Abstract
Posttraumatic epilepsy is a frequent consequence of brain trauma, but relatively little is known about how neuronal circuits are chronically altered after closed head injury. We examined whether local recurrent excitatory synaptic connections form between dentate granule cells in mice 8–12 wk after cortical contusion injury. Mice were monitored for behavioral seizures shortly after brain injury and ≤10 wk postinjury. Injury-induced seizures were observed in 15% of mice, and spontaneous seizures were observed weeks later in 40% of mice. Timm's staining revealed mossy fiber sprouting into the inner molecular layer of the dorsal dentate gyrus ipsilateral to the injury in 95% of mice but not contralateral to the injury or in uninjured controls. Whole cell patch-clamp recordings were made from granule cells in isolated hippocampal brain slices. Cells in slices with posttraumatic mossy fiber sprouting had an increased excitatory postsynaptic current (EPSC) frequency compared with cells in slices without sprouting from injured and control animals (P < 0.001). When perfused with Mg2+-free artificial cerebrospinal fluid containing 100 μM picrotoxin, these cells had spontaneous bursts of EPSCs and action potentials. Focal glutamate photostimulation of the granule cell layer evoked a burst of EPSCs and action potentials indicative of recurrent excitatory connections in granule cells of slices with mossy fiber sprouting. In granule cells of slices without sprouting from injured animals and controls, spontaneous or photostimulation-evoked epileptiform activity was never observed. These results suggest that a new regionally localized excitatory network forms between dentate granule cells near the injury site within weeks after cortical contusion head injury.
INTRODUCTION
Traumatic brain injury (TBI) is accompanied by a long-lasting increase in the risk for developing posttraumatic epilepsy (PTE) (Annegers et al. 1998; Caveness et al. 1979; Englander et al. 2003). However, treatment options for preventing or suppressing chronic seizures after head injury are limited and have been largely unsuccessful (Temkin 2009). Understanding basic mechanisms of posttraumatic epileptogenesis after experimental TBI, in comparison to other preclinical models, will provide important information necessary for developing cellular based therapeutic approaches for PTE.
In patients and experimental models of temporal lobe epilepsy, the generation of epileptic activity is associated with axon sprouting and reorganization of neuronal circuitry (Buckmaster et al. 2002; Dudek and Spitz 1997; Hunt et al. 2009; Shibley and Smith 2002; Sutula et al. 1989; Tauk and Nadler 1985). The dentate gyrus, which is particularly susceptible to injury, often undergoes structural reorganization, and it is a widely used model system for studying altered synaptic circuitry in epilepsy. Several studies suggest that dentate granule cells, which are not normally interconnected, sprout axon collaterals into the inner molecular layer (i.e., mossy fiber sprouting) to form functional recurrent excitatory connections with nearby granule cells during epileptogenesis and may contribute to network synchronization (Cronin and Dudek 1988; Cronin et al. 1992; Hunt et al. 2009; Lynch and Sutula 2000; Winokur et al. 2004; Wuarin and Dudek 1996, 2001). Mossy fiber sprouting has been reported weeks to months after experimental TBI in rodents (Golarai et al. 2001; Hunt et al. 2009; Kharatishvili et al. 2006; Santhakumar et al. 2001) and in temporal lobe epilepsy patients with a history of head injury (Swartz et al. 2006), but the functional implications of injury-induced regional alterations in neuronal circuitry after TBI have not been well described.
The degree of mossy fiber sprouting (MFS) after experimental TBI is qualitatively less than the robust, bilateral sprouting observed weeks after experimental status epilepticus. While less widespread axon reorganization is a more typical representation of the clinical setting, computational models have suggested that synchronous network activity may only occur if robust recurrent synaptic connections are present (Traub and Wong 1981, 1982). Studies using extracellular field recordings to examine network excitability in the dentate gyrus after experimental head injury have not consistently demonstrated epileptiform activity after TBI (Golarai et al. 2001; Hunt et al. 2009; Reeves et al. 1997; Santhumakar et al. 2001). Moreover, Santhakumar and colleagues (2001) reported a recovery within a month after fluid percussion injury from an early increase in extracellular excitability of the granule cell layer that may be related to mossy fiber sprouting. Therefore, mossy fiber sprouting may play a different functional role in the dentate gyrus after mechanical injury than in pharmacologically induced temporal lobe epilepsy models. The persistence of recurrent excitatory connections between granule cells after TBI has not been well established. Understanding how synaptic circuit reorganization may contribute to seizures or chronic changes in excitability after TBI should help to elucidate the importance of these cellular mechanisms in PTE.
Here, we performed controlled cortical impact (CCI) injury to test the hypothesis that mossy fiber sprouting forms an excitatory feedback circuit between granule cells after head injury. We specifically focused on three main questions: can an increase in excitatory synaptic input onto individual granule cells be detected after injury; do granule cells in slices with posttraumatic mossy fiber sprouting exhibit spontaneous epileptiform activity; and can excitatory synaptic events be elicited by local glutamate photostimulation at distant locations within the granule cell layer?
METHODS
Animals
Seven to eight week old adult male CD-1 mice (Harlan) weighing 28–35 g were housed under a normal 12 h/12 h light/dark cycle. Water and food were available ad libitum. Mice were housed for a minimum of 7 day prior to experimentation, and all procedures were first approved by the University of Kentucky Animal Care and Use Committee.
Head injury
Thirty-three mice were subjected to a severe unilateral cortical contusion by CCI injury as previously described (Hunt et al. 2009; Scheff et al. 1997). Briefly, mice were anesthetized by 2% isoflurane inhalation and placed in a stereotaxic frame. The skull was exposed by a midline incision, and a 4-mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap was removed without damage to the exposed underlying dura. The contusion device consisted of a computer-controlled, pneumatically driven impactor fitted with a beveled stainless steel tip 3 mm in diameter (Precision Systems and Instrumentation, Fairfax, VA). Brain injury was delivered using this device to compress the cortex to a depth of 1.0 mm at a velocity of 3.5 m/s and 400-ms duration. Surgical (Johnson and Johnson, Arlington, TX) was placed over the dura after injury, the incision sutured, and the animal was allowed to recover.
Seizure monitoring
Injured animals were monitored for immediate seizures (i.e., injury-induced) during a 90-min interval that began ∼90 min after CCI injury. We chose this time for practical reasons (i.e., to allow animals to fully recover from anesthesia and surgery) and based on a previous report that behavioral manifestations are not observed until ≥1 h post-CCI (Kochanek et al. 2006). Control and injured mice were subsequently observed 4–6 h/wk for spontaneous seizures during random 1– to 2-h intervals until 10 wk postinjury. Observation periods occurred during the light phase of the light/dark cycle, and seizures were rated from 1 to 5, with 5 being the most severe, according to a modified Racine scale (Racine 1972; Shibley and Smith 2002). To minimize subjectivity in seizure assessment, category one seizures (i.e., facial automatisms, increased grooming behaviors) were excluded from analysis. Posttraumatic seizures were classified based on the time postinjury in which they occurred: immediate, first 90-min monitoring session; early, 1–7 day postinjury; and late, >7 day after injury.
