Abstract
Cutaneous myelinated nociceptors are known to exhibit considerable heterogeneity in their response to noxious heat. In the present experiments, we studied heat sensitivity among myelinated nociceptors during early postnatal life to determine whether this heterogeneity is correlated with other physiological and anatomical properties. A total of 129 cutaneous myelinated nociceptors were recorded intracellularly and characterized using mechanical and thermal skin stimuli in ex vivo preparations from neonatal Swiss–Webster (SW) mice across postnatal ages P2–P10; physiologically identified cells were iontophoretically labeled with neurobiotin for analyses of dorsal horn terminations from heat-sensitive and heat-insensitive cells. Our results show that heat sensitivity is not strictly correlated with other physiological or anatomical properties, most notably mechanical threshold or laminar termination patterns, of myelinated nociceptors at these ages. Further, we found a marked decline in the number of heat-sensitive myelinated mechanonociceptors (A-mechanoheat nociceptors [AMHs]) during this early postnatal period. Indeed, 68% of myelinated nociceptors were AMHs between P2 and P5, whereas this percentage dropped to 36% between P6 and P10. Multiple independent lines of evidence suggest that this decrease reflects a change in phenotype in a subset of myelinated nociceptors that lose sensitivity to noxious heat in early postnatal life. Interestingly, evidence was also obtained for a significant strain difference since the early transient excess in the number of AMHs in P2–P5 SW neonates was not present in similarly aged neonates from the C57Bl/6 strain. Potential mechanisms underlying these postnatal changes in AMH number are discussed.
INTRODUCTION
Cutaneous myelinated nociceptors represent the peripheral neural apparatus underlying “first pain” and play an important role in protective mechanisms serving to safeguard the skin (Campbell and LaMotte 1983). This role may be especially important in newborn mammals, where immune and central nervous systems are not yet mature (Bona 2005) and damage to the skin may have both immediate impacts on survival and also long-term consequences for all aspects of somatosensory processing (Durham and Woolsey 1984; Torsney and Fitzgerald 2003). Viewed in this light, the marked hypersensitivity of neonates to external stimuli, seen through exaggerated withdrawal reflexes to stimulus intensities well below those that elicit similar reflexes in adults (Fitzgerald 2005), is not surprising because it likely represents a highly adaptive mechanism that has evolved to protect the skin during this vulnerable period.
Despite the importance of myelinated nociceptors to nociceptive processing in adulthood, little is known of their development. Recent work in neonatal mice has revealed that multiple classes of myelinated nociceptors can be distinguished on the combined basis of somal membrane properties, central anatomy, and peripheral responses to mechanical stimuli (Woodbury and Koerber 2003). Like low-threshold mechanoreceptors (LTMRs; Woodbury and Koerber 2007; Woodbury et al. 2001), the central termination patterns of myelinated nociceptors are adult-like shortly after birth (Boada and Woodbury 2008; Light and Perl 1979; Woodbury and Koerber 2003; Woodbury et al. 2004). As in adults, myelinated nociceptors in neonates exhibit a wide range of responses to mechanical stimuli; the activation of many by relatively low intensities may contribute to the hypersensitive reflexes of neonates (Woodbury and Koerber 2003).
In addition to mechanical hypersensitivity, neonates also show hypersensitivity to thermal stimuli (Conway et al. 1998; Falcon et al. 1996; Hiura et al. 1999; Hu et al. 1997). However, the afferent limb underlying this hypersensitivity is not clear. Although initial studies revealed that adult myelinated nociceptors normally do not respond to noxious heat (Beck et al. 1974; Burgess and Perl 1967; Fitzgerald and Lynn 1977; Lynn and Carpenter 1982; Perl 1968), myelinated nociceptors responding to both mechanical and heat stimuli (A-mechanoheat nociceptors [AMHs]) have been observed in many species (Cain et al. 2001; Georgopoulos 1976; Iggo and Ogawa 1971; Koltzenburg et al. 1997; LaMotte et al. 1982; Lawson et al. 2008; Treede et al. 1998; Woodbury et al. 2004). Little is known, however, about the development of heat sensitivity in myelinated nociceptors or AMH central terminations.
Toward this end, the present studies examined the heat sensitivity and central anatomy of myelinated nociceptors in neonatal Swiss–Webster mice using an ex vivo somatosensory system preparation. We found that a surprisingly large percentage of myelinated nociceptors exhibited sensitivity to noxious heat during the first week of life, but that heat sensitivity declined to adult levels in the second week. Multiple lines of evidence, both anatomical and physiological, suggest that this reduction reflects a loss of heat sensitivity among myelinated nociceptors, as opposed to a loss of mechanical sensitivity in AMH nociceptors or selective loss of the latter. In addition, evidence was obtained for a significant strain difference in mice, raising caution surrounding generalization of these findings outside this particular strain.
METHODS
The in vitro electrophysiological experiments described in the present study were conducted using an ex vivo somatosensory system preparation from postnatal (P) mice of either sex beginning on the second day after birth (P2). Throughout the text, “N” and “n” signify the number of experimental animals and recorded cells, respectively. Most experiments were conducted in neonates from the albino Swiss–Webster strain (SW; Charles River Laboratories, Wilmington, MA). In addition, experiments were also conducted in P2–P5 neonates from transient receptor potential vanilloid 1 (TRPV1) null-mutant mice for comparative purposes; controls for the latter were conducted in similarly aged neonates from the background strain (C57Bl/6; Jackson Laboratory, Bar Harbor, ME). All procedures conformed to National Institutes of Health guidelines and were approved by the University of Wyoming Animal Care and Use Committee.
Ex vivo somatosensory system preparation
The ex vivo back skin preparation used in this study was as detailed previously (Woodbury and Koerber 2003; Woodbury et al. 2001) with minor modification. Briefly, neonates ≤P5 were anesthetized by cooling on ice, whereas mice ≥P5 were anesthetized using ketamine and xylazine (90 and 10 mg/kg, respectively, administered intramuscularly); a series of control experiments revealed that these differences in anesthetic method did not influence our findings (see results). Anesthetized animals were quickly decapitated following transcardial perfusion with cold (12–15°C), oxygenated artificial cerebrospinal fluid (aCSF, in mM; 127.0 NaCl, 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 d-glucose; cf. Woodbury and Koerber 2003). The spinal cord, thoracic dorsal root ganglia (DRGs), dorsal cutaneous nerves (DCNs), and dorsolateral trunk skin on one side were dissected in continuity in a circulating bath of aCSF. Once isolated, the preparation was pinned out with epidermal surface of the skin facing upward and the aCSF was raised to 31–32°C (cf. Woodbury and Koerber 2003) for electrophysiological recording.
Physiological characterization
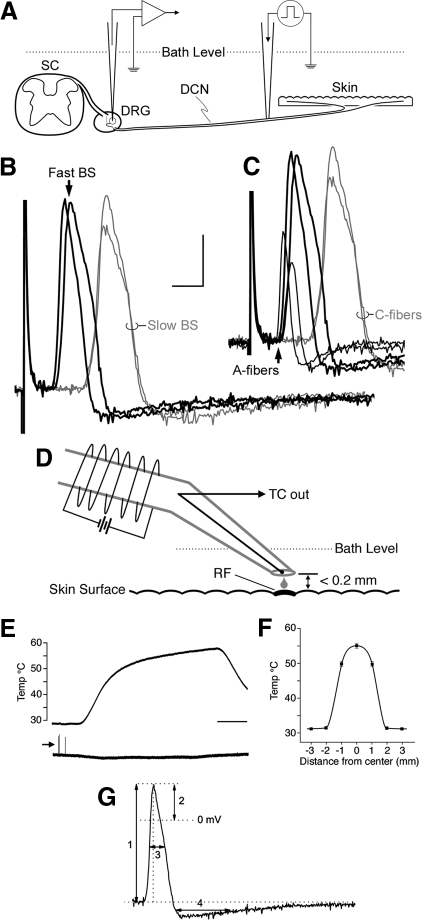
DRG somata were impaled with quartz (300–400 MΩ) or borosilicate micropipettes (90–200 MΩ) containing 20% neurobiotin (Vector Laboratories, Burlingame, CA) in 1 M potassium acetate. Electrical stimuli (50–500 μs at 0.5 Hz) were delivered to the DCN through an en passant suction electrode to locate DRG neurons with intact axons, as illustrated in Fig. 1A; care was taken during placement of this electrode to avoid conduction block. In accord with previous studies in which slightly lower temperatures were used (Woodbury and Koerber 2003), a broad spectrum of conduction velocities (CVs) was evident in each DCN studied regardless of age. With regard to cells exhibiting broad inflected somal spikes (i.e., putative nociceptors; e.g., Koerber et al. 1988), two distinct classes, referred to here as fast and slow broad-spiked (BS) cells, were evident even in the youngest neonates, as illustrated in Fig. 1B. In each nerve, fast BS cells exhibited CVs that were at least double those of slow BS cells and this difference in CV increased with age. Slow BS cells greatly outnumbered fast BS cells in each ganglion and the frequent encounters with the former provided a working reference in every nerve studied (average CV across P2–P10 = 0.42 ± 0.09 m/s; range = 0.23–0.58 m/s, n = 91); the slow BS cells that were characterized and labeled centrally exhibited properties diagnostic of C-polymodal nociceptors (e.g., moderate mechanical thresholds, sensitivity to noxious heat, and dense termination in lamina IIo; for mice, see Albers et al. 2006; Boada and Woodbury 2008; Woodbury et al. 2001, 2004). As seen in Fig. 1C, the CVs of fast BS cells clustered tightly with developing myelinated low-threshold mechanoreceptors (e.g., guard and down hair follicle afferents). To restrict analyses to future myelinated nociceptors, the present studies were restricted to fast BS cells as in a previous study (Woodbury and Koerber 2003). Peripheral CVs were subsequently calculated off-line using electrically evoked spike latency and the distance measured along the nerve between stimulating and recording electrodes; utilization time was not taken into account.
