Abstract
Activity-dependent alterations of synaptic transmission important for learning and memory are often induced by Ca2+ signals generated by depolarization. While it is widely assumed that Ca2+ is the essential transducer of depolarization into cellular plasticity, little effort has been made to test whether Ca2+-independent responses to depolarization might also induce memory-like alterations. It was recently discovered that peripheral axons of nociceptive sensory neurons in Aplysia display long-lasting hyperexcitability triggered by conditioning depolarization in the absence of Ca2+ entry (using nominally Ca2+-free solutions containing EGTA, “0Ca/EGTA”) or the absence of detectable Ca2+ transients (adding BAPTA-AM, “0Ca/EGTA/BAPTA-AM”). The current study reports that depolarization of central ganglia to ∼0 mV for 2 min in these same solutions induced hyperexcitability lasting >1 h in sensory neuron processes near their synapses onto motor neurons. Furthermore, conditioning depolarization in these solutions produced a 2.5-fold increase in excitatory postsynaptic potential (EPSP) amplitude 1–3 h afterward despite a drop in motor neuron input resistance. Depolarization in 0 Ca/EGTA produced long-term potentiation (LTP) of the EPSP lasting ≥1 days without changing postsynaptic input resistance. When re-exposed to extracellular Ca2+ during synaptic tests, prior exposure to 0Ca/EGTA or to 0Ca/EGTA/BAPTA-AM decreased sensory neuron survival. However, differential effects on neuronal health are unlikely to explain the observed potentiation because conditioning depolarization in these solutions did not alter survival rates. These findings suggest that unrecognized Ca2+-independent signals can transduce depolarization into long-lasting synaptic potentiation, perhaps contributing to persistent synaptic alterations following large, sustained depolarizations that occur during learning, neural injury, or seizures.
INTRODUCTION
The activity-dependent synaptic alterations thought to be important for learning, memory, and other types of neuronal plasticity are assumed—almost universally—to be induced by transient elevation of intracellular Ca2+ levels. Ca2+ is well suited for transducing electrical activity into cellular responses because the concentration of free intracellular Ca2+ is kept extremely low, its entry into the cytoplasm is enhanced dramatically by depolarization, and it can directly activate many enzymes and other protein effectors (Burgoyne 2007; Case et al. 2007; Hille 2001), thereby initiating functional changes within depolarized neurons. Ca2+ transients can also trigger exocytosis from depolarized neurons, releasing neuromodulators that alter synaptic strength (e.g., Kandel 2001). While the importance of Ca2+ as a trigger for activity-dependent plasticity is clear (e.g., Abrams et al. 1991; Malenka 1991; Rao and Finkbeiner 2007; Xu and Kang 2005; Zucker 1999), this does not mean that Ca2+-independent, depolarization-induced triggers of synaptic plasticity do not also exist and are not also important.
Two early and relatively neglected reports suggested that depolarization-induced, Ca2+-independent synaptic potentiation might exist. Potentiation of hippocampal synapses lasting >30 min was induced by 3-min treatment with a solution having high K+ and very low Ca2+ concentrations (HiK/0 Ca) following 4-min infusion of a nominally Ca2+-free solution (May et al. 1987). However, this study did not use Ca2+ chelators to further reduce Ca2+ levels in the “Ca2+-free solutions”. More convincing evidence came from the finding of persistent (>1 h) potentiation of the crayfish neuromuscular junction that was induced by 10 min of 20-Hz stimulation; this “long-term facilitation” was induced in a nominally Ca2+-free solution containing the membrane-permeant Ca2+ chelator, BAPTA-AM (Wojtowicz and Atwood 1988). Other studies have revealed nonsynaptic, memory-like plasticity involving Ca2+-independent induction signals during depolarization (see discussion). Of particular relevance for the possibility of Ca2+-independent, depolarization-induced synaptic potentiation was the demonstration that long-lasting hyperexcitability of Aplysia sensory neuron axons can be induced by intense 2-min depolarization (using HiK/0Ca solution) in the presence of the Ca2+ chelators, EGTA and BAPTA-AM, at concentrations sufficient to eliminate Ca2+ transients detectable with fura 2-AM imaging in the axons (and somata) during depolarization (Kunjilwar et al. 2009). Synaptic terminals of these and related sensory axons in Aplysia have been shown in a large and influential body of work to exhibit, short-, intermediate-, and long-term potentiation/facilitation induced both by activity-dependent signals (Antonov et al. 2001; Bailey et al. 2000; Eliot et al. 1994; Hawkins et al. 1983; Lin and Glanzman 1994; Schacher et al. 1997; Sutton and Carew 2000; Walters and Byrne 1983a, 1985), and by extrinsic modulators (reviewed by Byrne and Kandel 1996; Glanzman 2008; Kandel 2001; Reissner et al. 2006). Thus it was natural to investigate whether depolarization in the absence of detectable Ca2+ signals leads to potentiation of these highly plastic synapses.
Here we show that intermediate-term potentiation (ITP, lasting ≥1 h) and long-term potentiation (LTP, lasting 1 day) of sensorimotor synapses in Aplysia are induced by conditioning depolarization in conditions that eliminate detectable Ca2+ transients in sensory neuron processes and that this synaptic potentiation is associated with hyperexcitability of the central processes of the sensory neurons. These findings provide strong evidence for the existence of signals other than Ca2+ for transducing depolarization into long-lasting synaptic alterations.
METHODS
Nerve-ganglion preparation and solutions
Aplysia californica (90–250 g; from Alacrity Marine, Redondo Beach, CA or from the University of Miami—National Institutes of Health National Resource for Aplysia, Miami, FL) were kept in aerated artificial seawater (ASW, instant ocean) at 16°C on a 12-h light/dark cycle. After being anesthetized by injection of isotonic MgCl2 solution (383 mM), the pedal-pleural ganglia were excised and pinned in a chamber with the attached posterior pedal nerve, p9, threaded through a series of smaller wells (Fig. 1A) (Weragoda et al. 2004). The pleural and (in experiments on synaptic potentiation) the pedal ganglia were surgically desheathed in a 1:1 mixture of normal ASW [containing, in mM: 460 NaCl, 11 CaCl2, 10 KCl, 55 MgCl2, 10 Tris buffer (pH 7.6)] and isotonic MgCl2. This anesthetizing solution was then washed out with ASW. Experiments were conducted at room temperature (20–22°C). In all experiments, the ganglia were bathed ≥30 min prior to treatment (Fig. 1, B and C) with one of two “0 Ca” solutions. 0 Ca/EGTA solution contained (in mM) 460 NaCl, 10 KCl, 1 EGTA, 66 MgCl2, and 10 HEPES. This solution had ∼100 nM free Ca2+ as indicated by fura 2 imaging (see Kunjilwar et al. 2009). In many experiments, 10 mM BAPTA-AM was added to the HiDi solution (see following text) ≥15 min prior to beginning the pretests as well as to the 0 Ca/EGTA solution (i.e., 0 Ca/EGTA/BAPTA-AM). Thus the BAPTA-AM had ≥60–90 min to diffuse into the cells before HiK treatment. Conditioning depolarization (2 min) was produced by washing in “HiK” solutions that were identical to those applied immediately before treatment except that all of the NaCl was replaced by KCl; i.e., HiK/0Ca/EGTA or HiK/0Ca/EGTA/BAPTA-AM (Kunjilwar et al. 2009). Such HiK treatment depolarizes Aplysia neurons to ∼0 mV (Kunjilwar et al. 2009; Lin et al. 2003; Weragoda et al. 2004). Sham controls received identical treatment except that the same 0 Ca/EGTA solution was reintroduced rather than the corresponding HiK/0 Ca/EGTA solution. Treatment solutions were coded and applied in a blind procedure. Solutions were completely exchanged by infusion of a volume >10 times the volume of the chamber (1.5 ml) within 45 s. Excitatory postsynaptic potentials (EPSPs) were tested before and after exposure of the preparation to 0 Ca solutions. EPSPs were tested in a solution (see Fig. 1C) containing elevated concentrations of divalent cations (HiDi): ASW containing 2.2 × normal [Ca2+] (24.2 mM) and 2 × normal [Mg2+] (110 mM) with NaCl adjusted to maintain normal osmolarity. This solution effectively blocks polysynaptic components of connections between tail sensory neurons and tail motor neurons without altering the amplitude of the monosynaptic EPSP (Liao and Walters 2002). In long-term experiments, the excised ganglia and nerves were stored overnight at 16°C in ASW.
