Abstract
Background
The CALGB evaluated oral topotecan administered at two schedules and doses for MDS.
Methods
Patients with previously untreated primary or therapy-related MDS were eligible. Patients with refractory anemia (RA), RA with ringed sideroblasts (RARS), or refractory cytopenia with multilineage dysplasia (RCMD) were eligible only if red cell transfusion dependent, platelet count < 50,000/ul, or absolute neutrophil count < 1,000/ul with a recent infection requiring antibiotics.
Treatment
Patients were randomized to receive oral topotecan either at a dose of 1.2 mg/m2 twice daily for 5 days (Arm A) or once daily for 10 days (Arm B), repeated every 21 days for at least 2 cycles. Responding patients continued until progression, or unacceptable toxicity, or two cycles beyond a complete response.
Results
Ninety patients received treatment: 46 on Arm A and 44 on Arm B. Partial responses with improvement in all three cell lines occurred in 6 patients (7%) and hematologic improvement (in 1–2 cell lines) was seen in 21 patients (23%), for an overall response rate of 30%. Response duration was longer on Arm A (23 vs 14 months, p = 0.02). Seven out of fourteen patients with chronic myelomonocytic leukemia responded. There were 8 treatment-related deaths from infection (6) and bleeding (2). Diarrhea was the most frequent non-hematologic toxicity (Grade 3: 11%; Grade 4: 2%).
Conclusions
Oral topotecan in the dose and schedules evaluated in this trial demonstrated only a modest response rate with a troublesome toxicity profile in the treatment of MDS.
INTRODUCTION
The myelodysplastic syndromes (MDS) are clonal disorders characterized by cytopenias and evidence of morphologic dysplasia in one or more hematopoietic cell line. The French-American-British (FAB) classification categorized these syndromes into five subtypes, with differing morphologic features and prognoses (1). The more recent World Health Organization (WHO) classification divides MDS into eight entities based on morphologic, cytogenetic, and clinical parameters (2). An international prognostic scoring system (IPSS) has been developed that divides patients into outcome groups based on percentage of marrow blasts, karyotype, and degree and number of cytopenia (3). Patients often succumb to infection or complications of bleeding, or the disease evolves to acute myeloid leukemia (AML). The major goals of therapy in MDS are either to alter the natural history or to reduce the morbidity of the chronic cytopenias and attendant transfusion requirements. Many agents have been evaluated in MDS, including androgens, corticosteroids, cytokines (such as G-CSF, GM-CSF, and epoetin), vitamin D, and retinoids in an attempt to induce differentiation in the dysplastic cell lines (4,5, 6,7). More recent trials have evaluated the activity of novel agents such as thalidomide, tipifarnib, arsenic trioxide, and amifostine (8–11). None of these agents have demonstrated improved long term outcomes, although the use of cytokines can improve single lineage cytopenias transiently (12). A large randomized trial of low dose cytarabine compared to best supportive care yielded a complete remission (CR) rate of only 8%, with a median duration of 4 months. The median survival durations in the treated and untreated groups were 36 and 28 weeks respectively, which were not statistically significantly different (13).
Recently, both of the DNA methyltransferase inhibitors azacitidine and decitabine have been approved for MDS based upon data from randomized trials comparing one of these agents plus best supportive care to best supportive care alone (14–16). Most recently, lenalinomide was shown to yield cytogenetic CR and transfusion independence in the subset of MDS patients with del(5q) (17) and in a smaller number of patients without this cytogenetic abnormality (18). To date, only azacitidine and decitabine have been approved for all categories of MDS because they prolong the time to AML or death and reduce symptoms from cytopenias (14–16,19).
Investigators at the M.D. Anderson Cancer Center reported on the activity of topotecan, a topoisomerase I inhibitor, in patients with MDS (20). When given intravenously (IV) at 2 mg/m2/day for 5 days, 13 of 47 patients (28%) had a CR and 6 others (13%) had hematologic improvement. Because of its novel mechanism of action and acceptable toxicity profile following IV administration, the Cancer and Leukemia Group B (CALGB) investigated the effectiveness of oral topotecan in patients with MDS. Because of the older age of many patients with MDS, defining a well-tolerated, yet active, oral outpatient therapy would be an important advance. Certainly, the current availability of three new FDA approved therapies for MDS has resulted in better therapies for these patients. Yet, many patients with greater numbers of blasts and higher risk disease remain in need of therapy when they do not respond to these agents.