Fig. 1.
A: schematic of ex vivo preparation used in the present experiments with spinal cord (SC), dorsal root ganglia (DRG), dorsal cutaneous nerves (DCNs), and skin intact (modified from Woodbury et al. 2001). B: superimposed examples of electrically evoked somal action potentials (APs) with broad spikes (BSs) recorded sequentially from different neurons in the same DCN in a postnatal day 3 (P3) neonate; only 4 cells, lined up at the stimulus artifact, are shown for clarity. Note the clear separation into 2 discrete groups according to latency. In C, 2 APs recorded from physiologically identified low-threshold mechanoreceptors (LTMRs) in the same nerve have also been added, all evoked from the same point on the nerve. Note here that APs from 2 different myelinated nociceptors (large-amplitude/short-latency black traces) clustered tightly with the APs from developing myelinated LTMRs (smaller-amplitude/short-latency black traces; from left to right, guard and down hair follicle afferent), all of which were well separated in arrival time (bottom arrow) from the broad inflected APs of developing C-nociceptors (long-latency gray traces; calibration bar = 2.5 ms, 20 mV). D: schematic of heat stimulator used in the present experiments; RF, receptive field; TC, thermocouple. E: output temperature over time; arrow (bottom trace) indicates response to mechanical stimulus evoked while centering stimulator tip over the RF (calibration bar = 5 s). F: spatial distribution of temperature; gradient from tip center (“0”) was measured independently by a separate TC on the skin surface (methods). G: analyzed spike parameters: 1) amplitude, 2) overshoot, 3) spike duration at half-amplitude, 4) afterhyperpolarization (AHP) duration at half-amplitude.
Following stable impalement of fast BS cells, the skin was searched with a blunt glass probe (∼1-mm diameter) to locate the peripheral receptive field (RF). If an RF could not be located, the search was repeated by gentle probing with sharp watchmaker's forceps. Forces generated during the latter searches reached 50 mN (calculated pressure ≤4 bar, determined in a separate series of control experiments). These mechanical search protocols were sufficient to locate the RFs of roughly 90% of all fast BS cells with intact axons encountered in these experiments. Of the remaining fast BS cells in these nerves, it is unlikely that all were mechanically insensitive (i.e., “silent”) nociceptors (e.g., Treede et al. 1995) since RFs from about 10% of all intact cells with narrow uninflected spikes (NS; i.e., putative LTMRs; see Koerber et al. 1988; Woodbury and Koerber 2007; Woodbury et al. 2001) also could not be located, presumably due to damage during dissection. For mechanically sensitive cells, mechanical threshold and peripheral response properties were determined with calibrated von Frey filaments (Stoelting, Wood Dale, IL) delivered to the most sensitive location with the aid of a stereomicroscope; cells lacking a mechanically sensitive RF were not studied further.
NOXIOUS HEAT STIMULATION.
Following characterization with mechanical stimuli, RFs were exposed to noxious heating. The short length of neonatal nerves precluded raising the skin to the bath surface and thus the use of our feedback-controlled thermode as in adult in vitro preparations (Albers et al. 2006; Woodbury et al. 2004). We therefore used an in-line heater that delivered a fine stream of heated aCSF, as shown schematically in Fig. 1D. This device consisted of an insulated thin-walled glass tube wrapped with NiChrome wire across which variable DC voltage was applied to heat the internal gravity-fed flow; flow rate (∼0.3 ml/min) was adjusted to produce the maximal rate of heating without internal boiling. The outflow was fitted with tapered polyethylene tubing with a tip opening about 1-mm diameter and the temperature of the solution exiting the tip was monitored with an internally positioned calibrated thermocouple (IT-23, Physitemp, Clifton, NJ). In control experiments, the pH of aCSF exiting the tip ranged from 7.4 to 7.8 between 31 and 60°C and thermal expansion of the tip was ≤180 μm over this same temperature range.
To ensure optimal placement, the tip was brought into skin contact with a micromanipulator and used as a mechanical probe while carefully centering the tip over the area of greatest mechanical sensitivity (e.g., Figs. 1E and 2A). Prior to heating, the tip was raised until dimpling was no longer present (well beyond the point where mechanically induced firing ceased); further, to secure unimpeded flow of the solution throughout the heat trial and prevent spurious mechanical stimulation from thermal expansion (see preceding text), the tip was raised another 0.2 mm before heating commenced. Due to the high specific heat of water and substantial heat gradients along the submerged outlet tubing, the typical heat ramp was sigmoidal, with two roughly linear components (Fig. 1E); most heat responses occurred after the heat ramp reached its plateau phase (average ≃ 0.2°C/s). Heat trials were normally terminated when outflow temperatures reached 58°C; if no response occurred during this time, temperatures were maintained above 53°C for ≥30 s and allowed to exceed 60°C in some cases. Responses were occasionally seen at the latter extreme temperatures, although such afferents were normally rendered silent to subsequent mechanical stimuli and thus responses likely reflected injury discharge.
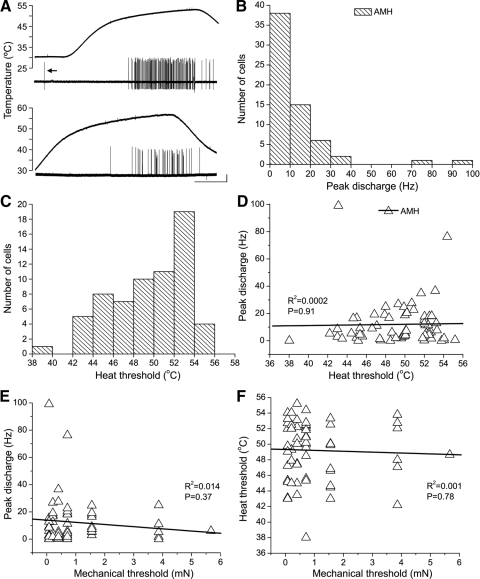
Fig. 2.
Heat sensitivity in neonatal A-mechanoheat nociceptors (AMHs). A: examples of the most vigorous responses to noxious heat observed in neonatal myelinated nociceptors, from P9 (top) and P3 (bottom) mice; both were laminae I–V nociceptors with low mechanical thresholds (0.08 mN for both); arrow (top example) shows mechanically evoked response elicited while centering heat stimulator on the RF. B: histogram showing frequency of peak discharge rates across the population of AMHs; Poisson-like distribution reveals the low probability of firing (i.e., sluggishness) to noxious heat overall. C: distribution of heat thresholds among AMHs. D: relationship between heat threshold and peak discharge rate. E: relationship between mechanical threshold and peak discharge rate; note that the highest discharge rates were seen in cells with the lowest mechanical thresholds. F: relationship between mechanical and heat thresholds among neonatal AMHs. Note lack of correlation. Calibration bar (A): 20 mV, 10 s.
Since previous heat exposure can alter nociceptor physiology (e.g., Thalhammer and LaMotte 1982; Yeomans and Proudfit 1996), exposed areas were marked with charcoal and any cells recorded subsequently with overlapping RFs were rejected. To estimate the actual temperature experienced by the epidermal surface during trials, heat dissipation from this point source (stabilized at 55°C) was measured in control experiments using a second calibrated IT-23 thermocouple positioned on the skin at varying distances from the tip along multiple radial directions. As seen in Fig. 1F, measurements obtained 0.2 mm directly below the tip were largely identical to those measured at the outflow (ΔT ≤0.5°C). Due to strong thermal buffering by the bath, however, temperatures dropped rapidly from the tip and equaled that of the surrounding bath within a 2-mm radius. Thus an area of skin about 3 mm in diameter was exposed to noxious heat during trials, well in excess of the size of most nociceptor RFs at these ages. In additional control experiments, this same technique resulted in an average heat threshold of 41.5°C (range: 37–43.7°C; n = 9) for C-polymodal nociceptors from submerged trunk skin in adult ex vivo preparations, indistinguishable from that seen using feedback-controlled Peltier stimulators on adult trunk skin held at the bath surface (Albers et al. 2006; Woodbury et al. 2004) or in vivo (Boada and Woodbury 2007, 2008).
