Abstract
The same visual stimulus evokes a different pattern of neural signals each time the stimulus is presented. Because this unreliability reduces visual performance, it is important to understand how it arises from neural circuitry. We asked whether different types of ganglion cell receive excitatory signals with different reliability and frequency content and, if so, how retinal circuitry contributes to these differences. If transmitter release is governed by Poisson statistics, the SNR of the postsynaptic currents (ratio of signal power to noise power) should grow linearly with quantal rate (qr), a prediction that we confirmed experimentally. Yet ganglion cells of the same type receive quanta at different rates. Thus to obtain a measure of reliability independent of quantal rate, we calculated the ratio SNR/qr, and found this measure to be type-specific. We also found type-specific differences in the frequency content of postsynaptic currents, although types whose dendrites branched at nearby levels of the inner plexiform layer (IPL) had similar frequency content. As a result, there was an orderly distribution of frequency response through the depth of the IPL, with alternating layers of broadband and high-pass signals. Different types of bipolar cell end at different depths of the IPL and provide excitatory synapses to ganglion cell dendrites there. Thus these findings indicate that a bipolar cell synapse conveys signals whose temporal message and reliability (SNR/qr) are determined by neuronal type. The final SNR of postsynaptic currents is set by the dendritic membrane area of a ganglion cell, which sets the numbers of bipolar cell synapses and thus the rate at which it receives quanta [SNR = qr × (SNR/qr)].
INTRODUCTION
The same visual stimulus evokes a different pattern of neural signals each time it is presented (de Ruyter van Steveninck et al. 1997). This variation occurs in part because of variation in the amount of transmitter released at chemical synapses due to the stochastic nature of calcium channel gating and the resulting fusion of synaptic vesicles. As a result, the synapse adds noise to the signal being transmitted and increases the variability of postsynaptic electrical signals (currents and voltages). Because an animal depends on these signals for the detection and discrimination of visual objects, this variability reduces visual performance (Borghuis et al. 2009).
Here we ask whether the reliability of excitatory postsynaptic currents (EPSCs) differs between different types of retinal ganglion cell. The reliability of postsynaptic signals recorded from fly visual neurons has been shown to differ significantly between different stages of visual processing (Simmons 1999; Simmons and de Ruyter van Steveninck 2005). Thus we thought it valuable to compare mammalian visual neurons at the same stage of processing but of distinct types. Our comparison was complicated by the fact that ganglion cells of the same type have different numbers of synapses and thus receive transmitter quanta at different rates. Because quanta are thought to follow Poisson statistics, the ratio of signal power to noise power (SNR) should rise proportionately with increasing quantal rate. Thus cells of the same type with different numbers of synapses were expected to show different SNRs. To tease out type-specific differences, we took the ratio of SNR to quantal rate, in this way providing a measure of reliability independent of the number of synapses and of quantal rate.
We also asked whether the frequency response of excitatory currents differs between different types of ganglion cell. It has been suggested that ganglion cells that branch in the middle of the inner plexiform layer (IPL) receive transient inputs; those that branch at either edge receive sustained inputs (Awatramani and Slaughter 2000; Roska and Werblin 2001). Transient and sustained may correspond to high-pass and broadband frequency responses. Thus here we explicitly map the frequency response of excitatory currents by using a noise stimulus with equal power at all the frequencies to which the ganglion cell can respond and by constructing impulse spectra. We found that the distribution of frequency response is more complex than previously appreciated, alternating between high-pass and broadband through the depth of the IPL.
METHODS
Visual stimulus
The stimulus was provided by a green light-emitting diode projected diffusely over the entire retinal preparation (556 nm). The mean intensity of the stimulus was 3 × 105 photons·μm−2·s−1, resulting in a photoisomerization rate for middle-wavelength cones of 3.3 × 104 R*·s−1·cone−1, which is a photopic illumination (λmax = 529 nm, outer segment: 8 μm × 3 μm2 [length × cross-sectional area]) (Yin et al. 2006). The stimulus lasted 50–60 s. The beginning of the stimulus was a white-noise sequence chosen at random from a Gaussian distribution at 1,000 Hz, but then filtered at 100 Hz (four-pole low-pass digital filter). The white noise was divided into four or five intervals each 10 s long. All intervals had the same mean intensity but each interval had a different SD, thus providing four or five different temporal contrasts in random order. The end of the stimulus consisted of a 5-s period of maximal intensity followed by a 5-s period of minimum intensity, used to record spontaneous excitatory postsynaptic currents (sEPSCs; see following text). This spatially homogeneous white-noise stimulus is effective despite center/surround antagonism because the surround is typically delayed and provides incomplete cancelation (Tokutake and Freed 2008).
Electrophysiology and imaging
An adult Hartley guinea pig (400–600 g, >8 wk old) was anesthetized with ketamine (133 mg kg−1), xylazine (13 mg kg−1), and pentobarbital (100 mg kg−1). An eye was removed and the animal was killed by anesthetic overdose. All procedures were performed according to University of Pennsylvania and National Institutes of Health guidelines. A piece of retina (∼1 cm2), attached to pigment epithelium, choroid, and sclera, was mounted in a chamber on an upright microscope with infrared differential interference contrast (IR-DIC) optics. All pieces were taken from that portion of the visual streak that lay in the superior retina. The tissue was superfused with Ames medium (Sigma-Aldrich, St. Louis, MO) that was saturated with 5% CO2-95% O2, adjusted with glucose to about 300 mOsm, and contained (in mM): 120 NaCl, 3.1 KCl, 0.5 KH2PO4, 23 Na2HCO3, 1.2 MgSO4, 1.15 CaCl2, plus amino acids and vitamins (pH 7.4, 34°C). In some experiments, 25 μM L-AP4 (l-2-amino-4-phosphonobutyrate; Tocris, Bristol, UK) was added to the superfusate.