METHODS
Patient Selection
Patients were considered eligible for the study if they had primary or therapy-related MDS that was previously untreated with any cytotoxic agent or cytokine therapy for MDS, except epoetin. Patients with refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEB/T) and chronic myelomonocytic leukemia (CMMoL) were eligibile independent of the presence or absence of cytopenias. Patients with refractory anemia (RA), refractory anemia with ringed sideroblasts (RARS), or refractory cytopenia with multilineage dysplasia (RCMD) were required to meet one of the following criteria: red blood cell (RBC) transfusion dependence, with greater than 4 units transfused in the 3 months prior to registration, platelet count less than 50,000/ul, or an absolute neutrophil count (ANC) less than 1000/ul with a recent infection requiring antibiotics. The FAB classification was utilized at patient enrollment with the category of RCMD added and defined in the protocol. Patients with prior chemotherapy or radiation therapy for a different disease were required to have completed such therapy at least 12 months prior to registration. Additional eligibility criteria were performance status of 0–3, bilirubin < 1.5 mg/dl, SGOT < 2 times the upper limit of normal, and creatinine < 1.5 mg/dl. Pregnant and nursing women were excluded. Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were peformed by the CALGB statisticians. Each participant signed an IRB-approved protocol-specific consent in accordance with federal and institutional guidelines.
Treatment Program and Evaluations
Registered patients were randomized to receive oral topotecan (Glaxo SmithKline) at a dose of 1.2 mg/m2 either twice daily for 5 days (Arm A) or once daily for 10 days (Arm B). Cycles were repeated every 21 days provided that the patient’s ANC and platelet count had recovered to pre-treatment levels, or to an ANC > 1500/µl and platelet count > 50,000/µl for patients with higher blood counts prior to starting therapy. Subsequent cycles were delayed until recovery of blood counts to these levels had occurred. Patients who never recovered their prior level of blood counts after two cycles were taken off of therapy and considered non-responders. Patients who developed grade 3 or 4 mucositis or diarrhea had therapy held until resolution, and all subsequent therapy was administered at 75% of the previous dose. Patients were first assessed for response after completing the first 2 cycles of therapy, and then after every 4 cycles. Therapy was continued for patients who had evidence of hematologic improvement (HI) or a partial response (PR) until progression or unacceptable toxicity was observed. Patients who achieved a CR received 2 additional cycles and were then followed without further therapy. Objectives of the study included the estimation of the response rates (CR, PR and hematologic improvement) as well as toxicity for the two schedules and doses of oral topotecan.
Central Review and Response Definitions
Bone marrow aspirate slides and biopsies obtained within 2 weeks prior to patient registration were submitted for central review (JWV). This review was not completed prior to patient disease stratification, randomization, or treatment. Pre-treatment bone marrow karyotypes underwent central cytogenetics review as part of CALGB study 8461.
Standard CALGB response criteria for MDS were used as in the previous azacitidine study (14). CR was defined as normalization of all cytopenias, marrow dysplasia, and blast count. PR was defined as > 50% improvement in all cytopenias, while HI was defined as > 50% improvement in the cytopenia existing in at least one lineage. Hematologic improvement was required to have been maintained without transfusion support for at least four weeks.
Statistical Considerations
The study design called for a total of 90 patients to be randomized with equal allocation to each of the two arms of this phase II trial, with an anticipated 10% of the patients being ineligible after Central Pathology review. The primary goal was to test within each of the two arms the null hypothesis that the CR+PR rate is ≤ 10% versus the alternative hypothesis that the CR+PR rate is ≥ 25% with 40 eligible patients. If 6 (15%) or fewer patients respond, the null hypothesis would not be rejected. If 7 (17.5%) or more patients respond, the null would be rejected and a phase III trial with this arm of the trial would be considered. This single-stage design had a power of 90% and a Type I error rate of 10%.
All patients who were treated on this protocol are included in the analysis. The intent-to-treat approach is adopted. Descriptive summary statistics are reported by arm on patient characteristics at baseline (e.g., demographics, disease characteristics and various risk or diagnostic categories, including both those based on central review and those based on institutional data). Outcome variables such as responses, overall survival (OS) and time to AML or death (PFS) are summarized both by arm and overall for the two arms combined, whenever appropriate, respectively. Analyses of the outcome endpoints by FAB and IPSS risk groups based on central review, institutional data, and central review supplemented with institutional data (the best available data) are performed and descriptively compared, even though only detailed results by the best available data are reported in the manuscript.
Response rates of CR + PR as well as CR+PR+HI are estimated along with their 95% confidence intervals (CIs), but only results on CR+PR+HI are reported here. OS and PFS are analyzed with the Kaplan-Meier product limit method and the median and its 95% CI are reported. Comparisons among various groups are performed with the Fisher’s exact test or Chi-square test for a discrete variable and the log-rank test for a time-to-event variable. Note that as a secondary analysis results within disease/risk subtypes are reported separately, although the sample size of this trial is too small for this analysis to be anything more than purely descriptive. Toxicities are tabulated by type and grade. A p-value of <0.05 is considered statistically significant. No multiple testing adjustments are made since most of the testing is of exploratory in nature.
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. The medical records of randomly selected patients at each participating institution were audited by members of the CALGB Data Audit Committee.