CELL IDENTIFICATION AND CLASSIFICATION.
As detailed previously (Woodbury and Koerber 2003), fast BS cells in neonates exhibited a large suite of physiological and anatomical traits diagnostic of adult myelinated nociceptors, including faithful encoding of stimulus intensity, generally moderate to high mechanical thresholds, and either large numbers of collaterals arborizing throughout all dorsal horn laminae or across the horizontal plane of the marginal zone (e.g., Boada and Woodbury 2008). For simplicity, these putative myelinated nociceptors are hereafter referred to simply as myelinated nociceptors; given the extensive overlap in physiological and anatomical properties between Aβ and Aδ nociceptors in adults (Djouhri and Lawson 2004; Boada and Woodbury 2008), further subdivision into Aβ/δ groupings was not attempted here due to minor differences in CV between myelinated populations at these ages (e.g., Fig. 1C). LTMRs were distinguished from myelinated nociceptors by the combined presence of narrow, uninflected somal spikes, mechanical thresholds ≤0.07 mN, and where available, characteristic central and peripheral anatomy as described in detail previously (Woodbury and Koerber 2007; Woodbury et al. 2001).
Myelinated nociceptors were subclassified according to their response to their first exposure to noxious heat. Cells were categorized as mechanoheat nociceptors (i.e., AMHs) if responses occurred within 30 s of temperatures ≥53°C (e.g., Treede et al. 1995, 1998). Cells that failed to respond within 30 s of temperatures ≥53 or up to 60°C were categorized as mechanoreceptors (i.e., A-mechanonociceptors [AMs]); occasional responses seen following the latter extremes typically accompanied complete silencing of the neuron (see earlier text) and were therefore interpreted as injury discharge rather than heat sensitivity. For the present purposes, cells were also classified as AMs if they developed spontaneous activity after prolonged exposures to temperatures >53°C and/or responded only to subsequent heat trials because such apparent heat sensitivity may instead reflect discharge coincidental to injury of terminals or surrounding nonneuronal cells (Davis et al. 1993). Unresponsive cells that did not experience temperatures ≥53°C for ≥30 s were excluded from analyses.
PHYSIOLOGICAL ANALYSES.
Cells satisfying minimum criteria of resting membrane potentials more negative than −40 mV and spike amplitudes >40 mV were included in analyses of somal action potential properties. Action potential parameters that were analyzed included the amplitude, overshoot, and duration (at half-amplitude; D50) of positive-going spikes, and the half-amplitude duration of afterhyperpolarizations (AHPs), as illustrated in Fig. 1G. Mechanical threshold was defined as the minimum force (from calibrated von Frey filaments) eliciting a response in multiple trials. Heat threshold was defined as the temperature corresponding to first spike; delay due to CV was not taken into account, but was likely inconsequential in view of the slow heating rate.
TISSUE PROCESSING AND HISTOLOGICAL ANALYSIS.
Following physiological characterization, cells were iontophoretically injected with neurobiotin (one cell/ganglion; 0.4–0.8 nA, 75% duty cycle, 0.3–20 nA·min total current). Three to 4 h later, the spinal cord was removed and immersion fixed in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) overnight at 4°C. The cord was then blocked, embedded in 10% gelatin, postfixed, and cryoprotected in 20% sucrose. Frozen transverse sections (40 μm) were serially collected in PB and reacted with standard avidin–biotinylated enzyme complex/3,3′-diaminobenzidine techniques (Elite ABC/DAB Kit; Vector Laboratories, Burlingame, CA). Sections were rinsed, mounted on slides, counterstained with 1% neutral red, dehydrated, cleared, and coverslipped. Labeled central processes were serially reconstructed and drawn with the aid of a camera lucida. Digital photomicrographs were obtained using a Retiga Exi (QImaging, Surrey, BC, Canada) and IPLab (Scanalytics, Rockville, MD). Images were manipulated only to normalize brightness and contrast using Photoshop (Adobe Systems, San Jose, CA).
STATISTICAL ANALYSES.
Afferents were categorized into functional groups on the basis of response properties to natural stimuli; such groupings were confirmed by additional information on somal membrane properties as well as central and peripheral anatomy in many cases. Spike parameters and most other physiological properties were compared between groups using two-sample t-tests after testing for normality; mechanical thresholds were not normally distributed even after log transformation and were compared using Mann–Whitney U tests. Potential changes in frequency distributions were tested using chi-square or Fisher's Exact tests, depending on sample size. Potential age-related changes in physiological parameters were evaluated with linear regression analyses. Statistical comparisons were performed using various software packages (MiniTab, State College, PA; OriginPro, Northampton, MA). P values <0.05 were considered significant. All group values are presented as means ± SD except where noted.
RESULTS
The present report is based on 129 myelinated nociceptors characterized using both mechanical and noxious heat stimuli in ex vivo back-skin preparations from neonatal Swiss–Webster (SW) mice across ages P2–P10 (N = 38). Samples recorded with quartz (n = 93) and borosilicate micropipettes (n = 36) were combined for analyses of mechanical thresholds, heat thresholds, and CV. However, because differences in somal spike properties were observed between samples obtained with different electrodes (data not shown), analyses of spike properties were restricted to the larger sample. In addition to myelinated nociceptors, 20 myelinated LTMRs were similarly characterized for comparison (data not shown). Further, multiple well-labeled collateral arbors were recovered from 34 myelinated nociceptors, 4 LTMRs, and 2 unmyelinated polymodal nociceptors that were iontophoretically stained with neurobiotin, providing additional information on afferent identity.
As in previous studies (Woodbury and Koerber 2003, 2007; Woodbury et al. 2001), myelinated nociceptors were readily distinguishable on both qualitative and quantitative grounds from myelinated LTMRs (the latter were identified on the combined basis of CV, spike shape, mechanical threshold, response properties, and in some cases central and peripheral anatomy). For example, somal action potentials of myelinated nociceptors exhibited an inflection on the falling phase at these temperatures (cf. Boada and Woodbury 2007), their spikes and AHPs were greater in both amplitude and duration than the narrow uninflected spikes of LTMRs (P < 0.0001 for both comparisons), they exhibited higher mechanical thresholds than those of LTMRs (P < 0.0001), and their slowly adapting responses became increasingly vigorous with increasing force; by contrast, the responses of LTMRs saturated at low intensities. The present findings therefore agree with those from a smaller sample characterized using mechanical stimuli only (Woodbury and Koerber 2003). The present studies further subdivided myelinated nociceptors based on the response to their first exposure to noxious heat.
Heat sensitivity among neonatal myelinated nociceptors
Slightly over half of the myelinated nociceptors in our sample responded to noxious heat (65/129; hereafter referred to as AMH), although this was strongly age dependent (see the following text). As shown in Fig. 2A, a few responded vigorously to RF heating, although most responded sluggishly, with low peak discharge rates (Fig. 2B) and/or small numbers of spikes (see also Fig. 4, A and B). Heat thresholds were <53°C for most (58/65; average = 49.4 ± 3.54°C; range = 38–55°C; Fig. 2C). The majority began responding during the plateau phase of the heat ramp (n = 50) and, although heating rates varied between trials, there was no correlation between peak discharge rate and heating rate (R2 = 0.002, P = 0.79; data not shown; cf. Tillman et al. 1995), possibly reflecting the relatively slow rates during this plateau phase (≤0.6°C/s). Contrary to findings in primates (Treede et al. 1998), peak discharge was also not correlated with either heat or mechanical threshold (Fig. 2, D and E) and there was also no correlation between heat and mechanical thresholds in AMHs (Fig. 2F).
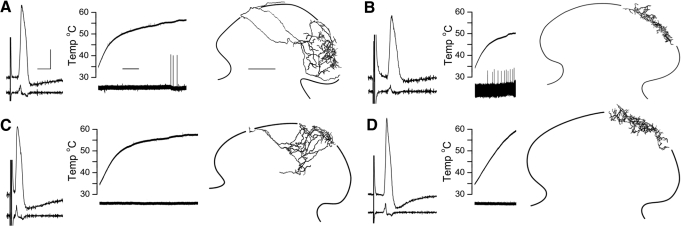
Fig. 4.