Slice preparation
Mice were deeply anesthetized by isoflurane inhalation and decapitated. The brain was removed and immersed in ice cold (2–4°C) oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 3 KCl, 1.3 CaCl2, 26 NaHCO3, 1.3 MgCl2, and 1.25 NaH2PO4 and equilibrated with 95% O2–5% CO2 (pH 7.2–7.4) with an osmalality of 290–305 mosM/kg. Brains were blocked and glued to a sectioning stage, and 400-μm-thick coronal slices were cut in cold, oxygenated ACSF using a vibrating microtome (Vibratome Series 1000; Technical Products International, St. Louis, MO). The hippocampus was isolated from all surrounding tissue, making sure to completely remove the entorhinal cortex. Slices were transferred to a storage chamber containing oxygenated ACSF at 34–36°C and their order maintained so that the location relative to the injury within each hippocampus was known. Some experiments were performed in Mg2+- free ACSF containing 100 μM picrotoxin (PTX) (see results, Figs. 4–7).
Fig. 4.
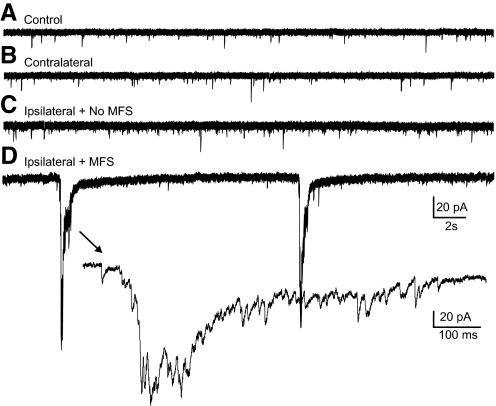
Spontaneous bursts of compound EPSCs in cells from slices with MFS ipsilateral to the injury. Representative whole cell voltage-clamp recordings of granule cells in a slice from an uninjured control mouse (A), a contralateral slice in an injured mouse (B), a slice ipsilateral to the injury without MFS (C), and a slice ipsilateral to the injury with MFS (D). →, expanded portion of the trace in D. Slices were incubated with Mg2+-free artificial cerebrospinal fluid (ACSF) and 100 μM PTX; Vm = −70 mV for all recordings.
Fig. 5.
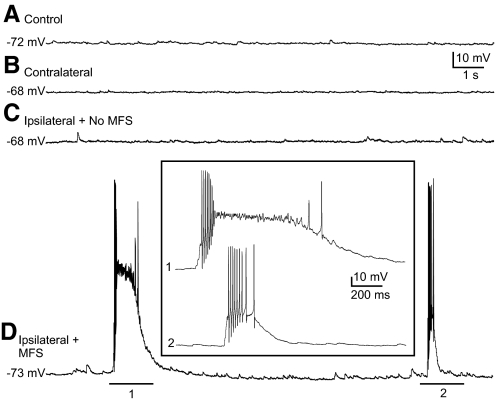
Spontaneous epileptiform bursts of action potentials in cells of slices with MFS ipsilateral to the injury. Representative whole cell current-clamp recordings of granule cells in a slice from an uninjured control mouse (A), a contralateral slice in an injured mouse (B), a slice ipsilateral to the injury without MFS (C), and a slice ipsilateral to the injury with MFS (D). Inset: expanded sections of the underlined portions of the trace in D labeled 1 and 2. Resting membrane potential is indicated for each trace.
Fig. 6.
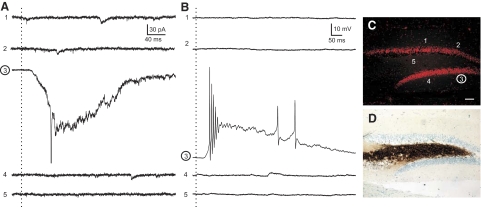
Granule cell—granule cell connections are not detected by glutamate photostimulation in cells from slices contralateral to the injury. A: voltage-clamp recordings at −70 mV from a granule cell. B: current-clamp recordings at resting membrane potential from the same cell as A. Dotted vertical lines, the time of stimulation. Numbers to the left of each trace indicate corresponding stimulus position shown in C. C: Nissl-stained image of the slice from which the recorded cell was obtained. Numbers correspond to the approximate locations along the granule cell layer that photostimulation was applied to give the numbered responses recorded in A and B. Stimulation position 3 (circled) is the approximate location of the recorded cell. Note that direct activation of the recorded cell induced an inward current and burst of action potentials. D: Timm's stain image of the same section in C indicating no MFS into the inner molecular layer.
Fig. 7.
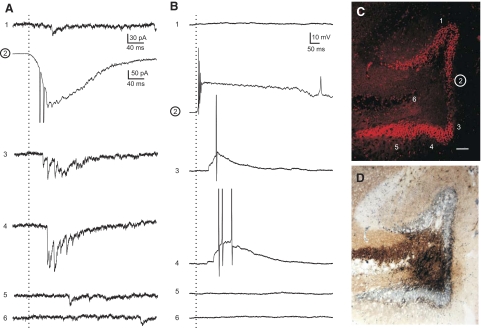
Granule cell—granule cell connections are detected by glutamate photostimulation in cells from slices ipsilateral to the injury with MFS. A: voltage-clamp recordings at −70 mV from a granule cell. B: current-clamp recordings at resting membrane potential from the same cell as A. Dotted vertical lines, the time of stimulation. C: Nissl-stained image of the slice from which the recorded cell was obtained. Numbers correspond to the approximate location along the granule cell layer that photostimulation was applied to give the responses recorded in A and B. Stimulation site number 2 (circled) is the approximate location of the recorded cell. Note that direct photoactivation of the recorded cell induced an inward current and burst of action potentials (A2 and B2). Activity induced in neurons at locations 3 and 4 resulted in synaptic responses (A3 and A4) and action potentials (B3 and B4) in the recorded granule cell. D: Timm's stain image of the same section in C. Note: MFS surrounds the position of the recorded cell.
Whole cell patch-clamp electrophysiology
After an equilibration period of ≥1 h, slices were transferred to a recording chamber on an upright, fixed-stage microscope equipped with infrared, differential interference contrast optics (Olympus BX50WI). Whole cell patch-clamp recordings were performed on visually identified granule cells. Patch pipettes were pulled from borosilicate glass (1.65 mm OD and 0.45 mm wall thickness, King Precision Glass, Claremont, CA) with a P-87 puller (Sutter Instruments). The intracellular solution contained (in mM) 140 K+ gluconate, 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 3 KOH, 2 ATP, and 0.2% biocytin (pH 7.15 – 7.3). Open tip resistance was 4–7 MΩ. Recordings were obtained with an Axopatch 200B amplifier (Axon Instruments, Foster City, CA), low-pass filtered at 2–5 kHz, digitized at 88 kHz (Neuro-corder; Cygnus Technology, Delaware Water Gap, PA), and recorded to pClamp 10.2 (Clampfit, Axon Instruments). Once in whole cell configuration, cells were voltage-clamped for ∼5 min at −70 mV (i.e., near resting membrane potential) to allow equilibration of intracellular and recording pipette contents. Resting membrane potential was determined by temporarily removing the voltage clamp and monitoring voltage. Spontaneous and evoked excitatory postsynaptic currents (s- and eEPSCs) were examined at a holding potential of –70 mV. Current-clamp (i.e., voltage) recordings were performed at resting membrane potential. Series resistance was monitored throughout the recordings, and data were only used for analysis if the series resistance remained <25 MΩ and changed by ≤20% during the recordings.