RESULTS
Patient Characteristics
One hundred patients with MDS were registered on CALGB 19803 between March 1999 and May 2000 from 21 main member institutions and their affiliates. Ten patients were registered but never received treatment; these patients are not included in any analyses. Eighty patients had adequate blood smears and bone marrow slides submitted that underwent central morphology review; 10 patients did not, and therefore the local institutional subclassification was used for them. After central review, 77 patients were diagnosed with one of 4 FAB-defined subsets (Table 1); 3 patients had AML or were unclassifiable. Results described below utilized the results obtained from central review primarily, supplemented by the institutional assessment where central review was lacking, so that all treated patients were evaluated. Eighty-one of the 90 treated patients had either central cytogenetic review (79) or institutional reports (2) for pre-treatment karyotypes so that an IPSS score could be assigned.
Table 1.
Patient Baseline Characteristics by Randomized Treatment Arm
| No. of Patients (%) | |||
|---|---|---|---|
| Variable | Arm A (n=46) | Arm B (n=44) | |
| Age, median | 69 (range, 49–81) | 71 (range, 32–85) | |
| Gender: | Male | 29(63) | 29(66) |
| Female | 17(37) | 15(34) | |
| Race/Ethnic: | White | 40(87) | 44(100) |
| Black | 4(9) | 0 | |
| Other | 2(4) | 0 | |
| Performance Status: | 0: Active | 17(37) | 14(32) |
| 1: Ambulatory | 25(54) | 24(55) | |
| 2: In Bed < 50% | 4(9) | 4(9) | |
| 3: In Bed > 50% | 0(0) | 2(5) | |
| FAB Subtype: | RAEB | 10(22) | 18(41) |
| (Central Review, n=77) | RAEB-T | 10(22) | 4(9) |
| CMML | 9(20) | 5(11) | |
| RA,RARS,RCMD | 13(28) | 8(18) | |
| Missing | 4(9) | 9(20) | |
| FAB Subtype: | RAEB | 16(35) | 19(43) |
| (Institutional Report, n=90) | RAEB-T | 7(15) | 9(20) |
| CMML | 7(15) | 3(7) | |
| RA,RARS,RCMD | 16(35) | 13(30) | |
| IPSS Risk Group: | Low | 4(9) | 3(7) |
| (Central, n=67) | Int-1 | 19(41) | 7(16) |
| Int-2 | 11(24) | 12(27) | |
| High | 4(9) | 7(16) | |
| Missing | 8(17) | 15(34) | |
| IPSS Risk Group: | Low | 6(13) | 5(11) |
| (Institutional, n=80) | Int-1 | 20(43) | 9(20) |
| Int-2 | 10(22) | 17(39) | |
| High | 4(9) | 9(20) | |
| Missing | 6(13) | 4(9) | |
| Hematologic Parameters (median; range; interquartile range) | |||
| No. of RBC units transfused* | 6 (0–39; 2–12) | 4 (0–20; 0–8) | |
| No. of Platelet transfusion events* | 0 (0–42; 0–0) | 0 (0–17; 0–0) | |
| Hemoglobin (gm/dl) | 9 (7–12; 8–10) | 9 (6–13; 8–11) | |
| Platelets (×103 /µl) | 102 (5–456; 49–165) | 64.5 (5–420; 26–173) | |
| WBC (×103 /µl) | 3.7 (1.1–74.2; 2.3–7.8) | 2.8 (1–19.3; 1.9–6) | |
| ANC (×103 /µl)* | 1.6 (.09–43.9; 0.7–4.3) | 1.4 (.02–9.5; 0.6–3.6) | |
One patient had missing data on number of RBC units transfused and ANC, and 8 patients had missing data on number of platelet transfusion events.
Interquartile range includes the 25th to 75th percentiles.
Ninety patients were randomized and treated on the study, with 46 on Arm A and 44 on Arm B. Median age at study entry was 70 years (range, 32–85). The 2 arms were balanced with respect to age, gender, race, performance status, FAB subtype, IPSS groups at baseline, and hematologic parameters at study registration (Table 1).
Responses
No complete remissions were observed. Partial remissions were seen in 6 patients (7%) and HI was seen in 21 patients (23%) for an overall response rate of 30%. The overall response rates observed on the 2 arms of the study were similar: 33% on Arm A and 27% on Arm B (Table 2). However, the response durations were significantly longer on Arm A than on Arm B; the median was 23 months (95% confidence interval (CI), 15–29 months) compared to 14 months (95% CI, 8–17 months; p = 0.02).
Table 2.
Best Overall Response by Randomized Treatment Arm
| Number (%) of Patients | ||
|---|---|---|
| Best Response | Arm A (n=46) | Arm B (n=44) |
| Partial Response | 4(9) | 2(5) |
| Hematologic Improvement | 11(24) | 10(23) |
| No response | 23(50) | 26(59) |
| Died with unknown marrow | 2(4) | 1(2) |
| Died with marrow failure | 2(4) | 1(2) |
| Other | 4(9) | 4(9) |
p = 0.91 for differences in overall response by treatment arm.