Patch electrodes (12 mΩ) were filled with a solution consisting of (in mM): 110 Cs gluconate, 10 NaCl, 1 EGTA·2.5Na, 10 HEPES, 10 lidocaine N-ethyl bromide, and 6 Lucifer yellow; this solution was adjusted with glucose to 310 mOsm and with gluconate to pH 7.2. The calculated reversal potential for glutamate channels (Eglut), with equal permeability to Cs+ and Na+, was about 2 mV and the calculated reversal potential for Cl− channels with equal permeability to Cl− and Br− (γ-aminobutyric acid, glycine, ECl) was about −49 mV. The access resistance (Ra) was 45 ± 3 MΩ and the holding current (Ihold) was −89 ± 40 pA, which resulted in a voltage error (Va) of −4 ± 2 mV. The resulting voltage-clamp cutoff frequency was about 70 Hz, above the nearly 20-Hz bandwidth of the recorded signals (fvc = [2πCmRa]−1, Cm = 50 pF). All voltages were corrected for a calculated junction potential of −13 mV but not for the access voltage (Va). The recordings were acquired with an AxoPatch 200B patch-clamp amplifier (eight-pole Bessel filter, fC = 1 kHz) and digitized on-line at 2 kHz using pClamp 7 (Axon Instruments, Foster City, CA).
During recording Lucifer yellow diffused from the pipette into the cell. After recording, and often while the patch pipette was still attached, cells were photographed with a cooled charge-coupled device camera, producing a stack of optical sections (Hamamatsu Photonics, Hamamatsu City, Japan) (×20 water-immersion lens, 0.5 numerical aperture [NA]). Dendrites were traced through the sections using the computer program Neuromantic (kindly provided by Dr. Darren Myatt) (Fig. 7A). Dendrites were also deblurred by deconvolving the sections with a point-spread function (Volocity, Improvision, Coventry, UK). Flare due to the bright soma and patch pipette was removed from individual sections with Photoshop (Adobe) and then sections were combined into a single image by projection of the maximum intensities (Fig. 1). To measure stratification depth in the IPL, a stack of about 10 sections was captured that included IR-DIC images of cell somas at both edges of the IPL and fluorescent dendrites within the IPL (×60 water, 0.9 NA). To quantify stratification, we counted the number of sections from the fluorescent dendrites to somas at either edge of the IPL.
Fig. 7.
SNR/contrast ratio is correlated with number of synapses. A: tracings of cells were made from optical sections. B: the ratio SNR/contrast was taken from the regression fits of Fig. 6 and plotted against total dendritic length taken from the tracing in A.
Fig. 1.
Ten types of ganglion cell whose excitatory postsynaptic currents were recorded. Cells are shown as projections from optical sections (methods). Some show a patch pipette in place (bright triangles, top left). Cell bodies appear larger because of fluorescent flare. A: 5 types of ganglion cell whose excitatory postsynaptic currents were measured for reliability and frequency response. B: 5 additional types of ganglion cells whose excitatory postsynaptic currents were measured for frequency response. Note that the scale bar in this figure is smaller than that in A; thus these cells have larger dendritic arbors (>250 μm, in this and subsequent figures: α, alpha; β, beta; δ, delta; LE, local edge).
Recordings typically lasted 40 min, after which the holding current increased and the amplitude of EPSCs decreased, presumably due to an observed decrease in cellular input resistance. To establish a criterion to halt analysis, we calculated the SD of currents during a stimulus repeat, then calculated the coefficient of variation of this measure across repeats (CVSD). We halted analysis of a recording after CVSD rose to >0.20. As a result, we analyzed intervals of recording that had a CVSD value of 0.08 ± 0.01 and that comprised 32 ± 3 stimulus repeats.
Identifying cell types
Guinea pig retina contains many cell types also found in cat and primate, with the closest homologies to cells found in rabbit (Fig. 1) (Koch et al. 2004; Manookin et al. 2008). Local edge (LE) cells (dendritic field diameter: 200–250 μm) have many short (<10 μm) secondary dendritic branches and stratify narrowly near the middle of the IPL (40–60% of the depth) (Amthor et al. 1989; van Wyk et al. 2006). LE cells probably correspond to the zeta cells of cat retina (Berson et al. 1998) and the G1 cells of rabbit retina (Rockhill et al. 2002). Beta cells (170–225 μm) have profuse fine dendrites and stratify diffusely near the middle of the IPL (Boycott and Wässle 1974). G5 cells (200–230 μm) have primary dendrites that fan out from the soma, have secondary dendrites that evenly fill the spaces between the primary dendrites, and stratify narrowly above the middle IPL (Rockhill et al. 2002). G2 cells (180–220 μm) have a branching pattern similar to that of the LE cells but stratify narrowly below the middle of the IPL (Rockhill et al. 2002). G5 and G2 cells probably correspond to the off and on parasol cells of Roska et al. (2006). on–off directionally selective (DS) cells (240–260 μm) have dendrites that form loops and stratify in both on and off layers of the IPL. Alpha cells (420–480 μm) have straight dendrites that radiate from the cell body; off and on alpha cells stratify narrowly above and below the middle zone, respectively. Delta cells (300–430 μm) somewhat resemble alpha cells, but have wavier dendrites that more often curve along a circle concentric to the soma; off and on delta cells stratify narrowly above the on and off alpha cells, respectively (Manookin et al. 2008; Rockhill et al. 2002). G8 cells (400–650 μm) have straight virtually unbranched dendrites and stratify narrowly close to the ganglion cell layer (Rockhill et al. 2002).
