Abstract
Recruitment and repetitive firing of spinal motoneurons depend on the activation of persistent inward calcium and sodium currents (PICs) that are in turn facilitated by serotonin and norepinephrine that arise primarily from the brain stem. Considering that in rats motoneuron PICs are greatly facilitated by increasing the presynaptic release of norepinephrine with amphetamine, we sought similar evidence for the modulation of PICs in human motoneurons. Pairs of motor units were recorded during a gradually increasing and then decreasing voluntary contraction. The firing frequency (F) of the lower-threshold (control) motor unit was used as an estimate of the synaptic input to the higher-threshold (test) motor unit. Generally, PICs are initiated during the recruitment of a motoneuron and subsequently provide a fixed depolarizing current that helps the synaptic input maintain firing until derecruitment. Thus the amplitude of the PIC in the test motor unit was estimated from the difference in synaptic input (ΔF) needed to maintain minimal firing once the PIC was fully activated (measured at the time of test unit derecruitment) compared with the larger synaptic input required to initiate firing prior to full PIC activation (measured at the time of test unit recruitment; ΔF = Frecruit − Fderecruit). Moreover, the activation time of the PIC was estimated as the minimal contraction duration needed to produce a maximal PIC (ΔF). In five subjects, oral administration of amphetamine, but not placebo, increased the ΔF by 62% [from 3.7 ± 0.6 to 6.0 ± 0.8 (SD) imp/s, P = 0.001] and decreased the time needed to activate a maximal ΔF from ∼2 to 0.5 s. Both findings suggest that the endogenous facilitation of PICs from brain stem derived norepinephrine plays an important role in modulating human motoneuron excitability, readying motoneurons for rapid and sustained activity during periods of high arousal such as stress or fear.
INTRODUCTION
The excitability of neurons in the spinal cord is not fixed but subject to pronounced modulation via monoamines released by axons descending from brain stem nuclei (reviewed in Heckman et al. 2005; Perrier et al. 2002). In the awake animal, activity of neurons in the raphe nuclei and locus coeruleus, the descending axons of which provide 90–95% of all spinal monoamines (Alvarez et al. 1998; Bjorklund and Skagerberg 1982; Patel et al. 1997), are modulated according to the arousal level of the animal. For example, high discharge rates in the locus coeruleus (descending norepinephrine system) occur during stressful situations, whereas low-level activity is observed during quiet rest and sleep, potentially to provide state-dependent modulation of spinal excitability (Abercrombie and Jacobs 1987; Rasmussen et al. 1986). The importance of descending serotonergic (5HT) and norepinephrine inputs on motoneuron excitability in particular is revealed by the acute abolishment of calcium and sodium persistent inward currents (PICs) following complete transection of the spinal cord (Harvey et al. 2006c; Hounsgaard et al. 1988). Activation of 5HT2 and α-1 receptors on the motoneuron facilitate these low-voltage activated PICs via Gq-coupled pathways to produce sustained depolarizations (plateau potentials) that facilitate motoneuron recruitment and tonic firing in the face of low-level ionotropic activity (Harvey et al. 2006a; Lee and Heckman 1999; Li et al. 2007). Likewise, administration of amphetamine, which increases the presynaptic release of norepinephrine (and to a lesser extent serotonin and dopamine), doubles the size of the calcium-mediated PIC (Rank et al. 2007, 2008).
In both animals and humans, evidence for the presence of PIC activation in motoneurons has been provided by recording sustained motor-unit activity during voluntary or reflex activation. For example, motor units that are recruited by brief muscle vibration (Gorassini et al. 1998; Kiehn and Eken 1997) or electrical stimulation (Collins et al. 2001, 2002) continue to discharge in a self-sustained manner following the removal of the excitatory stimulus. In addition, estimates of the amplitude of PIC contribution to motoneuron firing have been made via paired motor-unit recordings both in animal models (Bennett et al. 2001a; Powers et al. 2008) and in humans (Gorassini et al. 2002a,b, 2004; Mottram et al. 2009). To do this, the firing rate of the relatively lower-threshold (control) motor unit of the pair is used to estimate the synaptic drive to the motoneuron pool, including the synaptic input to the relatively higher-threshold test motor unit of the pair during a gradually increasing and then decreasing contraction. In animals, PICs are initiated at recruitment, produce a distinct acceleration in motoneuron firing as they are activated, and subsequently provide a relatively fixed depolarizing current that helps the synaptic input maintain firing until derecruitment (Li et al. 2004a). Thus after full activation, the PIC does not interfere with the linear relationship between synaptic input (current) and firing frequency of a motoneuron, allowing the firing rate of the control motor unit to provide a fairly accurate measure of synaptic input to the test motor unit (see discussion) (Heckman et al. 2005; Li et al. 2004a). Ultimately, the amount of depolarization provided by the PIC to the test unit can be estimated from the difference in synaptic input (or ΔF) needed to maintain minimal firing once the PIC is fully activated (measured at derecruitment), compared with the larger synaptic input required to initiate firing prior to full PIC activation (measured at recruitment; ΔF = Frecruit − Fderecruit). Thus the ΔF value corresponds to the reduction in synaptic input needed to counteract the depolarization provided by the PIC and has been directly verified by parallel motor unit and intracellular recordings in rat motoneurons (Bennett et al. 2001a,b).
Recent studies in humans have shown that increasing basal levels of serotonin and norepinephrine by oral caffeine increases the probability of evoking self-sustained discharge of motor units following their recruitment by brief muscle vibration (Walton et al. 2002). Although the increased likelihood of sustained unit discharge may have resulted from decreasing the threshold and/or increasing the amplitude of PIC activation, caffeine could have simply increased the resting membrane potential of the motoneuron to activate the PIC and associated self-sustained firing more readily (Harvey et al. 2006a; Mottram et al. 2009; Powers and Binder 2001; Ziskind-Conhaim et al. 1993), especially because caffeine also increases H-reflexes recorded at rest (Walton et al. 2003). Therefore in this study, we used the paired motor-unit analysis technique (ΔF) to test if amphetamine, which likely increases the presynaptic release of norepinephrine, increases our estimation of PIC amplitude in human motoneurons. Subjects were given high oral doses of amphetamine (20–25 mg), which acts more directly and potently than caffeine in releasing monoamines from the presynaptic terminal (norepinephrine in particular). A second advantage of using amphetamine is that we know, via direct intracellular recordings, that it increases the amplitude of calcium PIC activation and self-sustained firing of motoneurons (Rank et al. 2007). Changes in ΔF values were compared before and after placebo administration in the same group of subjects to determine if ΔF measures were sensitive to amphetamine and reproducible from day to day. Finally, we also examined if amphetamine decreased the activation time of the estimated PIC, which can take two or more seconds to fully activate when the amplitude of the PIC is small (Bennett et al. 2001a,b; Hounsgaard and Kiehn 1989; Li et al. 2004a).
