Abstract
The role of auditory cortex in sound localization and its recalibration by experience was explored by measuring the accuracy with which ferrets turned toward and approached the source of broadband sounds in the horizontal plane. In one group, large bilateral lesions were made of the middle ectosylvian gyrus, where the primary auditory cortical fields are located, and part of the anterior and/or posterior ectosylvian gyrus, which contain higher-level fields. In the second group, the lesions were intended to be confined to primary auditory cortex (A1). The ability of the animals to localize noise bursts of different duration and level was measured before and after the lesions were made. A1 lesions produced a modest disruption of approach-to-target responses to short-duration stimuli (<500 ms) on both sides of space, whereas head orienting accuracy was unaffected. More extensive lesions produced much greater auditory localization deficits, again primarily for shorter sounds. In these ferrets, the accuracy of both the approach-to-target behavior and the orienting responses was impaired, and they could do little more than correctly lateralize the stimuli. Although both groups of ferrets were still able to localize long-duration sounds accurately, they were, in contrast to ferrets with an intact auditory cortex, unable to relearn to localize these stimuli after altering the spatial cues available by reversibly plugging one ear. These results indicate that both primary and nonprimary cortical areas are necessary for normal sound localization, although only higher auditory areas seem to contribute to accurate head orienting behavior. They also show that the auditory cortex, and A1 in particular, plays an essential role in training-induced plasticity in adult ferrets, and that this is the case for both head orienting responses and approach-to-target behavior.
INTRODUCTION
The involvement of the auditory cortex in sound localization has been demonstrated by showing that removal or inactivation of the cortex in one hemisphere in carnivores and primates disrupts their ability to approach or discriminate sound sources in the contralateral hemifield (Heffner and Heffner 1990; Jenkins and Masterton 1982; Jenkins and Merzenich 1984; Kavanagh and Kelly 1987; Malhotra et al. 2004), whereas bilateral manipulations impair localization in both lateral hemifields (Heffner and Heffner 1990; Heffner and Masterton 1975; Kavanagh and Kelly 1987; Malhotra et al. 2004; Smith et al. 2004). However, the magnitude of the reported deficits depends on multiple factors, including the region and extent of the cortex affected and the method used to measure localization performance. Although these studies have consistently shown that the auditory cortex plays a critical role in the accuracy of locomotor responses to auditory targets, the effects of cortical lesions on reflexive head orienting responses are less clear cut (Beitel and Kaas 1993; Thompson and Masterton 1978). This could mean that independent neural circuits are responsible for reflexive orientation of the head toward a sound source and the ability to perceive a sound as coming from a particular location in space.
To maintain accurate sound localization in different acoustic environments, for example, when going from an open space to an enclosed room, it is essential that the neural processing of auditory spatial cues can be recalibrated according to the context in which the stimuli occur. Behavioral studies in humans (Hofman et al. 1998; Shinn-Cunningham et al. 1998) and ferrets (Kacelnik et al. 2006) have shown that considerable plasticity can also be induced by modifying the localization cue values. Although there is extensive evidence that auditory cortical response properties can be shaped by learning or by other changes in sensory inputs (Dahmen and King 2007), it is not yet known to what extent the spatial sensitivity of these neurons is plastic or whether the cortex is even required for bringing about adaptive changes in localization accuracy.
We have previously characterized sound localization behavior in ferrets by measuring both the head orienting and approach-to-target responses made by the animals following presentation of broadband sounds from loudspeakers covering the full 360° of sound azimuth (Kacelnik et al. 2006; Nodal et al. 2008; Parsons et al. 1998). In this study, we explore the neural circuitry responsible for translating auditory signals into motor commands by investigating the effects on the accuracy of both the initial head orienting movement and the subsequent locomotor response of bilaterally aspirating either the primary auditory cortex (A1) alone or larger areas of the cortex that also encompass nonprimary fields. We then used the same animals to determine the role of the cortex in the rapid, training-induced plasticity of auditory localization that has previously been described in ferrets (Kacelnik et al. 2006), by investigating the effects on these behaviors of reversibly occluding one ear. Our findings show that auditory cortex is required for both normal localization and its recalibration by experience.
METHODS
All experiments were carried out in accordance with the Animals (Scientific Procedures) Act 1986, and licensed by the UK Home Office and approved by the local ethical review committee at the University of Oxford. A total of 10 adult pigmented ferrets (Mustela putorius furo) from our breeding colony were used in this study, of which 7 received bilateral cortical lesions and therefore provided their own prelesion data. The remaining three ferrets provided control data for the earplugging experiment. The methods used to train the ferrets have been described in detail in previous reports (Kacelnik et al. 2006; Nodal et al. 2008) and are outlined briefly below.
Animal home environment and welfare
The ferrets were housed in standard laboratory cages and the environment was enriched with different objects such as balls, plastic tubes, and shelters. At least two times a week, the animals were allowed outside their cages so that they could interact with other ferrets and experience a more varied behavioral repertoire.
Each sound localization testing period lasted a maximum of 14 consecutive days, in which drinking water was provided only during the twice daily training sessions. Normal access to dry food was given in their cages. Average daily water consumption by ferrets was measured to be ∼60 ml/kg; when the total daily amount consumed during the testing sessions was less than this, supplementary fluid was provided at the end of each day's testing in the form of a puree made of ground food pellets and water. Body weights were recorded daily and compared with the baseline weight for each animal determined before the start of the water regulation paradigm. The maximum weight drop allowed during testing was 2 SD below their individual mean baseline weight. Between each 14-day testing period, animals were allowed breaks of ≥4 days, during which they had free access to water.
Apparatus and stimuli
The localization task was carried out in a circular arena of 70-cm radius enclosed by a hemispheric mesh dome, located inside a double-walled testing chamber. Twelve loudspeakers were positioned at 30° intervals around the perimeter of the arena and hidden from the animal by a muslin curtain. The animal had to stand on a raised platform near the center of the arena to initiate a trial by licking a centrally positioned waterspout. This ensured that its head was positioned at the center of the arena, facing the speaker at 0°, when sound stimuli were delivered at the beginning of each trial. Speakers to the animal's left were denoted by negative numbers and those to the right by positive numbers. A waterspout was also positioned below each of the speakers from which the animal received a small amount of water if it correctly judged the location of the sound source. Our software recorded which reward spout the animal licked first on each trial, thereby registering the magnitude and direction of the localization errors.
All stimuli were broadband noise bursts (with a low-pass cut-off frequency of 30 kHz) generated afresh on each trial using Tucker-Davis Technologies (Alachua, FL) System 2 hardware. The stimuli were filtered using the inverse transfer function for each speaker to obtain a flat spectrum and matched for overall level across the different speakers. The animals were initially trained to approach the speakers using continuous noise. Once the animals had learned the task, data were collected using sound durations of 2,000, 1,000, 500, 200, 100, and 40 ms. In each testing session, the sound duration was kept constant while the level was roved pseudorandomly from trial to trial in 7-dB steps from 56 to 84 dB SPL. This was done to disrupt potential “absolute level cues” arising from the acoustic shadowing caused by the animal's body and which might otherwise allow target localization based on the relative loudness of the stimulus.
Approach-to-target task
Naïve animals took about a week to learn the task. They were trained to stand on the central platform and lick the start spout continuously for 500–2,000 ms until the stimulus was presented from 1 of the 12 possible speaker locations. They were allowed ≤15 s to approach and lick 1 of the 12 corresponding reward spouts before the next trial could be started. Water rewards were delivered only if the animal made a correct response by licking the spout associated with the speaker from which the stimulus had been presented. To reduce the possibility of bias toward particular speaker locations, an incorrect response was followed by a correction trial (same stimulus and location) up to two times. If the animal continued to mislocalize the sound, an easy trial (comprising a continuous series of noise bursts from the same location) was presented. Neither the correction nor the easy trials were included in the analysis. Typically each 14-day testing period started with the longest sound duration (2,000 ms), which was gradually reduced after ≥300 trials had been performed at each of the stimulus durations.
Head orienting responses
In addition to the approach-to-target responses, we measured the change in head orientation following the presentation of the stimulus by tracking the movement of a self-adhesive reflective strip attached to an area of shaved skin along the midline of the animal's head. Using an overhead infrared-sensitive camera and video contrast detection device (HVS Image, Harlow, UK), the x-y-coordinates of the reflective strip were registered at a rate of 50 frames/s for 1 s after stimulus onset. From these coordinates, we calculated the angular extent of the orienting response relative to the initial head position. The animal was considered to have initiated an orienting response if a head movement in the same direction was recorded over three consecutive frames, with the time of the first frame taken as the latency of the movement. The initial head turn was considered over when a change in the direction of the movement was recorded. The time of the last frame was taken as end time. The final head bearing was calculated as the mean angle from the last three frames of the movement. Trials were excluded if the initial head angle (at the time of sound onset) deviated by >7° from straight ahead or if the head movement latency exceeded 500 ms.