Representative examples of response to first noxious heat exposure among anatomically identified myelinated nociceptors across different postnatal ages. For each cell, somal APs (and first derivatives, below), response to noxious heat, and camera lucida reconstructions of central anatomy (20 serial sections each) are shown from left to right. A: P4 laminae I–V AMH. B: P5 laminae I/IIo AMH. C: P9 laminae I–V AM. D: P9 laminae I/IIo AM. Note that the AM in C failed to respond to noxious heat despite stimulation for >30 s above 53°C, whereas the AM in D failed to respond despite stimulation ≤60°C. CV, mechanical threshold, and heat threshold for AMHs were, respectively, 1 m/s, 0.7 mN, 52°C (A) and 0.7 m/s, 1.56 mN, 45°C (B); CV and mechanical threshold for AMs were 1.3 m/s, 5.7 mN (C) and 0.7 m/s, 0.7 mN (D). Calibration bars = 20 mV and 5 ms (spikes, A–D), 10 s (heat traces, A–D), and 100 μm (camera lucida reconstructions, A–D).
HEAT-INSENSITIVE AFFERENTS.
Unlike the above, roughly half (64/129) of all myelinated nociceptors (and all LTMRs) failed to respond to noxious heating of the RF within 30 s of temperatures >53°C (hereafter referred to as AM; e.g., Fig. 1E). Many were exposed to temperatures exceeding 53°C for >40 s (n = 29) and/or with temperatures exceeding 60°C (n = 29) without effect (e.g., Fig. 4, C and D); as noted previously, the few that ultimately discharged but were also rendered mechanically inexcitable by such high temperatures (n = 6) were also included in this category.
Correlation between heat sensitivity and other nociceptor properties
PHYSIOLOGY.
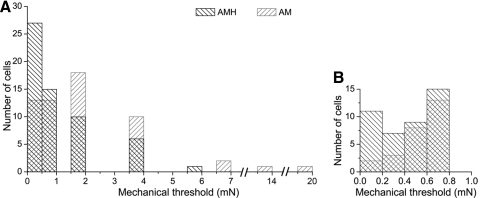
As a group, AMHs exhibited significantly lower mechanical thresholds (median = 0.71 mN; range = 0.08–5.7 mN, n = 59; Fig. 3) than AMs (median = 1.56 mN; range = 0.08–19.5 mN, n = 58; P < 0.01, Mann–Whitney U test), in contrast to findings in primates (Treede et al. 1998); for both, thresholds remained stable across this postnatal period (AMH: R2 = 0.0002, P = 0.92; AM: R2 = 0.008, P = 0.56; data not shown). The difference between groups reflected relatively high mechanical thresholds in a few AMs (Fig. 3A) and the greater prevalence among AMH (n = 18) than that among AM (n = 5) of relatively low thresholds (0.08–0.2 mN; Fig. 3B); interestingly, the most vigorous heat responses were observed among the latter (Fig. 2, A and E; cf. Treede et al. 1998). Outside mechanical thresholds, no other physiological differences were apparent between AMH and AM populations overall (spike amplitude: AMH, 69.6 ± 9.2 mV; AM, 69.1 ± 11.1 mV; overshoot: AMH, 15.0 ± 12.0 mV; AM, 13.3 ± 11.9 mV; spike duration: AMH, 1.5 ± 0.6 ms; AM, 1.5 ± 0.5 ms; AHP duration: AMH, 5.2 ± 2.6 ms; AM, 6.4 ± 5.5 ms), although this was also found to be age dependent (see following text).
Fig. 3.
A: superimposed histograms of AMH and A-mechanonociceptor (AM) mechanical thresholds from P2–P10 neonates; forces ≤1 mN are shown at expanded scale in B; note the prevalence of AMHs in this range.
CENTRAL ANATOMY.
As noted earlier, well-labeled central arbors were recovered from 34 myelinated nociceptors. Two populations could be readily distinguished in neonates as previously described (Woodbury and Koerber 2003); representative examples are shown in Fig. 4. The most numerous (n = 28, e.g., Fig. 4, A and C) projected in the dorsal column and gave rise to recurving, dorsally directed collaterals that arborized throughout laminae I–V, whereas a few (n = 6, e.g., Fig. 4, B and D) projected in Lissaur's tract and arborized in laminae I and IIo. The relative percentages of these two types, hereafter referred to as lamina I–V and I/IIo nociceptors, respectively, agree with previous mouse studies (Boada and Woodbury 2008; Woodbury and Koerber 2003).
Both laminae I/IIo and I–V nociceptors were represented within AMH and AM groups (Fig. 4) and thus there was no strict correlation overall between central anatomy and heat sensitivity. However, preliminary findings suggested this was age dependent. For example, 70% (9/13) of lamina I–V nociceptors were AMHs in neonates ≤P5 compared with only 27% (4/15) from neonates ≥P6 (Table 1), suggesting a loss of heat sensitivity in this anatomically identified population. Similarly, all (3/3) laminae I/IIo nociceptors were AMHs in neonates ≤P5 compared with only one (of 3) from neonates ≥P6 (Table 1), consistent with the absence of heat sensitivity in this population in adults (Boada and Woodbury 2008; Light and Perl 1979). Although neither age-related comparison reached statistical significance (lamina I–V, P = 0.055; lamina I/IIo, P = 0.4, Fisher's Exact two-tailed test), these results from anatomically identified subpopulations paralleled findings across our larger sample of physiologically characterized cells.
Table 1.
Laminar termination patterns of neonatal AMH and AM nociceptors
| AMH (n = 17) |
AM (n = 17) |
|||
|---|---|---|---|---|
| P2–P5 | P6–P10 | P2–P5 | P6–P10 | |
| Laminae I/IIo | 3 | 1 | 0 | 2 |
| Laminae I–V | 9 | 4 | 4 | 11 |
Postnatal reduction in AMH number
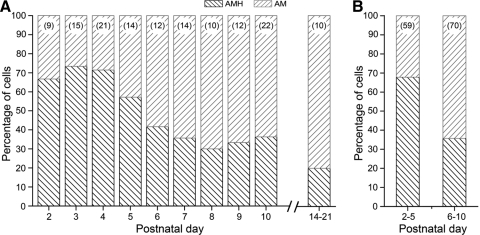
The relative percentages of AMHs and AMs encountered across different postnatal ages are shown in Fig. 5; myelinated nociceptors characterized in juvenile mice (P14–P21) in preliminary experiments are also included for comparison. As seen in Fig. 5A, AMHs predominated in younger neonates, whereas AMs predominated in older neonates, with the balance shifting between P5 and P6. Dividing the sample accordingly into two groups (i.e., P2–P5 and P6–P10, Fig. 5B) illustrates that AMHs represented about 68% (40/59) of the myelinated nociceptor population in P2–P5 neonates compared with about 36% (25/70) in P6–P10 neonates (P < 0.001, χ2 test). Both heat threshold and peak discharge rate remained stable across this postnatal period (P2–P10: threshold: R2 = 0.007, P = 0.51; peak rate: R2 = 0.013, P = 0.37; data not shown). The percentage of AMHs in P6–P10 neonates was similar to that in juveniles (Fig. 5A; P = 0.48, Fisher's Exact test) and in adult mouse hairy skin in vitro (Koltzenburg et al. 1997; Lawson et al. 2008; Stucky et al. 1999). It should be noted that these samples included all cases where sensitivity to noxious heat could be determined, regardless of membrane potential or spike cutoff criteria (methods). These results suggest that the majority of myelinated nociceptors are heat sensitive shortly after birth and that AMH numbers decline to adult levels near the start of the second postnatal week. However, various alternative explanations might also account for these findings.
Fig. 5.
A: percentages of AMHs and AMs encountered across different postnatal days; numbers in parentheses (top) represent total numbers of myelinated nociceptors (AMH and AM) characterized at each age. B: combined percentages of AMHs and AMs for age groups P2–P5 and P6–P10; note that AMH numbers show a significant decline in the 2nd postnatal week.
One possibility—that our sample from P2–P5 neonates included developing unmyelinated mechanoheat (e.g., C-polymodal) nociceptors—was effectively precluded during data collection by excluding the most slowly conducting population of cells in each nerve (methods; Fig. 1B). Although this could not be corroborated immunocytochemically (i.e., myelinated nociceptors show little if any neurofilament staining in early neonates; Lawson and Waddell 1991; C. Cassidy and C. J. Woodbury, unpublished observations), identity in many cases was confirmed independently through analyses of central anatomy. Overall, central projections were recovered from 30% (12/40) of P2–P5 AMHs (and 33% [10/30] of all AMHs with CVs ≤0.8 m/s, 5 of which had CVs <0.7 m/s). All exhibited termination patterns diagnostic of myelinated nociceptors as described earlier (see also Boada and Woodbury 2008; Woodbury and Koerber 2003); indeed, the slowest conducting AMH (0.61 m/s) was a typical laminae I–V nociceptor, with multiple dorsally recurving collaterals arising from parent axons in the dorsal column (for C-nociceptor central anatomy in neonates, see Fig. 7B in Woodbury et al. 2001).