METHODS
All experiments were approved by the Health Research Ethics Board of the University of Alberta and conformed to the Declaration of Helsinki. Five healthy subjects (1 male) aged 29–41 yr (34.6 ± 5.5) participated in this study. All subjects gave written informed consent prior to participation. The administration of Dextro-amphetamine (amphetamine for short) was supervised by a medical doctor.
Motor-unit recordings
Subjects were seated with their foot strapped securely onto a metal rest plate. Knee and ankle angles were set to ∼120° of flexion and 90°, respectively. The footplate was coupled to a force transducer to monitor both dorsi- and plantarflexion torques about the ankle joint. Subjects were given a visual display of their exerted torque on a computer screen, and they were instructed to track a triangular line drawn on a transparency overlain on the display using a dorsiflexion contraction. The offset, vertical and horizontal scale of the computer display was adjusted to modify the initial level, strength, and speed of the contraction, respectively. The strength of the contraction was adjusted so at least two motor units (control and test) were identified in the intramuscular electromyographic (EMG) signal during the ascending ramp of the contraction. The strength of the contraction was expressed as a percentage of the subject's maximum voluntary contraction (% MVC) obtained by averaging the maximum torque produced from three maximal contractions. Typically the contraction strength was ∼20% of MVC. Triangular torque contractions were separated by ≥30 s to avoid frequency-dependent facilitation of the motor units (Gorassini et al. 2002b; Hornby et al. 2003).
The intramuscular electrodes used for recording single motor-unit action potentials (MUAPs) were inserted into the tibialis anterior muscle using a 25-gauge needle that was removed after the wires were inserted (see Gorassini et al. 2002a for details). The intramuscular electrode consisted of three tightly wound and insulated 50-μm stainless steel wires that were bent at the end, covered with a small epoxy bead, and cut to give three small exposed recording sites. MUAPs were recorded differentially between the two recording sites that gave the best waveform discrimination. Surface EMGs from the tibialis anterior and soleus muscle were also recorded, the latter to monitor the amount of antagonist cocontraction that may affect torque measurements about the ankle joint. Intramuscular and surface EMG signals were fed to an isolated, high-impedance amplifier (Model 2024F Intronix Technologies, Bolton, ON, Canada) with a frequency response of DC to 10 kHz, containing an imbedded Butterworth filter with a 12 dB/octave cutoff. EMG signals were amplified by 5,000 times and high-pass filtered at 200 Hz for intramuscular EMG and band-pass filtered between 20 Hz and 2.5 kHz for surface EMG. EMG and torque signals were digitized using a Power 1401 A/D converter and Spike2 (Version 6) software (Cambridge Electronics Design, Cambridge, UK) using a sampling rate of 25 kHz for the intramuscular EMG, 5 kHz for the surface EMG, and 100 Hz for the torque signal.
Relationship between test unit activation time and ΔF
Intracellular data from rats, cats, and turtles indicate that the PIC (especially the calcium component) can take ≥2 s to activate, especially when the depolarizing inputs to the motoneuron are of low-amplitude or when the PIC itself is small (Bennett et al. 1998a, 2001a,b; Hounsgaard and Kiehn 1989). Thus we investigated if activating the test unit for a very brief period of time (<2 s, likely before full PIC activation) would affect the amplitude or stability of our PIC (ΔF) measurements. In addition, we examined if amphetamine, which increases the amplitude and decreases the activation time of the PIC (Rank et al. 2007), would also affect the activation time needed for the test unit to produce a stable ΔF measurement. To do this, the subjects performed a separate set of contractions where the duration of time that the test unit was activated before the subject relaxed the contraction (duration of increasing depolarization to the motoneuron) was varied between 0.5 and 10 s. Both the strength and rate of contraction/relaxation were kept constant. The duration of time that the test unit was active for during the ascending portion of the contraction was plotted against the size of the calculated ΔF for that trial. Trials without drug (before amphetamine or during pre and post placebo) that had matched rates of contraction and relaxation, as determined by visual inspection, were compared with trials after amphetamine intake.
Protocol of drug intake and measurement of blood pressure and heart rate
After recording a minimum of 15–20 triangular contractions, blinded subjects received either 20–25 mg of amphetamine or placebo (the male subject received a 25-mg dose due to his larger body mass). Amphetamine and placebo pills were concealed using a two-part telescoping starch capsule. The triangular contractions were repeated at 30, 60, and 90 min after drug intake. Heart rate and systolic/diastolic blood pressure were measured every 30 min before and after drug/placebo intake. Subjects were asked to report if there were any changes in psychological sensations after pill intake (light headedness, feelings of euphoria, tremor, palpitation, and tachycardia) regardless of whether placebo or amphetamine was administered. Subjects were asked to return to the laboratory no earlier than 1 wk after the first session where they received the other treatment. The order of placebo and amphetamine administration was randomized between subjects.
Data analysis
Data were analyzed off-line using commercial spike discrimination software (Spike2, Cambridge Electronic Design). The investigator (EU) who performed all data analysis was blinded as to whether placebo or amphetamine was used in a given experiment. Single MUAPs were first selected by setting a horizontal threshold, and then each waveform was inspected visually to identify a given motor unit based on waveform shape. When possible, the same motor-unit pair (control and test) was followed both before and after amphetamine/placebo administration (see values in legend of Fig. 2). However, due to electrode movement during the long experiment (typically 2.5–3 h), one or both of the motor-unit pairs could be lost, and new unit pairs were sampled after amphetamine/placebo intake. After obtaining the times of occurrences for each motor unit in Spike2, data were further analyzed in a custom-written Matlab program (The MathWorks, Natick, MA) as outlined in the following text.
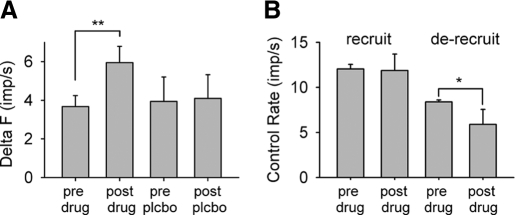
Fig. 2.