Responses were analyzed by treatment arm, FAB group, and IPSS score (Table 3) and by karyotype. Only FAB group and the absence of a cytogenetic abnormality correlated with the likelihood of response. Interestingly, patients with more advanced MDS, i.e., refractory anemia with excess blasts in transformation (RAEB-T) or CMML, were more likely to respond. IPSS score did not alter the likelihood of response. Thirteen of the 90 patients were under age 60. Their outcome was not significantly better than the overall group of patients with respect to any of the endpoints examined.
Table 3.
Responses by Treatment Arm, FAB Categories, and IPSS Score
| Treatment | No. of Patients (%) | 95% CI |
|---|---|---|
| Arm A (1.2mg BID × 5d) | 15/46 (33) | 20–48 |
| Arm B (1.2mg/day × 10d) | 12/44 (27) | 15–43 |
| Fisher's Exact Test p-value: 0.65 (2-sided) | ||
| FAB Category* | ||
| RAEB | 6/34 (18) | 7–35 |
| RAEB-T | 8/17 (47) | 23–72 |
| CMML | 7/14 (50) | 23–77 |
| RA,RARS,RCMD,MDS | 6/25 (24) | 9–45 |
| χ2p-value = 0.048 for differences among groups | ||
| IPSS Risk Group* | ||
| Low | 3/9 (33) | 7–70 |
| Int-1 | 9/31 (29) | 14–48 |
| Int-2 | 7/26 (27) | 12–48 |
| High | 5/15 (33) | 12–62 |
| Unknown | 3/9 (33) | 7–70 |
| χ2 p-value = 0.99 for differences among groups | ||
Based on supplemented centrally reviewed data
Cytogenetic risk group
Central cytogenetic review was available for 79 of the 90 randomized patients. No cytogenetic information was available for 6 patients on Arm A and 5 on Arm B. Clonal cytogenetic abnormalities were present in 42 patients (53%), while 37 patients had normal karyotypes. Patients with normal cytogenetics had a higher overall response rate (43%; 95% CI = 27–61%) than those with abnormal cytogenetics (19%; 95% CI = 9–34%). Overall response rates were very poor for the 2 most frequent abnormalities observed; none of 12 patients with del(5q) and only 1 of 10 patients with trisomy 8 responded. Only 1 of 5 patients (20%) with favorable cytogenetic abnormalities as defined by the IPSS [sole del(5q) in 2 patients and sole del(20q) in 3 patients] responded. The response rate was 26% for patients in the poor cytogenetic risk group as defined by the IPSS.
Survival
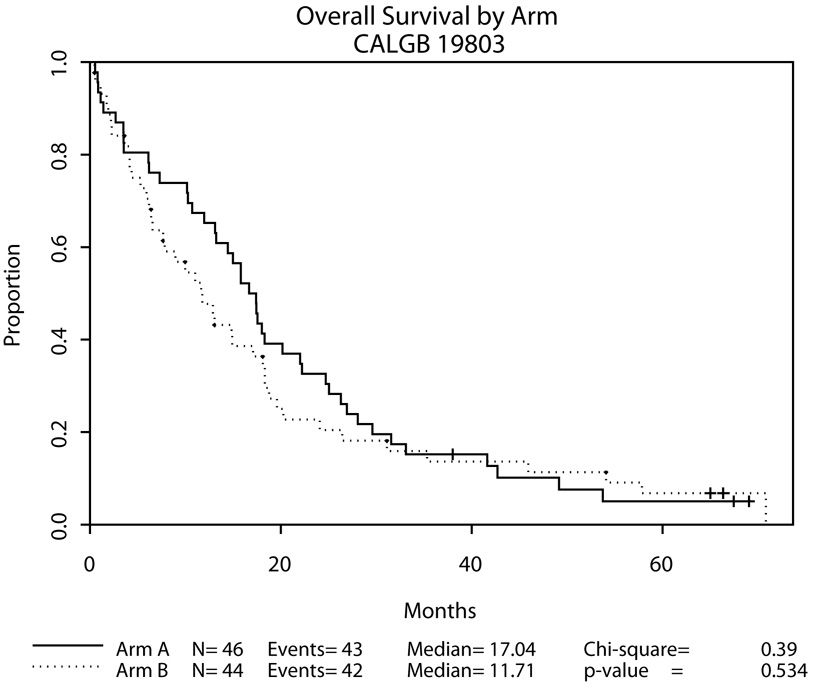
Patients on Arm A tended to survive longer than those on Arm B, but this difference was not statistically significant (p = 0.53, Figure 1). The median survivals were 17 months for patients on Arm A (95% CI, 13–22 months) and 12 months for patients on Arm B (95% CI, 7–18 months). Survival was significantly correlated with IPSS score, but not with FAB classification using the supplemented centrally reviewed data (Table 4).
Figure 1.
Table 4.