To evaluate the possibility that many AMHs lost mechanical sensitivity in older neonates (and were therefore overlooked given our dependence on mechanical search stimuli, e.g., Treede et al. 1998), the total number of silent myelinated “nociceptors” encountered (i.e., fast BS cells for which no cutaneous RF could be located) was monitored in a series of experiments (N = 13). We found no significant increase (relative to the total number of fast BS cells) in older neonates, as would be predicted if a large fraction of AMHs became mechanically insensitive in the second week [P2–P5: 3/34 (8.8%); P6–P10: 6/46 (13%); P = 0.73, Fisher's Exact test]. Moreover, identical results were obtained when the number of silent myelinated “LTMRs” was monitored in experiments [N = 26; i.e., fast uninflected narrow-spiked (NS) cells with no peripheral RF divided by all fast uninflected NS cells encountered; P2–P5: 3/32 (9.4%); P6–P10: 5/30 (16.7%); P = 0.71, Fisher's Exact test]. Although these combined findings suggest that silent cells in these experiments were afferents whose RFs were removed during dissection or were subsequently damaged, the existence of normally silent myelinated nociceptors and LTMR-like cutaneous afferents cannot be ruled out.
Additional control experiments found that AMHs constituted only 30% (8/27) of myelinated nociceptors in ice-anesthetized P6–P8 neonates (N = 8; data not shown), similar to pharmacologically anesthetized P6–P8 neonates (P = 0.59, χ2 test) and significantly less than ice-anesthetized P2–P5 neonates (see earlier text; P < 0.001, χ2 test), suggesting that the decline in AMHs did not reflect technical differences related to preanesthesia (methods). Further, results obtained in P2–P5 neonates from TRPV1 null mutants and C57Bl/6 controls (see following text) effectively preclude the possibility that these results might reflect technical differences in electrodes (e.g., differential biases toward AMHs and AMs at different ages) or the skin's insulative capacity in P2–P5 and P6–P10 neonates.
Phenotypic switch?
During the course of these experiments, we noticed that the spikes of AMs and AMHs often differed in appearance in younger neonates. In view of anatomical evidence suggesting a loss of heat sensitivity in identified subpopulations (see earlier text), we examined our larger physiological data set for evidence that might confirm or reject this putative change in phenotype.
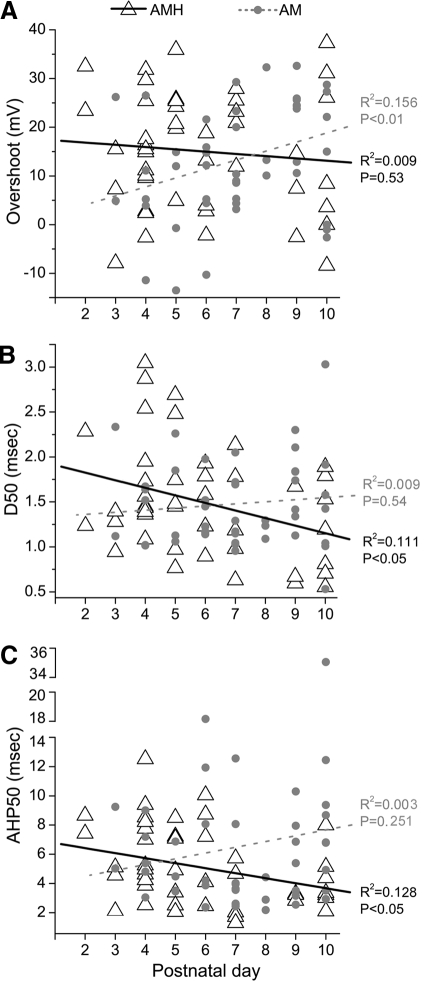
In analyses of action potential properties, spike overshoot was larger in AMHs than that in AMs in early neonates (P2–P5; Fig. 6A, Table 2), possibly indicating that AM somata were more easily damaged by penetration than AMH somata in young neonates; spike amplitude was also larger in P2–P5 AMHs relative to that in AMs, although this did not reach statistical significance (P = 0.09). Over time, however, this discrepancy between AMHs and AMs disappeared due to significant increases in AP amplitudes and overshoot among AMs in the P6–P10 group (Table 2); by contrast, spike amplitude and overshoot remained stable in AMHs, with no significant difference observed between P2–P5 and P6–P10 groups (Table 2), similar to LTMR controls (amplitude: R2 = 0.102, P = 0.16; overshoot: R2 = 0.128, P = 0.11; data not shown). Opposite trends were also observed in both spike (Fig. 6B) and AHP duration (Fig. 6C), both of which decreased over time in AMHs (Table 2), possibly reflecting better impalements in older cells; a decrease was also observed in LTMR spike (R2 = 0.463, P < 0.01) but not AHP duration (R2 = 0.007, P = 0.72; data not shown). By contrast, AM spike and AHP durations did not show this age-related decrease (Fig. 6, B and C); instead, the ranges of spike and AHP durations increased in the P6–P10 AM group (Table 2). That is, the P6–P10 AM category contained broader spikes than those seen in the P2–P5 group, a finding that runs counter to developmental predictions of spikes becoming narrower over time (discussion).
Fig. 6.
Developmental alterations in physiological properties of AMHs and AMs in early postnatal life. Regression analyses of age-related distributions of (A) spike overshoot, (B) spike duration at half-amplitude (D50), and (C) AHP duration at half-amplitude (AHP50). Note that developmental trends in AMs are opposite to those seen among AMHs (and LTMRs, data not shown; see text) and run counter to developmental predictions.
Table 2.
Postnatal physiological changes in AMH and AM nociceptors
| Factor | AMH |
AM |
||
|---|---|---|---|---|
| P2–P5 | P6–P10 | P2–P5 | P6–P10 | |
| Average postnatal age, days | 3.6–1.0; 40 | 8.2–1.6; 25 | 3.8–1.1; 19 | 8.3–1.5; 45 |
| Conduction velocity, m/s | 0.8 ± 0.2‡‡‡* (0.6–3.2; 40) | 1.3 ± 0.6 (0.6–3.2; 25) | 1.0 ± 0.4‡‡‡ (0.7–2.5; 19) | 1.6 ± 0.8 (0.6–4.9; 45) |
| Mechanical threshold, mN# | 0.71* (0.08–3.9; 36) | 0.71 (0.08–5.7; 23) | 1.13 (0.4–13.7; 16) | 1.56 (0.08–19.5; 42) |
| Heat threshold, °C | 49.7 ± 3.6 (42.2–55.2; 40) | 48.8 ± 3.7 (38.0–53.3; 25) | ||
| Spike amplitude, mV | 70.0 ± 10.1 (45.5–88.4; 25) | 69.2 ± 8.2 (54.5–86.4; 20) | 63.7 ± 12.0 (46.4–84.1; 11) | 70.8 ± 10.4 (40.8–93.6; 35) |
| Spike overshoot, mV | 16.5 ± 11.4* (−7.9–35.9; 25) | 13.2 ± 12.8 (−8.4–37.3; 20) | 7.2 ± 13.0‡ (−13.5–26.5; 11) | 15.2 ± 11.1 (−10.3–32.6; 35) |
| Spike duration (D50), ms | 1.7 ± 0.6‡ (0.8–3.0; 25) | 1.3 ± 0.5 (0.6–2.1; 20) | 1.5 ± 0.5 (1.0–2.3; 11) | 1.5 ± 0.5 (0.5–3.0; 35) |
| AHP duration (AHP50), ms | 5.9 ± 2.6‡ (2.1–12.5; 25) | 4.3 ± 2.5 (1.3–10.1; 20) | 5.6 ± 2.2 (3.1–9.2; 11) | 6.7 ± 6.2 (2.2–35.1; 35) |
Values represent means ± SD (range; n), except
(median, Mann–Whitney U test.
Difference (P < 0.05) within the same cell types (e.g., AMH or AM) across different age groups; ‡‡P < 0.01;
P < 0.001.
Difference (P < 0.05) across different cell types within the same age group.
The CVs of AMs also exhibited counterintuitive trends. At P2–P5, CVs of AMHs were significantly slower than AMs (Table 2). As expected, there was a strong positive correlation between age and CV in both AMHs and LTMRs (AMH: R2 = 0.372, P < 0.0001; LTMR: R2 = 0.463, P < 0.005; data not shown). However, a significant correlation between age and CV was not present in AMs (R2 = 0.052, P = 0.07); instead, the range of CVs among AMs increased with age since the older group contained afferents with CVs slower than those of the younger group (Table 2), findings that also run counter to expectation. Combined, these analyses suggest that the AM category in older neonates became populated by cells with broader spikes and slower conducting axons than those seen in younger AMs, properties characteristic of AMHs in younger neonates. Although these findings are only correlative, such counterintuitive developmental trends are consistent with the early loss of heat sensitivity among many AMHs suggested by anatomical analyses (see earlier text).