Group data. A: group mean (±SD) delta (Δ) F values for before (pre) and after (post) amphetamine (drug) and placebo (plcbo). In the amphetamine trials, 8 of the 16 motor-unit pairs were the same before and after drug administration, whereas 7 of the 11 motor-unit pairs were the same before and after placebo. On average, the test motor unit was recruited 2.9 ± 0.6 and 3.2 ± 0.5 s after the control motor unit before and after amphetamine, respectively, and 2.8 ± 0.9 and 2.8 ± 0.6 s before and after placebo, respectively. B: changes in the firing frequency of the control unit when the test unit was recruited (left bars) and de-recruited (right bars) before and after amphetamine. *P < 0.05, **P < 0.01
Estimation of PIC amplitude (ΔF)
The amplitude of PIC activation in a test motor unit (motoneuron) was estimated using the paired motor-unit analysis technique (see following text) (see also Gorassini et al. 2002a, 2004). The times of occurrences of each discriminated MUAP in Spike 2 were imported as a text file into Matlab where the instantaneous firing rate was calculated as the reciprocal of each interspike interval. The firing rate of a relatively lower-threshold control motor unit of the pair was used as a measure of synaptic input to a relatively higher-threshold test motor unit of the pair. To calculate the firing rate of the control unit at the time of test unit recruitment and de-recruitment, a fifth-order polynomial was used to smooth the firing rate profiles (see solid line in Fig. 1). The smoothed firing rate of the control unit at the time of test unit de-recruitment was automatically determined and subtracted from the smoothed firing rate of the control unit when the test unit was recruited (ΔF). As described in the introduction, the decrease in synaptic drive required to maintain a minimal discharge of the test unit compared with the synaptic drive needed to recruit the test unit initially (i.e., change in control unit frequency, ΔF) is proposed to reflect the amount of depolarization produced from the sustained activation of a PIC in the test motoneuron (Bennett et al. 2001b; Gorassini et al. 2002a, 2004).
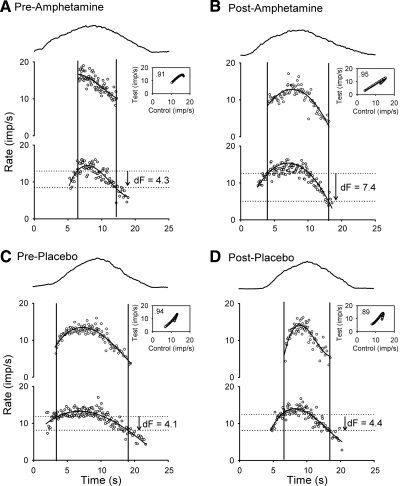
Fig. 1.
Single subject data. Representative data from a single subject showing torque (top graphs), test unit (middle graphs), and control unit (bottom graphs) profiles during triangular isometric contractions. A: before (pre) amphetamine, B: after (post) amphetamine, C: before (pre) placebo, and D: after (post) placebo. Note the firing rate of the control unit when the test unit was recruited was similar in all 4 cases; however, the firing rate of the control unit when the test unit was de-recruited was lower only after amphetamine to give a larger ΔF (dF). For all trials, the smoothed firing rate of the control unit was plotted against the smoothed firing rate of the test unit to give a rate-rate plot (see insets with corresponding r2 values). In all trials, the contraction torques reached ∼20% of the subject's maximum voluntary contraction (MVC).
In five subjects, a range of 5–10 contraction trials were used to calculate the average ΔF value for each experiment. An average of 6.4 ± 1.5 and 7.0 ± 1.4 trials were used to calculate the before and after amphetamine ΔF values, respectively, and an average of 6.4 ± 1.7 and 6.4 ± 1.1 trials were used to calculate the before and after placebo ΔF values, respectively. An average of 2.8 motor-unit pairs was used in each subject during the amphetamine experiments with 8 of the 16 pairs recorded both before and after amphetamine. In the placebo trials, an average of 2.1 unit pairs was used per subject with 7 of the 11 pairs being the same before and after placebo administration. The average postamphetamine ΔF was taken either at 60 (n = 3) or 90 (n = 2) minutes after pill intake depending on when an appreciable increase (≈ 10 mm/Hg) in blood pressure occurred. For the placebo trials, ΔF values were measured randomly across subjects at either 60 or 90 min after pill intake. The mean ΔF value for each subject was averaged across all subjects for a given condition. Only contraction trials with symmetrical torque profiles were included where the average rate of rise of the torque on the ascending arm of the contraction was equal and opposite to the average rate of decrease in torque on the descending (relaxation) arm of the contraction (see Table 1). Contractions with an abrupt increase or decrease in the torque profile, which typically occurred during the relaxation phase of the contraction, were rejected based on visual inspection of the data. In addition, only trials where the control unit fired for ≥2 s before the test unit was recruited were used (cf. Powers et al. 2008). This was done to ensure that the PIC was fully activated in the control motor unit given that the PIC, especially the slower calcium component, can take upward of 2 s to fully activate (Bennett et al. 2001a,b; Hounsgaard and Kiehn 1989; Li et al. 2004a). After full PIC activation, the firing rate of the control unit should then only reflect changes in synaptic drive (see discussion).
Table 1.
Firing rate properties of control and test units
| Median r2 Rate-Rate (Range) | Slope of Rate-Rate Regression Line | Control Mean Rate, imp/s | Test Mean Rate, imp/s | Contract Rate, %MVC/s | Relax Rate, %MVC/s | Peak Torque, %MVC | |
|---|---|---|---|---|---|---|---|
| Predrug | 0.92 (0.87-0.95) | 1.06 ± 0.24 | 11.0 ± 0.7 | 9.6 ± 1.8 | 2.1 ± 1.0 | 2.0 ± 0.9 | 18.5 ± 8.4 |
| Postdrug | 0.93 (0.87-0.94) | 0.80 ± 0.16 | 10.8 ± 1.2 | 10.1 ± 0.9 | 2.2 ± 1.6 | 1.8 ± 1.0 | 17.9 ± 9.5 |
| Preplacebo | 0.93 (0.87-0.95) | 1.30 ± 0.14 | 10.7 ± 1.6 | 10.8 ± 1.7 | 1.7 ± 0.9 | 1.7 ± 0.9 | 16.2 ± 13 |
| Postplacebo | 0.95 (0.86-0.97) | 1.22 ± 0.28 | 10.5 ± 1.7 | 10.2 ± 1.5 | 1.8 ± 1.0 | 1.7 ± 1.1 | 15.2 ± 9.4 |
Values (mean ± SD) are shown for before (pre) and after (post) amphetamine (drug) and placebo trials. Columns are described as follows: the median and range of r2 values for the rate-rate plots and the corresponding slope of the regression line fit through the data, mean rate of the control and test units measured throughout a contraction. Contract rate = the rate of rise of the contraction expressed in terms of the subject's maximum voluntary contraction (MVC). Relax rate = the rate of fall of the contraction expressed in terms of MVC. Peak torque = maximum torque reached in a contraction. t-tests used to compare values between treatments did not reach significance (all P > 0.05).