Experimental design
Seven ferrets received bilateral lesions of the auditory cortex. All but two of the animals (ferrets 9932 and 0318) were extensively trained on an azimuth sound localization task and transferred to an elevation discrimination task (Bizley et al. 2007) before receiving bilateral cortical lesions of different extents. In one group, we aimed to restrict the lesions to A1, whereas, in the other group, more extensive lesions were made that were intended to include both primary and nonprimary auditory cortical areas on the ectosylvian gyrus (EG) (Bizley et al. 2005).
After the lesions had been made, those ferrets that had been used in the vertical localization task were retrained in azimuth localization and the performance of all seven animals compared with that measured before surgery. We have previously found that ferrets readily switch between azimuth and elevation testing, and no differences in azimuth performance were apparent between animals that had performed the elevation task and those that did not. Once the postlesion data had been collected, we measured the capacity of the animals to relearn to localize sound in the horizontal plane after disrupting binaural cues by inserting an earplug in the left ear. This was done in the same way as described previously for animals with an intact auditory cortex (Kacelnik et al. 2006). The ferrets were sedated with medetomidine hydrochloride (domitor, 0.15 mg/kg; Pfizer, Sandwich, UK) and the external auditory meatus occluded with a foam plug (E.A.R., Boulder, CO). The concha of the external ear was filled with Otoform-K2 silicone impression material (Dreve Otoplastik, Unna, Germany). The sound attenuation produced by these earplugs has previously been estimated, using auditory brain stem response audiometry, to be ∼40 dB across a broad range of frequencies (Moore et al. 1989). Acoustical measurements from a number of different model ferret ears created from Otoform imprints of variously sized real ears confirmed that the earplugs produced 40–50 dB of attenuation at frequencies of >3.5 kHz, which gradually rolled off at lower frequencies. Otoscopic examination was used to confirm that the ear canals were unobstructed both before and after monaural occlusion.
Lesion procedure
The surgery to lesion the auditory cortex was performed under general anesthesia and aseptic conditions. All animals were administered atropine sulfate (0.06 mg/kg, Atrocare, Animal Care, York, UK) to minimize pulmonary secretions and dexamethasone (0.5 mg/kg, Dexadreson, Intervet UK, Milton Keynes, UK) to prevent brain edema, and received perioperative analgesia with Vetergesic (0.15 ml of buprenorphine hydrochloride, i.m.; Alstoe Animal Health, Melton Mowbray, UK).
For the extended lesions, three ferrets were anesthetized with an intramuscular (2 ml/kg) injection of Saffan (alphaxalone 9 mg/ml and alphadolone acetate 3 mg/ml, Schering-Plough, Hertfordshire, UK), with supplementary doses (1/3 of initial dose) administered to maintain a deep areflexic state throughout surgery. The animal was placed in a stereotaxic frame, the scalp was incised along the midline, and the temporal muscles were retracted bilaterally to allow access to the cranium over the EG. Once the EG was exposed by a craniotomy, the dura was retracted, and the lesions were made by gentle aspiration using a glass pipette connected to a suction pump. The extent of the area to be aspirated was judged intrasurgically from the sulcal pattern on the surface of the cortex. For the four animals that received restricted lesions of A1, the surgery was performed after intravenous administration of ketamine/medetomidine (Ketaset, 5 mg/kg/h, Fort Dodge Animal Health, Southampton, UK; Domitor, 22 μg/kg/h, Pfizer Animal Health, Sandwich, UK). Electrophysiological recordings were used to help determine the ventral limit of A1 on the caudal part of the middle EG (MEG). The lesion extended from here to the dorsal tip of the EG at its border with the suprasylvian sulcus. In these cases, care was taken to preserve the underlying white matter. The void space created by the aspiration of the cortex was filled with absorbable gel foam, the bone flap was put back, and the temporal muscle and scalp were sutured.
Behavioral testing started ∼2 wk after the cortical lesion surgery and, in most cases, was carried out over the next 3–6 mo. We observed no changes in performance during this time. In each case, the final experiment involved measuring the capacity of the ferrets to adapt to altered spatial cues produced by occluding one ear.
Electrophysiological recordings
In the animals with restricted lesions, electrophysiological recordings were carried out in a darkened anechoic chamber. After making an incision in the dura mater over the MEG, penetrations were made at several locations with a single tungsten electrode that was oriented perpendicular to the surface of the cortex. Broadband noise bursts or pure tones were presented using closed-field earphones (model RPHV 297, Panasonic, Bracknell, UK). Frequency response areas were constructed from the responses to pure tones (100-ms duration, presented pseudorandomly at a rate of 1 Hz from 500 Hz to 24 kHz in 1/3 octave steps and from 0 to 80 dB SPL in 10-dB increments). The tonotopic organization of A1 runs approximately dorso-ventrally (Bizley et al. 2005; Kowalski et al. 1995), so measurements of best frequencies and response latencies were used to determine the low-frequency ventral border of A1 with secondary areas on the posterior EG (PEG). The anterior border with the anterior auditory field (AAF) was estimated from previous recording studies (Bizley et al. 2005; Kowalski et al. 1995), whereas the dorsal and posterior borders of A1 were defined in relation to the sulcal pattern.
Histology
After behavioral testing was finished, the animals were overdosed with pentobarbital and perfused intracardially with 300 ml of 0.9% saline solution, followed by ≥1 liter of freshly made 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.2. The brains were dissected from the skull and embedded in sucrose solution (30% sucrose in 0.1 M PB). After photographs were taken, 50-μm coronal sections were cut at the level of the ectosylvian gyrus and the auditory thalamus. In the restricted lesion group, every section was Nissl stained with 0.5% cresyl violet. In the extensive lesion group, one half of the sections (1 every 100 μm) were Nissl stained, and in the other half, neurodegeneration was detected with a Nauta's selective silver-staining technique (Ye et al. 2001). Coronal sections immunostained for SMI32 (Bajo et al. 2007) taken from our archive were used to estimate the boundary between the MEG and PEG. Histological reconstructions and morphometric analysis were carried out using Neurolucida and StereoInvestigator software (MBF Bioscience, MicroBrightField, Williston, VT) in a Leica DMR microscope (Leica Microsystems, Heerbrugg, Switzerland).
Data analysis
Our software registered the reward spout licked by the animal and converted this to a percent correct-score and error magnitude and direction for each trial. These values, along with the head movement data and the associated stimulus parameters (location, duration, and level), were exported to Excel (Microsoft, Redmond, WA) for further analysis and presentation. The algorithms used to measure head movement latency and accuracy were implemented in advance and carried out automatically, therefore avoiding any possibility of subjective variations in the data analysis. The statistical analysis was done with SPSS software (SPSS, Chicago, IL).
The mutual information (MI) between the approach-to-target response location or final head bearing and the target location was calculated using the formula
where r is the response location or final head bearing, s is the target location, MI(r; s) is the MI between r and s, p(r, s) is the joint probability of r and s, and is equivalent to p(r|s) p(s), where p(s) and p(r) are obtained from the overall distribution of target locations and response (either approach-to-target or head bearing) locations, respectively.
RESULTS
Normal sound localization
All seven ferrets that received bilateral cortical lesions were tested for their ability to localize sound before carrying out the surgery to remove the auditory cortex. The prelesion localization behavior of these animals was entirely consistent with the data from other normal ferrets, which has been published in previous studies from this laboratory (Kacelnik et al. 2006; Nodal et al. 2008). As the stimulus duration was reduced from 2,000 to 40 ms, the accuracy of the approach-to-target responses declined, as shown by a progressive reduction in the proportion of trials in which a correct response was made (Fig. 1, A and B). In contrast, and again in keeping with previous studies (Nodal et al. 2008), localization accuracy was stable across the range of sound levels used (2-way ANOVA: duration F4,124 = 119.66, P < 0.001; level F4,124 = 0.61, P = 0.653; interaction F16,124 = 34.96, P = 0.868). The data collected at each stimulus duration were therefore pooled across sound level.
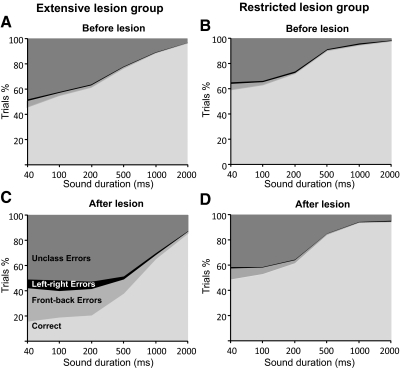
Fig. 1.