Mechanisms of heat loss
To begin to address potential mechanisms underlying these changes, experiments were conducted in early neonates from TRPV1 null-mutant mice (N = 4) to evaluate the possibility that the decline in AMHs might reflect transient expression of TRPV1 in a subset of myelinated nociceptors (e.g., Hjerling-Leffler et al. 2007). Interestingly, only 27% (6/22) of myelinated nociceptors were heat sensitive in P2–P5 TRPV1−/− neonates, indistinguishable from the percentages in older (i.e., P6–P10) SW neonates (see earlier text) and similar to percentages in TRPV1−/− adults (Lawson et al. 2008); heat thresholds and peak discharge were not significantly different from those of the samples from SW neonates (data not shown).
These findings suggest that TRPV1 is not required for heat sensitivity in myelinated nociceptors (see also Lawson et al. 2008). However, in sharp contrast to the results in P2–P5 SW neonates (see earlier text), only 29% (8/28) of myelinated nociceptors responded to heat in P2–P5 neonates from the background strain for TRPV1 null mutants (C57Bl/6; N = 5). The latter finding therefore signifies that TRPV1−/− mice shed little light on mechanisms underlying the loss of heat sensitivity in myelinated nociceptors in SW neonates. Such remarkable strain differences were unexpected and clearly warrant further investigation.
DISCUSSION
The present studies examined heat sensitivity across a large sample of cutaneous myelinated nociceptors in early postnatal life using ex vivo somatosensory system preparations from neonatal mice. In Swiss–Webster (SW) neonates, we found that heat-sensitive myelinated nociceptors exhibited diverse central morphologies and that heat sensitivity was not correlated with central anatomy at these ages, thus arguing against the possible existence of a separate divergent pathway (i.e., “labeled-line”) for heat-sensitive myelinated nociceptors. We also found that a surprisingly large number of myelinated nociceptors were heat sensitive within the first few days after birth but that this number dropped to adult levels near the end of the first postnatal week. All available evidence suggested that this decline reflects a loss of heat sensitivity among myelinated nociceptors. However, we also found that this early transient excess of heat-sensitive myelinated nociceptors appears to be strain dependent because it was not present in early C57Bl/6 neonates.
Heat sensitivity in myelinated nociceptors
The present findings of a dramatic postnatal decline in the percentage of AMHs in SW neonates are consistent with in vitro studies in adult mouse hairy skin, where only 21–30% of myelinated nociceptors responded to heat (Koltzenburg et al. 1997; Lawson et al. 2008; Stucky et al. 1999). Interestingly, in vivo studies of trunk hairy skin in adult SW mice have revealed fewer AMHs (<10%; Boada and Woodbury 2007, 2008), suggesting that prolonged aqueous immersion, as used for in vitro experiments, may have influenced these results. Regardless, these and other findings in mice contrast sharply with recent findings that most myelinated nociceptors are heat sensitive in primates (i.e., 80–86%; Treede et al. 1995, 1998). Although the simplest explanation for this discrepancy may be genuine species differences (see also Fitzgerald and Lynn 1977), it is important to note that few in vivo studies have been conducted in mice (Boada and Woodbury 2007; Cain et al. 2001) and none using laser-based heating protocols (Treede et al. 1995, 1998; cf. Perl 1968) that are critically dependent on wavelength (Iannetti et al. 2006). In the present in vitro studies, technical limitations prevented faster heating rates (methods), and whereas slow heating rates are optimal for estimating threshold (Bessou and Perl 1969; Tillman et al. 1995), it is well established that myelinated nociceptors respond better to faster rates (e.g., Yeomans and Proudfit 1996).
Heat sensitivity among neonatal nociceptors
The present studies found that the average heat threshold of myelinated nociceptors in neonates (∼49°C) was comparable to that of adult animals (Campbell and Meyer 1996). Despite the fact that neonatal rodents are unlikely to encounter such high temperatures and thus the adaptive significance of precocious development of adult heat thresholds in myelinated nociceptors remains unclear, it is notable that much lower heat thresholds have been observed in behavioral studies (≤40°C in Falcon et al. 1996; see also Conway et al. 1998; Hiura et al. 1999; Hu et al. 1997). Although heat thresholds of AMHs varied considerably across different ages, the lowest value (38°C) was obtained in an older (P10) neonate. Thus despite excess AMHs in young neonates, thresholds in our sample are too high to account for the marked thermal hypersensitivity seen in behavioral studies. Interestingly, preliminary findings suggest that C-polymodal nociceptors also cannot account for these behavioral thresholds because their average heat threshold in neonates (50.2°C; range = 46.7–56.1°C, n = 8) was higher than that in adults (41.5°C; methods). The population(s) of sensory neurons underlying the low withdrawal thresholds observed in behavioral studies therefore remains to be determined.
Postnatal changes in myelinated nociceptor phenotype
AM nociceptors displayed a number of developmental trends across diverse physiological properties that ran counter to predictions and suggested that the AM category was heterogeneous. For example, spike widths were narrower than those of AMHs in early neonates but became broader over time, opposite to the normal developmental trends seen in both LTMR controls and AMHs. Indeed, a postnatal decrease in spike duration in AMHs and LTMRs mirrors all previous studies of electrically excitable cells and is most clearly evident in studies of identified cells and/or homogeneous populations (e.g., Baccaglini and Spitzer 1977; Gao and Ziskind-Conhaim 1998; Kellerth et al. 1971; Tsuzuki et al. 1995). By contrast, maturation of spike properties in heterogeneous populations, especially DRG neurons that display diverse spike shapes that are correlated with function (Fang et al. 2005a; Koerber et al. 1988; Rose et al. 1986), has been difficult to address using unidentified cells (e.g., Baccaglini 1978; Fulton 1987; Lechner et al. 2009). The earliest recordings from functionally identified DRG neurons have found that spike properties are already adultlike at birth (Woodbury and Koerber 2003, 2007; Woodbury et al. 2001; cf. Fulton 1987), indicating that stereotypical spike properties are acquired prenatally (see Baccaglini 1978; Lechner et al. 2009). Interestingly, nociceptor spike width was recently suggested to increase during prenatal development (Lechner et al. 2009), providing a possible exception to the general rule and potential parallel with our observations in AMs. However, the findings of Lechner et al. (2009) are difficult to interpret within the context of known populations since the recorded samples (defined as nociceptors solely by the presence of inflected somal spikes) likely included a substantial fraction of C-LTMRs (Seal et al. 2009) as well as both myelinated and unmyelinated nociceptors; because the latter typically display broader inflected spikes than those of myelinated nociceptors or C-LTMRs (e.g., Fang et al. 2005a; Seal et al. 2009; Traub and Mendell 1988), the increase in average duration noted by Lechner et al. (2009) may instead reflect a numerical increase in C-nociceptors and/or differential rates of apoptosis across other populations at later embryonic stages (e.g., Lawson and Biscoe 1979; see reviews in Albers and Davis 2007; Snider and Silos-Santiago 1996); information on prenatal development of identified DRG populations will be needed to clarify this issue.
In addition to unexpected postnatal trends in spike duration among AMs (see earlier text), similar unexpected trends were also evident in AM spike amplitude and overshoot. Further, this change within AMs occurred simultaneously with the appearance of afferents with slower conducting peripheral axons. In combination, the simplest explanation is that AMHs in young neonates, which are characterized by slower conducting peripheral axons and broad, large-amplitude spikes, lost heat sensitivity over time and ultimately became classified as AMs. This idea is also supported by anatomical findings (results) and by similar age-related changes in the expression patterns of calcitonin gene-related peptide and transient receptor potential vanilloid 2 (TRPV2) between AMH and AM groups (Gutierrez et al. 2007). Thus multiple independent lines of evidence point to a phenotypic change in this population.
Mechanisms underlying loss of heat sensitivity
The cellular mechanisms underlying this early loss of heat sensitivity are not yet clear, although developmental changes in expression of noxious heat-sensitive TRP channels are the most obvious candidates (Caterina et al. 1999). Of these, the capsaicin receptor (TRPV1) would appear to represent the most viable candidate (Hjerling-Leffler et al. 2007) since TRPV2 is not correlated with heat sensitivity in myelinated nociceptors (Lawson et al. 2008; see also Gutierrez et al. 2007; Park et al. 2008). However, AMH number is unaffected in adult TRPV1 null-mutant mice (Lawson et al. 2008) and our results in P2–P5 TRPV1−/− neonates mirror this finding; combined, these results reveal that TRPV1 also does not normally play a significant role in myelinated nociceptor heat sensitivity, in accord with inferences based on immunocytochemistry (Caterina et al. 1999; Rashid et al. 2003) and primate studies in which capsaicin sensitivity is an unreliable predictor of heat sensitivity in myelinated nociceptors (Ringkamp et al. 2001).