Measure of common drive to control and test units
To ensure that the firing rate of the control motor unit was an accurate measure of synaptic drive to the test motor unit, i.e., that both units received a common synaptic drive, the degree of common firing rate modulation between each control and test motor-unit pair was measured. The smoothed firing rate of the control motor unit was plotted against the smoothed firing rate of the test unit at similar time points. To do this, a 50-point, fifth-order polynomial line was calculated for both the control and test units during the time interval that both units were activated to ensure that irrespective of how long a firing sequence was, a similar number of points was used in the linear regression (see Fig. 1, insets). When present, very fast accelerations in the initial firing rates of the test unit (≈3 imp/s or more), indicative of PIC activation at recruitment (a.k.a. secondary range firing, see discussion), were removed as done previously (Gorassini et al. 2002a; Kiehn and Eken 1997; Mottram et al. 2009). The slope of the regression line fit through the rate-rate plots and the co-efficient of determination (r2) were calculated (presented in Table 1). Only those trials where the r2 value was ≥0.7 were used to ensure that ≥70% of the rate modulation of the test unit could be accounted for by the rate modulation of the control motor unit.
Other measures of motor-unit firing rate and torque profiles
As shown in Table 1, various features of the firing rate profiles of the control and test motor units and of the contraction torque were calculated from the trials where the ΔF measurements were made: 1) the average firing rate of the control and test motor units throughout the entire contraction; 2) the average rate of rise of the torque profile during the ascending (contract) and descending (relax) phase of the contraction; and 3) the average peak torque during a contraction expressed as a percentage of the torque produced during a maximum voluntary contraction (%MVC).
Modulation in firing rate of the control motor unit
The extent of firing rate modulation of the control unit during a contraction, as measured from the maximum smoothed rate minus the minimum smoothed rate, was measured to determine if the ΔF values were constrained by how much the firing rate of the control unit changed in response to the change in synaptic drive (see also Powers et al. 2008). That is, if different control units modulated their firing rates by small amounts in response to the given synaptic drive, then the corresponding ΔF values should be small as well and vice versa for control units with large rate modulations. Thus the amount of firing rate modulation of the control unit was plotted against the corresponding ΔF value for a given contraction to determine if there was a relationship between the two and if there was any change in this relationship after amphetamine.
Statistics
Values are presented as means ± SD. The mean ΔF for each subject was found first (see Estimation of PIC amplitude) and then the mean ΔF across subjects (n = 5) was found. Normality of the data was tested with the Shapiro-Wilk test (SigmaPlot 11) with a t-test used for normal data and a Mann-Whitney rank sum test for nonnormal data. When applicable, unpaired Student's t-tests were used to compare the ΔF, the mean firing rate of the control or test unit during the contraction and the slope of the regression line fit through the rate-rate plots (see Table 1) before and after a treatment because the exact combination of motor-unit pairs was not always used. Paired t-test were used to compare the peak contraction torque reached before and after a treatment along with the average rate of rise or fall of the contraction (Table 1). Significance was set to P ≤ 0.05.
RESULTS
Effects of amphetamine and placebo on ΔF
SINGLE SUBJECT.
Figure 1 shows motor-unit activity during voluntary contractions of the tibialis anterior muscle from a representative subject both before and after intake of amphetamine on 1 day (20 mg, Fig. 1, A and B) and before and after placebo administration on another day (C and D). In the third trace of all graphs, the firing rate of an earlier-recruited control motor unit was used as an estimate of the synaptic input to the motoneuron pool and in particular to a later-recruited test motor unit (middle trace). Before amphetamine administration (Fig. 1A), the test unit was recruited when the smoothed firing rate of the control unit was 12.9 imp/s and de-recruited (stopped firing) when the control unit was 8.6 imp/s to produce a ΔF of 4.3 imp/s. At 2 h after amphetamine intake (Fig. 1B) for the same control-test motor-unit pair, the test unit was likewise recruited at a control unit rate of 12.7 imp/s but de-recruited when the rate of the control unit decreased to 5.3 imp/s to give a ΔF of 7.1 imp/s. The lower estimated synaptic input at de-recruitment of the test unit after amphetamine compared with before suggests that a larger PIC helped to sustain the discharge of the test unit further (see discussion). In this same subject tested on a different day, the firing rate of the control unit at test unit recruitment was similar before and after placebo (12.0 vs. 12.6 imp/s, same motor-unit pair tested) and unlike after amphetamine, the firing rate of the control unit at the time the test unit was de-recruited was similar after placebo compared with before (7.9 vs. 8.2 imp/s) to give similar ΔF values.
GROUP DATA.
Similar increases in ΔF by amphetamine were measured in the five subjects as amphetamine increased the average ΔF value by 62% from 3.7 ± 0.6 to 6.0 ± 0.8 imp/s (P = 0.001, Fig. 2A, unpaired t-test). In contrast, there were no increases in the ΔF values after placebo administration (3.9 ± 1.2 vs. 4.1 ± 1.2 imp/s, P = 0.8, Mann-Whitney rank sum test) with values similar to the before amphetamine trials and to values published previously (Gorassini et al. 2002a). The increase in the average ΔF by amphetamine was not a result of the average firing rate of the control unit increasing at the time of test unit recruitment (Fig. 2B, left bars, 12.0 ± 0.5 imp/s before vs. 11.9 ± 1.8 imp/s after drug, P = 0.85) but rather due to a decrease in the average firing rate of the control unit when the test unit was de-recruited (Fig. 2B, right bars; 8.4 ± 0.2 imp/s before vs. 5.9 ± 1.67 imp/s after, P = 0.01, unpaired t-test).