Overall Survival According to Randomized Treatment Arm, FAB Categories, and IPSS Risk Groups
| Total | Died | Median (mos) | 95% CI | p-value† | |
|---|---|---|---|---|---|
| By Arm | 0.53 | ||||
| Arm A | 46 | 43 | 17.0 | 13.1–22.0 | |
| Arm B | 44 | 42 | 11.7 | 6.5–18.1 | |
| FAB Categories* | 0.18 | ||||
| RAEB | 34 | 32 | 14.6 | 8.9–19.6 | |
| RAEB-T | 17 | 17 | 11.6 | 13.5–18.8 | |
| CMML | 14 | 13 | 13.1 | 10.0–18.1 | |
| RA, RARS, RCMD | 25 | 23 | 18.3 | 6.4–29.6 | |
| IPSS Risk Groups* | 0.004 | ||||
| Low | 9 | 8 | 18.3 | 10.0–28.1 | |
| Int-1 | 31 | 28 | 18.3 | 13.2–33.1 | |
| Int-2 | 26 | 25 | 14.9 | 7.7–18.1 | |
| High | 15 | 15 | 6.5 | 3.6–11.6 | |
| Unknown | 9 | 9 | 13.0 | 6.2–16.7 |
For differences among groups.
Based on supplemented centrally reviewed data
Time to AML or death
The median time to AML or death was 17 months for patients on Arm A and 11 months for patients on Arm B (p = 0.3, Table 5). This outcome parameter varied significantly (p = 0.001) with IPSS score, but not by FAB group using the supplemented centrally reviewed assignments. The median time to AML or death was 10 months for patients with abnormal cytogenetics (95% CI, 6–15) and 18 months for patients with normal cytogenetics (95% CI, 13–26; p = 0.45).
Table 5.
Time to AML or Death According to Randomized Treatment Arm, FAB Categories, and IPSS Risk Groups
| Total | Failed | Median (mos) | 95% CI | p-value† | |
|---|---|---|---|---|---|
| By Arm | 0.30 | ||||
| Arm A | 46 | 43 | 17.0 | 13.1–22.0 | |
| Arm B | 44 | 43 | 11.3 | 6.4–17.0 | |
| FAB Categories* | 0.14 | ||||
| RAEB | 34 | 33 | 13.6 | 7.8–18.3 | |
| RAEB-T | 17 | 17 | 11.6 | 3.5–18.7 | |
| CMML | 14 | 13 | 13.1 | 10.0–18.1 | |
| RA, RARS, RCMD | 25 | 23 | 18.3 | 6.4–29.5 | |
| IPSS Risk Group* | 0.001 | ||||
| Low | 9 | 8 | 18.3 | 10.0–28.0 | |
| Int-1 | 31 | 28 | 18.3 | 13.2–33.0 | |
| Int-2 | 26 | 26 | 13.9 | 7.3–17.4 | |
| High | 15 | 15 | 6.5 | 3.6–11.6 | |
| Unknown | 9 | 9 | 13.0 | 6.2–16.7 |
For differences among groups.
Based on supplemented centrally reviewed data
Toxicity
Toxicities were similar on the two treatment arms (Table 6). There were 8 treatment-related deaths with oral topotecan. Five of these resulted from documented infections and one from febrile neutropenia without a documented infection. There were also 2 deaths related to hemorrhage; one of these patients was found to be refractory to platelet transfusions after developing severe treatment-related thrombocytopenia. Neutropenia was common; grade 3 and 4 toxicities were reported in 17% and 65% of patients, respectively. Grade 3 and 4 thrombocytopenia were reported in 19% and 61% of patients, respectively. Diarrhea was the most common cause of grade 3 or 4 nonhematologic toxicity; 11% of patients experienced grade 3 and 2% grade 4 diarrhea.
Table 6.
Toxicities (Grade 3–5) by Arm*
| Arm | 3-Severe | 4-Life Thr | 5-Lethal | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Hematologic | ||||||||
| Leukocytes (total WBC) | 1 | 0 | 0% | 3 | 7% | 0 | 0% | |
| 2 | 4 | 9% | 1 | 2% | 0 | 0% | ||
| Hemoglobin (Hgb) for leukemia | 1 | 18 | 39% | 7 | 15% | 0 | 0% | |
| 2 | 11 | 25% | 7 | 16% | 0 | 0% | ||
| Neutrophils (ANC) | 1 | 7 | 15% | 32 | 70% | 0 | 0% | |
| 2 | 9 | 20% | 28 | 64% | 0 | 0% | ||
| Platelets for leukemia | 1 | 11 | 24% | 31 | 67% | 0 | 0% | |
| 2 | 7 | 16% | 26 | 59% | 0 | 0% | ||
| Gastrointestinal | ||||||||
| Diarrhea (without colostomy) | 1 | 7 | 15% | 0 | 0% | 0 | 0% | |
| 2 | 3 | 7% | 2 | 5% | 0 | 0% | ||
| Hemorrhage | ||||||||
| Epistaxis | 1 | 3 | 7% | 0 | 0% | 0 | 0% | |
| 2 | 0 | 0% | 0 | 0% | 0 | 0% | ||
| Hemorrhage/bleeding with grade | 1 | 5 | 11% | 2 | 4% | 1 | 2% | |
| 2 | 4 | 9% | 0 | 0% | 1 | 2% | ||
| Infection/febrile neutropenia | ||||||||
| Febrile neutropenia (fever of | 1 | 10 | 22% | 3 | 7% | 0 | 0% | |
| 2 | 9 | 20% | 0 | 0% | 0 | 0% | ||
| Infection documented | 1 | 13 | 28% | 2 | 4% | 3 | 7% | |
| 2 | 7 | 16% | 1 | 2% | 2 | 5% | ||
| Infection / Other | 1 | 0 | 0% | 0 | 0% | 1 | 2% | |
| 2 | 1 | 2% | 0 | 0% | 0 | 0% | ||
| Pulmonary | ||||||||
| Dyspnea (shortness of breath) | 1 | 0 | 0% | 2 | 4% | 0 | 0% | |
| 2 | 4 | 9% | 0 | 0% | 0 | 0% | ||
included are toxicities with a rate of > 5% on either arm.