Nevertheless, neonatal capsaicin is known to be neurotoxic to a relatively large fraction of thinly myelinated primary afferents (∼25–40%; Lawson 1987; Nagy et al. 1983), many of which are presumably nociceptors. It is conceivable that a similar fraction of myelinated nociceptors in SW mice transiently express TRPV1 in early postnatal life, a finding that could obviously account for the transient excess in AMH number in early neonates from this strain. Indeed, the age when the percentage of AMHs dropped to adult levels (P6/7) is in close agreement with the end of the critical period for capsaicin neurotoxicity (e.g., Saporta 1986). This possibility—that down-regulation of TRPV1 in a subset of AMH may define the critical period for capsaicin neurotoxicity in myelinated fibers—would be consistent with developmental trends in both anatomy and physiology found here (results). This would of course also argue against selective cell death of AMHs as the cause of their early decline (see also Berg and Farel 2000; Lawson 1979), although it remains to be determined whether neonatal capsaicin is neurotoxic to myelinated fibers in SW mice.
It is noteworthy in this regard that previous observations of neonatal capsaicin neurotoxicity on myelinated fibers were confined to rats and are not without controversy (cf. Nagy et al. 1983; Scadding 1980). Clearly, the finding that AMH number is unaffected in early TRPV1−/− neonates (results; see also Lawson et al. 2008) predicts that neonatal capsaicin should not be neurotoxic to myelinated primary afferents in C57Bl/6 mice, just as found in the CBA strain (Scadding 1980). Although this scenario is seemingly contradicted by recent findings of a postnatal decrease in capsaicin-sensitive IB4-negative DRG cells in C57Bl/6 mice (Hjerling-Leffler et al. 2007), cell identity was unknown in the latter studies and the observed decrease occurred a week later than that in the present study (i.e., P14; see also Saporta 1986).
Surprisingly, although early TRPV1−/− neonates failed to exhibit excess AMHs as in early SW neonates, the findings from P2 to P5 wildtype (C57Bl/6) control neonates were identical to those of TRPV1 knock-outs. Although these unanticipated results warrant further investigation, they effectively negate the possibility that our electrodes were biased against AMs in early neonates or that the decline in AMHs reflects physical changes in skin properties between P2–P5 and P6–P10. Indeed, taken together these findings contribute to mounting evidence of significant strain differences in mice (see also Mogil et al. 2005; Woodbury et al. 2004; cf. Milenkovic et al. 2008).
Whether this early strain difference simply reflects pigmentation state (e.g., SW mice are albino, whereas the C57Bl/6 strain is pigmented) is unclear at present. Although it is well established that mutations in stem cell factor (SCF) and/or c-kit signaling, recently discovered modulators of heat sensitivity (Milenkovic et al. 2007), produce albinism via loss of melanocytes, the underlying cause of albinism in SW mice reflects a defect in the C-locus (i.e., tyrosinase; melanocytes in the albino SW strain are amelanotic) and thus there is little evidence to suggest involvement of SCF/c-kit in the postnatal loss of heat sensitivity among myelinated nociceptors in SW mice. Further, with respect to differences in melanin content between strains, melanin's effects have been shown to be mainly photoprotective and it is well established that skin behaves as a nearly perfect black body for longer wavelengths, regardless of melanin content (Hardy 1934); importantly, the slow rates of conductive heat transfer in these experiments would likely have produced identical thermal gradients in the skin of both strains (Henriques and Moritz 1947). To our knowledge, additional strain differences in the developing skin of early neonates have not been documented, although future investigations into a growing list of potential candidates that actively modulate heat sensitivity, many involving signaling from nonneuronal cells (e.g., see Lumpkin and Caterina 2007; Malin et al. 2008; Mandadi et al. 2009; Negri et al. 2006; and references therein) may prove fruitful in this regard.
Ultimately, consideration of potential mechanisms underlying the reduction of AMH number in SW neonates must take into account known postnatal changes in trophic factor signaling. However, the most obvious candidate, nerve growth factor (NGF), cannot easily explain these findings. Thus although it is well established that myelinated nociceptors are extremely sensitive to alterations in NGF levels during this early postnatal period (Ritter et al. 1991) and that NGF exerts potent control over nociceptor heat sensitivity (Bennett et al. 1998; Lewin and Mendell 1994; Rueff and Mendell 1996), the latter effects do not manifest until P4 (Zhu et al. 2004), which coincidentally is when AMH numbers begin dropping sharply in SW neonates (results). Moreover, NGF levels in the skin of rats begin increasing over this period (Constantinou et al. 1994) and even excess NGF fails to alter the number of AMHs or their sensitivity to heat in mice (Stucky et al. 1999). This may signify that the direct actions of NGF on heat sensitivity (e.g., Zhang et al. 2005) are restricted to unmyelinated nociceptors; the same may also apply to SCF whose actions depend on TRPV1 (Milenkovic et al. 2007).
Nevertheless, postnatal changes in NGF signaling might be indirectly related to the present findings. An important clue stems from recent findings of trkA-negative myelinated nociceptors in adult rats (Fang et al. 2005b) and mice (Woodbury, unpublished observations), some of which appear to depend instead on neurotrophin-3/trkC signaling (McIlwrath et al. 2007; see also Tamura et al. 2005); in rats, the latter has no direct effect on nociceptor heat sensitivity (Malcangio et al. 1997; Shu et al. 1999) and, indeed, appears to abolish it (Wilson-Gerwing et al. 2005). In this light, the present findings could indicate that some trkA-negative myelinated nociceptors in adults switch trophic factor dependence in early postnatal life, down-regulating trkA as in many unmyelinated nociceptors (Molliver et al. 1997) and consequently losing noxious heat sensitivity through secondary effects on TRPV1 expression (Wilson-Gerwing et al. 2005). Additional information on trophic factor dependences of myelinated nociceptors throughout postnatal development will be needed to resolve these questions.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-44094 to C. J. Woodbury and National Center for Research Resources Grant RR-015640-.
ACKNOWLEDGMENTS
Present address of Y. Ye: Department of Oral and Maxillofacial Surgery, University of California San Francisco, 521 Parnassus Avenue, San Francisco, CA 94143.
REFERENCES
- Albers KM, Davis BM. The skin as a neurotrophic organ. Neuroscientist 13: 371–382, 2007 [DOI] [PubMed] [Google Scholar]
- Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci 26: 2981–2990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini PI. Action potentials of embryonic dorsal root ganglion neurones in Xenopus tadpoles. J Physiol 283: 585–604, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccaglini PI, Spitzer NC. Developmental changes in the inward current of the action potential of Rohon–Beard neurones. J Physiol 271: 93–117, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck PW, Handwerker HO, Zimmermann M. Nervous outflow from the cat's foot during noxious radiant heat stimulation. Brain Res 67: 373–386, 1974 [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Koltzenburg M, Priestley JV, Shelton DL, McMahon SB. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J Neurosci 10: 1282–1291, 1998 [DOI] [PubMed] [Google Scholar]
- Berg JS, Farel PB. Developmental regulation of sensory neuron number and limb innervation in the mouse. Dev Brain Res 125: 21–30, 2000 [DOI] [PubMed] [Google Scholar]
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol 32: 1025–1043, 1969 [DOI] [PubMed] [Google Scholar]
- Boada MD, Woodbury CJ. Physiological properties of mouse skin sensory neurons recorded intracellularly in vivo: temperature effects on somal membrane properties. J Neurophysiol 98: 668–680, 2007 [DOI] [PubMed] [Google Scholar]
- Boada MD, Woodbury CJ. Myelinated skin sensory neurons project extensively throughout adult mouse substantia gelatinosa. J Neurosci 28: 2006–2014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C. Neonatal Immunity Clifton, NJ: Humana Press, 2005 [Google Scholar]
- Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol 90: 541–562, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol 85: 1561–1574, 2001 [DOI] [PubMed] [Google Scholar]
- Campbell JN, LaMotte RH. Latency to detection of first pain. Brain Res 266: 203–208, 1983 [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Cutaneous nociceptors. In: Neurobiology of Nociceptors, edited by Belmonte C, Cervero F. Oxford, UK: Oxford Univ. Press, 1996, p. 117–145 [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441, 1999 [DOI] [PubMed] [Google Scholar]
- Constantinou J, Reynolds ML, Woolf CJ, Safieh-Garabedian B, Fitzgerald M. Nerve growth factor levels in developing rat skin: upregulation following skin wounding. Neuroreport 5: 2281–2284, 1994 [DOI] [PubMed] [Google Scholar]
- Conway CM, Martinez J, Lytle LD. Maturational changes in the thermal nociceptive responses of developing rats. Dev Psychobiol 33: 47–60, 1998 [PubMed] [Google Scholar]
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J Neurophysiol 69: 1071–1081, 1993 [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Aβ-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Rev 46: 131–145, 2004 [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol 223: 424–447, 1984 [DOI] [PubMed] [Google Scholar]