Control measures to ensure true increases in ΔF by amphetamine
The estimation of PIC amplitude relies on the assumption that the control motor unit is an accurate measure of synaptic input to the motoneuron pool and, more specifically, to the test motor unit. To ensure that the control and test motor units received similar synaptic inputs, the smoothed firing rate of a control unit was plotted against the smoothed firing rate of a test unit at similar points in time (see rate-rate plot insets of Fig. 1). For all experiments (before and after amphetamine or placebo), the median coefficient of determination (r2) for the fit of the regression line to these data were near 0.90 (Table 1), signifying that the majority of the firing rate of the test unit could be accounted for by the firing rate of the control motor unit. The slope of the regression fit through the rate-rate plots was close to 1.0 in all cases (Table 1), revealing that the modulation in firing rate of the control motor units occurred in proportion to the firing rate modulation of the test motor units. There was a small decrease in the rate-rate slope after amphetamine, but the difference was not significant (P = 0.08). The mean firing rates of the control and test motor units throughout a contraction were also similar in all experiments (Table 1). Thus although the test motor unit was recruited on average ≈3 s after the control unit during a contraction (see values in legend of Fig. 2), the test unit did not respond to the common synaptic input in a manner that was significantly different from the control motor units.
To ensure that the increase in ΔF values by amphetamine was not due to systematic changes in the speed at which the subjects increased or decreased their contractions, the rate of rise and fall of the dorsiflexion torque was compared across all conditions (Table 1). There were no differences in the rate of rise or fall of the contraction torque before or after amphetamine and placebo administration (all P > 0.4) nor were there any differences in the peak torque produced (all P > 0.7).
As a side note, in some trials, we were able to distinguish three different single motor units from a single intramuscular EMG trace with two of the earliest-recruited units acting as control units. When both control units were recruited ≥2 s before the test unit, the average absolute difference when measuring ΔF values from the same test unit (i.e., control unit 1 paired with test unit 1 vs. control unit 2 paired with test unit 1) was only 0.5 ± 0.3 imp/s (n = 11 trials). This is in contrast to the variable ΔF values recorded from cat motor units where only 0.5 s between the activation of the control and test motor units were controlled for (Powers et al. 2008).
Role of firing rate modulation of the control unit and ΔF
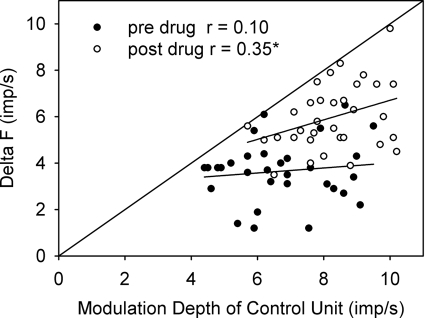
The magnitude of the ΔF values potentially could be constrained by the extent of firing rate modulation of the control unit in response to the changes in synaptic drive produced during the contraction (Powers et al. 2008). For instance, if the firing rate modulation of the control motor unit was small, the ΔF value must also be small correspondingly. However, when plotting the amount of firing rate modulation of the control unit (maximum smoothed rate − minimum smoothed rate) against the corresponding ΔF obtained for a given contraction trial, most of the points before amphetamine administration fell below the line of unity (Fig. 3, ●). Typically, the modulation in firing of the control unit was twice that of the ΔF (6.7 ± 1.5 imp/s modulation vs. 3.7 ± 1.5 imp/s ΔF) with no correlation between these two parameters (r = 0.1, P > 0.5), suggesting that the ΔF values were not constrained by or related to the firing rate responses of the control unit in response to the changing synaptic drive. Likewise, there was no significant correlation in the before (r = 0.27) or after (r = 0.18) placebo trials (all P > 0.1). In contrast, after amphetamine (Fig. 3, ○), more points fell close to the line of unity and a significant relationship emerged between the modulation in firing rate of the control unit and the ΔF (r = 0.35, P = 0.04). In addition, the average amount of control unit modulation was larger after amphetamine compared with before (after 8.3 ± 1.2 imp/s, P < 0.0001) along with the ΔF (6.0 ± 1.4 imp/s, P < 0.00001). The larger modulation of the control unit after amphetamine was due to the control unit reaching a lower minimum firing rate during the voluntary contraction (see values in legend of Fig. 3).
Fig. 3.
Modulation depth of control unit and relation to the delta (Δ)F. Relationship between the maximum excursion in firing rate of the control unit during a contraction (maximum smooth rate – minimum smooth rate) and the magnitude of the ΔF calculated for that contraction for all contraction trials used in Fig. 2. Before amphetamine (●) there was no significant relationship in contrast to after amphetamine (○) as reflected in the correlation coefficient values (r), suggesting the ΔF after amphetamine may have been slightly underestimated. The average maximum firing rate of the control unit (taken from the fitted polynomial) was 13.1 ± 1.5 imp/s before and 13.5 ± 1.8 imp/s after amphetamine (P = 0.36), whereas the average minimum rate was 6.4 ± 1.6 imp/s before and 5.2 ± 1.5 imp/s after amphetamine (P = 0.003). * P = 0.04.
Effect of amphetamine on test unit activation time and ΔF relationship
Similar to that shown in motoneurons of animals (see methods), the PIC in humans (likely the calcium component) appears to take a couple of seconds to activate when the depolarizing input to the motoneuron is of low amplitude. This is illustrated in Fig. 4A during a very weak contraction (∼1% MVC) for a test motor unit where the abrupt firing rate acceleration shortly after recruitment, indicative of a coincident PIC activation, occurred over a few seconds. Note that the rapid acceleration in firing rate of the test unit was likely not due to a rapid acceleration in synaptic input given that the firing rate of the earlier-recruited control motor unit (and torque) was smoothly modulated during this time. Thus in a separate series of trials, we varied the contraction time (i.e., duration of increasing depolarization to the motoneuron) to determine if this affected the secure recruitment of the PIC (and maximal ΔF). That is, if the PICs were not fully activated before the subject started to decrease the depolarizing drive to the test unit, then one would expect the PIC to inactivate sooner to produce a smaller ΔF. In trials without amphetamine (before amphetamine or placebo), ΔF values were smaller (2.1 ± 1.9 imp/s) when the test unit was only active for ≤2 s during the contraction (ascending) phase of the torque profile (Fig. 4B, ●). Beyond 2 s, the ΔF values leveled off to more typical and stable values (3.2 ± 0.8 imp/s, P < 0.00001) as indicated by the exponential rise to maximum fit line. In contrast, after amphetamine administration (Fig. 4B, ○), the ΔF values were high regardless of how long the contraction phase was (as little as 0.5 s), as shown for the near zero slope of the linear regression line fit through the data. Unlike before amphetamine administration, the ΔF values for test unit activation times <2 s (5.8 ± 1.8 imp/s) were not different from the values for test unit activation times >2 s (5.9 ± 1.4 imp/s, P = 0.8). In all contractions (short or long), the rate of rise and fall of the torque profile were kept constant at around 2% MVC/s.
