage and to better understand its cognitive correlates in children with TBI.
Several limitations to our current study must be identified and addressed in future research. First, the pre-injury white matter status, including FA, in the children in the TBI group compared to the control participants is unknown. The literature suggests that pre-morbid group differences may confound studies of the effects of TBI on brain structure and cognition (Donders & Strom, 2000). Previous investigations have shown increased incidence of pre-existing ADHD and learning problems in children who sustain TBIs (Fay et al., 1993). However, at least one recent study has addressed this question directly using twins. Ewing-Cobbs et al. (2006) studied pairs of identical twins who were discordant for TBI. They found that the children who sustained severe TBIs had reduced FA in all regions of the corpus callosum compared to their identical twins. This finding indicates that DTI measures are specifically sensitive to TBI-related changes in WM integrity and increases our confidence that the differences seen in group studies of children with TBI are not due solely to pre-morbid differences in the populations.
A second related issue that requires consideration in the current study as well as in future studies is the impact of pre-morbid ADHD in children with TBI. This issue is especially important given the large body of literature that reports executive dysfunction in this population, as well as a recent DTI study that found white matter differences in the frontal and cerebellar regions in children diagnosed with ADHD compared to controls (Ashtari et al., 2005). We did not exclude TBI subjects with pre-morbid ADHD in order to maximize the clinical relevance of the study. We then attempted to control for the inclusion of the one TBI subject with ADHD by including a matched control with ADHD. An alternative strategy for a future study might be to include an ADHD control group rather than matching individual subjects.
At this point, it is relatively clear that DTI is sensitive to both the acute WM damage in TBI as well as to changes in WM integrity over the long term (Arfanakis et al., 2002). Furthermore, there is evidence that DTI may provide an important metric of some aspects of the WM changes that take place following TBI (Arfanakis et al., 2002; Naganawa et al., 2004). Further research is needed to study the interaction between the normal developmental processes that affect WM development in children and the age at which the TBI occurs. Longitudinal studies, with DTI measures collected early in the course of injury and at follow-up, are needed. Careful integration with neuropsychological measures of working memory, processing speed, and other cognitive functions will result in a better overall understanding of prognosis and long-term outcome in pediatric TBI.
Acknowledgments
The authors would like to thank Rosemary Froehle for assistance with subject recruitment and data collection at Hennepin County Medical Center as well as Andrea Nugent for assistance in recruitment and data collection at Gillette Children’s Specialty Healthcare. Funding and research support was provided by the National Academy of Neuropsychology and the National Institutes of Health—P41RR008079 and P30NS057091.
References
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Attentional and processing skills following traumatic brain injury in early childhood. Brain Injury. 2005;19(9):699–710. doi: 10.1080/02699050400025281. [DOI] [PubMed] [Google Scholar]
- Anderson V, Fenwick T, Manly T, Robertson I. Attentional skills following traumatic brain injury in childhood: A componential analysis. Brain Injury. 1998;12(11):937–949. doi: 10.1080/026990598121990. [DOI] [PubMed] [Google Scholar]
- Anderson V, Morse SA, Catroppa C, Haritou F, Rosenfeld JV. Thirty month outcome from early childhood head injury: A prospective analysis of neurobehavioral recovery. Brain. 2004;127(Pt 12):2608–2620. doi: 10.1093/brain/awh320. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. American Journal of Neuroradiology. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- Asarnow RF, Satz P, Light R, Lewis R, Neumann E. Behavior problems and adaptive functioning in children with mild and severe closed head injury. Journal of Pediatric Psychology. 1991;16(5):543–555. doi: 10.1093/jpepsy/16.5.543. [DOI] [PubMed] [Google Scholar]
