Abstract
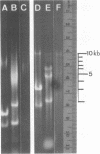
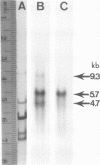
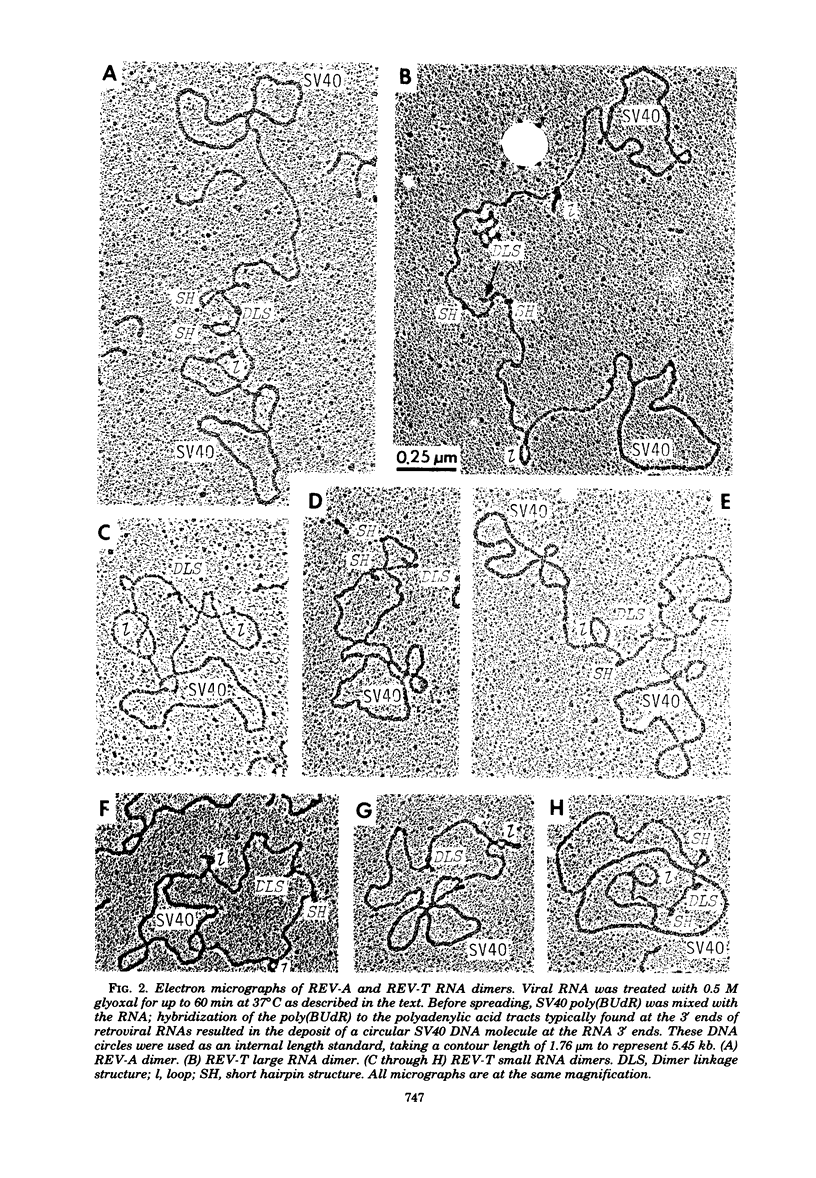
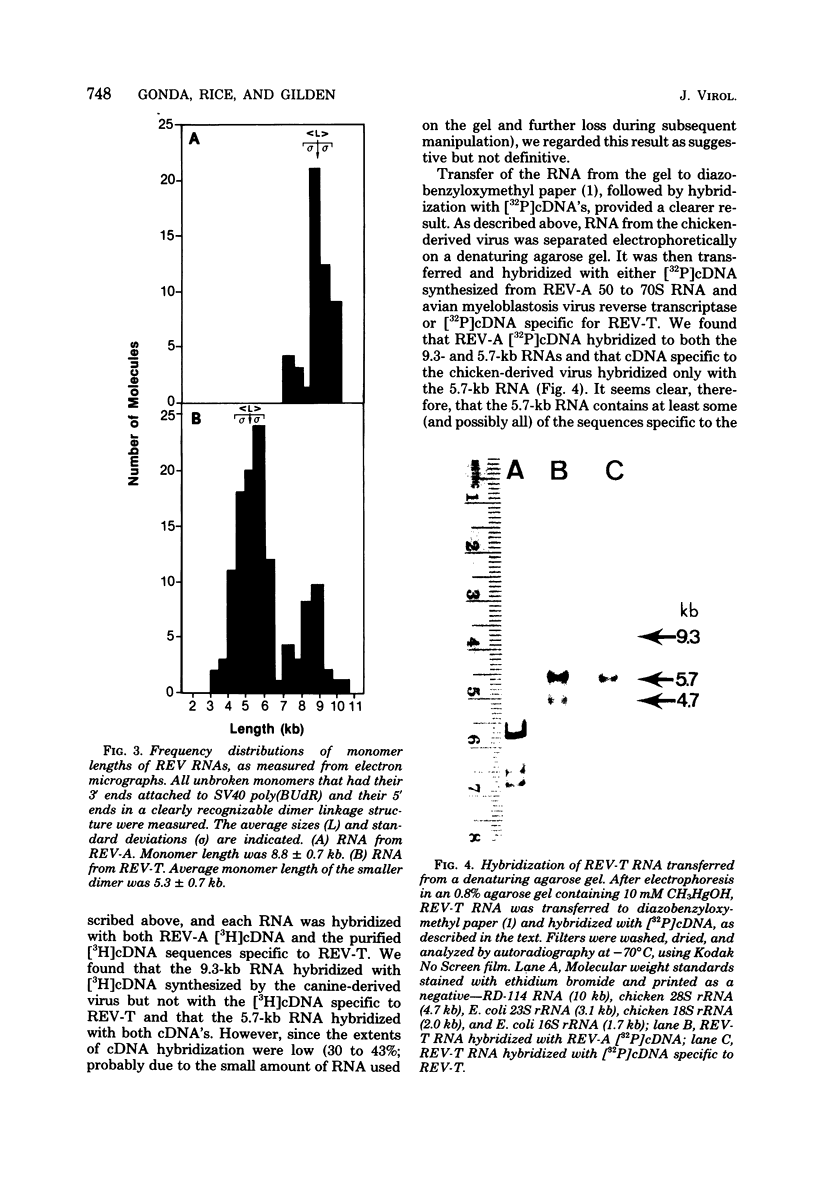
Reticuloendotheliosis virus strain T (REV-T) is a type C retrovirus known to transform avian fibroblasts, spleen cells, and bone marrow cells and to produce virulent reticuloendotheliosis in young chicks. Analysis of REV-T high-molecular-weight RNA by electrophoresis in denaturing gels and by electron microscopy revealed the presence of at least two classes of molecules. One class appeared in CH3HgOH gels to have a monomer length of 9.3 kilobases (kb); in electron microscopic spreads under mildly denaturing conditions, it existed as a typical retrovirus dimer, having a monomer length of 8.8 +/- 0.7 kb. The second class also existed as a dimer, with a monomer length of 5.7 kb in CH3HgOH gels. Hybridization with REVA-A 32P-labeled complementary DNA revealed a third size class of molecules (4.7 kb), which were not resolvable from the 5.7-kb class in electron microscope spreads and which comigrated with chicken 28S rRNA in denaturing gels. Only the 9.3-kb class was found in the reportedly nontransforming virus produced after infection of canine thymus cell line with REV-T. Thus, REV-T appears to be similar to the murine and feline sarcoma viruses and the avian acute leukemia viruses in that it consists of a nontransforming helper virus genome and a defective genome responsible for oncogenicity. Our previous results demonstrated the presence in REV-T and in uninfected chicken cellular DNA of some nucleotide sequences not found in virus produced by the canine line (S. Simek and N. Rice, J. Virol. 33:320--329, 1980). In this report we show by hybridization with highly specific 32P-labeled complementary DNAs that REV-T-specific sequences exist within the 5.7-kb genome. Since 32P-labeled complementary DNA synthesized from the canine-derived virus genome hybridized with all three classes of RNAs, we conclude that the 5.7-kb genome is a recombinant between some sequences found in the putative helper and some sequences specific to REV-T. As with the other oncogenic viruses mentioned above, these specific sequences appear to be derived from host DNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P., Goldfarb M. P., Weinberg R. A. A defined subgenomic fragment of in vitro synthesized Moloney sarcoma virus DNA can induce cell transformation upon transfection. Cell. 1979 Jan;16(1):63–75. doi: 10.1016/0092-8674(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bender W., Chien Y. H., Chattopadhyay S., Vogt P. K., Gardner M. B., Davidson N. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J Virol. 1978 Mar;25(3):888–896. doi: 10.1128/jvi.25.3.888-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Davidson N., Kindle K. L., Taylor W. C., Silverman M., Firtel R. A. The structure of M6, a recombinant plasmid containing Dictyostelium DNA homologous to actin messenger RNA. Cell. 1978 Nov;15(3):779–788. doi: 10.1016/0092-8674(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Bender W., Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976 Apr;7(4):595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- Benton C. V., Hodge H. M., Fine D. L. Comparative large-scale propagation of retroviruses from Old World (Mason-Pfizer monkey virus) and New World (squirrel monkey virus) primates. In Vitro. 1978 Feb;14(2):192–199. doi: 10.1007/BF02618222. [DOI] [PubMed] [Google Scholar]
- Breitman M. L., Lai M. M., Vogt P. K. The genomic RNA of avian reticuloendotheliosis virus REV. Virology. 1980 Jan 30;100(2):450–461. doi: 10.1016/0042-6822(80)90535-8. [DOI] [PubMed] [Google Scholar]
- Chien U. H., Lai M., Shih T. Y., Verma I. M., Scolnick E. M., Roy-Burman P., Davidson N. Heteroduplex analysis of the sequence relationships between the genomes of Kirsten and Harvey sarcoma viruses, their respective parental murine leukemia viruses, and the rat endogenous 30S RNA. J Virol. 1979 Sep;31(3):752–760. doi: 10.1128/jvi.31.3.752-760.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S., Kung H. J., Bender W., Davidson N., Ostertag W. Size, subunit composition, and secondary structure of the Friend virus genome. J Virol. 1976 Oct;20(1):264–272. doi: 10.1128/jvi.20.1.264-272.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Neubauer R. L., Fischinger P. J. Fractionation of DNA nucleotide transcripts from Moloney sarcoma virus and isolation of sarcoma virus-specific complementary DNA. J Virol. 1976 May;18(2):481–490. doi: 10.1128/jvi.18.2.481-490.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. B., Kang C. Y., Min-Min Wan K., Bose H. R., Jr Transformation of chick embryo fibroblasts by reticuloendotheliosis virus. Virology. 1977 Dec;83(2):313–321. doi: 10.1016/0042-6822(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Franklin R. B., Bose H. R., Jr Transformation by reticuloendotheliosis virus: development of a focus assay and isolation of a nontransforming virus. Virology. 1979 Feb;93(1):20–30. doi: 10.1016/0042-6822(79)90272-1. [DOI] [PubMed] [Google Scholar]