Light responses have been characterized for about half of these types. LE cells have sluggish firing patterns and respond to edges whose length confines them to the receptive field center (Amthor et al. 1989). Alpha and beta cells have brisk firing patterns and a conventional concentric receptive field center and surrounds. on–off DS and on delta cells are selective for the direction of moving stimuli (Amthor et al. 1984; He and Masland 1998). The physiologies of types G5, G2, G8, and off delta have not been thoroughly characterized.
To classify a cell, we measured its dendritic field diameters in two orthogonal dimensions and averaged them. We then determined whether the cell's dendritic arbor was larger or smaller than 250 μm, whether it stratified inside or outside the middle zone of the IPL (40–60% depth), and whether it was monostratified, diffusely stratified, or bistratified. This separated most of the cell types, but left alpha cells to be separated from delta cells and on–off DS from other bistratified cells by closer examination of dendritic branching and stratification. We have the impression that these other bistratified cells comprised multiple types but we did not divide them further. For all cells, we calculated the impulse response of excitatory currents evoked by white noise visual stimulation. An impulse response that was initially inward (at t = 0) identified an on cell; an impulse response that was initially outward identified an off cell. on cells stratified in the lower half of the IPL and off cells arborized in the upper half.
Calculating SNR and impulse spectrum
To calculate the ratio of signal power to noise power (SNR), we first constructed the SNR(f) spectrum. This spectrum was corrected for a finite number of stimulus repeats (i = 1 … m) because a finite number would allow some noise to be measured as signal (van Hateren and Snippe 2001)
| (1) |
where f is the frequency (in Hertz), S(f) denotes the Fourier transform of the signal, Ni(f) represents the Fourier transform of noise, * denotes the complex conjugate and 〈…〉i denotes averaging across repeats. We then integrated the spectrum from 0 to 100 Hz to calculate SNR. In practice, the currents were averaged over stimulus repeats to give the signal s(t) (Fig. 2C). The signal was then subtracted from current i to give a noise trace ni(t) (Fig. 2D). Then 4,096-point (2.048 s) windows of s(t) and ni(t) were Fourier transformed to give S(f) and Ni(f). The windows were overlapping and incremented by 820 points.
Fig. 2.
Measuring signal to noise ratio (SNR). A: an interval of the white noise stimulus (temporal contrast: mean/SD = 0.2). B: excitatory postsynaptic currents (EPSCs) evoked by repeated presentation of the stimulus (on LE cell). C: signal was EPSCs averaged over repeats. D: noise was the difference between the average EPSC and the EPSC evoked by a stimulus repeat. E: the power spectrum of signal-to-noise ratio, SNR(f), was integrated between 0 and 100 Hz to give the ratio of signal power to noise power (SNR).
To plot the frequency content of the currents, we calculated the impulse spectrum as the cross-correlation of the response and stimulus divided by the autocorrelation of the stimulus
| (2) |
where T(f) and R(f) are the Fourier transforms of the stimulus and recorded currents, respectively, and 〈…〉i,j denotes averaging across a series of overlapping 512-point windows incremented by about 50 points and then across stimulus repetitions. The impulse response was calculated as the inverse Fourier transform of the impulse spectrum.
Estimating quantal rate
Average quantal rate for a given stimulus contrast was estimated by dividing the average current for this contrast by the charge associated with a quantum (“quantal charge”). Because current is charge per unit time, dividing current by charge provided a rate. In practice, the quantal charge was taken from the integral of small sEPSCs during constant light intensity. Methods for identifying sEPSCs and estimating baseline current have been previously described (Freed 2005; Freed et al. 2003). sEPSCs were detected automatically by a “peak location” algorithm that smoothed the recording (5 ms) and found a local maximum (the leading edge) followed by a local minimum (the peak) followed by an exponential decay (criterion coefficient of determination for exponential fit: R2 = 0.7) (Tian et al. 1998). The beginning of each sEPSC was identified as a departure from baseline and the ending as a return to baseline. Threshold sEPSC amplitude was typically set at 3 pA to exceed the peak-to-peak noise level between sEPSCs (<1 pA).
To estimate quantal rate from excitatory currents required the derivation of a baseline current corresponding to a zero rate of light-evoked release. For a static stimulus, it would be appropriate to graph recorded current against contrast and determine where the current asymptotes at its outward limit. For the dynamic stimulus we used, the baseline was derived by graphing the recorded currents against a projection of stimulus contrast that was constructed by convolving the impulse response with the stimulus (for more details, see Freed 2005). The most outward asymptote of this plot was taken as the baseline. The baseline was then subtracted from the excitatory currents to remove the contributions of nonsynaptic and tonic synaptic conductances that occur in the absence of visual stimulation.