Glutamate photostimulation
Slices were perfused with γ-(carboxy-2-nitrobenzyl) ester, trifluoroacetic acid salt (i.e., CNB-caged glutamate, 250 μM; Molecular Probes, Eugene, OR) added to recirculating Mg2+-free ACSF and 100 μM PTX. Brief pulses of fluorescent light (30-ms exposure, UV filter, Chroma Technology, Rockingham, VT) were directed into the slice through the ×40 objective. The objective was initially positioned to “uncage” glutamate directly over the recorded cell. This consistently resulted in a large inward current in voltage-clamp or a large depolarization in current-clamp with three to six superimposed action potentials in all cells (n = 13). The objective was then moved away from the recorded cell by manually moving the microscope until a direct inward current after stimulation was no longer observed to establish the effective radius of stimulation (<50 μm). Photostimulation was applied to sites along the entire extent of the granule cell layer and in the hilus and CA3 region. A series of at least five stimuli were applied per stimulation location at 0.1-Hz stimulation frequency.
Timm's histochemistry
Coronal hippocampal slices used for recordings were placed in 0.37% sodium sulfide solution in 0.1M NaHPO4 for 20 min, followed by 4% paraformaldehyde in 0.15 M phosphate buffer overnight to fix the slices. Slices were then rinsed three times with phosphate-buffered saline (PBS; 0.01 M; pH 7.4) and placed in a 30% sucrose solution in PBS overnight or until they sank for cryoprotection. The slices were sectioned at 20 μm on a cryostat, rinsed, mounted on charged slides (Superfrost Plus; Fisher Scientific), and dried overnight. Sections were treated according to previous protocols using Timm's stain to reveal mossy fibers and Nissl counterstained with cresyl violet to visualize cell bodies (Hunt et al. 2009; Shibley and Smith 2002; Tauck and Nadler 1985). To semi-quantitatively assess the regional distribution of mossy fiber sprouting after CCI, sections from the ipsilateral and contralateral hemispheres were examined by an investigator, who was blind to the electrophysiological outcomes. Scores for sprouting were assigned based on the following scale of Tauck and Nadler (1985): 0, little to no Timm granules in the granule cell layer; 1, mild staining in the granule cell layer but not the inner molecular layer; 2, moderate continuous staining through the granule cell layer with discontinuous, punctuate staining in the inner molecular layer; and 3, continuous band of dense staining throughout the inner molecular layer. At least three sections from each slice were examined and the median score reported if variability between sections existed. Because Timm's staining between the blades of the granule cell layer was almost always asymmetric, Timm scores were assigned based on the region of the granule cell layer in which the recorded cell was obtained (i.e., inner blade, apex, or outer blade). Regions of the dentate gyrus with Timm scores >1 were considered to have an abnormal degree of mossy fiber sprouting (Hunt et al. 2009; Patrylo and Dudek 1998; Shibley and Smith 2002). The degree of structural damage to the dentate gyrus was analyzed at the injury site in the same Timm scored sections by switching the filter from bright field to fluorescence. Cresyl violet emits red fluorescence under a TRITC filter (Alvarez-Buylla et al. 1990).
Data analysis and statistics
Data analysis was performed using pClamp 10.2 (Clampfit, Axon Instruments), MiniAnalysis 6.0 (Synaptosoft, Leonia, NJ), and Instat (GraphPad software, San Diego, CA) programs. A 2-min sample recording per cell was used for measuring sEPSC frequency and amplitude. Events characterized by a typical fast rising phase and exponential decay phase were manually detected using MiniAnalysis, and only currents with amplitudes greater than three times the root mean square (RMS) noise level were included for analysis. Event frequency and mean amplitude were averaged across neurons (i.e., n = neurons) and groups were compared by one-way ANOVA followed by a Tukey's post hoc test. Spearman rank correlation was used for the nonparametric assessment of the relationship between Timm score and EPSC frequency. To analyze differences in spontaneous epileptiform burst responses, two-tailed Fisher's exact test was used to compare cells in slices with mossy fiber sprouting versus slices without sprouting. For photostimulation experiments, EPSC frequency was analyzed every 100 ms for 500 ms prior to and after each stimulation trial and averaged for each stimulus location (i.e., n = stimulation sites). A response at a given location was considered to be positive (i.e., a local synaptic connection was evoked) if the following criteria were satisfied: 1) the number of EPSCs in at least one of the first three 100-ms segments after stimulation was greater than the mean number of events per 100 ms prior to stimulation +3 SD; and 2) a response was observed in at least three trials with relatively consistent delay between stimulation and response, demonstrating that the response was repeatable. Responses with <10-ms latency between photostimulation and response were considered to be direct (i.e., due to postsynaptic activation of the recorded neuron) and were excluded from further analysis such that it would interfere with detection of synaptic responses (Calloway and Katz 1993; Schubert et al. 2001). All stimulus locations in which the selection criteria were not met were considered to have a negative response. The number of eEPSCs for each stimulation site was calculated by subtracting the number of EPSCs in the first 300 ms before stimulation from the number of EPSCs in the first 300 ms after stimulation. We chose this relatively short time window to limit confounding effects of polysynaptic activation and because we previously found that field potential bursts indicative of recurrent excitatory connections between granule cells occur within the first 300 ms after an initial antidromically evoked population spike (Hunt et al. 2009). Data are expressed as means ± SD, and significance was set at P < 0.05.
RESULTS
Posttraumatic seizures
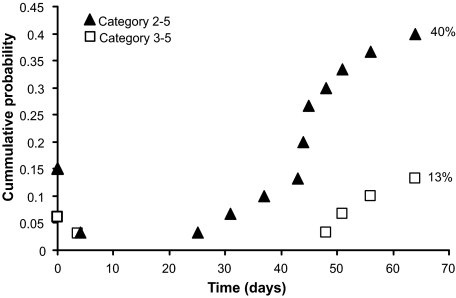
Mice were monitored for behavioral seizures from the time of injury until 10 wk postinjury. During the first 90-min monitoring period following injury, 5 of 33 mice (15%) were observed to have at least one injury-induced seizure. The majority of these mice had only one or two seizures; one mouse had three category 3–5 seizures during this period. One mouse (3%) that did not have immediate seizures had a category 3 seizure at 3 day postinjury (i.e., early seizure). Spontaneous category 2–4 seizures were observed in 12 of 30 mice (40%), and category 3–4 seizures were observed in 4 of 30 mice (13%) (Fig. 1). The average latency from injury to first observed spontaneous seizure was 6.5 ± 1.3 wk after injury. This latent period is considered to be an estimate because we did not monitor continuously. Of the five mice that had immediate seizures, three (60%) were observed to have spontaneous seizures.
Fig. 1.