Overall performance on the approach-to-target task for both groups before (A and B) and after (C and D) either extensive (A and C) or restricted (B and D) cortical lesions were made. Each panel shows the cumulative percentages of trials for the different sound durations used. Trials are sorted into those in which correct responses were made and, for the incorrect responses, are further subdivided into left-right errors (responses made to the hemifield contralateral to the target), front-back errors (responses made to the anterior hemifield following presentation of sounds in the ipsilateral posterior hemifield and vice versa), and unclassified (i.e., all other) errors. Note that the proportion of incorrect responses made by the animals in the 12-speaker task increased after the lesions had been made, particularly at shorter sound durations, but a much greater impairment was observed in the extensive lesion group.
Although the prelesion data for the two groups showed the same pattern of localization behavior, the ferrets in the extensive cortical lesion performed slightly less well, achieving scores that were 5–10% lower than those in the restricted lesion group (Fig. 1, A and B). This mainly reflected a difference in performance between the two groups for frontal speaker locations (compare left and right panels in the top rows of Fig. 2, A and B) and explained the lower values of the MI between the response and target locations for all sound durations (Fig. 3). These differences almost certainly reflect a difference in the way in which these two groups of ferrets were initially trained; a grid was attached to the central platform through which the ferrets in the extensive lesion group had to insert their heads to trigger a trial by licking the central spout, whereas this grid was not used to train the animals in the restricted lesion group. This also likely explains why the animals in the extensive lesion group took longer to reach the reward spouts (mean ± SD: 2.52 ± 1.15 s) than those in the restricted lesion group (1.87 ± 0.90 s). Consequently, we did not combine the data from these two groups and instead examined the effects of the lesions on sound localization by comparing the performance of the animals with that obtained within each group before surgery.
Fig. 2.
Sound localization performance in the approach-to-target task for 2 representative cases (F0140, left column, which received extensive bilateral cortical lesions, and F0317, right column, which received smaller bilateral lesions that were restricted to the primary auditory cortex). Data were obtained at 2 different sound durations: 1,000 (A) and 40 ms (B). The size of the dots indicates, for each of the 12 target locations, the proportion of responses to different loudspeaker/reward-spout locations. For each plot, the value of the overall mutual information (MI) between response and target location is shown at the bottom right. A: localization performance at 1,000 ms was slightly impaired after extensive cortical lesions, but remained unaltered after restricted lesions. B: this difference was also apparent at 40 ms, where a modest disruption was seen after restricted cortical lesions and a much more substantial impairment was found after extensive cortical lesions.
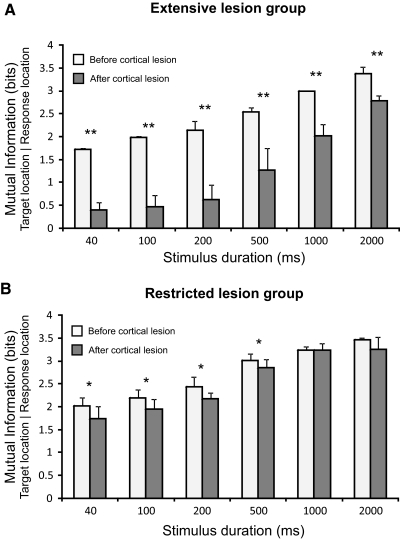
Fig. 3.
The performance of the animals in the approach-to-target task was quantified by measuring the mutual information between the target and response locations. The mean and SD of these values are plotted across sound duration for the extensive lesion (A) and restricted lesion (B) groups both before (white bars) and after (gray bars) the cortex had been aspirated. In keeping with the change in the percentage of correct responses, the MI values were lower for shorter sound durations. The MI values were significantly reduced in both groups following the lesions, but a much larger effect was found in the extensive lesion group. **P < 0.01, *P < 0.05.
At the longest stimulus durations, prelesion performance was very consistent across different target locations. This is shown for noise bursts of 1,000 ms in duration in Fig. 2A. A reduction in stimulus duration primarily affected performance at lateral and posterior locations, as shown for 40-ms noise bursts in Fig. 2B. The effect of stimulus duration on auditory localization behavior is shown by the statistically significant differences in the MI between the target and response locations as the duration was reduced (Fig. 3; extensive lesion group F5,12 = 40.6, P < 0.001; restricted lesion group F5,6 = 12.47, P = 0.004). Post hoc Scheffé tests showed that significant differences were found in the extensive lesion group between the distribution of approach-to-target responses at the three shortest stimulus durations (40, 100, and 200 ms) and that obtained at 2,000 ms, whereas, in the restricted lesion group, significant differences were found between the performance at the three shortest durations and that measured for all stimulus durations ≥500 ms.
Although the proportion of errors increased as the stimulus duration was reduced, their magnitude remained fairly consistent. Most of the incorrect responses were made to the adjacent reward spout, which were therefore categorized as an error of 30°, the smallest value that could be recorded in our 12-speaker setup. Incorrect responses were divided into left-right errors (responses made to the hemifield contralateral to the target), front-back errors (responses made to the anterior hemifield following presentation of sounds in the ipsilateral posterior hemifield and vice versa), and unclassified (i.e., all other) errors. Around 90% of the errors made before aspirating the cortex were unclassified and include those of 30° in size. The incidence of left-right errors varied significantly but inconsistently with stimulus duration (F11,18 = 2.954, P = 0.02), ranging from only 0.08 ± 0.12% of all trials at 2,000 ms to 1.40 ± 0.62% at 40 ms. A clearer trend was seen with the front-back errors, which increased in incidence as the stimulus duration was reduced (F11,29 = 4.92, P = 0.001) from 0.48 ± 0.59% of all trials at 2,000 ms to 5.52 ± 2.19% at 40 ms (Fig. 1, A and B).
We also recorded the head orienting responses made during the first second after the onset of the stimulus as the animals turned toward the target. Consistent with the paucity of left-right errors, the animals almost always turned toward the appropriate side of the midline and a clear correlation was apparent between the final bearing and the location of the target (Fig. 4). Although the head orienting response typically undershot the target location, the amplitude of these movements increased with the eccentricity of the target up to target locations at 90°, after which no further increases were observed, presumably marking the point at which the animals left the central platform so that whole body could be turned toward the sound source. The effect of stimulus duration on the head orienting responses was quantified by measuring the MI between the final head bearing and the target location. In contrast to the accuracy of the prelesion approach-to-target responses, these values remained unchanged as the stimulus duration was altered (Fig. 5; extensive lesion group F5,6 = 1.66; P = 0.275; restricted lesion group F5,12 = 0.06; P = 0.997). This highlights the stereotyped nature of the head orienting responses, which are most likely triggered by the onset of the sound and less dependent on how long it lasts.
Fig. 4.
Effects of cortical lesions on sound-evoked head orienting responses. Final bearing of the initial head movement for the same representative cases shown in Fig. 2 (F0140 and F0317) in response to sounds of 2 different durations, 1,000 (A) and 40 ms (B), before and after the lesions were made. Each plot shows in grayscale the conditional probability of different final head bearings, with a bin size of 7.5°, for each target location. For each plot the value of the overall mutual information between final bearing and target location is shown at the bottom right. As with the approach-to-target responses, little effect was seen for long duration noise bursts (A), whereas for brief sounds, the clear correlation observed between the final head bearing and target location in the prelesion data was seriously disrupted after extensive cortical lesions, but only slightly affected after restricted lesions (B).
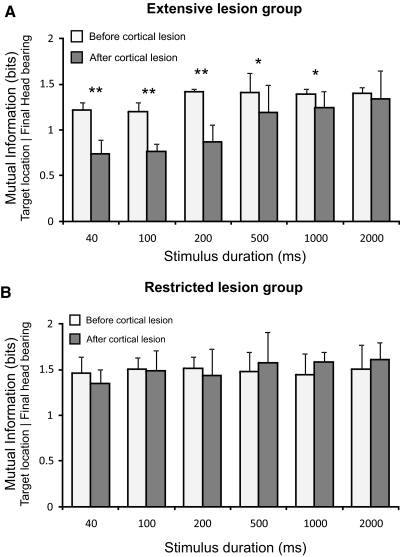
Fig. 5.
Mean and SD of the mutual information values between target location and final head bearing across sound duration for the extensive lesion (A) and restricted lesion (B) groups both before (white bars) and after (gray bars) the cortex had been aspirated. In contrast to the percent correct responses in the approach-to-target task, the MI values for the prelesion data were relative constant across sound duration. Extensive cortical lesions caused a significant reduction of the MI values for sounds of <500 ms in duration (A), whereas restricted cortical lesions did not alter the MI values (B). **P < 0.01, *P < 0.05.