- Falcon M, Guendellman D, Stolberg A, Frenk H, Urca G. Development of thermal nociception in rats. Pain 67: 203–208, 1996 [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN. TrkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci 25: 4868–4878, 2005b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol 565: 927–943, 2005a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci 6: 507–520, 2005 [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Lynn B. The sensitization of high threshold mechanoreceptors with myelinated axons by repeated heating. J Physiol 265: 549–563, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton BP. Postnatal changes in conduction velocity and soma action potential parameters of rat dorsal root ganglion neurones. Neurosci Lett 73: 125–130, 1987 [DOI] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol 80: 3047–3061, 1998 [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol 39: 71–83, 1976 [DOI] [PubMed] [Google Scholar]
- Gutierrez S, Ye Y, Woodbury CJ. Heat sensitivity among neonatal myelinated nociceptors is not correlated with either TRPV2 or CGRP. Soc Neurosci Abstr 33: 722.3, 2007 [Google Scholar]
- Hardy JD. The radiation of heat from the human body: III. The human skin as a black-body radiator. J Clin Invest 13: 615–620, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques FC, Jr, Moritz AR. Studies of thermal injury. I. The conduction of heat to and through skin and the temperatures attained therein. A theoretical and an experimental investigation. Am J Pathol 23: 530–549, 1947 [PMC free article] [PubMed] [Google Scholar]
- Hiura A, Nakagawa H, Koshigae Y, Yoshizako A, Kubo Y, Ishizuka H. Age-related changes in the response to thermal noxious heat and reduction of C-fibers by neonatal treatment with capsaicin. Somatosens Mot Res 16: 115–121, 1999 [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Al-Qatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci 27: 2435–2443, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Hu R, Berde CB. Neurologic evaluation of infant and adult rats before and after sciatic nerve blockade. Anesthesiology 86: 957–965, 1997 [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Tracey I. Similar nociceptive afferents mediate psychophysical and electrophysiological responses to heat stimulation of glabrous and hairy skin in humans. J Physiol 577: 235–248, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Ogawa H. Primate cutaneous thermal nociceptors. J Physiol 216: 77–78, 1971 [PubMed] [Google Scholar]
- Kellerth JO, Mellström A, Skoglund S. Postnatal excitability changes of kitten motoneurones. Acta Physiol Scan 83: 31–41, 1971 [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol 60: 1584–1596, 1988 [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol 78: 1841–1850, 1997 [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Thalhammer JG, Torebjork HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci 2: 765–781, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain 9: 298–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol 8: 275–294, 1979 [DOI] [PubMed] [Google Scholar]
- Lawson SN. The morphological consequences of neonatal treatment with capsaicin on primary afferent neurones in adult rats. Acta Physiol Hung 69: 315–321, 1987 [PubMed] [Google Scholar]
- Lawson SN, Biscoe TJ. Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J Neurocytol 8: 265–274, 1979 [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 435: 41–63, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Frenzel H, Wang R, Lewin GR. Developmental waves of mechanosensitivity acquisition in sensory neuron subtypes during embryonic development. EMBO J 28: 1479–1491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Mendell LM. Regulation of cutaneous C-fiber heat nociceptors by nerve growth factor in the developing rat. J Neurophysiol 71: 941–949, 1994 [DOI] [PubMed] [Google Scholar]
- Light AM, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol 186: 133–150, 1979 [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature 445: 858–865, 2007 [DOI] [PubMed] [Google Scholar]
- Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hind limb. Brain Res 238: 29–43, 1982 [DOI] [PubMed] [Google Scholar]
- Malcangio M, Garrett NE, Cruwys S, Tomlinson DR. Nerve growth factor- and neurotrophin-3-induced changes in nociceptive threshold and the release of substance P from the rat isolated spinal cord. J Neurosci 17: 8459–8467, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KA, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain 138: 484–496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflügers Arch 458: 1093–1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci 26: 1801–1812, 2007 [DOI] [PubMed] [Google Scholar]
- Milenkovic N, Frahm C, Gassmann M, Griffel C, Erdmann B, Birchmeier C, Lewin GR, Garratt AN. Nociceptive tuning by stem cell factor/c-Kit signaling. Neuron 56: 893–906, 2007 [DOI] [PubMed] [Google Scholar]
- Milenkovic N, Wetzel C, Moshourab R, Lewin GR. Speed and temperature dependences of mechanotransduction in afferent fibers recorded from the mouse saphenous nerve. J Neurophysiol 100: 2771–2783, 2008 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Heiko A, Hofmann HA, Hordo C, Messlinger K, Nemmani KVS, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci USA 102: 12938–12943, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19: 849–861, 1997 [DOI] [PubMed] [Google Scholar]
- Nagy JI, Iversen LL, Goedert M, Chapman D, Hunt SP. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci 3: 399–406, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Colucci M, Margheriti F, Melchiorri P, Vellani V, Tian H, De Felice M, Porreca F. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci 26: 6716–6727, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park U, Guan Y, Raja SN, Caterina MJ. In vivo function of TRPV2 in nociception. Soc Neurosci Abstr 34: 265.4, 2008 [Google Scholar]
- Perl ER. Myelinated afferent fibres innervating the primate skin and their response to noxious stimuli. J Physiol 197: 593–615, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Inoue M, Bakoshi S, Ueda H. Increased expression of vanilloid receptor 1 on myelinated primary afferent neurons contributes to the antihyperalgesic effect of capsaicin cream in diabetic neuropathic pain in mice. J Pharmacol Exp Ther 306: 709–717, 2003 [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci 21: 4460–4468, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AM, Lewin GR, Kremer NE, Mendell LM. Requirement for nerve growth factor in the development of myelinated nociceptors in vivo. Nature 350: 500–502, 1991 [DOI] [PubMed] [Google Scholar]
- Rose RD, Koerber HR, Sedivec MJ, Mendell LM. Somal action potential duration differs in identified primary afferents. Neurosci Lett 63: 259–264, 1986 [DOI] [PubMed] [Google Scholar]
- Rueff A, Mendell LM. Nerve growth factor and NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophysiol 76: 3593–3596, 1996 [DOI] [PubMed] [Google Scholar]
- Saporta S. Loss of spinothalamic tract neurons following neonatal treatment of rats with the neurotoxin capsaicin. Somatosens Res 4: 153–173, 1986 [DOI] [PubMed] [Google Scholar]
- Scadding JW. The permanent anatomical effects of neonatal capsaicin on somatosensory nerves. J Anat 131: 471–482, 1980 [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462: 651–655, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu XQ, Llinas A, Mendell LM. Effects of trkB and trkC neurotrophin receptor agonists on thermal nociception: a behavioral and electrophysiological study. Pain 80: 463–470, 1999 [DOI] [PubMed] [Google Scholar]
- Snider WD, Silos-Santiago I. Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Philos Trans R Soc Lond B Biol Sci 351: 395–403, 1996 [DOI] [PubMed] [Google Scholar]
- Stucky CL, Koltzenburg M, Schneider M, Engle MG, Albers KM, Davis BM. Overexpression of nerve growth factor in skin selectively affects the survival and functional properties of nociceptors. J Neurosci 19: 8509–8516, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S, Morikawa Y, Senba E. TRPV2, a capsaicin receptor homologue, is expressed predominantly in the neurotrophin-3-dependent subpopulation of primary sensory neurons. Neuroscience 130: 223–228, 2005 [DOI] [PubMed] [Google Scholar]
- Thalhammer JG, LaMotte RH. Spatial properties of nociceptor sensitization following heat injury of the skin. Brain Res 231: 257–265, 1982 [DOI] [PubMed] [Google Scholar]
- Tillman DB, Treede RD, Meyer RA, Campbell JN. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: estimates of receptor depth and threshold. J Physiol 485: 753–765, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, Fitzgerald M. Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. J Physiol 550: 255–261, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ, Mendell LM. The spinal projection of individual identified A-δ- and C-fibers. J Neurophysiol 59: 41–55, 1988 [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol 80: 1082–1093, 1998 [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 483: 747–758, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki S, Yoshida S, Yamamoto T, Oka H. Developmental changes in the electrophysiological properties of neonatal rat oculomotor neurons studied in vitro. Neurosci Res 23: 389–397, 1995 [DOI] [PubMed] [Google Scholar]
- Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VMK. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci 25: 758–767, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J Neurosci 23: 601–610, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Koerber HR. Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. J Comp Neurol 505: 547–561, 2007 [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, Koerber HR. Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J Comp Neurol 436: 304–323, 2001 [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci 24: 6410–6415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Woodbury CJ. Acute effects of neonatal inflammation on peripheral properties of myelinated but not unmyelinated cutaneous nociceptors. Soc Neurosci Abstr 34: 170.8, 2008 [Google Scholar]
- Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain 68: 141–150, 1996 [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J 24: 4211–4223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol 92: 3148–3152, 2004 [DOI] [PubMed] [Google Scholar]