Mice develop injury-induced and spontaneous seizures after severe controlled cortical impact (CCI) injury. Cumulative probability plot of the 1st observed seizure after CCI injury (time 0). Seizure counts were reset after 1 and 7 day to separate immediate, early, and spontaneous seizures.
Injury-induced mossy fiber reorganization
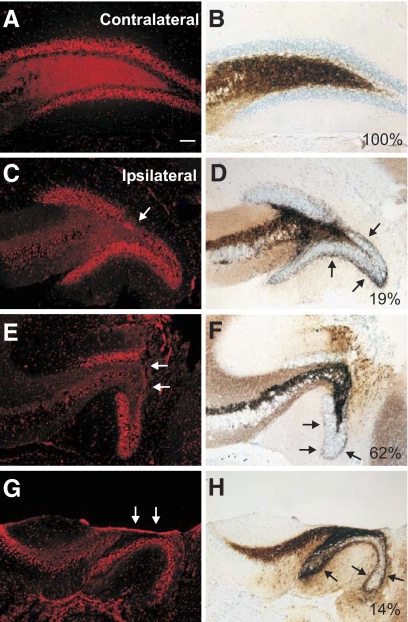
Coronal brain sections of the dorsal hippocampus used for recordings in 21 mice were examined for gross anatomical damage in the hippocampus and mossy fiber sprouting at the injury site. Damage through the entire depth of the cortex directly below the impact site and hippocampal distortion were observed in all injured mice examined 8–12 wk post-TBI, as previously described (Hall et al. 2005; Saatman et al. 2006; Tong et al. 2002). In 20 of 21 mice (95%), the cortical cavitation at the injury site extended into the hippocampus, and a separation of the dorsal and ventral blades of the granule cell layer was visible. Mossy fiber sprouting was observed in sections ipsilateral to the injury in all 20 mice. One mouse had hippocampal and granule cell layer distortion, but cavitation was restricted to the cortex. Mossy fiber sprouting was not present in this mouse. Figure 2 shows the range of lesion to the dentate gyrus and degree of mossy fiber sprouting under the injury. No gross structural damage was observed in any section contralateral to the injury (n = 21) or in control mice (n = 15 animals), and these sections were also devoid of abnormal mossy fiber organization (i.e., all Timm's scores ≤1).
Fig. 2.
Cavitation into the hippocampus and posttraumatic mossy fiber sprouting 8–12 wk after severe CCI injury. A: representative Nissl stain of the dentate gyrus contralateral to the injury site. B: Timm's stain of the same section in A shows the absence of mossy fiber sprouting in the inner molecular layer. C, E, and G: representative Nissl-stained images of the ipsilateral dentate gyrus at the injury site. →, indicate severe thinning of the granule cell layer. D, F, and H: Timm's stain of the same sections in C, E, and G. Note the presence of moderate mossy fiber sprouting in all sections (→). Percentage of mice with each type of lesion/Timm stain at the injury site is indicated. Scale bar is 100 μm.
Excitatory input to dentate granule cells
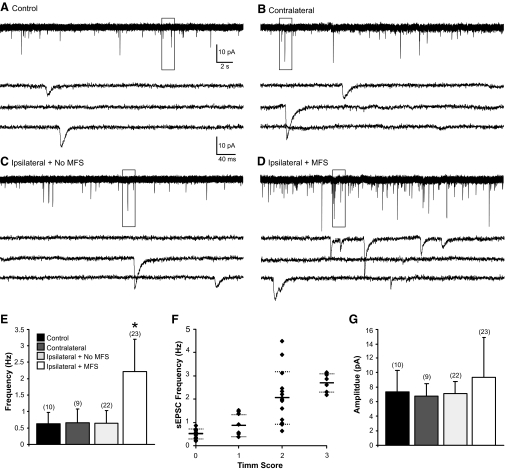
To analyze whether excitatory synaptic input to dentate granule cells was increased after CCI injury, whole cell voltage-clamp recordings of sEPSCs were obtained from granule cells in four treatment groups based on post hoc identification of mossy fiber sprouting: ipsilateral slices with mossy fiber sprouting (n = 23 cells in 20 slices from 13 animals) and ipsilateral slices without mossy fiber sprouting (n = 22 cells in 18 slices from 14 animals), contralateral slices (n = 9 cells in 8 slices from 7 animals), and uninjured controls (n = 10 cells in 9 slices from 6 animals). Recordings were made from cells in the apex or outer blade regions of the granule cell layer where Timm's staining was typically most robust. The average resting membrane potential for these cells was: controls, −75 ± 3.8 mV; contralateral, −76 ± 2.9 mV; ipsilateral with no MFS, −75 ± 5.3 mV; and ipsilateral with MFS, −79 ± 4.4 mV. These values are consistent with previously reported ranges of −65 to −85 mV (Fricke and Prince 1984; Staley et al. 1992; Wuarin and Dudek 1996), and one-way ANOVA found no difference between groups [F(3,64) = 2.03, P > 0.05].
sEPSCs were recorded at a holding potential of −70 mV. At this potential, outward currents were not typically present. Application of 1 mM kynurenic acid blocked inward events, indicating that these currents were mediated by glutamate receptors. Representative recordings for each group are shown in Fig. 3, A–D. As shown in Table 1, EPSC kinetics (i.e., 10–90% rise time and decay time constant) were comparable to previous reports (Keller et al. 1991; Staley and Mody 1992), and significant differences were not observed between groups (P > 0.05). A significant difference in the mean event frequency between treatment groups was detected by one-way ANOVA [control: 0.64 ± 0.3 Hz, contralateral: 0.66 ± 0.4 Hz, ipsilateral without MFS: 0.65 ± 0.4 Hz, ipsilateral with MFS: 2.2 ± 0.9 Hz; F(3,64) = 27.1, P < 0.001; Fig. 3E]. Post hoc comparisons revealed that granule cells from ipsilateral slices with MFS had a significantly higher frequency of sEPSCs versus other groups. To determine whether EPSC frequency was associated with the density of Timm's staining, a Timm score was obtained for each slice in which a recording was obtained. The average sEPSC frequency for each cell in slices ipsilateral to the injury was plotted as a function of Timm score (Fig. 3F), and a Spearman's rank correlation analysis indicated a significant positive relationship between EPSC frequency and Timm's score (rs = 0.82, P < 0.001). This is consistent with previous reports that suggest a relationship between EPSC frequency and degree of MFS in kainate-treated rats (Wuarin and Dudek 2001). The mean amplitude of sEPSCs for each treatment group was: control, −7.4 ± 3.0 pA; contralateral, −6.8 ± 1.7 pA; ipsilateral without MFS, −7.1 ± 1.7 pA; and ipsilateral with MFS, −9.4 ± 5.4 pA. One-way ANOVA did not indicate significant differences between groups [F(3,64) = 1.9, P > 0.05; Fig. 3G].
Fig. 3.