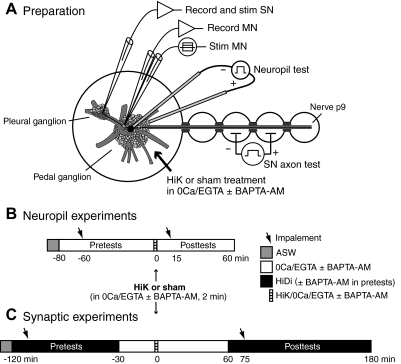
Fig. 1.
Preparation and experimental sequences. A: nerve-ganglia preparation consisting of excised pedal and pleural ganglia with the attached tail nerve (p9). Extracellular test stimuli were applied to the nerve to identify tail sensory neurons and then across the pedal ganglion (“neuropil test”) to determine the thresholds of their central processes in the vicinity of sensorimotor synapses (monitored by intracellular recording of evoked spikes conducted to the sensory neuron soma). Monosynaptic excitatory postsynaptic potentials (EPSPs) were tested by stimulating a tail sensory neuron (SN) and recording its synaptic response in a tail motor neuron (MN) that was manually clamped to −70 mV. MN input resistance (Rin) was tested by intracellular stimulation through a separate stimulating electrode. Neuropil test stimulation and MN recordings were performed in separate experiments. B: sequence of solution changes and microelectrode impalements into sensory neurons in experiments examining excitability of processes in the neuropil. The times indicated are relative to the offset of the 2-min high K+ (HiK) treatment. C: solution changes and SN and MN impalements in experiments examining synaptic potentiation. When present, BAPTA-AM was applied before and during the pretests, and was also included in the 0Ca/EGTA and high divalent cation (HiDi) solutions. In long-term experiments, the sequence was the same except that the posttests in HiDi were conducted 18–24 h after treatment.
Electrophysiological measurements
Short-term (∼15 min) and intermediate-term (1–3 h) hyperexcitability (STH and LTH) of sensory neuron processes within the pedal ganglion neuropil were examined by determining the threshold of action potentials evoked by constant current test pulses passed between a 1.5-mm-diam Pt-Ir electrode, insulated except for the tip, placed underneath the center of the ventral surface of the fully sheathed pedal ganglion and an identical electrode pressed against the dorsal surface, ∼2–3 mm posterior to the root of the pleural-pedal connective. This is near the region occupied by the somata of identified tail motor neurons (Fig. 1A) (Walters et al. 1983) and includes the region where synapses between tail sensory and tail motor neurons are concentrated (Wainwright et al. 2002; Zhang et al. 2003). To identify tail sensory neurons, extracellular test stimuli were also applied peripherally to segments of the posterior pedal nerve (p9) ∼2 cm from the pedal ganglion, using described methods (Kunjilwar et al. 2009; Weragoda et al. 2004). Sensory neuron spike thresholds were obtained with ascending series of 5-ms pulses delivered first to the nerve and then across the pedal ganglion with the evoked action potential monitored by intracellular electrodes in somata in the pleural ganglion (Figs. 1A and 2, A and B). Intracellular recordings from tail sensory neurons and tail motor neurons were made with glass capillary microelectrodes (35–50 MΩ) filled with 3 mM potassium acetate. Neurons were impaled for pretests, the electrodes removed before the washes associated with treatment, and then the same neurons reimpaled for posttests. Motor neurons were impaled with separate electrodes for measuring membrane potential and passing current. EPSP recordings were made in HiDi solution (see preceding text), with the motor neuron manually clamped to −70 mV. In all cases, the short-latency (4–8 ms) EPSP associated with the first observed action potential in the sensory neuron was measured. This often occurred during impalement of the sensory neuron (Fig. 3A); otherwise during injection of 20-ms depolarizing currents into the sensory neuron soma (Fig. 3B), which also provided a measure of the action potential threshold in the soma. Motor neuron input resistance, Rin, was tested in HiDi solution with descending series of 1-s current pulses while manually clamped at −70 mV. Rin was determined from the linear portion of the resulting V-I curve.
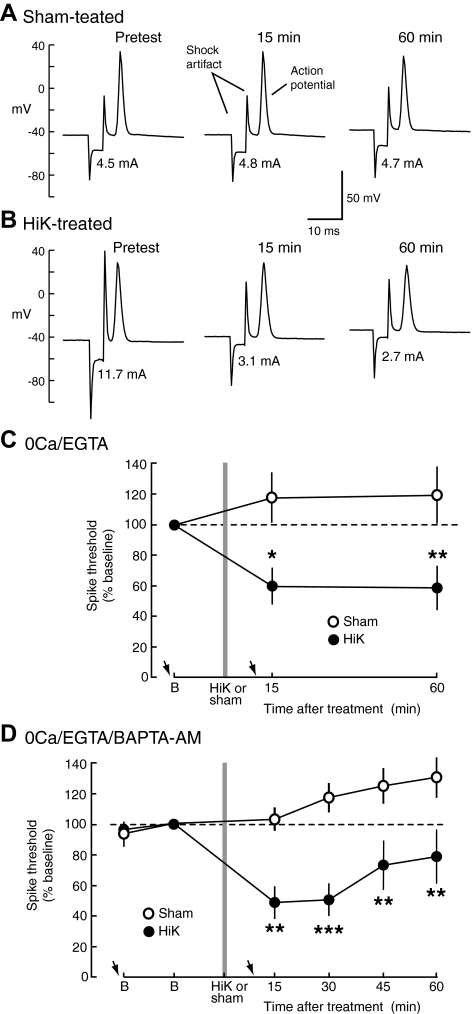
Fig. 2.
Hyperexcitability of sensory neuron processes near central synaptic terminals induced by 2-min depolarization develops and is maintained in 0Ca/EGTA and 0Ca/EGTA/BAPTA-AM solutions. A and B: examples of sensory neuron action potentials evoked by threshold stimuli (stimulus current is indicated below each spike) before and after sham (A) and HiK (B) treatment. C: short- and intermediate-term hyperexcitability (STH/ITH) of sensory neuron processes in the pedal ganglion induced by 2-min treatment with HiK/0Ca/EGTA and maintained for ≥60 min in 0Ca/EGTA solution. Means of the median spike thresholds per preparation are normalized to the baseline (B) trial during the pretest phase (sham, n = 4 preparations with 10 sensory neurons; HiK, n = 4 preparations with 9 sensory neurons). Arrows indicate approximate times of microelectrode impalement. D: STH/ITH of sensory neuron processes in the pedal ganglion induced by treatment with HiK/0Ca/EGTA/BAPTA-AM and maintained in 0Ca/EGTA/BAPTA-AM solution (sham, n = 7 preparations with 11 sensory neurons; HiK, n = 5 preparations with 11 sensory neurons). For all excitability (and synaptic) data presented in this paper, each preparation contributed a single data point for each test (the median value from 1 to 4 sensory neurons tested per preparation) for statistical analyses. Differences between sham and HiK treatment outcomes were assessed with 2-way, repeated-measures ANOVA followed by Bonferroni posttests. *, P < 0.05; **, P < 0.01.
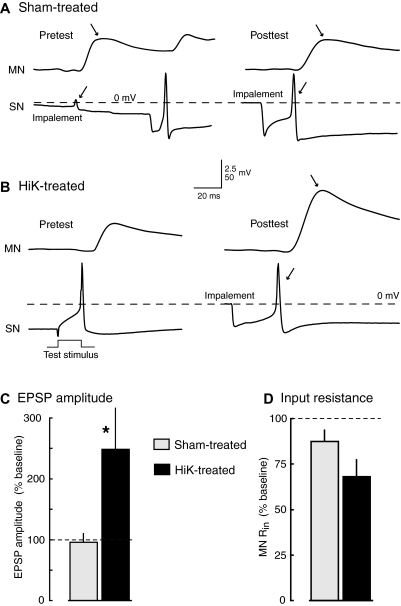
Fig. 3.