Quantal charge was verified by ensemble fluctuation (noise) analysis. Consider that each EPSC has a charge Q, which is the linear sum of n quanta, where n has a Poisson probability distribution (Freed 2005; Freed et al. 2003). Due to such stochastic processes as, e.g., variation in vesicle size, binding of transmitter molecules to postsynaptic receptors, and gating of postsynaptic currents, quantal charge has a Gaussian distribution with mean 〈q〉 and a coefficient of variance CVq2. It can be shown (Freed 2005) that the quantal charge is
| (3) |
where 〈Q〉 and σQ2 are the mean and variance of an EPSC's charge across stimulus repetitions. In practice, we identified an EPSC in the average current using the peak detection algorithm and found its beginning and ending times (Fig. 5A). We then estimated 〈Q〉 for each stimulus repetition by integrating the resulting currents from this beginning time to this ending time. Then for each ESPC we calculated σQ2 and 〈Q〉. The coefficient of variation for quantal charge CVq2 was estimated from the distribution of sEPSCs recorded from the same cell as the light-evoked EPSCs. For each ganglion cell, quantal charge was averaged across contrasts; to ensure accuracy, however, we selected contrasts for which the number of detected ESPCs was >15 and the correlation between σQ2 and 〈Q〉 was greater than R = 0.60 (Fig. 5B). We did not use the results of noise analysis to estimate quantal rate because noise analysis uses the same basic measurements that the calculation of SNR requires, i.e., the mean and variance of recorded currents. We thus avoided the implication that any proportionality between SNR and quantal rate was a trivial result of mathematical identity.
Fig. 5.
Verifying quantal charge with ensemble noise analysis. A: EPSCs were detected in currents evoked by white noise (same cell as in Fig. 3). B: each point represents a detected EPSC. The charge variance across stimulus repetitions σQ2 is proportional to the average charge 〈Q〉, consistent with Poisson statistics. Substituting variance and average into the equation of ensemble analysis (Eq. 3) provides an estimate of quantal charge q*. C: each point represents a ganglion cell. The points fall along the line q* = q′, indicating that light-evoked EPSCs are composed of quanta with the same average charge as that calculated from the sEPSCs.
RESULTS
Different types of ganglion cell have excitatory postsynaptic signals of different reliability
We selected for study five types of ganglion cells that had small dendritic arbors (<250 μm), with relatively few synapses, and thus received quanta at rates low enough to be amenable to our analysis (see following text) (Fig. 1A). Cells were identified by their dendritic diameter, dendritic stratification in the IPL, and morphology (see methods). The following description is based on six LE, four off beta, six on beta, six G5, and three G2 cells.
To measure SNR at different quantal rates, we presented a white noise stimulus that had four or five different temporal contrasts (Fig. 2A) (methods). We voltage clamped at ECl to nullify the driving force for inhibitory postsynaptic currents (Freed 2005). The resulting excitatory currents were trains of EPSCs; each EPSC was a sudden downward deflection from a common baseline and an exponential decay back to baseline (Fig. 2B). When the stimulus was repeated, the timing and amplitude of these EPSCs were repeated with small variations (Freed 2005; Murphy and Rieke 2008). Signal was taken as the train of EPSCs averaged over repeats and noise was taken as the difference between each train and the average train (Fig. 2, C and D). The spectrum of the ratio of signal power to noise power was constructed and then integrated to derive SNR (methods) (Fig. 2E).
Quantal rate was calculated for each contrast by dividing the average current for the 10-s interval by the charge transfer associated with a single quantum (quantal charge). Quantal charge was calculated by recording spontaneous miniature EPSCs (mEPSCs), placing their peaks in register, averaging them, and then integrating this average (Fig. 3, A and B). Quantal charge differed between cell types (Fig. 3C and Table 1): the off beta cell had the largest quantal charge and the G2 the smallest (113 ± 15 and 40 ± 17 pA·ms, respectively).
Fig. 3.
Charge transfer from spontaneous (s)EPSCs. A: sEPSCs were detected by their onset ( ), peak (✚), and exponential decay (solid lines). B: sEPSCs were averaged and then integrated to give quantal charge q′. C: average quantal charge for 6 types of retinal ganglion cell.
), peak (✚), and exponential decay (solid lines). B: sEPSCs were averaged and then integrated to give quantal charge q′. C: average quantal charge for 6 types of retinal ganglion cell.
Table 1.
Comparison of reliability and quantal charge between different types of retinal ganglion cells
| LE | ||||
| off β | RQ | off β | ||
| on β | R | on β | ||
| G5 | Q | G5 | ||
| G2 | R | Q | R |
Pairs that have an “R” at their intersection have significantly different ratios of SNR to quantal rate; pairs with a “Q” at their intersection have significantly different quantal charges (Tukey–Kramer HSD, α = 0.05).
The result was that for each cell, SNR was proportional to quantal rate (Fig. 4). The relationship between SNR and quantal rate was highly linear, with a correlation coefficient of 0.97 ± 0.01, consistent with Poisson statistics. The average x-axis intercept for all cells was 0.1 ± 0.4 quanta/s that, compared with an average maximal quantal rate of 228 ± 49, is close to zero.
Fig. 4.