Increased spontaneous excitatory postsynpatic currents (sEPSCs) in slices of the ipsilateral dentate gyrus with mossy fiber sprouting (MFS). A–D: representative whole cell patch-clamp recordings of granule cells in slices from control (A), contralateral (B), and ipsilateral (C) without MFS and ipsilateral with MFS (D). Boxed areas of each trace are enlarged below. E: average EPSC frequency for cells in each treatment group. The numbers of cells are indicated in parentheses above each bar. F: EPSC frequency plotted as a function of Timm score. Solid lines, mean EPSC values; dotted lines, ±S D. G: average amplitude for cells in each of the 4 treatment groups. Error bars indicate mean ± SD. Asterisk, P < 0.001.
Table 1.
Passive membrane properties of granule cells after CCI injury
| Average Timm Score | RMP, mV | 10–90% EPSC RT (ms) | EPSC Decay τ (ms) | |
|---|---|---|---|---|
| Control | 0 | −75 ± 3.8 | 1.8 ± 0.1 | 7.6 ± 0.6 |
| Contralateral | 0 | −76 ± 2.9 | 1.8 ± 0.2 | 7.8 ± 0.5 |
| Ipsilateral (No MFS) | 0.4 ± 0.5 | −75 ± 5.3 | 1.9 ± 0.4 | 7.9 ± 1.2 |
| Ipsilateral (MFS) | 2.4 ± 0.5 | −79 ± 4.4 | 1.7 ± 0.3 | 7.3 ± 1.3 |
Values are means ± SD; CCI, controlled cortical impact; RMP, resting membrane potential; EPSC, excitatory postsynaptic current; RT, rise time; MSF, mossy fiber sprouting.
Spontaneous epileptiform burst activity in slices with MFS
When surgically isolated from afferent inputs, dentate granule cells normally do not fire spontaneous bursts of action potentials at resting membrane potential even when inhibition is depressed (Cronin et al. 1992; Fricke and Prince 1984; Wuarin and Dudek 1996). However, in conditions of reduced inhibition and increased excitability, spontaneous burst responses can be observed in slices from kainate-treated rats with MFS but not in controls (Cronin et al. 1992). We tested whether spontaneous epileptiform burst activity occurred in granule cells after injury. Slices were perfused with Mg2+-free ACSF containing 100 μM PTX to unmask NMDA receptor-mediated excitatory synapses and block GABAA-mediated inhibition. Recordings were first obtained from granule cells in voltage-clamp at a holding potential of −70 mV. Spontaneous bursts of repetitive or compound EPSCs were never observed in 42 granule cells in slices from injured and control slices without MFS (n = 5, control; n = 14, contralateral; and n = 23, ipsilateral without MFS). In contrast, 34 of 51 cells (67%) in 34 slices with MFS from 19 injured animals had spontaneous bursts of large-amplitude compound EPSCs (Fig. 4). This difference was found to be significant (P < 0.001; Fisher's exact test). Some of the cells examined for EPSCs were also examined for spontaneous epileptiform bursts of action potentials by switching to current-clamp mode and recording activity at resting membrane potential. Action potentials were not observed in any of 17 cells from slices without MFS from injured and control animals (n = 5, control; n = 5, contralateral; and n = 7, ipsilateral without MFS). In contrast, 65% of cells in slices with MFS displayed bursts of action potentials (n = 17 of 26, Fig. 5). This difference was statistically significant (P < 0.001; Fisher's exact test). Action potential bursts were only observed in cells that also had bursts of EPSCs. Spontaneous burst activity was therefore only observed in granule cells of slices with MFS from injured animals and was never observed in slices without MFS from control or injured animals.
Excitatory synaptic connections between granule cells after CCI
Localized glutamate stimulation has been used to elicit responses that reflect synaptic connections between granule cells in slices with MFS after pilocarpine or kainate treatment (Lynch and Sutula 2000; Winokur et al. 2004; Wuarin and Dudek 1996, 2001). We used glutamate photolysis to test the hypothesis that synaptic connections between granule cells can be evoked in slices with MFS ispilateral to the injury and not in the contralateral dentate gyrus where MFS was absent. The presence (or absence) of synaptic connections was assessed by examining two postsynaptic responses: photolysis-evoked EPSCs were first recorded at a holding potential of −70 mV and photolysis-evoked action potentials were recorded at resting membrane potential. Stimuli applied directly to the recorded cell evoked a large-amplitude inward current with occasional superimposed sodium currents (voltage-clamp) or a large depolarization with superimposed action potentials (current-clamp), confirming that the stimulation parameters were capable of evoking action potentials in the recorded cells (Fig. 6, A and B).
An increase in evoked synaptic activity was not observed in cells from slices contralateral to the injury (Fig. 6); i.e., the average number of EPSCs in any given 100-ms segment after stimulation was not greater than the average number of EPSCs before stimulation +3 SD at any stimulation site contralateral to the injury (n = 42 stimulus locations in 5 cells from 3 animals). In current-clamp recordings, photostimulation at the same locations did not evoke excitatory postsynaptic potentials (EPSPs) or action potentials at any site except when applied directly to the recorded neuron. Therefore glutamate photostimulation failed to elicit a synaptically mediated response at any location along the granule cell layer or in the hilus/CA3 region in slices contralateral to the injury.
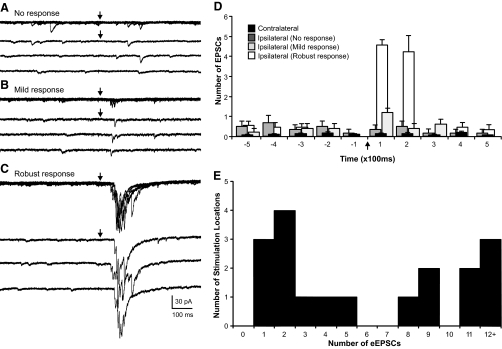
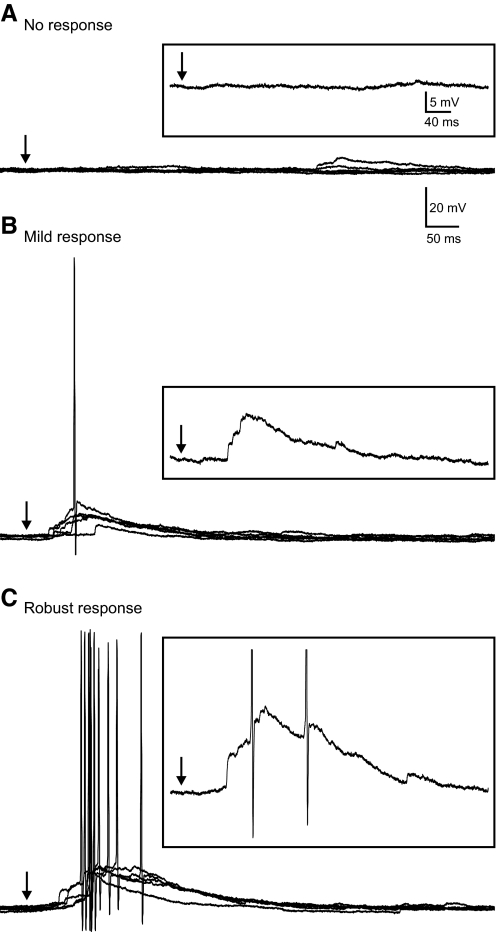
In contrast, photostimulation of the granule cell layer evoked a synaptic response and an increase in activity in six of eight cells (75%) from eight ipsilateral slices with MFS (n = 66 stimulus locations, 5 animals; Fig. 7). A total of 18 stimulation locations (1–6 locations per cell) were determined to give a positive response to photostimulation. Figures 8 and 9 show the variability of evoked responses in the granule cell layer ipsilateral to the injury site. A frequency histogram was constructed to show the distribution of the average number of eEPSCs for locations that had a positive response to photostimulation (Fig. 8E). In current-clamp mode, EPSPs were consistently evoked and often reached action potential threshold (66%). Photostimulation evoked action potentials in nearly all trials (96%) at locations that had at least four eEPSCs in voltage-clamp mode. Only 24% of stimulations evoked action potentials at locations that had less than four eEPSCs. The remaining stimulus locations (n = 48), including all 17 locations in the hilus and CA3, had no response to photostimulation. An increase in the number of EPSCs after stimulation was not observed and EPSPs were not evoked by photostimulation at these locations.