- Ashikaga R, Araki Y, Ishida O. MRI of head injury using FLAIR. Neuroradiology. 1997;39(4):239–242. doi: 10.1007/s002340050401. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, et al. Attention-deficit/hyperactivity disorder: A preliminary diffusion tensor imaging study. Biological Psychiatry. 2005;57(5):448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: Theory, experimental design and data analysis—a technical review. NMR in Biomedicine. 2002;15(7–8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Bawden HN, Knights RM, Winogron HW. Speeded performance following head injury in children. Journal of Clinical and Experimental Neuropsychology. 1985;7(1):39–54. doi: 10.1080/01688638508401241. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, et al. Normal white matter development from infancy to adulthood: Comparing diffusion tensor and high b value diffusion weighted MR images. Journal of Magnetic Resonance Imaging. 2005;21(5):503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. 3rd ed. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Bigler ED. Neuroimaging in pediatric traumatic head injury: Diagnostic considerations and relationships to neurobehavioral outcome. Journal of Head Trauma Rehabilitation. 1999;14(4):406–423. doi: 10.1097/00001199-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, et al. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: Correlation with neuropsychological outcome. American Journal of Neuroradiology. 1997;18:1–10. [PMC free article] [PubMed] [Google Scholar]
- Catroppa C, Anderson V. Recovery in memory function in the first year following TBI in children. Brain Injury. 2002;16(5):369–384. doi: 10.1080/02699050110104444. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Traumatic brain injury. 2006;Vol. 2006 [Google Scholar]
- Culbertson WC, Zillmer EA. Tower of London, Drexel University. Toronto: Multi-Health Systems; 2000. [Google Scholar]
- Dennis M, Guger S, Roncadin C, Barnes M, Schachar R. Attentional-inhibitory control and social-behavioral regulation after child-hood closed head injury: Do biological, developmental, and recovery variables predict outcome? Journal of the International Neuropsychological Society. 2001;7(6):683–692. doi: 10.1017/s1355617701766040. [DOI] [PubMed] [Google Scholar]
- Donders J, Strom D. Neurobehavioral recovery after pediatric head trauma: Injury, pre-injury, and post-injury issues. Journal of Head Trauma Rehabilitation. 2000;15(2):792–803. doi: 10.1097/00001199-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Donders J, Warschausky S. WISC-III factor index score patterns after traumatic head injury in children. Child Neuropsychology. 1997;3(1):71–78. [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex. 2007 doi: 10.1093/cercor/bhm003. doi:10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J. Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. American Journal of Neuroradiology. 2006;27(4):879–881. [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Levin HS, Fletcher JM. Neuropsychological sequelae after pediatric traumatic brain injury. In: Ylvisaker M, editor. Traumatic brain injury rehabilitation: Children and adolescents. Boston: Butterworth-Heinimann; 1998. pp. 11–26. [Google Scholar]
- Ewing-Cobbs L, Miner ME, Fletcher JM, Levin HS. Intellectual, motor, and language sequelae following closed head injury in infants and preschoolers. Journal of Pediatric Psychology. 1989;14(4):531–547. doi: 10.1093/jpepsy/14.4.531. [DOI] [PubMed] [Google Scholar]
- Ezaki Y, Tsutsumi K, Morikawa M, Nagata I. Role of diffusion-weighted magnetic resonance imaging in diffuse axonal injury. Acta Radiologica. 2006;47(7):733–740. doi: 10.1080/02841850600771486. [DOI] [PubMed] [Google Scholar]
- Farmer MY, Singer HS, Mellits ED, Hall D, Charney E. Neurobehavioral sequelae of minor head injuries in children. Pediatric Neuroscience. 1987;13(6):304–308. doi: 10.1159/000120348. [DOI] [PubMed] [Google Scholar]
- Fay GC, Jaffe KM, Polissar NL, Liao S, Martin KM, Shurtleff HA, et al. Mild pediatric traumatic brain injury: A cohort study. Archives of Physical Medicine and Rehabilitation. 1993;74(9):895–901. [PubMed] [Google Scholar]
- Fenwick T, Anderson V. Impairments of attention following childhood traumatic brain injury. Child Neuropsychology. 1999;5(4):213–223. doi: 10.1076/0929-7049(199912)05:04;1-R;FT213. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg HM. Behavioral changes after closed head injury in children. Journal of Consulting and Clinical Psychology. 1990;58(1):93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, Roberts GW, Gennarelli TA, Maxwell WL, Adams JH, Kerr S, et al. Axonal injury: A universal consequence of fatal closed head injury? Acta Neuropathologica. 1995;89(6):537–543. doi: 10.1007/BF00571509. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function (BRIEF) Lutz, FL: Psychological Assessment Resources, Inc; 2000. [Google Scholar]