DISCUSSION
Topotecan interacts with the enzyme topoisomerase I in a manner that stabilizes topoisomerase I-DNA complexes and brings about cell death. The target enzyme is more abundantly expressed in neoplastic cells than in normal cells (21). Objective antileukemia activity was observed in two phase I studies of intravenous topotecan in patients with refractory acute leukemia (22,23). Subsequently, a phase II trial of topotecan 2 mg/m2 IV daily for 5 days in 47 patients with MDS (including 25 with CMML) yielded a CR rate of 28% and hematologic improvement in 13% of patients. Among 8 patients with clonal cytogenetic abnormalities who achieved a CR, all became cytogenetically normal, including 5 with abnormalities of chromosomes 5 or 7. Fever and mucositis were the most common side effects. The one year survival rate was 38%, and the median survival time was 10.5 months (24). In a subsequent trial, the dose of topotecan was lowered to 1.25 mg/m2/day given by continuous infusion over 5 days but together with cytarabine at 1 gm/m2 over 2 hours daily for the same 5 days (25). Thirty-five patients were treated (3 with RAEB, 18 with RAEB-T, and 14 with CMML); 46% were over 65 years old, and 51% had cytogenetic abnormalities. Twenty-two patients (63%) had a CR. Fever occurred in 59% of the induction courses and documented infections in 48%, but severe mucositis was reported in only 3%.
MDS is a disease mostly of older patients, many of whom are not candidates for aggressive therapeutic interventions (26). Many have co-morbid disorders that impact on outcome. In the current study, we evaluated the efficacy and toxicity of oral topotecan for MDS patients with fair to good performance status. We also studied and previously reported on the pharmacokinetics of oral topotecan in these patients and their adherence to an oral chemotherapy regimen (27). When administered as an oral agent on a 5 day schedule, others have shown that the maximum tolerable dose (MTD) of topotecan for solid tumor patients was 2.3 mg/m2 given once per day (28), similar to the total doses used on both arms in this study. We observed primarily minor responses when patients with MDS were treated with oral topotecan. This was accompanied by significant toxicities related to the therapy. Responses were more commonly seen in patients with CMML or RAEB-T and in those with normal cytogenetics. The median survival for all patients treated with oral topotecan on the study was 15 months, which is comparable to the survival of the very similar population of patients who were randomized to the observation plus supportive care arm of CALGB study 9221, our randomized study of azacitidine in MDS (14,15). The rate of response to topotecan did not differ across the IPSS risk groups, but we observed that overall survival, as well as the time to AML or death, was significantly shorter in patients with a higher IPSS score at study entry.
We did observe responses in 50% of patients with CMML treated on this study. It is worth noting that patients with this diagnosis also had a higher response rate in the trial reported by Beran et al, using the combination of topotecan and cytarabine, compared to other categories of MDS. We also noted a response rate of 47% in patients with RAEB-T treated on this trial. Thus, this agent’;s greatest activity may be in the more severe or proliferative forms of MDS. However, neither of these subtypes is still included among the myelodysplastic syndromes in the current WHO classification.
Our findings also emphasized the importance of expert morphology review in MDS trials. As shown in Table 1, there was often discordance between the local institutional assessment of MDS subgroup and that determined after expert central review. In most cases, more advanced disease due to a higher percentage of blasts or more extensive dysplasia was noted during the central review. These discordant interpretations can influence the IPSS score. However in this case, whether we analyzed the results using only centrally review data or only institutional assessments or both, the clinical outcomes in this study were essentially the same.
The present study demonstrated minimal activity and troublesome toxicity from oral topotecan for the treatment of MDS. It is possible that a lower dose of topotecan might have yielded a similar response rate with a lower rate of toxicity. The use of lower doses of topotecan might allow the use of this agent in combination with other active agents for patients with advance MDS. New therapies with enhanced activity are needed for patients with MDS.