Fig. 8.
Variation in evoked EPSC (eEPSC) responses in granule cells from slices ipsilateral to the lesion. A–C: representative responses in a single neuron at 3 different stimulation locations in the granule cell layer. Each response shows 5 consecutive overlapping responses with 3 consecutive individual traces separately shown below. A: photostimulation did not evoke a response. B: a mild response of 1–2 EPSCs was consistently evoked in each of 5 trials. C: a more robust response that consisted of 5–9 eEPSCs in each of 5 trials after photostimulation at a different site in the granule cell layer. D: number of EPSCs per 100 ms before and after stimulation for each representative response in A–C. Contralateral responses are averaged across all stimulation locations in 5 neurons. →, the time of stimulation. E: frequency histogram shows the distribution of the average number of eEPSCs at 18 stimulation sites that had a positive response.
Fig. 9.
eEPSPs and action potentials in granule cells ipsilateral to CCI injury after photostimulation at distant locations in the granule cell layer. A–C: representative responses in a single neuron at 3 different stimulation locations of the granule cell layer. Each response shows 5 consecutive overlapping responses. A: no response evoked by photostimulation. B: a mild response was evoked with 1 of 5 responses reaching action potential threshold. Photostimulation at this location evoked an average of 3 EPSCs in voltage-clamp mode. C: a more robust evoked response that consisted of a small burst of action potentials in each of 5 trials. Photostimulation at this location evoked an average of 10 EPSCs in voltage-clamp mode. Insets show expanded portion of one trace. →, the time of stimulation.
DISCUSSION
Cellular mechanisms underlying the increased risk of epilepsy after head injury are not well understood. Here, we examined local network interactions in dentate granule cells from mice with posttraumatic MFS and seizures. The goal of this study was to test the hypothesis that regionally localized MFS after experimental cortical contusion injury was sufficient to produce a recurrent excitatory circuit in granule cells. An increase in excitatory input to individual granule cells was detected in slices with posttraumatic MFS. In conditions of increased excitation and decreased inhibition, these cells displayed spontaneous epileptiform burst activity. Responses that reflect synaptic connections between granule cells were activated by glutamate photostimulation at some locations of the granule cell layer but never in the hilus or CA3. These data are consistent with the development of a local recurrent excitatory circuit (Lynch and Sutula 2000; Miles and Wong 1987; Miles et al. 1986; Traub and Wong 1981, 1982; Winokur et al. 2004; Wuarin and Dudek 2001).
An important unresolved question regarding MFS after TBI is whether it is associated with a relevant functional alteration in neuronal circuitry. A dominant hypothesis has been that MFS after epileptogenic insult forms a positive feedback circuit between granule cells. Another hypothesis proposes that sprouted mossy fibers preferentially form synaptic connections with inhibitory interneurons and may act to bolster recurrent inhibition (Kotti et al. 1997; Sloviter 1991, 1992). Studies that have examined chronic changes in excitability after TBI have primarily used extracellular field recordings and have not consistently identified synchronous network activity indicative of a recurrent excitatory circuit. This may be because many of these experiments were not performed in the presence of GABAA-receptor antagonists (Reeves et al. 1997; Santhakumar et al. 2001), which is necessary for revealing recurrent excitatory circuits in the dentate gyrus (Cronin et al. 1992). However, Santhakumar et al. (2001) reported that evoked population activity in the chronically injured dentate gyrus with MFS after moderate fluid percussion injury was similar to controls even in conditions of disinhibition. Because only minimal Timm granules are detected in the inner molecular layer months after moderate fluid percussion injury (Santhakumar et al. 2001; Shumate et al. 1995), the failure to detect an increase in recurrent excitability by extracellular recordings from granule cells may also be due to the paucity of new connections in that model. A basic property of recurrent excitatory circuits is that there must be a sufficient number of excitatory interconnections between cells in order for activity to spread through the entire network (Miles and Wong 1983; Traub and Wong 1981, 1982). Other studies report that spontaneous electrographic seizures or MFS do not develop until 7–12 mo after severe fluid percussion injury in rats (Kharatishvili et al. 2006, 2007). On the other hand, we recently demonstrated spontaneous population activity indicative of synchronous network activation in slices with more robust MFS after severe CCI (Hunt et al. 2009), a focal injury model.
In the present study, we provide relatively direct evidence for increased granule cell—granule cell connections after TBI. Several independent studies have indicated that recurrent excitatory circuits are normally masked by recurrent inhibitory circuits, even in animals with frequent spontaneous seizures, and can be revealed in conditions of reduced inhibition and/or increased excitation (Cronin et al. 1992; Hunt et al. 2009; Lynch and Sutula 2000; Patrylo and Dudek 1998; Smith and Dudek 2001, 2002; Winokur et al. 2004; Wuarin and Dudek 1996, 2001). Therefore reorganized circuits are proposed to form the basis from which functional electrical discharges periodically arise in context of other abnormalities. We used Mg2+-free ACSF containing 100 μM PTX in the present study to unmask NMDA-mediated excitatory synapses and block GABAA-mediated inhibition. These conditions were sufficient to reveal new excitatory connections in slices from injured animals with MFS that were absent in control and injured slices without MFS under identical recording conditions. It is unknown whether similar results would be obtained in the presence of PTX alone. Glutamate photostimulation is useful for identifying local synaptic connections because it allows for focal stimulation of cell bodies and dendrites without activating axons of passage (Calloway and Katz 1993). Evoked responses to photostimulation in some areas of the granule cell layer—but not in other areas—suggests an underlying local circuitry. Moreover, we observed variation in these responses (i.e., mild to robust responses), indicating that not all regions of the granule cell layer contribute equally to the newly formed network. Despite reports that indicate mossy cell-granule cell connections can sometimes be observed in slice preparations (Buckmaster et al. 1992; Scharfman 1995), we did not find evidence of a photolysis-evoked excitatory synaptic connection between the hilus or CA3 region and any of our recorded granule cells. This is not surprising due to the relatively low probability of finding connections between the hilus and granule cells in vitro (Scharfman 1995).