- Golden C, Freshwater S, Golden Z. The stroop color and word test: Children’s version. Wood Dale, IL: Stoelting; 1998. [Google Scholar]
- Graham DI, Ford I, Adams JH, Doyle D, Lawrence AE, McLellan DR, et al. Fatal head injury in children. Journal of Clinical Pathology. 1989;42(1):18–22. doi: 10.1136/jcp.42.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. A manual for the wisconsin card sorting test. Odessa FL: Psychological Assessment Resources, Inc; 1981. [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, et al. Pediatric diffusion tensor imaging: Normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29(2):493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hoffman N, Donders J, Thompson EH. Novel learning abilities after traumatic head injury in children. Archives of Clinical Neuropsychology. 2000;15(1):47–58. [PubMed] [Google Scholar]
- Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. American Journal of Neuroradiology. 2004;25(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- Hurley RA, McGowan JC, Arfanakis K, Taber KH. Traumatic axonal injury: Novel insights into evolution and identification. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(1):1–7. doi: 10.1176/jnp.16.1.1. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. Journal of Neurosurgery. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Knights RM, Ivan LP, Ventureyra EC, Bentivoglio C, Stoddart C, Winogron W, et al. The effects of head injury in children on neuropsychological and behaviural functioning. Brain Injury. 1991;5(4):339–351. doi: 10.3109/02699059109008107. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the united states: Differences by race. Journal of Head Trauma Rehabilitation. 2005;20(3):229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. Journal of Head Trauma Rehabilitation. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lee ZI, Byun WM, Jang SH, Ahn SH, Moon HK, Chang Y. Diffusion tensor magnetic resonance imaging of microstructural abnormalities in children with brain injury. American Journal of Physical Medicine and Rehabilitation. 2003;82(7):556–559. doi: 10.1097/01.PHM.0000073830.15643.6A. [DOI] [PubMed] [Google Scholar]
- Levin HS. Neuroplasticity following non-penetrating traumatic brain injury. Brain Injury. 2003;17(8):665–674. doi: 10.1080/0269905031000107151. [DOI] [PubMed] [Google Scholar]
- Levin HS, Benavidez DA, Verger-Maestre K, Perachio N, Song J, Mendelsohn DB, et al. Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology. 2000;54(3):647–653. doi: 10.1212/wnl.54.3.647. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G. Executive functions after traumatic brain injury in children. Pediatric Neurology. 2005;33(2):79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Chang CC, Zhang L, Schachar R, Ewing-Cobbs L, et al. Working memory after traumatic brain injury in children. Annals of Neurology. 2002;52(1):82–88. doi: 10.1002/ana.10252. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Zhang L, Swank PR, Ewing-Cobbs L, Dennis M, et al. Changes in working memory after traumatic brain injury in children. Neuropsychology. 2004;18(2):240–247. doi: 10.1037/0894-4105.18.2.240. [DOI] [PubMed] [Google Scholar]
- Levin HS, Mendelsohn D, Lilly MA, Yeakley J, Song J, Scheibel RS, et al. Magnetic resonance imaging in relation to functional outcome of pediatric closed head injury: A test of the ommaya-gennarelli model. Neurosurgery. 1997;40(3):432–440. doi: 10.1097/00006123-199703000-00002. discussion 440–431. [DOI] [PubMed] [Google Scholar]
- Levin HS, Williams DH, Valastro M, Eisenberg HM, Crofford MJ, Handel SF. Corpus callosal atrophy following closed head injury: Detection with magnetic resonance imaging. Journal of Neurosurgery. 1990;73(1):77–81. doi: 10.3171/jns.1990.73.1.0077. [DOI] [PubMed] [Google Scholar]
- Levin HS, Zhang L, Dennis M, Ewing-Cobbs L, Schachar R, Max J, et al. Psychosocial outcome of TBI in children with unilateral frontal lesions. Journal of the International Neuropsychological Society. 2004;10(3):305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York: Oxford University Press, Inc; 2004. [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: Diffuse axonal injury-associated traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2001;82(10):1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176(2):439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Naganawa S, Sato C, Ishihra S, Kumada H, Ishigaki T, Miura S, et al. Serial evaluation of diffusion tensor brain fiber tracking in a patient with severe diffuse axonal injury. American Journal of Neuroradiology. 2004;25(9):1553–1556. [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Yasokawa YT, Miwa K, Yoshimura SI, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77(7):850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to congress on mild traumatic brain injury in the united states: Steps to prevent a serious public health problem; Centers for Disease Control and Prevention; Atlanta, GA. 2003. [Google Scholar]
- Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain—a technical review. NMR in Biomedicine. 2002;15(7–8):543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Povlishock JT. Pathophysiology of neural injury: Therapeutic opportunities and challenges. Clinical Neurosurgery. 2000;46:113–126. [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tucson, AZ: Neuropsychological Press; 1985. [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior assessment system for children. 2nd ed. Circle Pines, MN: AGS Publishing; 2004. [Google Scholar]
- Roncadin C, Guger S, Archibald J, Barnes M, Dennis M. Working memory after mild, moderate, or severe childhood closed head injury. Developmental Neuropsychology. 2004;25(1–2):21–36. doi: 10.1080/87565641.2004.9651920. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, et al. Diffusion tensor imaging in chronic head injury survivors: Correlations with learning and memory indices. Neuroimage. 2006;29(1):117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Salorio CF, Slomine BS, Grados MA, Vasa RA, Christensen JR, Gerring JP. Neuroanatomic correlates of CVLT-C performance following pediatric traumatic brain injury. Journal of the International Neuropsychological Society. 2005;11(6):686–696. doi: 10.1017/S1355617705050885. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. Journal of Head Trauma Rehabilitation. 2003;18(4):307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Takaoka M, Tabuse H, Kumura E, Nakajima S, Tsuzuki T, Nakamura K, et al. Semiquantitative analysis of corpus callosum injury using magnetic resonance imaging indicates clinical severity in patients with diffuse axonal injury. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;73(3):289–293. doi: 10.1136/jnnp.73.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG. Research on outcomes of pediatric traumatic brain injury: Current advances and future directions. Developmental Neuropsychology. 2004;25(1–2):199–225. doi: 10.1080/87565641.2004.9651928. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Verger K, Junque C, Levin HS, Jurado MA, Perez-Gomez M, Bartres-Faz D, et al. Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Injury. 2001;15(3):211–221. doi: 10.1080/02699050010004059. [DOI] [PubMed] [Google Scholar]
- Warschausky S, Kay JB, Chi P, Donders J. Hierarchical linear modeling of California verbal learning test-children’s version learning curve characteristics following childhood traumatic head injury. Neuropsychology. 2005;19(2):193–198. doi: 10.1037/0894-4105.19.2.193. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3rd ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4th ed. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Lim KO. Advances in white matter imaging: A review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience and Biobehavioral Reviews. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO. Closed head injury. In: Yeates KO, Ris MD, Taylor HG, editors. Pediatric neuropsychology: Research, theory, and practice. New York: Guilford; 2000. pp. 92–116. [Google Scholar]
- Yeates KO, Armstrong K, Janusz J, Taylor HG, Wade S, Stancin T, et al. Long-term attention problems in children with traumatic brain injury. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(6):574–584. doi: 10.1097/01.chi.0000159947.50523.64. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Luria J, Bartkowski H, Rusin J, Martin L, Bigler ED. Postconcussive symptoms in children with mild closed head injuries. Journal of Head Trauma Rehabilitation. 1999;14(4):337–350. doi: 10.1097/00001199-199908000-00003. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG. Neurobehavioral outcomes of mild head injury in children and adolescents. Pediatric Rehabilitation. 2005;8(1):5–16. doi: 10.1080/13638490400011199. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Drotar D, Wade S, Stancin T. Neurobehavioral symptoms in childhood closed-head injuries: Changes in prevalence and correlates during the first year post-injury. Journal of Pediatric Psychology. 2001;26:79–91. doi: 10.1093/jpepsy/26.2.79. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. Aprospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology. 2002;16(4):514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]