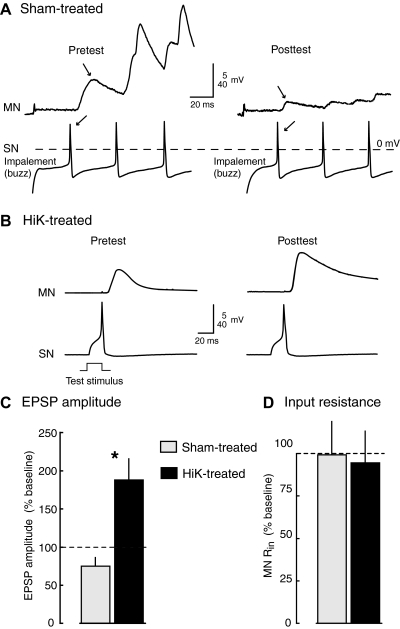
Potentiation of monosynaptic connections between tail sensory neurons and tail motor neurons is induced by 2-min conditioning depolarization under conditions that eliminate the driving force for Ca2+ entry into cells. A and B: examples of EPSPs evoked by the 1st action potential in sensory neurons stimulated in HiDi solution before (pretest) and ∼90 min after (posttest) treatment in sham (A) or HiK (B) solutions containing 0Ca/EGTA. In A, the 1st action potentials (←) and resulting EPSPs (→) are produced by impalement of the sensory neuron. No action potential occurred during impalement of the sensory neuron shown in B during the pretest, so the 1st action potential occurred when 20-ms pulses were delivered to the soma to determine soma spike threshold. C and D: significant potentiation of EPSP amplitude by HiK/0Ca/EGTA treatment. Graphs show means ± SE of the median test responses per preparation in each condition normalized to the 1st EPSP evoked by each sensory neuron during the pretest (sham, n = 9 preparations with 24 sensory neurons and 11 motor neurons; HiK, n = 7 preparations with 19 sensory neurons and 10 motor neurons). *, P < 0.05; **, P < 0.01, 2-tailed, unpaired t-test comparing the changes in responses (from pre- to posttest) between sham- and HiK-treated preparations.
Data analysis
Data are reported as means ± SE with n indicating the number of preparations tested in each condition. Multiple sensory neurons (and in some cases, motor neurons) were usually tested in each preparation and averaged, with the median of the measurements per preparation used as a single data point. Even when the number of cells is also reported, the statistical tests were performed using each preparation, not each cell, as the statistical unit. Measurements were performed with blind procedures. Comparisons between treatments of unpaired preparations were made with unpaired t-tests. Comparisons of a single group before and after treatment were made with paired t-tests. Comparisons of two groups across multiple tests utilized two-way ANOVA with repeated measures. If there was a significant overall effect (P < 0.05), this was followed by Bonferroni post hoc tests. Differences in sensory neuron survival rates were assessed with Fisher's exact tests. Statistically significant differences (P < 0.05) are indicated by asterisks in each figure (see figure legends for details of the statistical analyses).
RESULTS
Conditioning depolarization induces STH and ITH of sensory neuron processes near synaptic terminals in 0Ca/EGTA and 0Ca/EGTA/BAPTA-AM solutions
We first asked if depolarization-induced hyperexcitability could be triggered and maintained in the absence of Ca2+ entry within compartments of tail sensory neurons located in the region of the pedal ganglion where these cells synapse onto tail motor neurons (Wainwright et al. 2002; Zhang et al. 2003). Intense 2-min depolarization of peripheral axons of these neurons in a solution (HiK/0Ca/EGTA) that eliminates the driving force for Ca2+ entry into the cell is known to induce axonal STH, ITH, and LTH (Kunjilwar et al. 2009). To determine whether hyperexcitability occurs in sensory neuron processes near synaptic terminals after HiK treatment, we tested spike threshold by passing test currents across the pedal ganglion through electrodes placed below and above the tail motor neuron region while recording from sensory neuron somata in the pleural ganglion (Fig. 1A). Because the sensory axons and processes are primarily in the neuropil within the pedal ganglion (e.g., Wainwright et al. 2002), and the Ca2+-free solutions block synaptic transmission (and therefore any indirect excitation of the sensory neurons), we assume that the sites of sensory action potential generation elicited by these test stimuli are within the neuropil. After bathing the pedal and pleural ganglia in 0Ca/EGTA for >60 min (Fig. 1B), we applied HiK/0Ca/EGTA solution to the ganglia for 2 min and found that the threshold for action potential generation in sensory neuron processes within the pedal ganglion decreased markedly. Examples of threshold responses in the neuropil (monitored in sensory neuron somata, see Fig. 1A) are shown in Fig. 2, A and B, before, 15 min, and 60 min after HiK or sham treatment. The 2-min depolarization produced by treatment with HiK/0Ca/EGTA significantly decreased the threshold for sensory neuron spike generation in the neuropil compared with the effects of sham treatment 15 min (STH) and 60 min (ITH) following treatment (Fig. 2C, see legend for statistics).
We next asked if STH and ITH of sensory neuron processes in the pedal ganglion neuropil could be induced and maintained by conditioning depolarization under conditions in which elevation of intracellular free Ca2+ from both extra- and intracellular sources was minimized. Kunjilwar et al. (2009) showed that addition of the membrane-permeant Ca2+ chelator, BAPTA-AM (10 μM), to 0Ca/EGTA solution prevented detectable Ca2+ transients during HiK treatment of the axons of dissociated sensory neurons (although the same exposure to BAPTA-AM in ASW was not sufficient to effectively chelate the enormous amounts of Ca2+ that enter the cell during prolonged depolarization in the presence of normal extracellular [Ca2+]o). Using 0Ca/EGTA/BAPTA-AM and HiK/0Ca/EGTA/BAPTA-AM solutions, we found that 2-min depolarization under conditions of extracellular and probable intracellular Ca2+ chelation still produced significant STH and ITH of sensory neuron processes within the neuropil (Fig. 2D). To compare our results more closely to the effects on peripheral axons reported by Kunjilwar et al. (2009), test stimuli were applied every 15 min before and for 60 min after treatment. These results show that intense depolarization for 2 min under conditions associated with little or no Ca2+ signaling produces STH and ITH of tail sensory neuron processes in the vicinity of their central synapses onto tail motor neurons. This hyperexcitability is similar in magnitude and time course to that seen in the sensory neurons' peripheral axons (Kunjilwar et al. 2009).
In both sets of experiments summarized in Fig. 2, we also tested the excitability of tail sensory neuron axons in nerve p9, ∼15 mm from the main chamber that received HiK treatment. No significant changes in axonal spike threshold were found after HiK treatment [the mean axonal thresholds in HiK-treated preparations in both 0Ca/EGTA (n = 4) and 0Ca/EGTA/BAPTA-AM (n = 7) across all the posttests remained within 95–100% of the baseline values], and no significant differences were seen between HiK-treated and sham-treated preparations in either study. This indicates that depolarization-induced, Ca2+-independent STH and ITH are restricted to compartments of the sensory neuron that receive the conditioning depolarization, reminiscent of the site specificity of LTH found in peripheral axonal segments after localized treatment of nerve segments with HiK (Kunjilwar et al. 2009; Weragoda et al. 2004) or 5-HT (Weragoda and Walters 2007).