Reliability of excitatory signals to different types of retinal ganglion cell. Each graph shows SNR vs. quantal rate for cells of the same type; each symbol and the associated regression fit represent a cell. The histogram (bottom right) is a summary of the reliability of each cell type, where reliability is measured as the ratio of SNR to quantal rate, taken from the regression fits in the graphs.
To produce a measure of reliability that was independent of quantal rate, we took the ratio of SNR to quanta rate from the slope of the regression fit between SNR and quantal rate (Fig. 4). An ANOVA showed that this measure differed significantly between cell types (P = 0.004). The LE cells had the most reliable synaptic input and the G2 the least reliable (SNR·quanta−1·s−1 = 1.2 ± 0.24 vs. 0.19 ± 0.04) (Fig. 4, bottom right). The difference in reliability between LE and G2 was statistically significant, as were differences in reliability between other cell types (Tukey–Kramer Honestly Significant Difference, corrected for multiple comparisons, α = 0.05) (Table 1).
Verification of quantal charge
Our estimate of quantal rate depends on the assumption that the light-evoked EPSCs are composed of quanta equal in charge to sEPSCs. To check this assumption, we subjected the light-evoked EPSC to ensemble noise analysis: we followed an EPSC through the stimulus repetitions and calculated its charge by integrating it from beginning to end (Fig. 5A). We then calculated the variance σQ2 and the average 〈Q〉 of this charge across repetitions. The variance and average were proportional, consistent with Poisson statistics governing the occurrence of quanta (R = 0.73 ± 0.02) (Fig. 5B).
Quantal charge q* was calculated by substituting σQ2 and 〈Q〉 into the equation for ensemble analysis (Eq. 3 in methods). Another parameter necessary for this equation, the coefficient of variation for quantal charge CVq2, was taken from the distribution of sEPSCs (methods). Across ganglion cells, the quantal charge from ensemble analysis was strongly correlated with the quantal charge from sESPCs (R = 0.91) (Fig. 5C). This apparent match between quantal charges indicates light-evoked EPSCs are composed of quantal charges similar to sEPSCs. Quanta are likely to be the result of the fusion of single synaptic vesicles, although it is possible that they are the result of the coordinated fusion of multiple vesicles (Glowatzki and Fuchs 2002; Singer et al. 2004).
Ratio of SNR to contrast is correlated with the number of synapses
The relationship between SNR and contrast was highly linear, with an average correlation coefficient of R = 0.99 + 0.01 (Fig. 6). Presumably this linear behavior was obtained because the contrasts used were moderate; we did not try higher contrasts because they truncate the Gaussian distribution of intensities, although we expect that at higher contrasts the SNR would saturate. Cells of the same type showed diverse slopes for the linear relationship between SNR and contrast (SNR/contrast). A diversity of SNR/contrast ratios within a type was supported by an ANOVA that showed that the variance between types was not significantly greater than the variance within a type (P = 0.57).
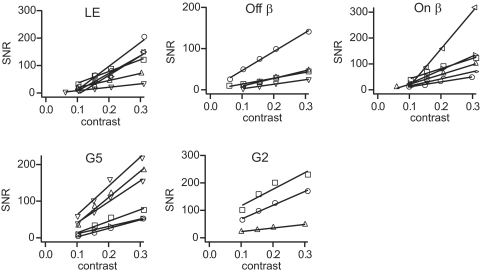
Fig. 6.
SNR of excitatory input to different types of ganglion cell plotted against contrast. Contrast of white-noise stimulus was varied from mean/SD = 0.1 to 0.3. Each symbol and associated regression line are from a single cell. Note that ganglion cells of the same type have different slopes, indicating different SNR/contrast ratios.
To explain the diversity of SNR/contrast ratios for cells of the same type, we considered how the morphology of a ganglion cell might influence the SNR of its excitatory postsynaptic currents. A ganglion cell receives synapses from an array of bipolar cells of the same type (in some cases a ganglion cell receives synapses from multiple arrays, but each array can be considered separately) (Freed and Sterling 1988). The stimulus has equal intensity across the retina and thus at each contrast, all synapses release quanta at equal rates. Yet ganglion cells of the same type have a distribution of dendritic diameters. As dendritic diameter increases, so would the number of synapses and thus the rate at which the ganglion cell receives quanta (Freed 2000). Because the SNR of excitatory currents is proportional to quantal rate, the ratio SNR/contrast should be proportional to the number of synapses.
To test the prediction that the ratio of SNR to contrast is proportional to the number of synapses, we approximated the number of synapses on a ganglion cell by measuring the total length of dendrites and then by multiplying this by the density of synapses per unit length of dendrite. The total length of dendrites, obtained by tracing them through optical sections, ranged from 1,216 to 2,432 μm for LE cells and 1,139 to 6,395 μm for beta cells (Fig. 7A). For LE cells the density of synapses per unit length is 0.95 ± 0.06, giving 1,155 to 2,311 synapses (Xu et al. 2008). For beta cells the density of synapses is 1.00 ± 0.22, giving 1,139 to 6,395 synapses. The result was a clear correlation between SNR/contrast and number of synapses for both LE and beta cells (R = 0.84 for LE and 0.77 for beta) (Fig. 7B). Apparently as predicted, the number of synapses that a ganglion cell receives sets the ratio of SNR to contrast for its excitatory currents.