The degree of neuronal circuit reorganization necessary to produce a seizure focus remains unresolved. It seems reasonable to suggest that in a network configuration consisting of the appropriate principle cells interconnected by regionally localized recurrent excitatory connections, activating these particular circuits under appropriate conditions (e.g., a transient failure of inhibition) may engage an entire population of neurons and contribute to seizure generation (Miles and Wong 1983; Traub and Wong 1981). Activating nearby areas without interconnections may not lead to synchronous network activation. The presence of regionally localized MFS and recurrent excitation in mice with spontaneous seizures after CCI injury is consistent with this proposed mechanism. More extensive synaptic reorganization may thus increase the probability of seizure generation or seizure severity. However, this relationship is likely indirect (Buckmaster and Dudek 1997). Development of seizure foci in other susceptible brain regions, such as CA1 (Scheff et al. 2005; Smith and Dudek 2001, 2002) or neocortex (Salin et al. 1995), may also undergo synaptic reorganization independent of MFS. Moreover, chronic injury-induced dysfunction of inhibitory circuitry may compromise the ability of these circuits to mask new excitatory connections. The net effect of new localized recurrent excitatory circuits in combination with altered synaptic inhibition after head injury may contribute to the generation of spontaneous seizures. Interestingly, we previously reported an interval-specific alteration in extracellular paired-pulse responses, an indirect measure of synaptic inhibition, selective for ipsilateral slices with MFS (Hunt et al. 2009). Future studies aimed at more thoroughly investigating altered inhibition after CCI are necessary to resolve this issue.
The abnormal electrophysiological responses in granule cells after head injury were qualitatively less robust and less widespread than reports at similar time points after pharmacologically induced status epilepticus (Lynch and Sutula 2000; Winokur et al. 2004; Wuarin and Dudek 1996, 2001). This may be due to less robust Timm staining in the inner molecular layer or less extensive synaptic network remodeling after head injury (i.e., only a portion of the dentate gyrus forms a recurrent excitatory circuit). Mechanisms other than MFS and formation of new excitatory connections, such as altered glutamate receptor pharmacology (Meldrum et al. 1999) or altered ion channel function (Steinlein 2004), could also contribute to the abnormal responses observed after injury and cannot be ruled out. However, if these were sufficient features, it would then be expected that epileptiform activity be present in the injured dentate gyrus independent of MFS. We found a relationship between the degree of Timm granules in the inner molecular layer and EPSC frequency as previously reported after kainate-treatment in rats (Wuarin and Dudek 2001). Slices from injured animals that were devoid of MFS in the inner molecular layer acted as internal controls, and responses in these slices were similar to those from uninjured controls even in ipsilateral slices from animals with regionally localized sprouting in adjacent sections of the dentate gyrus. This supports the hypothesis that the presence of epileptiform activity is associated with MFS rather than injury.
Structural damage and MFS after CCI injury remains relatively localized to areas near the injury site, even in mice that have spontaneous seizures. This is different from induction paradigms that use status epilepticus and typically result in widespread damage that includes bilateral lesion of the hippocampus. Severe fluid percussion injury, which primarily produces a concussive injury, also results in a variable degree of bilateral damage and MFS (Kharatishvili et al. 2006). The present results suggest that widespread damage and synaptic reorganization is not an obligatory component of recurrent circuit formation or spontaneous seizure generation, but at least some degree of synaptic reorganization appears to be requisite for seizure generation. We report here a gross anatomical description of cortical damage after CCI injury to provide a qualitative representation of the hippocampal damage observed in mice used in this study. Other reports have provided more detailed analyses of the degree of injury and cell loss produced by severe CCI (Hall et al. 2005; Saatman et al. 2006; Tong et al. 2002). In the present study, a lesion to the granule cell layer at the injury site and MFS was observed in nearly all mice. While MFS is more robust after severe versus moderate contusion injury (Hunt et al. 2009), it is unlikely that a granule cell layer lesion is necessary to elicit MFS after TBI (Kharatishvili et al. 2006).
Sprouting after CCI was typically most robust in sections just temporal to the injury site and became progressively less robust with increased distance away from the injury. In contrast, sprouting was generally absent in sections septal to the injury. While not always detected, granule cell loss has been reported in some temporal lobe epilepsy patients (Houser 1990), and reductions in hippocampal volume have been reported after TBI (Bigler et al. 1997). Likewise, a recent study reported MFS in at least a portion of the dentate gyrus—but not all areas—of resected tissue from all temporal lobe epilepsy patients with a history of head injury (Swartz et al. 2006). Therefore, localized MFS is likely a relevant marker of TBI-induced epilepsy associated with mesial temporal lobe sclerosis.
Seizure detection was limited to infrequent behavioral monitoring, and many seizures likely went undetected. Moreover, nonconvulsive seizures and other electrographic abnormalities (i.e., interictal spiking) cannot be accounted for. Statler et al. (2009) recently reported abnormal spontaneous electrographic activity and recurrent seizures in rats after CCI. Convulsive seizures were accompanied by behavioral manifestations similar to those described in the present study. Therefore, convulsive seizures associated with abnormal EEG activity are apparent in this model. While the monitoring method employed in the present study is widely used and allows for comparison with earlier reports using models of status epilepticus (Buckmaster and Dudek 1997; Cronin and Dudek 1988; Patrylo and Dudek 1998; Racine 1979; Shibley and Smith 2002; Sloviter 1992; Wuarin and Dudek 2001), additional studies that use continuous EEG monitoring are necessary to provide more quantitative analyses of posttraumatic seizures. Regardless, spontaneous convulsive seizures are apparent in many mice after severe CCI injury.
The development of PTE in humans is highly variable and likely depends on injury characteristics such as severity, location, and injury type in addition to genetic background and acute and long-term treatment (Pitkanen and McIntosh 2006). Therefore it is impossible to experimentally reproduce all of the manifestations of human PTE. The lack of a reliable animal model in which PTE is easily and consistently reproducible has complicated studies aimed at elucidating cellular mechanisms of epileptogenesis after closed head TBI. The present study demonstrates that severe CCI injury is an advantageous model of PTE as many mice develop chronic seizures and localized synaptic circuit reorganization in the dentate gyrus.
GRANTS
This work was supported by an Epilepsy Foundation training fellowship to R. F. Hunt and National Institutes of Health Grants AG-21981 to S. W. Scheff and NS-052302 to B. N. Smith.