Acknowledgments
The research for CALGB 19803 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). Oral topotecan and support for pharmacokinetic studies were received from Glaxo-Smith-Kline. \ The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, M.D., supported by CA32291
Dartmouth Medical School - Norris Cotton Cancer Center, Lebanon, NH–Marc S. Ernstoff, M.D., supported by CA04326
Duke University Medical Center, Durham, NC–Jeffrey Crawford, M.D., supported by CA47577
Greenville CCOP, Cancer Centers of the Carolinas, Greenville, SC–Jeffrey K. Giguere, M.D, supported by CA29165
Illinois Oncology Research Association, Peoria, IL–John W. Kugler, M.D., supported by CA35113
Massachusetts General Hospital, Boston, MA–Michael L. Grossbard, M.D., supported by CA12449
Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, M.D., supported by CA04457
Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, M.D., supported by CA02599
State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, M.D., supported by CA21060
The Ohio State University Medical Center, Columbus, OH–Clara D Bloomfield, M.D., supported by CA77658
University of California at San Diego, San Diego, CA–Joanne Mortimer, M.D., supported by CA11789
University of Chicago, Chicago, IL –Gini Fleming, M.D., supported by CA41287
University of Illinois MBCCOP, Chicago, IL–Lawrence E. Feldman, M.D., supported by CA74811
University of Iowa, Iowa City, IA–Gerald Clamon, M.D., supported by CA47642
University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, M.D., supported by CA31983
University of Minnesota, Minneapolis, MN–Bruce A Peterson, M.D., supported by CA16450
University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C Perry, M.D., supported by CA12046
University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, M.D., supported by CA47559
University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, M.D., supported by CA47555
Wake Forest University School of Medicine, Winston-Salem, NC–David D Hurd, M.D., supported by CA03927
Weill Medical College of Cornell University, New York, NY–Scott Wadler, M.D., supported by CA07968
REFERENCES
- 1.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–199. [PubMed] [Google Scholar]
- 2.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 3.Mufti GJ, Steven JR, Oscier DG, Hamblin TJ, Machin D. Myelodysplastic syndromes: a scoring system with prognostic significance. Br J Haematol. 1985;59(3):425–433. doi: 10.1111/j.1365-2141.1985.tb07329.x. [DOI] [PubMed] [Google Scholar]
- 4.Piedras J, Hernandez G, Lopez-Karpovitch X. Effect of androgen therapy and anemia on serum erythropoietin levels in patients with aplastic anemia and myelodysplastic syndrome. Blood. 1995;86:338a. doi: 10.1002/(sici)1096-8652(199802)57:2<113::aid-ajh4>3.0.co;2-z. #1341. [DOI] [PubMed] [Google Scholar]
- 5.Negrin RS, Haeuber DH, Nagler A, Olds LC, Donlon T, Souza LM, Greenberg PL. Treatment of myelodysplastic syndromes with recombinant human granulocyte colony-stimulating factor. A phase I-II trial. Ann Intern Med. 1989;110:976. doi: 10.7326/0003-4819-110-12-976. [DOI] [PubMed] [Google Scholar]
- 6.Vadhan-Raj S, Keeting M, LeMaistre A, Hittelman WN, McCredie K, Trujillo JM, Broxmeyer HE, Henney C, Gutterman JU. Effects of recombinant human granulocyte-macrophage colony stimulating factor in patients with myelodysplastic syndromes. N Engl J Med. 1987;317:1545. doi: 10.1056/NEJM198712173172501. [DOI] [PubMed] [Google Scholar]
- 7.Koeffler HP, Heitjan D, Mertelsmann R, Kolitz JE, Schulman P, Itri L, Gunter P, Besa E. Randomized study of 13-cis retinoic acid vs. placebo in the myelodysplastic disorders. Blood. 1988;71(3):703–708. [PubMed] [Google Scholar]
- 8.Zorat F, Shetty V, Dutt D, Nascimben F, Allampallam K, Dar S, York A, Gezer S, Venugopal P, Raza A. The clinical and biological effects of thalidomide in patients with myelodysplastic syndromes. Br J Haematol. 2001;115(4):881–894. doi: 10.1046/j.1365-2141.2001.03204.x. [DOI] [PubMed] [Google Scholar]
- 9.List AF, Brasfield F, Heaton R, Glinsmann-Gibson B, Crook L, Taetle R, Capizzi R. Stimulation of hematopoiesis by amifostine in patients with myelodysplastic syndrome. Blood. 1997;90(9):3364–3369. [PubMed] [Google Scholar]
- 10.Fenaux P, Raza A, Mufti GJ, Aul C, Germing U, Kantarjian H, Cripe L, Kerstens R, De Porre P, Kurzrock R. A multicenter phase 2 study of the farnesyltransferase inhibitor tipifarnib in intermediate- to high-risk myelodysplastic syndrome. Blood. 2007;109(10):4158–4163. doi: 10.1182/blood-2006-07-035725. [DOI] [PubMed] [Google Scholar]