Conditioning depolarization induces synaptic ITP in 0Ca/EGTA and 0Ca/EGTA/BAPTA-AM solutions
To investigate whether depolarization-induced potentiation of sensorimotor synapses could be triggered in the absence of Ca2+ entry, we applied HiK/0Ca/EGTA solution to the pedal and pleural ganglia and measured monosynaptic EPSPs between tail sensory neurons and tail motor neurons before and 75–180 min after HiK or sham treatment (Fig. 1C). For 30 min prior to and 60 min after treatment, the main chamber was bathed in 0Ca/EGTA solution. EPSPs at these synapses can only be elicited in solutions containing sufficient Ca2+, but in normal Ca2+ concentrations, single action potentials in Aplysia sensory neurons often evoke polysynaptic as well as monosynaptic EPSPs (Walters and Cohen 1997). Therefore we tested the EPSPs in a solution (HiDi) containing elevated concentrations of the divalent cations, Ca2+ and Mg2+ that not only permits synaptic transmission but also greatly reduces the incidence polysynaptic EPSPs evoked by single spikes in the sensory neuron (Liao and Walters 2002). This solution was not washed in until the preparation had been in 0Ca/EGTA solution for 60 min after the 2 min HiK treatment (Fig. 1C), so no residual elevation of K+ would have been present when Ca2+ was reintroduced. Examples of EPSPs before and after HiK or sham treatment are shown in Fig. 3, A and B. In each case, the first EPSP observed during sampling of each sensory neuron was measured. This avoided possible posttetanic potentiation or depression of the synapses by bursts of spikes that were often elicited by impalement of the sensory neuron. As illustrated, membrane potential could change gradually or abruptly during impalement (but was stable afterwards), and the first spike and consequent EPSP were sometimes elicited before the electrode had completely penetrated the cell (Fig. 3A, left). In other cases, the first spike was evoked by a test pulse injected into the soma during the determination of soma spike threshold (Fig. 3B, left).
The 2-min depolarization by HiK/0Ca/EGTA treatment caused a 2.5-fold increase in mean EPSP amplitude 75–180 min following treatment (Fig. 3C), significantly increasing it compared with the effects of sham treatment. Interestingly, both HiK and sham treatments under these conditions tended to decrease input resistance (Rin) in the motor neuron (Fig. 3D). Rin decreased from pre- to posttest in 9 of 11 motor neurons tested in sham-treated preparations (from a mean of 42 to 37 MΩ) and in 8 of 9 motor neurons in HiK-treated preparations (from a mean of 33 to 24 MΩ). These decreases in Rin suggested that the effects on synaptic transmission might be even greater than indicated by the measured EPSP values (which would be reduced by decreased postsynaptic Rin – see discussion). No significant differences were found between the pretest values of EPSPs in HiK and sham-treated groups, and in sham-treated preparations no significant changes in EPSP values were found between the pre- and posttest. In addition, no significant differences were found in the resting membrane potentials (RMP) of sensory neuron somata (means for pre- and posttest values, respectively: sham, −38.2 and −40.3 mV, n = 24; HiK, −40.6 and 42.9 mV, n = 19).
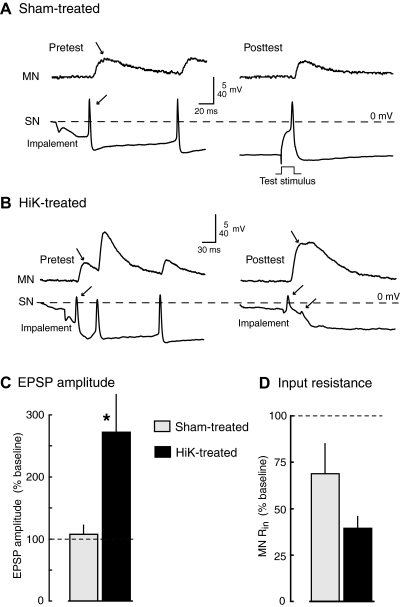
To determine whether depolarization-induced potentiation of sensorimotor synapses could be triggered under conditions in which elevation of intracellular free Ca2+ from both extra- and intracellular sources is minimized, we repeated the study just described but applied 0Ca/EGTA/BAPTA-AM and HiK/0Ca/EGTA/BAPTA-AM solutions to the pedal and pleural ganglia. In addition, BAPTA-AM was included in the HiDi solution used during synaptic testing, to provide additional time (60–90 min total) for diffusion across synaptic membranes prior to HiK treatment. Examples of EPSPs before and after HiK or sham treatment are shown in Fig. 4, A and B. Again, the first EPSP observed during sampling of each sensory neuron was measured. As illustrated here, the first EPSPs were often evoked by action potentials generated by impalement of the sensory neuron. No obvious effects of the BAPTA-AM were evident on synaptic transmission, consistent with the minor effect on Ca2+ transients observed in sensory neuron axons when BAPTA-AM application was not combined with 0Ca/EGTA (Kunjilwar et al. 2009). Two-minute depolarization again significantly increased EPSP amplitude compared with the effects of sham treatment 60–180 min following treatment with the induction occurring this time in the presence of both intracellular and extracellular Ca2+ chelators (Fig. 4C). Both HiK and sham treatments under these conditions tended to decrease Rin in the motor neuron (Fig. 4D) with Rin decreasing from pretest to posttest in 5 of 6 motor neurons in the sham-treated preparations (from a mean of 17 to 11 MΩ) and in 4 of 4 motor neurons in the HiK-treated preparations (from a mean of 32 to 12 MΩ). In this synaptic potentiation study, we utilized one-tailed, rather than two-tailed, unpaired t-tests to assess statistical significance because the directions of change were predicted on the basis of the effects of HiK treatment found in the prior study using 0Ca/EGTA solutions (Fig. 3). As described in the following text, the very high mortality of the sensory neurons treated in 0Ca/EGTA/BAPTA-AM and tested in HiDi solution made it impractical to achieve sample sizes large enough for more conservative statistical tests. Sensory neuron somata surviving after prolonged 0Ca/EGTA/BAPTA-AM exposure were often relatively inexcitable and had reduced action potential amplitudes (see Fig. 4, A and B, right). However, no significant differences between HiK and sham groups were found in the RMPs of sensory neuron somata (means for pre- and posttest values, respectively: sham, −38.7 and −36.2 mV, n = 13; HiK, −35.6 and 33.8 mV, n = 7). No significant changes in EPSPs were found between the pre- and posttest in sham-treated preparations.
Fig. 4.
Potentiation of monosynaptic connections between tail sensory neurons and tail motor neurons is induced by 2-min conditioning depolarization under conditions likely to eliminate Ca2+ transients during depolarization. A and B: examples of EPSPs evoked in HiDi solution before and ∼90 min after treatment in sham (A) or HiK (B) solutions containing 0Ca/EGTA/BAPTA-AM. In most cases, the 1st EPSP (→) was caused by sensory neuron spikes generated during impalement (←). EPSPs tested in HiDi solution before and 60–90 min after HiK/0Ca/EGTA/BAPTA-AM treatment showed significant potentiation compared with those tested after 0Ca/EGTA/BAPTA-AM treatment (C), while motor neuron input resistance tended to decrease (D; sham, n = 4 preparations with 13 sensory neurons and 6 motor neurons; HiK, n = 4 preparations with 7 sensory neurons and 4 motor neurons). *, P < 0.05, 1-tailed, unpaired t-test comparing the changes in responses (from pre- to posttest) between sham- and HiK-treated preparations. Graphs show means ± SE of the median test responses per preparation. One-tailed tests were utilized because the direction of the effect was predicted by the experiments summarized in Fig. 3 (see text).