Bandwidth of SNR spectrum does not explain type-specific differences in reliability
So far we have found that SNR grows proportionally with quantal rate but grows faster for some cell types than for others. Because SNR is the integral of the SNR(f) spectrum, this differential growth could be achieved by having the bandwidth of this spectrum grow or shrink as quantal rate increases. To test for this, we quantified the shape of the SNR spectrum by measuring the frequency at its peak and the upper cutoff frequency at which it declined to one half of its peak. We also measured amplitudes at 1 and at 20 Hz as percentages of the peak amplitude. All of these measures had a weak and nonsignificant correlation with quantal rate, indicating no change in the shape of the SNR spectrum (ANOVA, P > 0.26, n = 76 spectra). Plotting each measure at one quantal rate against the same measure at the next highest quantal rate gave data points that followed a diagonal, confirming no consistent change in the shape of the spectrum (Fig. 8, B and C). Thus as quantal rate increased the shape of the SNR spectrum was constant.
Fig. 8.
SNR spectrum scales with quantal rate. A: SNR spectra normalized by divided by the quantal rate. Note that resulting normalized spectra b(f) are similar, indicating that the spectrum retains a constant shape as it scales with quantal rate. B: plotting peak frequency (Fpeak) and upper cutoff frequency (Fhi) at one quantal rate (F[i]) against the same measure at the next highest quantal rate (F[i+1]) gives data points that follow the diagonal (F[i] = F[i+1]), indicating no consistent change in the shape of the spectrum. C: plotting the amplitude of the SNR spectrum at 1 Hz (Vlow) and at 20 Hz (Vhi) at one quantal rate (V[i]) against the next highest quantal rate (V[i+1]) gives data points that follow the diagonal, confirming no consistent change in shape. D: bandwidth: the difference between the low-frequency cutoff (Flow) and the high-frequency cutoff (Fhi) for the different ganglion cells types. There were no statistically significant differences in bandwidth.
To further test for shape constancy, we normalized the SNR spectrum by quantal rate: we took a series of spectra from a single cell, obtained at a series of contrasts, and then divided these spectra by the corresponding quantal rates. This normalization resulted in a series of very similar spectra, confirming that the shape of the SNR(f) spectrum does not change with quantal rate (Fig. 8A).
SNR growing faster with quantal rate for some types than for others could also be achieved by having some types with bandwidth greater than that of others. To test this, bandwidth was measured by taking the difference between the low-frequency cutoff of the spectrum where it declines to 70% of its peak value and the high-frequency cutoff where it declines to one half its peak value. An ANOVA failed to demonstrate significant differences between the bandwidth of different types of ganglion cell (P = 0.29, n = 76 spectra) (Fig. 8D).
An orderly distribution of frequency response through the depth of the inner plexiform layer
Because we wanted a fuller representation of frequency response across all strata of the IPL, we added five types of ganglion cell with large dendritic arbors to the five smaller types already used for analysis of SNR. Thus the following description of frequency responses is based on 13 LE, 6 off beta, 9 on beta, 8 G5, 6 G2, 6 off alpha, 5 on alpha, 6 off delta, 6 on delta, and 4 G8 cells. We also included 9 small bistratified cells that send separate dendritic arbors to on and off layers. So that we could ascribe the response of a small bistratified ganglion cell to a single stratum, we blocked the on input with the mGluR6 agonist L-AP4. We averaged spectra derived from stimuli of all contrasts (SD/mean = 0.1 to 0.4).
Figure 9 shows the resulting distribution of frequency response across the depth of the IPL. All spectra had two peaks, one at low frequency and another at high frequency (3.5 ± 0.1 and 16.3 ± 0.1 Hz). These peaks are unlikely to be an artifact because the stimulus had equal power for all frequencies to which the cells could respond (0–100 Hz). Because bipolar cells provide excitatory inputs to ganglion cells, the two peaks suggest two classes of bipolar cell that respond to low and high frequencies, respectively.
Fig. 9.
Stratification of frequency responses through the depth of the inner plexiform layer (IPL). To the left are the dendritic stratifications of identified types of ganglion cell, showing SE as error bars. Small bistratified cells are represented by 2 filled circles connected by a dotted line. To the right are the impulse spectra from the same cell types, showing SE as confidence bands (gray). Impulse spectra from the small bistratified cell are from recordings during which on bipolar cells were blocked with L-AP4.
To gauge how frequency response distributed across the depth of the IPL, we considered that a concentration of power in the high-frequency peak indicates a high-pass signal, but an approximately equal distribution of power between high and low frequencies indicates a broadband signal. At the scleral (upper) edge of the inner plexiform layer, off delta and G4 cells showed broadband signals (although the G5 could be called low-pass because it concentrates power in the low-frequency peak). Moving toward the vitreous (downward), the off alpha cell and small bistratified cell showed high-pass signals. Still lower, at the middle of the IPL (40–60%), the LE and off beta cells showed broadband signals. Moving lower into the on layer (50–100%), types G2, on delta, on beta, and on alpha showed high-pass signals, although the on beta had slightly more power at low frequencies than did the on alpha. Finally, the most vitreal (lowest) cell type, the G8, showed a high-pass signal. Thus going from scleral to vitreal (downward) there seems to be an orderly transition from broadband, to high-pass, to broadband, then to high-pass.