In keeping with the differences observed in their approach-to-target behavior, the prelesion head orienting responses were found to differ somewhat between the two groups of ferrets (Fig. 5). Thus the MI values between head bearing and target location were lower (1.34 ± 0.12 bits) and the latency of these responses longer (262.74 ± 173.43 ms) in the extensive lesion group than in ferrets in which restricted cortical lesions were to be made (MI 1.48 ± 0.17 bits; latency, 186.88 ± 115.15 ms). Again, this can be attributed to differences in the training strategies used in the two groups.
Sound localization after cortical damage
All animals with cortical lesions showed a deficit in sound localization relative to their performance before surgery. These deficits were apparent in both the approach-to-target and head orienting responses. Despite some differences in the extent of the lesions, the performance of the animals within each group was consistent with more pronounced deficits observed in the ferrets with extensive cortical lesions (Fig. 6) and for shorter stimulus durations (Fig. 6; Table 1). Because bilateral lesions were made, we expected to see equivalent deficits on the left and right sides of space. This was confirmed by comparing the percentage of correct responses in each hemifield for the three shortest stimulus durations (40–200 ms; repeated-measures ANOVA; extensive lesion group F2,6 = 3.196; P = 0.114; restricted lesion group F3,8 = 1.402; P = 0.311). In line with prelesion data, midline target positions (0 and 180°) were localized more accurately than lateral positions in the ferrets with restricted lesions, whereas this difference was less evident after the extensive lesions (Table 1).
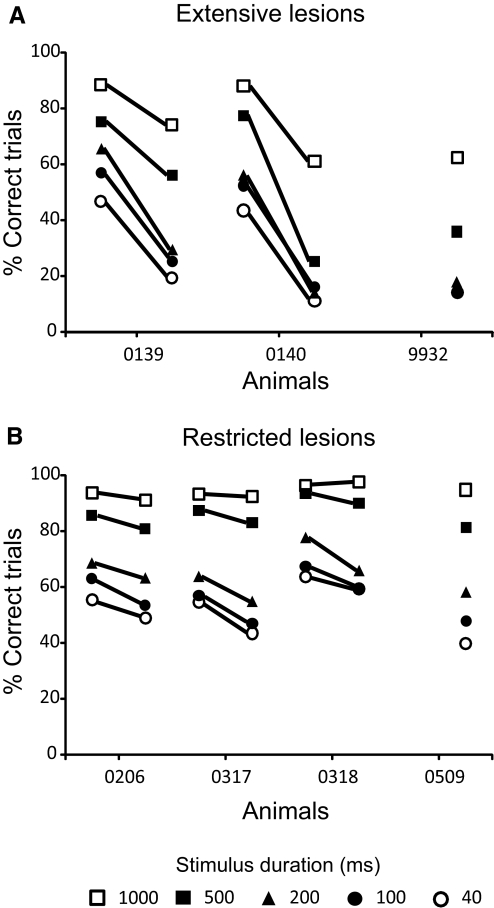
Fig. 6.
Summary of the effects of extensive (A) or restricted (B) cortical lesions on sound localization accuracy. Mean prelesion and postlesion percent correct scores (averaged across all speaker locations) are shown for the individual animals in each group at different sound durations. Note the much larger drop in performance in the extensive lesion group. In 2 cases, equivalent prelesion data were not available because the animals had previously been used in a different auditory task. The postlesion scores of these animals were, however, very similar to those obtained for the other ferrets in each group.
Table 1.
Percentage of correct approach-to-target responses by individual ferrets after bilateral lesions of the auditory cortex
| 40 ms |
100 ms |
200 ms |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Animal | Left | Right | Midline | Left | Right | Midline | Left | Right | Midline |
| 0206 | 36.8 | 48.3 | 80.9 | 44.2 | 47.8 | 91.3 | 59.7 | 60.5 | 80.6 |
| 0317 | 36.2 | 38.4 | 75.0 | 37.72 | 47.3 | 70.8 | 45.2 | 57.0 | 77.0 |
| 0318 | 36.8 | 37.6 | 54.3 | 40.6 | 48.2 | 64.3 | 64.1 | 53.4 | 56.8 |
| 0509 | 54.2 | 53.1 | 89.0 | 45.9 | 63.7 | 80.9 | 62.0 | 62.9 | 86.0 |
| Restricted lesions | 41.0 ± 8.8 | 44.3 ± 7.6 | 74.8 ± 14.8 | 42.1 ± 3.8 | 51.8 ± 8.0 | 76.9 ± 11.8 | 57.8 ± 8.5 | 58.4 ± 4.14 | 75.1 ± 12.7 |
| 9932 | 15.5 | 15.1 | 8.5 | 17.0 | 21.5 | 13.2 | 42.4 | 38.6 | 13.8 |
| 0139 | 15.0 | 15.9 | 40.9 | 23.4 | 20.6 | 40.6 | 29.1 | 24.3 | 43.0 |
| 0140 | 10.2 | 12.1 | 11.8 | 13.3 | 17.0 | 19.8 | 12.56 | 17.06 | 11.1 |
| Extensive lesions | 13.5 ± 2.9 | 14.4 ± 2.1 | 20.4 ± 17.9 | 17.0 ± 5.5 | 17.8 ± 2.5 | 22.7 ± 16.6 | 19.6 ± 8.6 | 21.0 ± 3.7 | 22.4 ± 17.9 |
Values are means ± SD or percentage.
Approach-to-target behavior
After large aspiration lesions encompassing both primary and nonprimary areas of the auditory cortex, the time taken to make an approach-to-target response increased (F1,22938 = 172.935; P < 0.001) and became more variable (before lesion 2.53 ± 1.16 s; after lesion 2.82 ± 1.63 s). Compared with the responses measured before surgery, localization accuracy was reduced at all sound durations tested (Fig. 1, A and C). Nevertheless, it is clear from Fig. 2A that, at least for long sound durations, a correlation remains between the target location and the approach-to-target response made by the animals, suggesting that the removal of large regions of the auditory cortex did not impair their ability to execute the task. Instead, the lower percentage correct scores reflect impaired precision in the localization responses.
At the shortest sound durations, the ferrets with extensive cortical lesions performed very poorly, and their percentage correct scores were only just above the level expected by chance (Fig. 1C). The proportion of all three types of localization error increased, with the largest change being in the incidence of front-back errors, which increased from 4.99 ± 0.16% to 26.84 ± 1.12% for stimuli of 40 ms in duration. Before the cortex was aspirated, these animals made significantly more front-back errors at 40 ms than at other sound durations, whereas, after the lesions, the proportion of front-back errors was higher for all sound durations <500 ms than for longer sounds (F5,17 = 32.146; P < 0.001). Although there was also some increase in left-right errors (Fig. 1C), the animals generally responded to the correct lateral hemifield. This can be clearly observed in the distribution of responses for each target location (Fig. 2B, left column).
The change in localization performance was quantified by measuring the MI between the target and response locations (Fig. 3). The MI values were significantly reduced following the extensive cortical lesions (F1,6 = 113.19; P < 0.001). This was the case at all stimulus durations [t(3) >3.47; P = 0.026], although the largest differences were observed for the briefest sounds (Fig. 3A). These results indicate that the ferrets could still localize long duration sounds, albeit less precisely than before the cortex was lesioned, but could do no more than indicate whether brief sounds were located on the left or right of the midline (Fig. 2B, left column).
Sound localization abilities of the animals with aspiration lesions restricted to A1 were essentially unchanged compared with their prelesion performance at the longest sound durations (≥1,000 ms; Figs. 1, B and D, 2A, right column, and 3B). With briefer noise bursts, however, small deficits were apparent, as indicated by a lower percentage of correct responses (Fig. 1, B and D), and less precise approach-to-target responses (Fig. 2B, right column). The MI values were also significantly reduced after A1 lesions (F1,12 = 25.57; P < 0.001; Fig. 3B). These impairments were found at all target locations but were most pronounced for lateral and posterior stimuli (Fig. 2B, right column). This suggests that lesions of A1 affect localization performance only in the more demanding circumstances, for brief stimuli and for lateral sound directions where spatial acuity is normally not as good as it is at the midline (Table 1). The increase in error magnitude following A1 lesions was in part caused by a significant increase in the proportion of front-back errors (F1,5 = 5.221; P = 0.030), which, at 40 ms, rose from 5.87 ± 3.02% with the cortex intact to 9.18 ± 4.12% after its removal (Fig. 1, B and D). In contrast, no change was found in the incidence of left-right errors. Response times were unaffected by the A1 lesions (prelesion, 1.87 ± 0.90 s; after lesion, 1.84 ± 0.88 s).