Fig. 4.
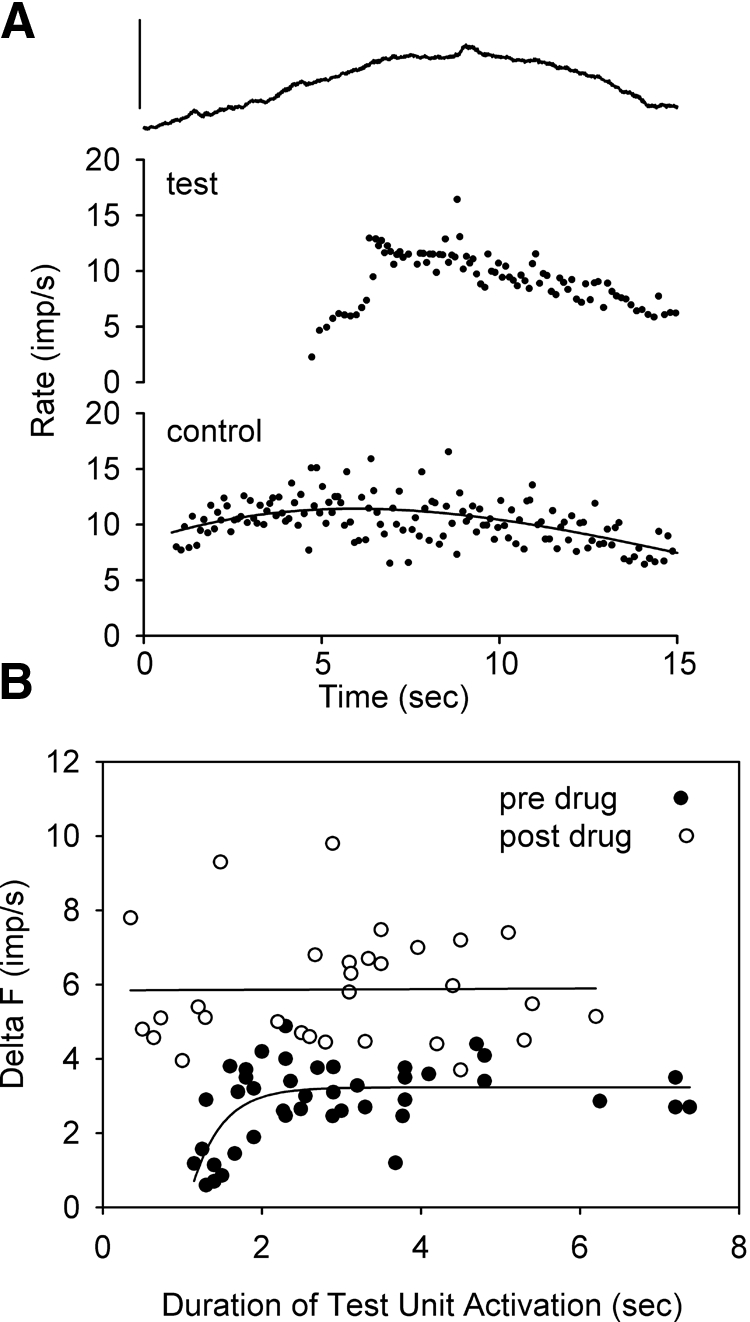
Activation time of the persistent inward current (PIC). A: example of fast acceleration in firing onset of test unit (middle graph) when the control unit (bottom graph) and torque profile (top graph) gradually increased to signify activation of PIC (secondary range firing) in test unit. B: duration of time that test unit was active for during the ascending portion of the contraction plotted against the size of the calculated ΔF for that trial. Experiments without amphetamine intake (no drug: preamphetamine or placebo trials: ●) and trials after amphetamine intake (post drug: ○) in 3 subjects for whom activation time of the test unit was systematically varied. Torque calibration in A = 1% MVC
Effects of amphetamine and placebo on heart rate and blood pressure
To determine if amphetamine had other physiological effects beyond the changes in motoneuron ΔF values, we also measured the subject's resting blood pressure and heart rate for both the amphetamine and placebo trials. The average systolic/diastolic blood pressure before amphetamine intake was 114.1 ± 5.7/75.0 ± 5.9 mm/Hg (average of 2 measurements) and was similar to the blood pressure recorded in these same subjects before placebo administration on a separate experimental day (114.2 ± 5.9/75.9 ± 7.0 mm/Hg). The increase in systolic pressure averaged over the 60- to 90-min time period after amphetamine intake, where a majority of the ΔF measures were made (see methods), was 9.1 ± 9.1 mm/Hg and was greater than the small average decrease in blood pressure measured after placebo intake (−4.1 ± 5.9 mm/Hg, P = 0.003). Likewise the increase in diastolic pressure after amphetamine (6.8 ± 4.7 mm/Hg) was larger compared with the increase after placebo (0.3 ± 5.3 mm/Hg, P = 0.02). The average heart rate was similar before amphetamine (76.3 ± 8.1 beat/min) and placebo (71.1 ± 8.8 beat/min) intake; however, the small increase in heart rate after amphetamine (2.8 ± 9.4 beat/min) was not significantly greater than the small increase that occurred after placebo intake (1.3 ± 7.7 beat/min, P = 0.72).
DISCUSSION
Intake of a 20- to 25-mg dose of oral amphetamine, but not placebo, enhanced motoneuron PIC activation as reflected by a 62% increase in ΔF values measured from pairs of motor units activated during triangular voluntary contractions. The increase in ΔF was due to the test unit of the pair being able to fire at much lower estimates of synaptic input as measured by the firing rate profile of an earlier-recruited control motor unit. In addition, amphetamine shortened the amount of time that a test unit needed to be activated to produce a maximal ΔF. Both of these findings suggest that amphetamine increased the amplitude of PIC activation in the motoneuron. We discuss in the following text the validity of using the ΔF as a relative, indirect measure of PIC amplitude and the effects of possible increases in norepinephrine drive by amphetamine on motoneuron function.