LTP of sensorimotor synapses that persists for at least a day is induced by depolarization in 0Ca/EGTA solution
To see if depolarization-induced synaptic potentiation lasting 1 day can be induced in the absence of Ca2+ entry, we tested monosynaptic EPSPs in HiDi solution (pretest), incubated the preparation in 0Ca/EGTA solution for ≥30 min prior to 2 min HiK or sham treatment and for ≥1 h afterward, kept the preparations overnight in ASW, and tested the EPSPs in HiDi solution the next day (posttest). Examples of EPSPs before and 1 day after HiK or sham treatment are shown in Fig. 5, A and B. Elimination of Ca2+ entry at the time of depolarization failed to prevent synaptic LTP assessed 18–24 h later as revealed by significantly greater EPSP amplitudes after HiK treatment than sham treatment (Fig. 5C). In contrast to the decrease in motor neuron Rin observed in the ITP studies, neither the HiK- nor sham-treated preparations showed any significant change in Rin when tested the day after treatment (Fig. 5D). No significant changes in EPSP or Rin values were found between the pre- and posttest in sham-treated preparations. Only a minority of sensory neurons survived into the 1-d posttest; 17% (7 of 42 cells) in the sham-treated group, and 25% (16 of 65) in the HiK-treated group. These survival rates did not differ significantly from each other. Because of the high mortality rates associated both with long-term testing and with synaptic testing following exposure to 0Ca/EGTA/BAPTA-AM (see following text), we did not investigate possible long-term synaptic effects of HiK treatment conducted in 0Ca/EGTA/BAPTA-AM.
Fig. 5.
Long-term synaptic potentiation is induced by 2-min conditioning depolarization under conditions that eliminate the driving force for Ca2+ entry. A and B: examples of EPSPs evoked in HiDi solution before and ∼20 h after treatment in sham (A) or HiK (B) solutions containing 0Ca/EGTA. The 1st sensory neuron spike (←) in each example in A was evoked during impalement facilitated by “buzzing” the microelectrode (oscillating the capacitance compensation circuit), which caused the artifacts at the beginning of each record. EPSPs (C) tested in HiDi solution before and 18–24 h after HiK/0Ca/EGTA treatment showed significant potentiation compared with those tested after sham treatment. Motor neuron Rin (D) was not altered in HiK- or sham-treated preparations in the long-term tests (sham, n = 3 preparations with 9 sensory neurons and 5 motor neurons; HiK, n = 4 preparations with 7 sensory neurons and 6 motor neurons). Graphs show means ± SE of the median test responses per preparation. *, P < 0.05, 2-tailed, unpaired t-test comparing the changes in responses overnight between sham- and HiK-treated preparations.
Sensory neuron mortality is increased by re-exposure to extracellular Ca2+ following prolonged conditions that reduce Ca2+ signaling
The prolonged exposure of neurons to very low Ca2+ conditions in our experiments—in many cases followed by exposure to elevated Ca2+ levels (HiDi solution)—may produce cellular stresses that potentially interact with the depolarizing treatment, which in principle might contribute to differences in EPSP amplitude between HiK- and sham-treated preparations. To begin to address this question, we compared the survival rates of sensory neurons that had been tested and treated under each of our major experimental conditions. Even under optimal conditions, prolonged or repeated intracellular impalement with a sharp microelectrode will kill some of these sensory neurons (unpublished observations). We found that 43% of sensory neurons bathed in 0Ca/EGTA solution for ∼90 min (between the 1st impalement in the synaptic pretests and the 2nd impalement in posttests, both conducted in HiDi solution) survived through their synaptic posttests, and no significant difference was found between the survival rates of HiK-treated and sham-treated neurons (Fig. 6A, left). In contrast, only 19% of sensory neurons bathed in 0Ca/EGTA/BAPTA-AM (between impalements and synaptic tests conducted in HiDi solution) survived through their posttests, while, again, no significant difference was found between the survival rates of HiK- and sham-treated neurons (Fig. 6A, right). The percentage of sensory neurons surviving through their posttests was significantly lower in the 0Ca/EGTA/BAPTA-AM groups (HiK and sham, combined) than the groups impaled, tested and treated identically without the addition of BAPTA-AM (Fig. 6A). This indicates that prolonged intracellular chelation of Ca2+ increases the death of sensory neurons in these experiments but that the conditioning HiK treatment has no major effect on survival of sensory neurons bathed for 90 min in either 0Ca/EGTA/BAPTA-AM or just 0Ca/EGTA.
Fig. 6.
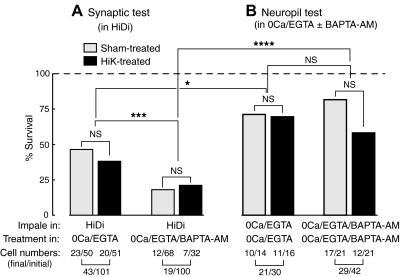
Conditions that reduce Ca2+ signaling increase sensory neuron mortality after re-exposure to high levels of extracellular Ca2+. A: survival rates of sensory neurons given synaptic tests in HiDi solution before and after exposure to low-Ca2+ solutions are sensitive to the degree of Ca2+ chelation but not to HiK treatment. Differences in the survival of HiK- and sham-treated sensory neurons were not sigifnicantly different (NS) in either condition, but the combined samples (HiK plus sham) of sensory neurons exposed to 0Ca/EGTA showed greater survival through the posttests than those exposed to 0Ca/EGTA/BAPTA-AM (43 of 101 vs. 19 of 100 cells, respectively, P < 0.001, ***, Fisher's exact test). B: survival rates of sensory neurons exposed to 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM but not re-exposed to high levels of extracellular Ca2+ (during excitability tests in the neuropil, see Fig. 2). These were higher than those re-exposed to high Ca2+ levels after 0Ca/EGTA (B vs. A, left; 21 of 30 vs. 43 of 101 cells, respectively, P < 0.05, *) or 0Ca/EGTA/BAPTA-AM (B vs. A, right; 29 of 42 vs. 19 of 100 cells, respectively, P < 0.0001, ****). No significant differences were found in the survival rates of HiK- vs. sham-treated sensory neurons in 0Ca/EGTA or in 0Ca/EGTA/BAPTA-AM. These data were obtained from the experiments summarized in Figs. 2–4.
In the experiments that examined sensory action potential thresholds in the neuropil (Fig. 2), sensory neurons remained in 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM solution continuously for the duration of the experiment (see Fig. 1B). They were impaled twice in these “Ca2+-free” conditions, once for the two pretests, and again for the two to four posttests, but were never exposed to HiDi solution (or other solutions containing high levels of Ca2+) because synaptic tests were not conducted. Again no significant effect of HiK treatment on survival was found under either condition (Fig. 6B), although the survival rate of HiK-treated sensory neurons in 0Ca/EGTA/BAPTA-AM appeared somewhat lower. Comparing the survival rates of sensory neurons treated in 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM but tested in HiDi (Fig. 6A) versus, respectively, neurons treated and tested in either 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM (Fig. 6B), the sensory neurons impaled and tested in HiDi displayed significantly less survival than those impaled and tested in the “0 Ca2+” solutions (i.e., comparing Fig. 6A, left, to B, left, and A, right, to B, right). These differences indicate that prolonged exposure to 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM by itself causes relatively little cell death in the time frame of these experiments; however, re-exposure to high extracellular Ca2+ levels following prolonged Ca2+ depletion (perhaps in combination with reimpalement of the neuron) is associated with substantial neuronal death in our synaptic experiments.