Head orienting responses
The head orienting responses made by the animals in the extensive lesion group were also abnormal. The latency of these responses on trials in which the animal approached the correct loudspeaker location increased significantly from 300.66 ± 181.74 to 358.82 ± 206.67 ms for 40-ms sounds [t(544) = 3.63; P < 0.001] and from 267 ± 182.59 to 304.85 ± 194.18 ms at 100 ms [t(623) = 2.49; P = 0.006]. Although the animals usually turned toward the appropriate side (Fig. 4, left column), the head bearing MI values were significantly reduced after the cortical lesion (F11,29 = 4.68; P = 0.002). This effect was most pronounced for stimulus durations of <500 ms (Fig. 5A), at which the distribution of the orienting responses was particularly variable (Fig. 4B, left column). The duration dependent effect of large cortical lesions on orientating behavior is shown by the presence of significant differences in the MI values across stimulus duration after the lesion (F5,17 = 4.363; P = 0.017), whereas this was not the case beforehand (F5,11 = 1.66; P = 0.275).
On the other hand, the overall pattern of head orienting responses was unaffected by A1 lesions, although these responses were more variable, particularly at shorter stimulus durations, than in the prelesion measurements (Fig. 4, right column). A small but significant decrease in the latency of these responses was observed following the lesions (from 186.89 ± 115 to 171.65 ± 90.22 ms; F1, 28650 = 137.945, P < 0.001). The values of the MI between final head bearing and the location of stimuli <500 ms in duration also tended to be more variable after the lesions, but the mean values remained almost unaltered with no significant differences between the prelesion and postlesion data (Fig. 5B; F11,41 = 0.423; P = 0.934). This suggests that the final head bearing is equally informative about target location before and after aspirating A1.
Adaptation to altered localization cues after cortical damage
Unilateral occlusion of one ear alters the localization cues available and, in normal ferrets, impairs their ability to localize both long- and short-duration sounds (Kacelnik et al. 2006). Accurate sound localization can rapidly recover, however, as long as the animals are provided with appropriate training. This plasticity is seen for broadband sounds of different duration, indicating that relearning can take place under both closed-loop (where the animals could potentially use dynamic spatial cues or make corrective adjustments in their responses) and open-loop conditions (where the sound is over before the head starts to move) (Kacelnik et al. 2006). We examined the ability of the animals with cortical lesions to adapt to a unilateral earplug along with an additional group of three normal control ferrets. Because of the deficits observed with brief sounds, we carried out this part of the study using noise bursts of 1,000 ms in duration to ensure that all the ferrets with cortical lesions could localize fairly accurately before the earplug was inserted.
As expected, localization accuracy was much poorer after plugging the left ear. The percentage of correct scores dropped (Fig. 7A) and the head orienting responses were much less accurate (Fig. 8, middle panels). These deficits were most apparent for stimuli presented on the side of the earplug (Fig. 7B), which the ferrets tended to mislocalize to the other side of the midline, although performance was impaired throughout the horizontal plane. The scores achieved on the day the earplug was inserted were much lower in the ferrets with extensive cortical lesions than in those with restricted lesions or in animals with an intact auditory cortex (Fig. 7). Indeed, with the earplug in place, these ferrets often scored at chance levels (8.3% for a 12-speaker task), indicating a complete inability to localize sound in the presence of abnormal spatial cues. The performance of both groups of ferrets with cortical lesions then remained at these levels despite twice-daily sound localization training over the next 8 days while the earplug was still in place. Regression lines fitted to the data obtained during the period of earplug wearing for both groups resulted in slopes of around zero (b = 0.327, R2 = 0.057 for the extensive cortical lesion group; b = 0.296, R2 = 0.030 for the restricted lesion group), which did not differ from one another (F1,13 = 0.507; P = 0.490). We further analyzed the data from the animals with A1 lesions, which scored above chance in the presence of the unilateral earplug. No improvement was observed on either side of space; indeed, the regression lines fitted to the percent correct scores over the period of plugging had negative slopes (left hemifield: b = 1.39, R2 = 0.468; right hemifield: b = 0.71, R2 = 0.064). This lack of improvement was seen not only in the percent correct scores, but also in the size of the errors made, which remained high in both lateral hemifields. This lack of recovery contrasts with the steady improvement in scores over the same period of monaural occlusion in the three control ferrets with an intact cortex (shown by the gray bars in Fig. 7A).
Fig. 7.
Plasticity of sound localization depends on the auditory cortex. A: percent correct scores (averaged across all speaker locations) measured every day over a 10-day period. The performance is shown 1 day before (Pre) and on each of the 8 days after insertion of an earplug in the left ear (Left ear plugged), as well as on the day on which the earplug was removed (Post). The stimuli were 1,000-ms noise bursts. The gray bars depict the range of values obtained from 3 other ferrets with an intact auditory cortex. In these control animals, the percentage of correct responses falls dramatically when the ear is first plugged but recovers toward preplug levels with daily behavioral training. The different symbols correspond to the ferrets with cortical lesions, with the open symbols showing the data from the ferrets with restricted cortical lesions (the dashed line represents the mean scores for this group) and the filled symbols showing the data from 2 of the ferrets with extensive cortical lesions (the solid black line indicates the mean performance; the third animal in this group could not do the task with the earplug in place). In contrast to the controls, the localization abilities of the animals with cortical lesions did not improve during the period of time over which the earplug was worn. B: stimulus-response plots showing the approach-to-target performance before (Pre Plug) and after plugging the left ear (Plugged days 1–3 and Plugged days 7–9) and after removal of the earplug (Post Plug). The size of the dots indicates, for each of the 12 target locations, the proportion of responses to different loudspeaker/reward-spout locations, and the corresponding percent correct scores are given to the right of each panel. Monaural occlusion disrupted sound localization accuracy at all locations tested, but particularly on the side of the earplug. No improvement in performance was seen with daily localization training (middle panels). After earplug removal, sound localization accuracy returned to preplug levels.
Fig. 8.
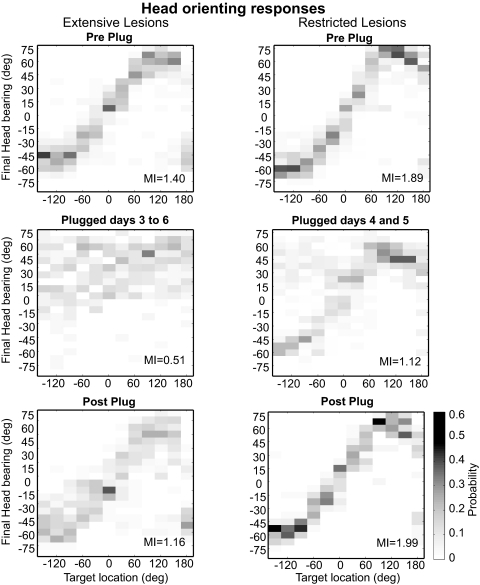
Effect of monaural occlusion on acoustic head orienting behavior in the ferrets with cortical lesions. Each plot shows in grayscale the conditional probability of different final head bearings, with a bin size of 7.5°. These data were obtained from the same trials used to show the approach-to-target responses in Fig. 7. The correlation between final bearing and target location observed before inserting the ear plug (top row) was completely disrupted in the extensive lesion group when the left ear was occluded, with the animals turning toward the right side irrespective of the target location (left middle panel). A smaller bias to the right was also observed in the animals with restricted cortical lesions (right middle panel). In neither group did the accuracy of the orienting responses improve during the period of earplugging. Removal of the earplug resulted in a complete return to normal head orienting behavior (Post Plug).
The recovery of sound localization accuracy with behavioral training in normal ferrets is also seen in the head orienting responses (Kacelnik et al. 2006). Again, however, no improvement was found in the animals with cortical lesions (Fig. 8). Both animals with extensive lesions that were able to perform the task after the left ear was plugged showed the same trend in their head movements, which were characterized by a consistent mislocalization toward the right, unplugged side. As with the approach-to-target behavior, the most dramatic change in the head orienting responses occurred for target positions on the plugged side, but responses to targets located on the right were also affected (Fig. 8, middle panel). The failure to adjust these responses is reflected by the MI between target location and final head bearing. Because only two animals in the extensive lesion group were able to perform sufficient trials in the presence of the earplug, we pooled together the data from these ferrets to calculate the MI. The MI value was greatly reduced on insertion of the earplug, from 1.40 to 0.65 bits (based on trials performed during the initial 4 days with the earplug), and remained at this level (0.57 bits on days 7–8) until the plug removed, whereupon performance immediately improved with the MI value jumping back to 1.16 bits.
Head orienting responses were also disrupted by occluding one ear in all the animals with restricted cortical lesions. The change in orienting responses was, however, less dramatic and was largely restricted to stimuli presented on the side of the earplug, in response to which the animals often turned toward the right (Fig. 8, middle panel). In contrast, the distribution of head orienting responses for stimuli presented on the unplugged side remained correlated with target location. Overall, the MI values dropped from 1.89 ± 0.07 bits before earplug insertion to 1.38 ± 0.21 bits on the first day with the earplug. As with the ferrets with more extensive cortical lesions, those values did not improve over time with daily training while the earplug was in place (1.23 ± 0.29 bits on the last day) and returned to preplug levels only when the earplug was removed (1.99 ± 0.28 bits).