Validity of ΔF in estimating PIC
Intracellular recordings have shown that during a triangular current injection, indirectly measuring the amplitude of the PIC by subtracting the amount of current at the time a motoneuron stops firing from the amount of current needed to recruit the motoneuron (ΔI) gives a smaller measure of PIC amplitude compared with when this current is directly measured during voltage clamp (Li and Bennett 2003). Despite this, the ΔI measure is very sensitive to changes in PIC amplitude following the application of various antagonists that block either the sodium or calcium component of the PIC. Although measuring the PIC amplitude in current clamp may underestimate the PIC measured via voltage clamp, it is very sensitive to relative changes in PIC amplitude (Harvey et al. 2006a; Li and Bennett 2003; Li et al. 2007). Importantly, the PIC estimated from the ΔI is nearly identical to the PIC estimated from the ΔF when computed from the firing rate response of pairs of motor units in the same rats (Bennett et al. 2001a,b). In fact, it is possible to convert the ΔF value to a ΔI value because of the linear frequency-current (F-I) relationship of motoneurons (see Fig. 2 in Bennett et al. 2001a and Fig. 1 in Gorassini et al. 2004). A linear F-I relationship holds true for moderate firing rates of the motoneuron (5–20 Hz) when activated for short periods of time (seconds), regardless of whether the current reaching the soma is supplied by intracellular current injection or from synaptic inputs (Bennett et al. 1998a, 2001a; Lee et al. 2003; Li et al. 2004a; Prather et al. 2001).
Based on the intracellular findings in the preceding text, it is reasonable to assume that the firing rate of an earlier-recruited control motor unit can provide a relatively accurate measure of synaptic input to a later-recruited test motor unit. We know that the control units in this study were receiving similar synaptic inputs as the test units given that >90% of the firing rate of the test units could be accounted for by the firing rate modulations of the control motor units as reflected in the r2 values of the rate-rate plots (≈0.9, Table 1). A tight and linear co-modulation was preserved even after amphetamine, suggesting that increases in PIC amplitude via a possible increase in activation of the norepinephrine receptor did not induce appreciable nonlinearities in the input-output properties of both the control and test motoneurons. This is similar to findings in rat motoneurons where changes in PIC amplitude via blockage or facilitation of monoamine receptors (Harvey et al. 2006a,c) does not change the linearity of the F-I relationship of motoneurons. Thus even after amphetamine, the control motor unit likely continued to be a fairly accurate estimate of synaptic input to the test motor unit to provide relatively accurate estimates of ΔF and PIC amplitudes in the motoneuron. Importantly, our finding that the ΔF measurements were sensitive to amphetamine also suggests that the paired motor-unit recording technique is a fairly sensitive indicator of relative changes in PIC amplitude given that PICs in rat motoneurons are likewise modulated by amphetamine (Rank et al. 2007).
As mentioned in the preceding text, for moderate and short activation times, motoneurons respond linearly to their inputs. However, at the onset of cell firing, a motoneuron can exhibit a few seconds of high gain firing (referred to as “secondary range” firing) (Li et al. 2004a) if the PIC is being activated when the motoneuron first begins to fire (e.g., Fig. 4A) (Lee and Heckman 1998; Mottram et al. 2009). This is why we only chose trials where ≥3 s occurred between the activation of the control and test units to ensure the control motor unit was past any potential, high gain firing that could contaminate the estimated measure of synaptic input to the test motor unit. Although the control and test motor units were recruited a couple of seconds from one another, they had very similar mean firing rates throughout the contraction with a mean slope of the rate-rate regression line close to 1 (see Table 1), suggesting that the control and test units were very similar in their transduction properties. There was, however, a small but nonsignificant decrease in the slope of the rate-rate regression line after amphetamine which may have resulted from the greater number of lower firing rates at the end of the contraction.
Finally, the possibility of spike-frequency adaptation, where a reduction in the firing rate of the motoneuron occurs during a constant input (Zeng et al. 2005), could have affected the ΔF measurement. For instance, a larger spike-frequency adaptation of the control unit compared with the test unit would lead to an overestimation of the ΔF (and PIC amplitude). However, appreciable spike-frequency adaptation was unlikely given that in all control and test motor units studied, we ensured that during a steady contraction effort, the firing rate of the units (at 10–12 imp/s) remained stable for ≥10 s (see also Bawa and Murnaghan 2009). This suggests that at the low and relatively brief (<10 s) contractions used in this study, there was likely very little spike-frequency adaptation of the units. If there was spike-frequency adaptation, it likely occurred to a similar extent in both the control and test units given the parallel rate modulations between the two units as seen for the high r2 values in the rate-rate plots, thus canceling out any potential spike-frequency adaptation effects on the ΔF.
Modulation depth of units
The extent of firing rate modulation of the control units (∼7 imp/s) before amphetamine and during the placebo trials was typically twice that of the measured ΔF (∼3.5 imp/s) with no relationship between the two. This suggests that the measurement of ΔF was not constrained by or related to the extent of firing rate modulation of the control unit produced by changes in synaptic drive during the voluntary contraction. This is in contrast to data from the decerebrate cat where ΔF's were measured in medial gastrocnemius motor units primarily activated by pinching the skin around the ankle (Powers et al. 2008). Much of the positive relationship in the cat data was produced by a small percentage of units (7/48) having large firing rate modulations of the control unit (>10 imp/s) with no relationship for units having modulation depths of <10 imp/s (r = −0.03, n = 43) (R. Powers, personal communication). In fact, this is similar to our human data where the modulation depths of the control unit were all ≤10 imp/s. It was only after amphetamine when the ΔF's became bigger that a positive relationship emerged and in several of the trials, the modulation depth of the control unit was similar to the ΔF value itself. Thus for very large ΔF values (>6 imp/s), the ability of the control unit to change its firing rate to synaptic inputs may underestimate the amplitude of the PIC.