DISCUSSION
SNR is proportional to quantal rate
Quantal release at synapses driven by slow potentials is commonly thought to follow Poisson statistics and thus finding that SNR is proportional to quantal rate was not entirely unexpected. However, this common idea has rarely been specifically tested and, when it has, the results have not supported it. First, noise does not increase with quantal rate for postsynaptic signals from fly visual neurons, which is inconsistent with Poisson statistics (Simmons 1999; Simmons and de Ruyter van Steveninck 2005). Second, constant noise with rising quantal rate has been observed in postsynaptic currents from hair cell afferents, again inconsistent with Poisson statistics (Glowatzki and Fuchs 2002). Third, in the mammalian retina, glutamate and protons are coreleased from cone photoreceptor vesicles; the protons inhibit calcium currents and delay the release of subsequent vesicles (DeVries 2001). Proton inhibition would result in a refractory period that would constitute a negative dependence between quanta, which is inconsistent with Poisson statistics. Thus it is not yet clear whether, for release driven by slow potentials, Poisson statistics are the exception or the rule.
Retinal ganglion cells of the same type are thought to share morphology, synaptic inputs, and intrinsic spiking properties. To these shared features the present results add the reliability of excitatory input. To demonstrate this required that two sources of variability within a type be normalized. First, we normalized SNR by quantal rate to control for differences in dendritic length within a type. Second, we normalized currents by quantal charge to control for differences in quantal charge. Without normalizing SNR by these means, we could find no significant differences in SNR between cell types.
Our measurement of quantal rate included light-evoked release but not spontaneous or tonic release (methods). Yet we think that tonic release rate is likely to be low for three reasons. First, the x-axis intercepts of the SNR versus quantal rate were very close to zero (Fig. 4), suggesting that tonic release adds minimal noise. Second, sEPSCs occur at a sufficiently low rate that they were detectable (Fig. 3A). Third, in a separate study we find that the bipolar cell's membrane potential is close to the threshold for transmitter release, thus minimizing tonic rate (Liang and Freed, unpublished data).
How retinal circuitry sets the reliability of excitatory synaptic input to ganglion cells
Why do ganglion cell types have postsynaptic currents with different reliability? One possible answer—that different types have different bandwidth—was eliminated in results (Fig. 8D). Another possible answer—that reliability depends on noise intrinsic to the ganglion cell—seems equally unlikely because nonsynaptic ion channels in the ganglion cell membrane are the predominate source of intrinsic noise but we reduced their contribution by substituting gluconate for K+ in the pipette solution to block K+ channels, by adding QX-314 to block Na+ channels, and by voltage clamping to block voltage-activated channels. Most likely, reliability is affected by noise sources in the circuitry that convey signals from photoreceptors to ganglion cells. Such “network noise” is transmitted through excitatory synapses on a ganglion cell and appears in the EPSCs (Murphy and Rieke 2006). Possibly different circuits have different noise levels because they contain different amacrine and bipolar cell types and their overall three-dimensional structures have different degrees of convergence and divergence (Sterling et al. 1988).
To estimate the number of synapses on a cell, we took advantage of the fact that we could accurately measure dendritic length, then used a different linear density of synapses for different cell types. Yet the area density—the number of synapses per dendritic membrane area—appears to be an essential metric in the construction of ganglion cells because it has the same value for LE, beta, alpha, and on–off DS cells (Xu et al. 2008). The area synaptic density combined with the present results suggest how a ganglion cell's dendritic morphology sets the SNR of its EPSCs in four functional steps 1) the size and branching pattern of the dendritic arbor set the membrane area; 2) because area density of synapses is constant, the membrane area sets the number of synapses; 3) neuronal type determines the quantal rate at a synapse and thus the rate at which a ganglion cell receives quanta; and finally, 4) the quantal rate sets the SNR of its EPSCs.
Mapping of frequency response across depth of IPL
It was previously proposed that transient bipolar cells segregate to the middle of the IPL and sustained bipolar cells to the edges, thus leading to a similar distribution of ganglion cells with transient and sustained spike activity (Awatramani and Slaughter 2000; Roska and Werblin 2001). A known exception to this proposal is the local edge cell, which ramifies in the middle of the IPL and exhibits sluggish spike activity and more sustained excitatory inputs than those of either alpha or beta cells (Roska et al. 2006; van Wyk et al. 2006) (Fig. 9). Yet the general idea that the temporal properties of excitatory input determine that of the spike train holds up. Alpha cells show high-pass inputs consistent with their brisk-transient spike activity. Delta and beta cells receive high-pass inputs with slightly more power at low frequencies than alpha cells, consistent with their brisk-sustained activity (He and Masland 1998). Local edge cells receive broadband inputs, consistent with their sluggish activity. One discrepancy is the off beta cell whose excitatory input is as broadband as the sluggish local edge cell, but whose spike activity is much more transient (Fig. 9). This discrepancy may be due to inhibitory inputs to the off beta cell that modify the temporal properties of the spike train (van Wyk et al. 2006).
Although we did not confirm the previous idea that transient and sustained signals segregate to the middle and edges of the IPL, we did find an orderly transition of temporal signals through its depth, suggesting 4 or 5 different levels (Fig. 8). Because different bipolar cell types end at different levels, this suggests that temporal classes of bipolar cell number 4 or 5. These numbers are less than the 9 to 13 distinct types of bipolar cell found in various retinas (e.g., Wässle et al. 2009), suggesting that our method of recording excitatory currents in ganglion cells may provide a coarse sampling of bipolar cell types or else bipolar cells with similar temporal properties end in nearby levels of the IPL.