DISCUSSION
Sensorimotor synapses exhibit Ca2+-independent, depolarization-induced potentiation
The present results provide strong evidence that conditioning depolarization can trigger long-lasting potentiation of plastic synapses without using Ca2+ as the major induction signal. Synaptic potentiation lasting 1–3 h (ITP) was induced under conditions that prevent Ca2+ influx (0Ca/EGTA solution) and also under the conditions (0Ca/EGTA/BAPTA-AM solution) that we showed, using fura 2-AM imaging, to prevent both Ca2+ influx and detectable Ca2+ transients in the processes and somata of dissociated Aplysia sensory neurons (Kunjilwar et al. 2009). The lengthy exposure to 0Ca/EGTA (>30 min) and to the membrane permeant Ca2+ chelator, BAPTA-AM (60–90 min), prior to HiK treatment in the present study suggests that Ca2+ would have been effectively chelated in extra- and intracellular compartments in the desheathed ganglia preparations utilized, and thus that neuronal Ca2+ transients would have been effectively blocked, as they were in dissociated neurons (Kunjilwar et al. 2009). As discussed in the following text, effects of BAPTA-AM on neuronal survival also indicate that intracellular Ca2+ was effectively chelated in the present experiments, although we cannot rule out spatially or temporally restricted Ca2+ transients that would be below the limits of detection in our fura 2-AM experiments (Kunjilwar et al. 2009). Depolarization to ∼0 mV for ∼2 min under these conditions induced long-lasting hyperexcitability (STH and ITH) of sensory neuron processes within the pedal ganglion (Fig. 2) in the region of the potentiated synapses (Wainwright et al. 2002; Zhang et al. 2003). In magnitude and time course, this central hyperexcitability is quite similar to that found after HiK treatment of peripheral sensory axons under the same conditions (Kunjilwar et al. 2009). Hyperexcitability of sensory processes close to their synaptic terminals onto motor neurons suggests that Ca2+-independent, depolarization-induced synaptic potentiation is correlated with some presynaptic alterations. Additional evidence for alterations of the presynaptic cell came from observations of modest hyperexcitability in sensory neuron somata after HiK treatment, but the effects of local and distant depolarization on soma properties, which are complex and of uncertain relevance to the synaptic alterations, will be described elsewhere (R. Crook, F. Reyes, K. Kunjilwar, Q. Yang, E. Walters, unpublished observations). The ITP induced by conditioning depolarization in 0Ca/EGTA and in 0Ca/EGTA/BAPTA-AM cannot be explained by an increase in postsynaptic input resistance, which instead decreased significantly, as monitored in motor neuron somata following HiK treatment and, to a lesser extent, following sham treatment (Figs. 3 and 4). As a crude estimate of the change in synaptic transmission corrected for the observed decreases in postsynaptic Rin, we used Ohm's Law and the measured EPSP and Rin values to calculate an apparent excitatory postsynaptic current (EPSC) (see also Cleary et al. 1998) for each synaptic connection before and after HiK treatment. HiK treatment in 0Ca/EGTA and in 0Ca/EGTA/BAPTA-AM produced a five- to sevenfold increase in the mean estimated EPSC versus the 2.5-fold increase in the mean measured EPSP for the same connections during ITP. Although the actual increases in synaptic currents are unknown, this simplified calculation indicates that the large increases in EPSP amplitude could substantially underestimate the robust increases in synaptic strength that were produced by 2-min depolarization in the absence of detectable Ca2+ signals.
The sites (or site) responsible for these synaptic alterations remain to be determined. Potential postsynaptic contributions to depolarization-induced ITP/LTP do not include increases in input resistance of the motor neuron (Figs. 3D, 4D, and 5D), or increases in its soma excitability (unpublished observations). However, other postsynaptic alterations, such as increased insertion of glutamate receptors (e.g., Glanzman 2008), might be critical for the observed synaptic potentiation. It will be important to investigate possible roles of the motor neuron, and see if depolarization also fails to evoke detectable Ca2+ transients in motor neuron processes under these conditions. Interestingly, ITP induced in 0Ca/BAPTA-AM at the crayfish neuromuscular junction depends on presynaptic mechanisms (Wojtowicz and Atwood 1988). Together with suggestive findings from mammalian hippocampal synapses (May et al. 1987) as well as the crustacean neuromuscular junction (Wojtowicz and Atwood 1988), our results indicate that Ca2+-independent, depolarization-induced ITP mechanisms exist and involve, at least in part, presynaptic alterations. In addition to ITP, we found that depolarization in 0Ca/EGTA produced LTP that lasted ≥1 day. Addressing important questions about the possible dependence of Ca2+-independent, depolarization-induced ITP and LTP on protein synthesis and gene transcription will be challenging because the combined stresses of Ca2+ depletion, Ca2+ re-exposure, pharmacological inhibition of translation or transcription, and prolonged maintenance of ganglia ex vivo substantially decrease neuronal survival (see also Kunjilwar et al. 2009).
Cell mortality but not synaptic potentiation appears to be increased by re-exposure to Ca2+ after Ca2+ depletion
In a prior study, we were impressed at how well Aplysia sensory neurons tolerate extreme reduction of extra- and intracellular free Ca2+ levels (Kunjilwar et al. 2009), which should disturb Ca2+-dependent membrane repair and other cellular processes (Fishman and Bittner 2003; McNeil and Terasaki 2001). Except for the long-term (1 day) experiments, which typically have higher mortality rates, these earlier experiments were performed in the continuous presence of 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM. In the present study, experiments on neuropil excitability also showed relatively high rates of sensory neuron survival for ≥60 min after HiK or sham treatment in the continuous presence of 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM (Fig. 6B). It is, however, impossible to test conventional chemical synaptic transmission without sufficient extracellular Ca2+. Transmission at Aplysia sensorimotor synapses requires far more extracellular Ca2+ than was present in these solutions. For example, a solution containing 1% of normal [Ca2+], a much higher concentration than in the solutions used here, blocks detectable transmission at Aplysia sensorimotor synapses (Weragoda et al. 2004). Moreover because we needed to largely eliminate polysynaptic components of the tested synaptic connections, we performed our synaptic tests in HiDi solution containing approximately twice the normal extracellular concentrations of Ca2+ and Mg2+ (Liao and Walters 2002). Thus following 90 min in 0Ca/EGTA or 0Ca/EGTA/BAPTA-AM, the neurons were abruptly exposed to high levels of extracellular Ca2+ in the HiDi solution. Similarly, in long-term experiments the neurons were abruptly exposed to high levels of Ca2+ in the ASW used to maintain the ganglia overnight. In all of these experiments, the survival rates of sensory neurons were significantly lower than those observed when sensory neurons were reimpaled for posttests in 0Ca/EGTA or in 0Ca/EGTA/BAPTA-AM solutions that had been continuously present (see Fig. 6). Thus it seems likely that re-exposure to high levels of extracellular Ca2+, perhaps in combination with reimpalement by a microelectrode, severely reduces survival of the sensory neurons. The mechanisms are unknown, but one possibility is that store-operated Ca2+ currents, known to be activated by depletion of intracellular Ca2+ stores in Aplysia neurons (Kachoei et al. 2006), combine with other Ca2+ currents and leakage of Ca2+ at the site of impalement to bring Ca2+ concentrations to toxic levels after high extracellular Ca2+ levels are restored. Our experimental design did not allow us to measure effects of interactions among Ca2+ depletion, Ca2+ reexposure, and reimpalement on motor neuron survival. However, the relative decreases in motor neuron input resistance found in HiDi solution after depolarization in 0Ca/EGTA and 0Ca/EGTA/BAPTA-AM are consistent with the possibility that these interactions produce considerable cellular stress in motor neurons as well.
Two important implications for Ca2+-independent, depolarization-induced plasticity come from our survival observations in the sensory neurons. First, these observations provide independent evidence that BAPTA-AM penetrated the sensory neurons sufficiently to alter cellular function. There was significantly lower survival after 0Ca/EGTA/BAPTA-AM exposure than 0Ca/EGTA exposure (Fig. 6A), indicating that BAPTA-AM had a biological effect on the sensory neuron, presumably by chelating intracellular Ca2+. This adds, in the ex vivo ganglion preparation, to evidence for the effectiveness of BAPTA-AM treatment found by imaging free Ca2+ in dissociated sensory neurons (Kunjilwar et al. 2009) and strengthens our conclusion that depolarization-induced synaptic potentiation occurs in the absence of effective Ca2+ signaling. Second, we found no statistically significant effects of conditioning HiK treatment on neuronal survival, although in two of the studies shown in Fig. 6 there appeared to be a trend for lower survival in the HiK groups. Under the most severe conditions (synaptic tests in HiDi following 0Ca/EGTA/BAPTA-AM exposure), any Ca2+-independent depolarization effects on mortality showed no indication of adding to the Ca2+-dependent mortality in these cells (Fig. 6A, right). Thus 2-min depolarization did not protect against toxic effects of Ca2+ depletion and repletion. This argues against the possibility that better health of the HiK-treated preparations partially accounted for the differential effect on EPSP amplitudes measured after depolarization in the 0Ca/EGTA/BAPTA-AM solution.