Together, these data show that both groups of ferrets with cortical lesions were still able to localize long duration sounds accurately, provided that they received normal binaural input, but were not able to compensate for altered spatial cues over a time course in which substantial recovery is observed in ferrets with an intact auditory cortex.
Extent of the lesions in auditory cortex
In the three ferrets with extensive cortical lesions, the region aspirated included the MEG, where the primary areas, A1 and AAF, are located (Fig. 9). In addition, at least part of the PEG and/or anterior EG (AEG) was missing too, although the precise extent of the damage to the higher-level cortical fields found there varied from case to case. These lesions often included much of the underlying white matter and occasionally extended dorsally over the suprasylvian sulcus to encroach on the suprasylvian gyrus. When the white matter beneath the cortex was removed (as shown by the black regions in the schematics of the two hemispheres in Fig. 9), the space created by the lesion became continuous with the lateral ventricle (LV), as shown in the drawings of coronal sections in Fig. 9. However, apart from one case (left lesion in F0140; Fig. 9B), this did not affect other structures in close vicinity to the LV, such as the hippocampus.
Fig. 9.
Location and extent of the cortical lesions in the 3 animals (A, B, and C) classified as the “extensive lesion group.” For each case, a diagram of the cerebral cortex shows the lesioned area marked in gray, whereas areas in black represent where the underlying white matter was also aspirated. The coronal sections drawn at the level of the middle ectosylvian gyrus, as indicated by the line in A, show the total loss of the cortical layers and, in most cases, the underlying white matter. The approximate limits of the different auditory cortical areas described in the ferret are marked on a photomicrograph of the left ectosylvian gyri. AEG, anterior ectosylvian gyrus; ADF, anterior dorsal field; AVF, anterior ventral field; AAF, anterior auditory field; A1, primary auditory cortex; Hp, hippocampus; ls, lateral sulcus; LV, lateral ventricle; MEG, middle ectosylvian gyrus; PEG, posterior ectosylvial gyrus; PPF, posterior pseudosylvian field; PSF, posterior suprasylvian field; SSG, suprasylvian gyrus; sss, suprasylvian sulcus; pss, pseudosylvian sulcus; rf, rhinal fissure. Calibration bars represent 2 mm.
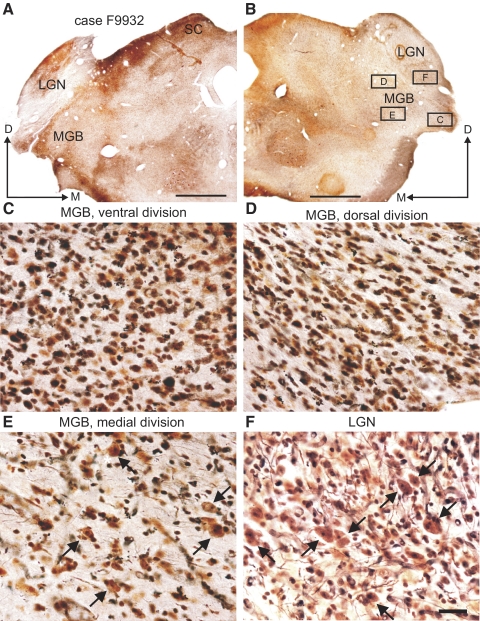
Case F9932 had large lesions that included both the AEG and PEG, with more extensive damage found on the left side (Fig. 9A). Degeneration was observed in all three subdivisions of the MGB (Fig. 10), and only the medial division of the MGB contained cellular elements with a normal appearance (Fig. 10E, arrows). The density, size, and morphology of neurons in the lateral geniculate nuclei (LGN; Fig. 10F, arrows) were similar to those seen in normal control ferrets, and there were few signs of degeneration. This indicates that, although the lesions were deep, any damage to fibers of passage in the optic radiation was minor. In case F0140, the lesion in the right cortex occupied the MEG and much of the AEG, whereas the PEG was more intact (Fig. 9B). On the left side of this animal, the lesion encompassed all three subdivisions of the EG and it was the only case in which the hippocampus was affected by the aspiration. In the third case, F0139, the lesions included smaller portions of the AEG and PEG (Fig. 9C). Again, there was some asymmetry in the extent of the lesions, with damage to the right cortex found mainly in the MEG and AEG, whereas, on the left side, the aspirated area was larger and more posterior. Although the lesions on both sides of this animal reached the lateral ventricle, the void space was smaller than in the other two cases. The suprasylvian sulcus was also preserved on both sides in case F0139 (Fig. 9C). In the thalamus of this ferret, degeneration was restricted to the ventral division of the MGB on the right side, but extended to the dorsal division on the left. None of the ventral division cells had a normal appearance and a lot of dark glial elements were found there.
Fig. 10.
Degeneration in the thalamus after extensive lesions of the auditory cortex. Photomicrographs of coronal sections at the level of the medial geniculate body (MGB) and lateral geniculate nucleus (LGN) stained with a silver degeneration technique. Cortical lesions for this animal (F9932) are shown in Fig. 9A. A and B: low-power views of both sides of the brain. C–E: high-power photomicrograph of the different subdivisions of the right MGB. F: high-power photomicrograph of the right LGN. Squares in B represent the locations where each of these higher-magnification pictures was taken. Arrows in E and F indicate cells with a normal appearance that were observed in the medial division of the MGB and in the LGN. Calibration bars are 5 mm in A and B and 0.1 mm in F; calibration bar in F also applies to C–E.
In the four ferrets with restricted lesions, the aspirated area was centered on A1 (Fig. 11A). These lesions were typically quite superficial near their edges and gradually extended deeper toward the underlying white matter at their centers, where more of the cortical layers were missing (Fig. 11B). The amount of tissue lost relative to the total volume of the MEG, which includes both A1 and AAF (Fig. 11A), was estimated to be ∼30%. In two cases (F0206 and F0317), the locations and extent of the lesions were very similar on the left and right sides; although the lesions were larger in F0317, they were deemed to occupy most of A1 based on the surrounding cortical landmarks and recording data. In the other two cases (F0318 and F0509), the histological reconstructions showed that the lesions were more prominent on the right side. However, in those two cases, the left A1 showed clear signs of atrophy (Fig. 11C). In both animals, A1 was 20–50% thinner than in other parts of the auditory cortex, indicating considerable loss of the supragranular layers, and, in case F0509, the overall laminar organization of A1 was disrupted on the left side. Thus, although there was some variation in the amount of cortical tissue that had been removed, all four animals showed clear damage to A1 on both sides, in keeping with the bilateral localization deficits observed in each case.
Fig. 11.
Location and extent of the cortical lesions in the 4 animals in the “restricted cortical lesion” group. A: histological reconstructions of the cerebral cortex are shown schematically for each case. Note that the lesions were focused on the primary auditory cortex, which makes up only part of the MEG. In 2 animals, largely symmetrical lesions were made on each side; in the other 2, a greater proportion of the right cortex was aspirated, although the caudal part of the MEG, where A1 is located, was damaged on the left side also. In case F0509, the lesion also included part of the left suprasylvian gyrus. Coronal section in B shows for case F0317 that nearly all the cortical layers had been removed in the central part of the lesions and that there was virtually no encroachment on the underlying white matter. C: atrophy at the level of the A1 in the left hemisphere in cases F0318 and F0509. Compared with the control case shown in the left panel (F0640), the cortex is clearly damaged, with atrophy of the supragranular layers in F0318 (middle panel) and of all the layers in F0509 (right panel). Calibration bars are 2 mm in A and B and 0.5 mm in C. ps, pial surface; wm, white matter.
DISCUSSION
In this study, we used two measures of spatial hearing, the initial orienting response and the selection of which loudspeaker location to approach to receive a reward, to assess the effects of aspiration lesions of the auditory cortex on the ability of ferrets to localize broadband sounds in the horizontal plane. Our findings confirm previous studies in showing that an intact auditory cortex is required for normal localization, particularly for brief sounds. However, we found that the two measures of localization behavior could be dissociated according to which regions of the cortex had been aspirated. After extensive lesions of the auditory cortex, similar deficits were observed in the head orienting and approach-to-target responses. In contrast, lesions of A1 alone produced a small impairment in approach-to-target behavior, without affecting the accuracy of sound-evoked head orienting. We also found that the auditory cortex plays a critical role in the ability of ferrets to adjust with behavioral training to an imbalance in inputs between the two ears, showing its importance in adaptive plasticity.