- Hoelzer J. D., Lewis R. B., Wasmuth C. R., Bose H. R., Jr Hematopoietic cell transformation by reticuloendotheliosis virus: characterization of the genetic defect. Virology. 1980 Jan 30;100(2):462–474. doi: 10.1016/0042-6822(80)90536-x. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Lai M. M., Vogt P. K. Genome of avian myelocytomatosis virus MC29: analysis by heteroduplex mapping. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1265–1268. doi: 10.1073/pnas.76.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Wong T. C., Holmes K. V. Comparative ultrastructural study of four reticuloendothelias viruses. J Virol. 1975 Oct;16(4):1027–1038. doi: 10.1128/jvi.16.4.1027-1038.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. Structure, subunit composition, and molecular weight of RD-114 RNA. J Virol. 1975 Aug;16(2):397–411. doi: 10.1128/jvi.16.2.397-411.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. J., Hu S., Bender W., Bailey J. M., Davidson N., Nicolson M. O., McAllister R. M. RD-114, baboon, and woolly monkey viral RNA's compared in size and structure. Cell. 1976 Apr;7(4):609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- Maisel J., Bender W., Hu S., Duesberg P. H., Davidson N. Structure of 50 to 70S RNA from Moloney sarcoma viruses. J Virol. 1978 Jan;25(1):384–394. doi: 10.1128/jvi.25.1.384-394.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R., Jr Group-specific antigen shared by the members of the reticuloendotheliosis virus complex. J Virol. 1976 Mar;17(3):983–990. doi: 10.1128/jvi.17.3.983-990.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Rup B. J., Spence J. L., Hoelzer J. D., Lewis R. B., Carpenter C. R., Rubin A. S., Bose H. R., Jr Immunosuppression induced by avian reticuloendotheliosis virus: mechanism of induction of the suppressor cell. J Immunol. 1979 Sep;123(3):1362–1370. [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Donner L., Turek L. P. Restriction endonuclease mapping of unintegrated proviral DNA of Snyder-Theilen feline sarcoma virus: localization of sarcoma-specific sequences. J Virol. 1979 Dec;32(3):860–875. doi: 10.1128/jvi.32.3.860-875.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek S., Rice N. R. Analysis of the nucleic acid components in reticuloendotheliosis virus. J Virol. 1980 Jan;33(1):320–329. doi: 10.1128/jvi.33.1.320-329.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]