Conclusion
By recording postsynaptic excitatory currents from ganglion cells of different types, we add to an emerging picture of parallel channels that convey information about different temporal aspects of the visual stimulus. This picture is substantially complete from what is known about bipolar cells. The midget and blue bipolar cell types select among cone photoreceptors and thus convey chromatic signals, although the majority of bipolar types are diffuse and contact essentially all cones within the reach of their dendrites and are spectrally nonselective. Because diffuse types are spectrally nonselective and because they all have roughly equivalent receptive field sizes, they are unlikely to be distinguished by marked differences in chromatic coding or spatial selectivity. Instead, diffuse bipolar types are likely to convey different temporal signals. In support of temporal differences, bipolar types have dendrites that bear glutamate receptors with distinct kinetics that can shape the temporal properties of their excitatory inputs from cones (DeVries 2000). Moreover, bipolar cell types receive inhibitory input from distinct amacrine circuits that sharpen the temporal differences between them (Roska and Werblin 2001). We add to this picture of parallel channels by showing that they convey information with different amounts of reliability. Finally, we suggest that distinct channels segregate into at least four different levels of the IPL.
GRANTS
This work was supported by National Eye Institute Grant R01-EY-013333 to M. A. Freed.
REFERENCES
- Amthor FR, Oyster CW, Takahashi ES. Morphology of on-off direction-selective ganglion cells in the rabbit retina. Brain Res 298: 187–190, 1984 [DOI] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J Comp Neurol 280: 97–121, 1989 [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM. Origin of transient and sustained responses in ganglion cells of the retina. J Neurosci 20: 7087–7095, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Pu M, Famiglietti EV. The zeta cell: a new ganglion cell type in cat retina. J Comp Neurol 399: 269–288, 1998 [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Sterling P, Smith RG. Loss of sensitivity in an analog neural circuit. J Neurosci 29: 3045–3058, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol 240: 397–419, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruyter van Steveninck RR, Lewen GD, Strong SP, Koberle R, Bialek W. Reproducibility and variability in neural spike trains. Science 275: 1805–1808, 1997 [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28: 847–856, 2000 [DOI] [PubMed] [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron 32: 1107–1117, 2001 [DOI] [PubMed] [Google Scholar]
- Freed MA. Parallel cone bipolar pathways to a ganglion cell use different rates and amplitudes of quantal excitation. J Neurosci 20: 3956–3963, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed MA. Quantal encoding of information in a retinal ganglion cell. J Neurophysiol 94: 1048–1056, 2005 [DOI] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Timing of quantal release from the retinal bipolar terminal is regulated by a feedback circuit. Neuron 38: 89–101, 2003 [DOI] [PubMed] [Google Scholar]
- Freed MA, Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci 8: 2303–2320, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5: 147–154, 2002 [DOI] [PubMed] [Google Scholar]
- He S, Masland RH. ON direction-selective ganglion cells in the rabbit retina: dendritic morphology and pattern of fasciculation. Vis Neurosci 15: 369–375, 1998 [DOI] [PubMed] [Google Scholar]
- Koch K, McLean J, Berry M, Sterling P, Balasubramanian V, Freed MA. Efficiency of information transmission by retinal ganglion cells. Curr Biol 14: 1523–1530, 2004 [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci 28: 4136–4150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron 52: 511–524, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci 11: 318–326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci 22: 3831–3843, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space–time patterns of excitation and inhibition form the spiking output. J Neurophysiol 95: 3810–3822, 2006 [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410: 583–587, 2001 [DOI] [PubMed] [Google Scholar]
- Simmons PJ. The performance of synapses that convey discrete graded potentials in an insect visual pathway. J Neurosci 19: 10584–10594, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons PJ, de Ruyter van Steveninck R. Reliability of signal transfer at a tonically transmitting, graded potential synapse of the locust ocellar pathway. J Neurosci 25: 7529–7537, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Lassova L, Vardi N, Diamond JS. Coordinated multivesicular release at a mammalian ribbon synapse. Nat Neurosci 7: 826–833, 2004 [DOI] [PubMed] [Google Scholar]
- Sterling P, Freed MA, Smith RG. Architecture of rod and cone circuits to the On-beta ganglion cell. J Neurosci 8: 623–642, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Hwang TN, Copenhagen DR. Analysis of excitatory and inhibitory spontaneous synaptic activity in mouse retinal ganglion cells. J Neurophysiol 80: 1327–1340, 1998 [DOI] [PubMed] [Google Scholar]
- Tokutake Y, Freed MA. Retinal ganglion cells: spatial organization of the receptive field reduces temporal redundancy. Eur J Neurosci 28: 914–923, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci 26: 13250–13263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci 29: 106–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Vasudeva V, Vardi N, Sterling P, Freed MA. Different types of ganglion cell share a synaptic pattern. J Comp Neurol 507: 1871–1878, 2008 [DOI] [PubMed] [Google Scholar]
- Yin L, Smith RG, Sterling P, Brainard DH. Chromatic properties of horizontal and ganglion cell responses follow a dual gradient in cone opsin expression. J Neurosci 26: 12351–12361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]