Auditory cortex and sound localization
It has previously been shown that bilateral lesions of the auditory cortex impair sound localization within each lateral hemifield, while preserving some ability to distinguish between sources located on either side of the midline (Heffner 1978; Heffner and Heffner 1990; Heffner and Masterton 1975; Kavanagh and Kelly 1987). In those experiments, animals had to discriminate between just two source locations on each trial. In contrast, we adopted a 12-speaker task so that localization accuracy and precision could be measured throughout the horizontal plane. This allowed us to examine the distribution of responses rather than simply how well the animals could discriminate between two sound source locations. In keeping with the earlier reports, we found that ferrets with both extensive and restricted cortical lesions exhibited deficits on both sides of space, although interestingly, the animals with bilateral A1 lesions were still able to localize sounds presented on the midline. As in lesion studies in other species, those deficits were greatest under the open-loop conditions present for the localization of brief sounds. This could mean that localization deficits occurred only when the animals had to remember the source of the sound, as reported in a study of role of the barn owl's forebrain in auditory spatial working memory (Knudsen and Knudsen 1996). However, it seems more likely that, at longer durations, the animals might have benefited from the availability of dynamic spatial cues that potentially become available once the head starts to move or made corrective adjustments in their responses as they tracked the longest duration sounds to their source.
Some studies have reported that the magnitude of the deficits varied with lesion size, with larger effects produced by lesions that extended beyond A1 (Heffner 1978; Heffner and Masterton 1975; Kavanagh and Kelly 1987). We also found that ferrets made fewer correct responses and larger localization errors after lesions that included both A1 and other cortical fields than if we attempted to restrict the aspirated area to A1. This reflects a general effect on their ability to localize sound, as these animals also exhibited deficits in vertical localization that scaled in magnitude with lesion size (Bizley et al. 2007). Both groups of ferrets generally responded to the correct side, indicating that they could lateralize the sound. However, whereas the ferrets with extensive cortical lesions could do little more than this when brief sounds were used, a clear correlation remained between target and response location after removing A1 alone. This accords with the effects of reversibly inactivating A1 in ferrets using a slow-release polymer containing muscimol; those animals were still able to approach the appropriate region of space on most trials, but did so less accurately than before the period of cortical inactivation (Smith et al. 2004).
Jenkins and Merzenich (1984) found that partial A1 lesions in cats resulted in frequency-specific sound-localization deficits, and that the performance of the animals was normal for tone frequencies represented within intact regions of this cortical field. Consequently, it is possible that sparing of a small region of A1 may have contributed to the fairly modest impairment exhibited by the ferrets in their ability to localize broadband noise. However, we think this is unlikely for the following reasons. First, like Jenkins and Merzenich (1984), we used electrophysiological recordings to guide the placement of the lesions. Unlike the cat, isofrequency bands in ferret A1 appear to be continuous with those in the neighboring anterior auditory field (Bizley et al. 2005), making it more difficult to identify the border between these two primary areas. However, in cats, activity in AAF is not required for normal sound localization (Lomber and Malhotra 2008), so any impact of the lesions on this field is unlikely to have a bearing on our results. Second, the deficits observed after making bilateral lesions of A1 closely resemble those produced by reversible inactivation using muscimol-Elvax implants, which were shown to be effective in silencing cortical neurons beneath the implants and to a restricted extent in the surrounding tissue (Smith et al. 2004). It is also unlikely that the magnitude of the localization deficits changed over time. In other studies in which A1 was aspirated, persistent localization deficits were found over the various testing periods used (Heffner and Masterton 1975; Jenkins and Merzenich 1984; Kavanagh and Kelly 1987). This contrasts with temporary impairments produced by muscimol–Elvax implants, which become less pronounced as activity in the cortex returns (Smith et al. 2004).
We therefore believe that the most likely explanation for the consistent observation that lesions affecting larger regions of the auditory cortex results in greater deficits than those focused on A1 is that other cortical fields also contribute to sound localization. Cooling studies in cats have shown that, in addition to the well-established effects of silencing A1, inactivation of the posterior auditory field (Lomber and Malhotra 2008; Malhotra and Lomber 2007), anterior ectosylvian sulcus (Malhotra and Lomber 2007), or dorsal zone (DZ) of the auditory cortex (Malhotra et al. 2008) results in localization deficits. On the other hand, cooling of other cortical areas, such as the secondary auditory cortex or anterior auditory field, does not, suggesting that only part of the auditory cortex is critically involved in spatial processing. Nevertheless, the finding that activity in each of several different areas is needed for normal sound localization could explain why the magnitude of the localization deficits scales with lesion size. Indeed, Malhotra et al. (2008) reported a greater impairment when they deactivated both A1 and DZ than either area by itself. Comparison of the extent of the extensive lesions with electrophysiological data from other ferrets (Bizley et al. 2005, 2009) suggests that nonprimary areas containing neurons whose responses are informative about sound source location would have been removed in each of the animals in this group.
Separate pathways for orienting responses and approach-to-target behavior?
As far as we are aware, our study provides the first attempt to measure the effects of cortical lesions on both the initial head movement and the subsequent approach-to-target response made by the animals. The head orienting responses provide a more absolute measure of localization accuracy and were evenly distributed within a ±60° range of the anterior midline. In control animals, we observed a close correspondence with the approach-to-target responses and have previously reported that when ferrets approach an incorrect target location, the preceding head turn is more closely correlated with that response than with the target location (Nodal et al. 2008). However, to determine whether the same neural processing strategies are responsible for the two measures of localization behavior, we have to compare how each is affected by silencing specific brain regions.
Although consistent deficits have been reported in approach-to-target responses following either lesions or inactivation of the auditory cortex, the effects of cortical lesions on acoustic orientation behavior are more varied. Thompson and Masterton (1978) found that fewer orienting responses were made by cats with large bilateral cortical lesions, but they did not report any change in the accuracy of those movements. A lower probability of reflexive head orienting responses was also seen in cats with bilateral cortical lesions by Beitel and Kaas (1993). In this case, however, the head movements had longer latencies and were less accurate than those made by control animals. This result accords with our own findings in ferrets with extensive cortical lesions, where we found that the latency of the head movements was increased and their accuracy substantially reduced compared with measurements made prior to the lesions. In contrast, much more modest changes were caused by bilateral lesions restricted to A1. Although head orienting responses were more variable, their latencies were actually reduced slightly and the MI between target location and final head bearing was unaltered, indicating the animals' ability to turn toward the appropriate region of space.
Several studies have shown that damage to (Knudsen et al. 1993; Thompson and Masterton 1978; Wagner 1993) or reversible inactivation (Lomber et al. 2001) of parts of the midbrain leads to at least temporary impairments in acoustic orientation behavior. Those circuits therefore seem to be sufficient to guide orienting responses. Our findings suggest that A1 does not play an essential part in this aspect of sound localization, whereas nonprimary auditory areas do seem to be involved. Although we cannot rule out the possibility that the deficits observed in animals with extensive cortical lesions arose from the resulting degeneration of thalamocortical neurons, this seems unlikely given that previous studies have emphasized the importance of the midbrain, which does not project directly to the cortex, in sound localization. The contribution of different neural circuits to normal sound-evoked orienting behavior has been shown in barn owls, where inactivation of both midbrain and forebrain pathways is required to prevent the animals from localizing sound, with much smaller deficits observed when either pathway is targeted independently (Knudsen et al. 1993).
Although distinct parallel pathways could be involved in different aspects of spatial hearing, it seems more likely that they interact to translate auditory signals into motor commands. It has been suggested that head orienting deficits produced by cortical lesions might arise from the loss of descending corticofugal neurons (Beitel and Kaas 1993; Thompson and Masterton 1978). The finding that auditory localization deficits produced in cats by unilateral ablation of the auditory cortex can be reversed by cooling the contralateral superior colliculus (SC) (Lomber et al. 2007) provides evidence that the cortex and midbrain act together to influence spatial hearing. Although the neural circuitry underlying this result has yet to be elucidated, the loss of descending projections from auditory cortex to the SC could certainly play a part in the deficits observed in this study. In ferrets, projections to the SC arise from AES and from the posterior suprasylvian field on the PEG, both of which were damaged in the animals with extensive cortical lesions, but not from A1 (Bajo et al. 2007). This might therefore account for why the ability to turn the head toward a sound source was largely spared following A1 lesions, but not when the lesions also included these more ventral regions of auditory cortex.
Plasticity of sound localization requires the auditory cortex
It is well established that the sensitivity of auditory cortical neurons, most commonly studied in A1, exhibits plasticity across multiple timescales (Dahmen and King 2007). For example, learning in various auditory tasks is accompanied by changes in A1 response properties (Polley et al. 2006; Recanzone et al. 1993; Schnupp et al. 2006). This raises the possibility that plasticity of cortical spatial response properties could underlie the capacity to relearn to localize sound in the presence of substantially altered auditory spatial cues (Hofman et al. 1998; Kacelnik et al. 2006). Our finding that ferrets are unable to adapt to the altered spatial cues produced by occluding one ear if the auditory cortex is lesioned supports this notion. Importantly, no recalibration was observed with sounds that were long enough for the ferrets to localize them with near normal accuracy before the ear was occluded, indicating that the task was sufficiently easy for the animals with cortical lesions to perform as well as control ferrets. It is possible that some improvement in performance would have been seen had the animals been trained with the earplug in place for longer. However, no change was observed over an 8-day period of monaural occlusion during which the performance of control animals recovers with the same amount of behavioral training to near preplug levels.