Whether the depolarization effects and Ca2+ re-exposure effects are related bears on the general question of whether some of the effects of conditioning depolarization induced under conditions of little or no Ca2+ signaling involve a subsequent Ca2+-dependent step to complete the induction process. Because of the requirement for Ca2+ influx in the tests of conventional synaptic transmission that we conducted, a late Ca2+-dependent step cannot be excluded as a contributor to the observed synaptic potentiation. However, other Ca2+-independent, depolarization-induced effects in the sensory neurons—hyperexcitability of peripheral axons and central processes—are expressed in the absence of re-exposure to Ca2+. Furthermore, by itself re-exposure to Ca2+ after Ca2+ depletion induced little or no potentiation of sensorimotor synapses, as shown by the lack of significant EPSP potentiation compared with pretest values in sham-treated preparations (Figs. 3 and 4). Thus although Ca2+ influx through store-operated channels has been suggested to contribute to LTP induction in mammalian hippocampus (Baba et al. 2003; Mellentin et al. 2007), mechanisms activated by Ca2+ depletion and re-exposure cause relatively little potentiation of Aplysia sensorimotor synapses under our experimental conditions. Also unlikely as a major induction signal is delayed Ca2+ influx via exchange for Na+ that might have accumulated during intense depolarization (e.g., Misler and Hurlbut 1983) because the HiK solutions we used for conditioning depolarization had virtually all Na+ replaced by K+.
Most important, none of these potential Ca2+-dependent induction mechanisms could have operated until Ca2+ signaling was restored 60 min after depolarizing treatment. Therefore mechanisms must exist in or near these synapses that can sense intense, 2-min depolarization in the probable absence of Ca2+ signaling and that induce an influential trace of this depolarization in the sensory neurons and their synapses—a trace that in our experiments persisted for ≥60 min. This Ca2+-independent depolarization signal by itself induces hyperexcitability of the sensory neuron and by itself or in series with subsequent Ca2+ signals is sufficient to trigger long-lasting synaptic potentiation. We do not know whether the Ca2+-independent sensor(s) of depolarization is located in the sensory neuron, motor neuron, or in other cells that release signals that alter the sensory neuron and sensorimotor synapse. If the depolarization sensor is extrinsic to the sensory neuron, during the phases of our experiments that Ca2+ is effectively absent, this voltage sensor would have to communicate with the sensory neuron without using Ca2+ signaling, perhaps by voltage-dependent, Ca2+-independent exocytosis of a neuromodulator (e.g., Bernath 1992; Zhang and Zhou 2002).
Possible significance of Ca2+-independent, depolarization-induced synaptic potentiation
Nearly all investigations into the induction of activity- or depolarization-dependent synaptic plasticity have focused on Ca2+ as a necessary and sufficient molecular trigger (e.g., Abrams et al. 1991; Malenka 1991; Rao and Finkbeiner 2007; Xu and Kang 2005; Zucker 1999). Few investigators have considered the possibility that Ca2+-independent triggers may be activated by depolarization to act in parallel with Ca2+ signals to induce some forms of synaptic potentiation. Interestingly, the three studies that have supported this possibility thus far examined synapses in three different phyla: Chordata (May et al. 1987), Arthropoda (Wojtowicz and Atwood 1988), and Mollusca (this study), suggesting that Ca2+-independent, depolarization-induced synaptic potentiation is widespread. There is growing evidence for diverse mechanisms that can couple depolarization to cellular signaling pathways in a Ca2+-independent manner. These include nonconducting functions of voltage-gated channels (Kaczmarek 2006), such as the depolarization-dependent, conduction-independent activation of a p38 MAP kinase by a voltage-gated K+ channel (Hegle et al. 2006). Voltage sensitivity has been implicated in various membrane-associated proteins, including a G-protein-coupled receptor (Ben-Chaim et al. 2006), a phosphatidylinositol phosphatase (Iwasaki et al. 2008), and a proton permeation protein (Okamura 2007). Moreover, depolarization-dependent, Ca2+-independent transcriptional regulation of a hypoxia-inducible factor occurs in some cancer cells (Lan et al. 2007). Thus there may exist numerous Ca2+-independent transducers of depolarization, some of which might contribute to depolarization-induced synaptic potentiation. An interesting question is where (or whether) Ca2+-independent signals of depolarization converge with Ca2+-dependent pathways to engage mechanisms of synaptic potentiation. Given the similarities between our results and those of Wojtowicz and Atwood (1988), it is interesting that intermediate- and long-term synaptic potentiation/facilitation at both the crayfish neuromuscular junction (Dixon and Atwood 1989) and Aplysia sensorimotor synapses (e.g., Bergold et al. 1992; Schacher et al. 1988) involve activation of a cAMP-PKA pathway. This pathway and others are potential sites of convergence of Ca2+-independent and Ca2+-dependent signals.
Another unanswered question is whether Ca2+-independent, depolarization-induced synaptic potentiation might have functions distinct from the well-known Ca2+-dependent forms of potentiation. An initial clue may come from the patterns of conditioning depolarization used thus far to induce this potentiation; we used 2-min continuous depolarization to ∼0 mV caused by HiK treatment; May et al. (1987) used 3–4 min HiK treatment (40–80 mM) that probably depolarized the cells into a range between −30 and −10 mV, and Wojtowicz and Atwood (1988) used a 10 min train of high-frequency (20 Hz), intracellular pulses of 3- to 5-ms duration that in TTX depolarized the motor neuron terminal sufficiently to evoke substantial transmitter release. Thus each induction protocol involved intense depolarization of presynaptic terminals that was much more prolonged than the transient depolarizations produced in experimental models of learning and memory, such as LTP (e.g., Glanzman 2008; Malenka 1991). Conditions of intense and prolonged local depolarization may occur in a variety of normal as well as clinically important contexts. In nociceptive Aplysia sensory neurons, intense depolarization occurs during strong pinching stimulation of their receptive fields with each pinch inducing high-frequency activation and afterdischarge that can last many seconds (Clatworthy and Walters 1993; Illich and Walters 1997; Walters et al. 1983, 2004). In addition to both peripheral depolarization caused directly by membrane damage and central depolarization occurring during resulting trains of action potentials, resting depolarization of the sensory neuron soma lasting many minutes follows bursts of high-frequency action potentials (Walters and Byrne 1983b, 1985). Although the amplitude observed in the soma of this sustained postburst depolarization is modest (<10 mV), it might be larger in the presynaptic terminals. More generally, large sustained depolarizations in synaptic regions might occur in the mammalian brain during some forms of learning (Destexhe et al. 2003) and are likely during certain pathological states, including spinal cord injury (Park et al. 2004), traumatic brain injury (e.g., Shaw 2002), and epileptic seizures (e.g., de Curtis and Avanzini 2001). The potential involvement of Ca2+-independent, depolarization-induced synaptic potentiation in these normal and abnormal neural states encourages a search for its underlying mechanisms, which today remain largely unknown.
GRANTS
This work was supported by National Institute Neurological Disorders and Stroke Grant NS-35979 to E. T. Walters.
ACKNOWLEDGMENTS
We thank K. Kunjilwar, R. Crook, H. Fishman, R. Grill, R. Heidelberger, and R. O'Neil for useful comments and discussions and L. Klaassen for help with blind procedures.
Present address of F. D. Reyes: Dept. of Anesthesiology and Pain Medicine, The University of Texas-MD Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030.