- 11.Raza A, Buonamici S, Lisak L, Tahir S, Li D, Imran M, Chaudary NI, Pervaiz H, Gallegos JA, Alvi MI, Mumtaz M, Gezer S, Venugopal P, Reddy P, Galili N, Candoni A, Singer J, Nucifora G. Arsenic trioxide and thalidomide combination produces multi-lineage hematological responses in myelodysplastic syndromes patients, particularly in those with high pre-therapy EVI1 expression. Leuk Res. 2004;28(8):791–803. doi: 10.1016/j.leukres.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Hellstrom-Lindberg E, Gulbrandsen N, Lindberg G, Ahlgren T, Dahl IM, Dybedal I, Grimfors G, Hesse-Sundin E, Hjorth M, Kanter-Lewensohn L, Linder O, Luthman M, Lofvenberg E, Oberg G, Porwit-MacDonald A, Radlund A, Samuelsson J, Tangen M, Winquist I, Wisloff F the Scandinavian MDS Group. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol. 2003;120(6):1037–1046. doi: 10.1046/j.1365-2141.2003.04153.x. [DOI] [PubMed] [Google Scholar]
- 13.Miller KB, Kim K, Morrison FS, Winter JN, Bennett JM, Neiman RS, Head DR, Cassileth PA, O’Connell MJ. The evaluation of low-dose cytarabine in the treatment of myelodysplastic syndromes: a phase III intergroup study. Ann Hematol. 1992;65(4):162–168. doi: 10.1007/BF01703109. [DOI] [PubMed] [Google Scholar]
- 14.Silverman LR, Demakos EP, Peterson BL, et al. A randomized controlled trial of azacytidine in patients with the myelodysplastic syndrome: A study of the Cancer and Leukemia Group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 15.Silverman LR, McKenzie DR, Peterson BL, Backstrom JT, Beach CL, Larson RA. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921 and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R, 3rd, Shen L, Nimer SD, Leavitt R, Raza A, Saba H. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer. 2006;106(8):1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 17.List A, Dewald G, Bennett J, Giagoundis A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, Reeder C, Wride K, Patin J, Schmidt M, Zeldis J, Knight R Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 18.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, Dreisbach L, Schiffer CA, Stone RM, Greenberg PL, Curtin PT, Klimek VM, Shammo JM, Thomas D, Knight RD, Schmidt M, Wride K, Zeldis JB, List AF. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 19.Kornblith AB, Herndon JE, 2nd, Silverman LR, Demakos EP, Odchimar-Reissig R, Holland JF, Powell BL, DeCastro C, Ellerton J, Larson RA, Schiffer CA, Holland JC. Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: a Cancer and Leukemia Group B study. J Clin Oncol. 2002;20(10):2441–2452. doi: 10.1200/JCO.2002.04.044. [DOI] [PubMed] [Google Scholar]
- 20.Beran M, Kantarjian H, O’Brien S, Koller C, et al. Topotecan, a topoisomerase I inhibitor, is active in the treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 1996;88(7):2473–2479. [PubMed] [Google Scholar]
- 21.Rowinsky EK, Grochow L, Hendricks C, et al. Phase I and pharmacologic study of topotecan: A novel topoisomerase-1 inhibitor. J Clin Oncol. 1992;10(4):647–656. doi: 10.1200/JCO.1992.10.4.647. [DOI] [PubMed] [Google Scholar]
- 22.Rowinsky EK, Adjei A, Ross C, et al. Phase I and pharmacologic study of the topoisomerase I-inhibitor topotecan in patients with refractory acute leukemia. J Clin Oncol. 1994;12(10):2193–2203. doi: 10.1200/JCO.1994.12.10.2193. [DOI] [PubMed] [Google Scholar]
- 23.Kantarjian HM, Beran M, Ellis A, Zwelling L, et al. Phase I study of topotecan, a new topoisomerase I inhibitor, in patients with refractory or relapsed acute leukemia. Blood. 1993;81:1146. [PubMed] [Google Scholar]
- 24.Beran M, Kantarjian H, O’Brien S, Koller C, et al. Topotecan, a topoisomerase I inhibitor, is active in the treatment of myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 1996;88(7):2473–2479. [PubMed] [Google Scholar]
- 25.Beran M, Kantarjian H. Results of topotecan-based combination therapy in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Semin Hematol. 1999;36 Suppl 8:3–10. [PubMed] [Google Scholar]
- 26.Larson RA. Myelodysplasia: When to treat and how? Best Practices & Research Clin Haematol. 2006;19:293–300. doi: 10.1016/j.beha.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Klein CE, Kastrissios H, Miller AA, Hollis D, Yu D, Rosner GL, Grinblatt DL, Larson RA, Ratain MJ. Pharmacokinetics, pharmacodynamics and adherence to oral topotecan in myelodysplastic syndromes: a Cancer and Leukemia Group B study. Cancer Chemotherapy & Pharmacology. 2006;57(2):199–206. doi: 10.1007/s00280-005-0023-6. [DOI] [PubMed] [Google Scholar]
- 28.Gerrits CJH, Burris H, Schellens JHM, Eckardt J, et al. Oral topotecan given once or twice daily for ten days: a phase I pharmacology study in adult patients with solid tumors. Clin Ca Res. 1998;4:1153–1158. [PubMed] [Google Scholar]