REFERENCES
- Alvarez-Buylla A, Ling CY, Kirn JR. Cresyl violet: a red fluorescent Nissl stain. J Neurosci Methods 33: 129–133, 1990 [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser A, Coan SP, Rocca WP. A population-based study of seizures and traumatic brain injuries. N Eng J Med 338: 20–24, 1998 [DOI] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B. Hippocampal volume in normal aging and traumatic brain injury. Am J Neuroradiol 18: 11–13, 1997 [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kaintate treated rats. J Comp Neurol 385: 385–404, 1997 [PubMed] [Google Scholar]
- Buckmaster PS, Stowbridge BW, Kunkel DD, Schmiege DL, Schwartzkroin PA. Mossy cell axonal projections to the dentate gyrus molecular layer in the rat hippocampal slice. Hippocampus 2: 349–362, 1992 [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci 22: 6650–6658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci USA 90: 7661–7665, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caveness WF, Meirowski AM, Rish BL, Mohr JP, Kistler JP, Dillon JD, Weiss GH. The nature of posttraumatic epilepsy. J Neurosurg 50: 545–553, 1979 [DOI] [PubMed] [Google Scholar]
- Cronin J, Dudek FE. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res 474: 181–184, 1988 [DOI] [PubMed] [Google Scholar]
- Cronin J, Obenaus A, Houser CR, Dudek FE. Electrophysiology of dentate granule cells after kainite-induced synaptic reorganization of the mossy fibers. Brain Res 573: 305–310, 1992 [DOI] [PubMed] [Google Scholar]
- Dudek FE, Spitz M. Hypothetical mechanisms for the cellular and neurophysiologic basis of secondary epileptogenesis: proposed role of synaptic reorganization. J Clin Neurophysiol 14: 90–101, 1997 [DOI] [PubMed] [Google Scholar]
- Englander JE, Bushnik T, Duong TT, Cifu DX, Zafonte R, Wright J, Hughes R, Bergman W. Analyzing the risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil 84: 365–373, 2003 [DOI] [PubMed] [Google Scholar]
- Fricke RA, Prince DA. Electrophysiology of dentate granule cells. J Neurophysiol 51: 195–209, 1984 [DOI] [PubMed] [Google Scholar]
- Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci 21: 8523–8537, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma 22: 252–265, 2005 [DOI] [PubMed] [Google Scholar]
- Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res 535: 195–204, 1990 [DOI] [PubMed] [Google Scholar]
- Hunt RF, Scheff SW, Smith BN. Posttraumatic epilepsy after controlled cortical impact injury in mice. Exp Neurol 215: 243–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharatishvili I, Immonen R, Gröhn O, Pitkänen A. Quantitative diffusion MRI of hippocampus as a surrogate marker for post-traumatic epileptogenesis. Brain 130: 3155–3168, 2007 [DOI] [PubMed] [Google Scholar]
- Kharatishvili I, Nissinen JP, McIntosh TK, Pitkänen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience 140: 685–697, 2006 [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knock-out mice develop lethal status epilepticus after experimental traumatic brain injury. J Cerebral Blood Flow Met 26: 565–575, 2006 [DOI] [PubMed] [Google Scholar]
- Keller BU, Konnerth A, Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol 435: 275–293, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotti T, Reikkinen PJ, Miettinen R. Characterization of target cells for aberrant mossy fiber collaterals in the dentate gyrus of epileptic rat. Exp Neurol 146: 323–330, 1997 [DOI] [PubMed] [Google Scholar]
- Lynch M, Sutula T. Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol 83: 693–704, 2000 [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Akbar MT, Chapman AT. Glutamate receptors and transporters in genetic and acquired models of epilepsy. Epilepsy Res 36: 189–204, 1999 [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RKS. Single neurons can initiate synchronized population discharge in the hippocampus. Nature 306: 397–418, 1983 [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RKS. Inhibitory control of local excitatory circuits in the guinea pig hippocampus. J Physiol 388: 611–629, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R, Wong RKS, Traub RD. Synchronized afterdischarges in the hippocampus: contribution of local synaptic interactions. Neuroscience 12: 1179–1189, 1986 [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol 79: 418–429, 1998 [DOI] [PubMed] [Google Scholar]
- Pitkanen A, McIntosh TK. Animal models of posttraumatic epilepsy. J Neurotrauma 23: 241–261, 2006 [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizures. Electrorencephalogr Clin Neurophysiol 32: 281–294, 1979 [DOI] [PubMed] [Google Scholar]
- Reeves TM, Lyeth BG, Phillips LL, Hamm RJ, Povlishock JT. The effects of traumatic brain injury on inhibition in the hippocampus and dentate gyrus. Brain Res 1: 119–132, 1997 [DOI] [PubMed] [Google Scholar]
- Saatman KE, Feeco KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma 23: 1241–1253, 2006 [DOI] [PubMed] [Google Scholar]
- Salin P, Tseng GF, Hoffman S, Parada I, Prince DA.Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci 15: 8234–8245, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff ADH, Jeng J, Toh Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol 50: 708–717, 2001 [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J Neurophysiol 74: 179–194, 1995 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma 14: 615–627, 1997 [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Hicks RR, Baldwin SA, Robinson S, Brackney C. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma 22: 719–732, 2005 [DOI] [PubMed] [Google Scholar]
- Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy Res 49: 109–120, 2002 [DOI] [PubMed] [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kotter R, Ziles K, Luhmann HJ. Layer-specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci 21: 3580–3592, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus 1: 41–66, 1991 [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Possible functional consequences of synaptic reorganization in the dentate gyrus of kainite-treated rats. Neurosci Lett 137: 91–96, 1992 [DOI] [PubMed] [Google Scholar]
- Shumate M, Rafiq A, Lyeth B, Gong QZ, Coulter DA. Traumatic brain injury produces loss of neurons in hilus and CA3 but no significant mossy fiber sprouting in hippocampus (Abstract). Epilepsia 36: 118, 1995 [Google Scholar]
- Smith BN, Dudek FE. Short- and long-term changes in CA1 network excitability after kainate treatment in rats. J Neurophysiol 85: 1–9, 2001 [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Network interactions mediated by new excitatory connections between CA1 pyramidal cells in rats with kainite-induced epilepsy. J Neurophysiol 87: 1655–1658, 2002 [DOI] [PubMed] [Google Scholar]
- Staley KJ, Mody I. Integrity of perforant path fibers and the frequency of action potential independent excitatory and inhibitory synaptic events in dentate gyrus granule cells. Synapse 9: 219–224, 1992 [DOI] [PubMed] [Google Scholar]
- Staley KJ, Otis TS, Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrodes and whole cell recordings. J Neurophysiol 67: 1346–1357, 1992 [DOI] [PubMed] [Google Scholar]
- Statler KD, Scheerlinck P, Pouliot W, Hamilton M, White HS, Dudek FE. A potential model of pediatric posttraumatic epilepsy. Epilepsy Res 86: 221–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci 5: 400–408, 2004 [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 26: 321–330, 1989 [DOI] [PubMed] [Google Scholar]
- Swartz BE, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia 47: 1373–1182, 2006 [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci 5: 1016–1022, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RKS. Penicillin induced epileptiform activity in the hippocampal slice: a model of synchronization of CA3 pyramidal cell bursting. Neuroscience 6: 223–230, 1981 [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RKS. Cellular mechanisms of neuronal synchronization in epilepsy. Science 216: 745–747, 1982 [DOI] [PubMed] [Google Scholar]
- Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia 50: 10–13, 2009 [DOI] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferreiro DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp Neurol 176: 105–116, 2002 [DOI] [PubMed] [Google Scholar]
- Winokur RS, Kubal T, Liu D, Davis SF, Smith BN. Recurrent excitation in the dentate gyrus of a murine model of temporal lobe epilepsy. Epilepsy Res 58: 93–105, 2004 [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rat. J Neurosci 16: 4438–4448, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol 85: 1067–1077, 2001 [DOI] [PubMed] [Google Scholar]