Neither the extensive nor the restricted lesion group was able to relearn to localize sound with one ear occluded. No plasticity was observed for either the initial head orienting response or the approach-to-target behavior, although both measures of sound localization show adaptive improvements in ferrets with an intact auditory cortex (Kacelnik et al. 2006). Thus even though the integrity of A1 is not required for accurate sound-evoked head movements in ferrets with normal binaural inputs, it does play a vital role in allowing orienting, as well as approach-to-target, responses to be adjusted by experience when those inputs are altered. Descending projections from A1 appear to be key to this, because ferrets with unilateral lesions of layer V neurons that project to the inferior colliculus show impaired adaptive plasticity in the contralateral hemifield (Bajo et al. 2009). These findings extend those observations by showing that the ability to adapt to altered cues is lost throughout the horizontal plane if the cortex is lesioned bilaterally and that this is equally the case irrespective of whether the lesion is restricted to A1 or includes other cortical areas as well. Although layer 5 corticocollicular projection neurons are critical for learning-induced localization plasticity, the loss of those neurons has no effect on the normal ability of the animals to localize sound at any of the durations tested (Bajo et al. 2009). This is in contrast to the present study, where lesions that extended to other cortical layers produced a duration-dependent deficit in approach-to-target response accuracy. Thus processing within A1 and other parts of the auditory cortex is required for normal localization accuracy, whereas signals conveyed to the midbrain by the descending corticocollicular pathway appear to enable learning-induced changes in spatial hearing to be brought about, most likely by reweighting the contribution of different spatial cues (Kacelnik et al. 2006). Although it remains unclear whether the changes in neuronal spatial response properties that accompany sound-localization plasticity originate in the cortex or the midbrain, these findings highlight the importance of interactions between these different levels of processing in enabling animals to maintain localization accuracy in different acoustic conditions.
GRANTS
This study was supported by the Wellcome Trust through a Wellcome Principal Research Fellowship to A. J. King.
ACKNOWLEDGMENTS
We thank S. Spires, R. Campbell, and D. Kumpik for assistance with the data collection and P. Keating for measuring the acoustical effects of the earplugs.
Present address for D. R. Moore: MRC Institute of Hearing Research, University Park, Nottingham NG7 1BD, UK.
REFERENCES
- Bajo VM, Nodal FR, Bizley JK, Moore DR, King AJ. The ferret auditory cortex: descending projections to the inferior colliculus. Cereb Cortex 17: 475–491, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci 2009December27 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel RE, Kaas JH. Effects of bilateral and unilateral ablation of auditory cortex in cats on the unconditioned head orienting response to acoustic stimuli. J Neurophysiol 70: 351–369, 1993 [DOI] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Nelken I, King AJ. Functional organization of ferret auditory cortex. Cereb Cortex 15: 1637–1653, 2005 [DOI] [PubMed] [Google Scholar]
- Bizley JK, Nodal FR, Parsons CH, King AJ. Role of auditory cortex in sound localization in the midsagittal plane. J Neurophysiol 98: 1763–1774, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizley JK, Walker KM, Silverman BW, King AJ, Schnupp JWH. Interdependent encoding of pitch, timbre, and spatial location in auditory cortex. J Neurosci 29: 1064–1075, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, King AJ. Learning to hear: plasticity of auditory cortical processing. Curr Opin Neurobiol 17: 456–464, 2007 [DOI] [PubMed] [Google Scholar]
- Heffner H. Effect of auditory cortex ablation on localization and discrimination of brief sounds. J Neurophysiol 41: 963–976, 1978 [DOI] [PubMed] [Google Scholar]
- Heffner H, Masterton B. Contribution of auditory cortex to sound localization in the monkey (Macaca mulatta). J Neurophysiol 38: 1340–1358, 1975 [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol 64: 915–931, 1990 [DOI] [PubMed] [Google Scholar]
- Hofman PM, Van Riswick JG, Van Opstal AJ. Relearning sound localization with new ears. Nat Neurosci 1: 417–421, 1998 [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Masterton RB. Sound localization: effects of unilateral lesions in central auditory system. J Neurophysiol 47: 987–1016, 1982 [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM. Role of cat primary auditory cortex for sound-localization behavior. J Neurophysiol 52: 819–847, 1984 [DOI] [PubMed] [Google Scholar]
- Kacelnik O, Nodal FR, Parsons CH, King AJ. Training-induced plasticity of auditory localization in adult mammals. PLoS Biol 4: e71, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh GL, Kelly JB. Contribution of auditory cortex to sound localization by the ferret (Mustela putorius). J Neurophysiol 57: 1746–1766, 1987 [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Disruption of auditory spatial working memory by inactivation of the forebrain archistriatum in barn owls. Nature 383: 428–431, 1996 [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF, Masino T. Parallel pathways mediating both sound localization and gaze control in the forebrain and midbrain of the barn owl. J Neurosci 13: 2837–2852, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski N, Versnel H, Shamma SA. Comparison of responses in the anterior and primary auditory fields of the ferret cortex. J Neurophysiol 73: 1513–1523, 1995 [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S. Double dissociation of ‘what’ and ‘where’ processing in auditory cortex. Nat Neurosci 11: 609–616, 2008 [DOI] [PubMed] [Google Scholar]
- Lomber SG, Malhotra S, Sprague JM. Restoration of acoustic orienting into a cortically deaf hemifield by reversible deactivation of the contralesional superior colliculus: the acoustic “Sprague Effect”. J Neurophysiol 97: 979–993, 2007 [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Cornwell P. Role of the superior colliculus in analyses of space: superficial and intermediate layer contributions to visual orienting, auditory orienting, and visuospatial discriminations during unilateral and bilateral deactivations. J Comp Neurol 441: 44–57, 2001 [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol 92: 1625–1643, 2004 [DOI] [PubMed] [Google Scholar]
- Malhotra S, Lomber SG. Sound localization during homotopic and heterotopic bilateral cooling deactivation of primary and nonprimary auditory cortical areas in the cat. J Neurophysiol 97: 26–43, 2007 [DOI] [PubMed] [Google Scholar]
- Malhotra S, Stecker GC, Middlebrooks JC, Lomber SG. Sound localization deficits during reversible deactivation of primary auditory cortex and/or the dorsal zone. J Neurophysiol 99: 1628–1642, 2008 [DOI] [PubMed] [Google Scholar]
- Moore DR, Hutchings ME, King AJ, Kowalchuk NE. Auditory brain stem of the ferret: some effects of rearing with a unilateral ear plug on the cochlea, cochlear nucleus, and projections to the inferior colliculus. J Neurosci 9: 1213–1222, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodal FR, Bajo VM, Parsons CH, Schnupp JW, King AJ. Sound localization behavior in ferrets: comparison of acoustic orientation and approach-to-target responses. Neuroscience 154: 397–408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CH, Lanyon RG, Schnupp JW, King AJ. Effects of altering spectral cues in infancy on horizontal and vertical sound localization by adult ferrets. J Neurophysiol 82: 2294–2309, 1998 [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci 26: 4970–4982, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13: 87–103, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnupp JWH, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci 26: 4785–4795, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn-Cunningham BG, Durlach NI, Held RM. Adapting to supernormal auditory localization cues. I. Bias and resolution. J Acoust Soc Am 103: 3656–3666, 1998 [DOI] [PubMed] [Google Scholar]
- Smith AL, Parsons CH, Lanyon RG, Bizley JK, Akerman CJ, Baker GE, Dempster AC, Thompson ID, King AJ. An investigation of the role of auditory cortex in sound localization using muscimol-releasing Elvax. Eur J Neurosci 19: 3059–3072, 2004 [DOI] [PubMed] [Google Scholar]
- Thompson GC, Masterton RB. Brain stem auditory pathways involved in reflexive head orientation to sound. J Neurophysiol 41: 1183–1202, 1978 [DOI] [PubMed] [Google Scholar]
- Wagner H. Sound-localization deficits induced by lesions in the barn owl's auditory space map. J Neurosci 13: 371–386, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Carp RI, Schmued LC, Scallet AC. Fluoro-Jade and silver methods: application to the neuropathology of scrapie, a transmissible spongiform encephalopathy. Brain Res Brain Res Protoc 8: 104–112, 2001 [DOI] [PubMed] [Google Scholar]