REFERENCES
- Abrams TW, Karl KA, Kandel ER. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci 11: 2655–2665, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonov I, Antonova I, Kandel ER, Hawkins RD. The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia. J Neurosci 21: 6413–6422, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y. Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci 23: 7737–7741, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Zhu H, Chen M, Kandel ER. A novel function for serotonin-mediated short-term facilitation in Aplysia: conversion of a transient, cell-wide homosynaptic hebbian plasticity into a persistent, protein synthesis-independent synapse-specific enhancement. Proc Natl Acad Sci USA 97: 11581–11586, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of “gating charge” is coupled to ligand binding in a G-protein-coupled receptor. Nature 444: 106–109, 2006 [DOI] [PubMed] [Google Scholar]
- Bergold PJ, Beushausen SA, Sacktor TC, Cheley S, Bayley H, Schwartz JH. A regulatory subunit of the cAMP-dependent protein kinase down-regulated in Aplysia sensory neurons during long-term sensitization. Neuron 8: 387–397, 1992 [DOI] [PubMed] [Google Scholar]
- Bernath S. Calcium-independent release of amino acid neurotransmitters: fact or artifact? Prog Neurobiol 38: 57–91, 1992 [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci 8: 182–193, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci 16: 425–435, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium 42: 345–350, 2007 [DOI] [PubMed] [Google Scholar]
- Clatworthy AL, Walters ET. Rapid amplification and facilitation of mechanosensory discharge in Aplysia by noxious stimulation. J Neurophysiol 70: 1181–1194, 1993 [DOI] [PubMed] [Google Scholar]
- Cleary LJ, Lee WL, Byrne JH. Cellular correlates of long-term sensitization in Aplysia. J Neurosci 18: 5988–5998, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol 63: 541–567, 2001 [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4: 739–751, 2003 [DOI] [PubMed] [Google Scholar]
- Dixon D, Atwood HL. Adenylate cyclase system is essential for long-term facilitation at the crayfish neuromuscular junction. J Neurosci 9: 4246–4252, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot LS, Hawkins RD, Kandel ER, Schacher S. Pairing-specific, activity-dependent presynaptic facilitation at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci 14: 368–383, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman HM, Bittner GD. Vesicle-mediated restoration of a plasmalemmal barrier in severed axons. News Physiol Sci 18: 115–118, 2003 [DOI] [PubMed] [Google Scholar]
- Glanzman DL. New tricks for an old slug: The critical role of postsynaptic mechanisms in learning and memory in Aplysia. Prog Brain Res 169C: 277–292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science 219: 400–405, 1983 [DOI] [PubMed] [Google Scholar]
- Hegle AP, Marble DD, Wilson GF. A voltage-driven switch for ion-independent signaling by ether-a-go-go K+ channels. Proc Natl Acad Sci USA 103: 2886–2891, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes Sunderland, MA: Sinauer, 2001 [Google Scholar]
- Illich PA, Walters ET. Mechanosensory neurons innervating Aplysia siphon encode noxious stimuli and display nociceptive sensitization. J Neurosci 17: 459–469, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Murata Y, Kim Y, Hossain MI, Worby CA, Dixon JE, McCormack T, Sasaki T, Okamura Y. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA 105: 7970–7975, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachoei BA, Knox RJ, Uthuza D, Levy S, Kaczmarek LK, Magoski NS. A store-operated Ca2+ influx pathway in the bag cell neurons of Aplysia. J Neurophysiol 96: 2688–2698, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek LK. Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7: 761–771, 2006 [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038, 2001 [DOI] [PubMed] [Google Scholar]
- Kunjilwar KK, Fishman HM, Englot DJ, O'Neil RG, Walters ET. Long-lasting hyperexcitability induced by depolarization in the absence of detectable Ca2+ signals. J Neurophysiol 101: 1351–1360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan M, Shi Y, Sun L, Liu L, Guo X, Lu Y, Wang J, Liang J, Fan D. KCl depolarization increases HIF-1 transcriptional activity via the calcium-independent pathway in SGC7901 gastric cancer cells. Tumour Biol 28: 173–180, 2007 [DOI] [PubMed] [Google Scholar]
- Liao X, Walters ET. The use of elevated divalent cation solutions to isolate monosynaptic components of sensorimotor connections in Aplysia. J Neurosci Methods 120: 45–54, 2002 [DOI] [PubMed] [Google Scholar]
- Lin H, Bao J, Sung YJ, Walters ET, Ambron RT. Rapid electrical and delayed molecular signals regulate the serum response element after nerve injury: convergence of injury and learning signals. J Neurobiol 57: 204–220, 2003 [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proc Biol Sci 255: 113–118, 1994 [DOI] [PubMed] [Google Scholar]
- Malenka RC. The role of postsynaptic calcium in the induction of long-term potentiation. Mol Neurobiol 5: 289–295, 1991 [DOI] [PubMed] [Google Scholar]
- May PB, Goh JW, Sastry BR. Induction of hippocampal long-term potentiation in the absence of extracellular Ca2+. Synapse 1: 273–278, 1987 [DOI] [PubMed] [Google Scholar]
- McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nat Cell Biol 3: E124–129, 2001 [DOI] [PubMed] [Google Scholar]
- Mellentin C, Jahnsen H, Abraham WC. Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices. Neuropharmacology 52: 118–125, 2007 [DOI] [PubMed] [Google Scholar]
- Misler S, Hurlbut WP. Post-tetanic potentiation of acetylcholine release at the frog neuromuscular junction develops after stimulation in Ca2+-free solutions. Proc Natl Acad Sci USA 80: 315–319, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y. Biodiversity of voltage sensor domain proteins. Pfluegers 454: 361–371, 2007 [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21: 754–774, 2004 [DOI] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci 30: 284–291, 2007 [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Shobe JL, Carew TJ. Molecular nodes in memory processing: insights from Aplysia. Cell Mol Life Sci 63: 963–974, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacher S, Castellucci VF, Kandel ER. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science 240: 1667–1669, 1988 [DOI] [PubMed] [Google Scholar]
- Schacher S, Wu F, Sun ZY. Pathway-specific synaptic plasticity: activity-dependent enhancement and suppression of long-term heterosynaptic facilitation at converging inputs on a single target. J Neurosci 17: 597–606, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw NA. The neurophysiology of concussion. Prog Neurobiol 67: 281–344, 2002 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Carew TJ. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron 26: 219–231, 2000 [DOI] [PubMed] [Google Scholar]
- Wainwright ML, Zhang H, Byrne JH, Cleary LJ. Localized neuronal outgrowth induced by long-term sensitization training in Aplysia. J Neurosci 22: 4132–4141, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Bodnarova M, Billy AJ, Dulin MF, Diaz-Rios M, Miller MW, Moroz LL. Somatotopic organization and functional properties of mechanosensory neurons expressing sensorin-A mRNA in Aplysia californica. J Comp Neurol 471: 219–240, 2004 [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science 219: 405–408, 1983a [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH. Slow depolarization produced by associative conditioning of Aplysia sensory neurons may enhance Ca2+ entry. Brain Res 280: 165–168, 1983b [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci 5: 662–672, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol 50: 1522–1542, 1983 [DOI] [PubMed] [Google Scholar]
- Walters ET, Cohen LB. Functions of the LE sensory neurons in Aplysia. Invert Neurosci 3: 15–25, 1997 [DOI] [PubMed] [Google Scholar]
- Weragoda RM, Ferrer E, Walters ET. Memory-like alterations in Aplysia axons after nerve injury or localized depolarization. J Neurosci 24: 10393–10401, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weragoda RM, Walters ET. Serotonin induces memory-like, rapamycin-sensitive hyperexcitability in sensory axons of Aplysia that contributes to injury responses. J Neurophysiol 98: 1231–1239, 2007 [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Atwood HL. Presynaptic long-term facilitation at the crayfish neuromuscular junction: voltage-dependent and ion-dependent phases. J Neurosci 8: 4667–4674, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang J. The mechanisms and functions of activity-dependent long-term potentiation of intrinsic excitability. Rev Neurosci 16: 311–323, 2005 [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhou Z. Ca2+-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nat Neurosci 5: 425–430, 2002 [DOI] [PubMed] [Google Scholar]
- Zhang H, Wainwright M, Byrne JH, Cleary LJ. Quantitation of contacts among sensory, motor, and serotonergic neurons in the pedal ganglion of Aplysia. Learn Mem 10: 387–393, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol 9: 305–313, 1999 [DOI] [PubMed] [Google Scholar]