Change in firing rate behavior of motor units after amphetamine
Although amphetamine allowed the motor units to fire at much lower levels of estimated synaptic input, there was no change in the mean firing rates of the units during the matched voluntary contractions (see Table 1). In animal experiments, it has been shown that PIC activation lowers the F-I slope of the motoneuron likely by increasing the conductance of the cell, making it harder to depolarize the motoneuron and increase the firing rate with further injected current (Li et al. 2004a). Likewise, reducing the size of the PIC by blocking the norepinephrine α1 receptor increases the firing rate of the motoneuron to a matched level of injected current (Harvey et al. 2006c). Although α1 receptor facilitation may enhance the amplitude of the PIC to help secure recruitment of the motoneuron and allow it to fire at low levels of synaptic input, it may also reduce the firing rate response of the cell to a given synaptic input. It is difficult to assess in humans if any change in firing response to a given synaptic input did occur after amphetamine because subjects were instructed to match the same torque profile both before and after amphetamine. Thus any possible increases in descending or afferent (Jankowska 2001) synaptic drive required to produce the same torque profile may have masked any decreases in firing response of the motoneuron induced by amphetamine.
Activation time of the PIC
Intracellular experiments have shown that when the amplitude of the PIC is small or of moderate size (≤1 nA), it can take a few seconds of depolarization for the PIC to activate stably (Bennett et al. 1998b, 2001a; Li et al. 2004a). This is similar to human data where motor units that continue to discharge in a self-sustained manner following the removal of an excitatory input require the excitatory stimulus to be applied for ≥2 s (Collins et al. 2001, 2002; Gorassini et al. 1999; Walton et al. 2002). Likewise, in the present study, contractions where the test motor unit was activated for ≤2 s before the subject began to decrease their voluntary drive produced ΔF values that were smaller compared with trials where voluntary drive continued to increase for ≥2 s (Fig. 4B). However, when the amplitude of a PIC is increased, either through repeated cell activation (warm-up) or with monoamine receptor agonists, the time to full activation of the PIC is shortened to speed up secure recruitment and self-sustained firing of the motoneuron (Bennett et al. 1998b; Rank et al. 2007). Such findings may explain why, after amphetamine, stable and larger ΔF's were obtained for trials when subjects began to relax their contraction after ≤2 s. It is possible that the larger PIC after amphetamine helped to recruit securely the motoneuron to self-sustained firing even when the synaptic input was lowered sooner during the relaxation phase of the contraction. A more abrupt activation of the PIC was also observed by a reduction in the fast firing rate accelerations of the test unit at the onset of cell firing (i.e., secondary range firing as shown for the test unit in Fig. 4A), which may have contributed to the lower slope of the rate-rate plots for some of the units after amphetamine (see Table 1).
Functional effects of increased monoamine drive on motoneuron behavior
As mentioned in the introduction, norepinephrine drive to the spinal cord is increased during high arousal states such as stress (Abercrombie and Jacobs 1987; Rasmussen et al. 1986). Pharmacologically, we tried to induce a state of heightened norepinephrine drive by increasing the presynaptic release of endogenous norepinephrine via amphetamine. In addition to producing elevations in resting blood pressure, the putative increase in norepinephrine drive by amphetamine also increased the estimated amplitude of the motoneuron PIC as reflected by a 62% increase in ΔF values. A consistent increase occurred in all subjects with significance being reached in a sample size of only five subjects. This is not surprising given that amphetamine produces a three- to fourfold increase in PIC-mediated long-lasting reflexes in a small number of animals (n = 4–5) (Rank et al. 2007, 2008). In addition, amphetamine is more potent in facilitating the motoneuron PIC compared with norepinephrine agonists potentially because it increases the presynaptic release of norepinephrine close to the receptor and does not rely on exogenous diffusion of a drug, as occurs with agonist application. In this study, our estimation of PIC enhancement allowed for a faster and longer recruitment of self-sustained firing to help boost and sustain synaptic activation of the motoneuron. Securing recruitment of the motoneuron and its continual discharge during high arousal states such as stress or fear may help to execute a proper and timely motor response.
GRANTS
Funding for this work was provided by the Canadian Institutes of Health Research, National Institutes of Neurological Disorders and Stroke Grant R01 NS-048170, and the Alberta Heritage Foundation for Medical Research. E.Udina was recipient of a postdoctoral fellowship from the Spanish ministry of Education and Sciences. J. D'Amico was supported by a studentship from the Natural Sciences and Engineering Research Council of Canada.
ACKNOWLEDGMENTS
We thank Dr. Liu Shi Gan for helpful support with the Matlab programming. We thank Dr. David Bennett for helpful comments on the final draft of the manuscript.
REFERENCES
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci 7: 2837–2843, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha- motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393: 69–83, 1998 [PubMed] [Google Scholar]
- Bawa P, Murnaghan C. Motor unit rotation in a variety of human muscles. J Neurophysiol 102: 2265–2272, 2009 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuck B, Gorassini MA. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998a [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuck B, Gorassini MA. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2038–2045, 1998b [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001a [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001b [DOI] [PubMed] [Google Scholar]
- Björklund A, Skagerberg G. Descending monoaminergic projections to the spinal cord. In: Brain Stem Control of Spinal Mechanisms, edited by Sjolund B, Bjorklund A. Amsterdam: Elsevier Biomedical, 1982, p. 55–88 [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci 21: 4059–4065, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behavior in human motoneurones. J Physiol 538: 289–301, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kuhn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol 82: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Bennett DJ, Yang JF. Self-sustained firing of human motor units. Neurosci Lett 247: 13–16, 1998 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasticity after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002a [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol 87: 1859–1866, 2002b [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. 5-ht2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170a, 2006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol 96: 1141–1157, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol 89: 416–426, 2003 [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405: 345–367, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurons caused by a nifedipine-sensitive calcium plateau potential. J Physiol 414: 265–282, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335–378, 1992 [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol 533: 31–40, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998 [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol 81: 2164–2174, 1999 [DOI] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol 89: 27–39, 2003 [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97: 1236–1246, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004a [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ. Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol 91: 2236–2246, 2004b [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER. Selective action of noradrenaline and serotonin on neurons of the spinal superficial dorsal horn in the rat. J Physiol 582: 127–136, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Nakae A, Ballou T, Vavrek R, Fouad K, Rank MM, Stephens M, Anelli R, Harvey PJ, Heckman CJ, Bennett DJ. Role of constitutively active 5-HT2C receptors following spinal cord injury in rats. Soc Neurosci Abstr 76.8, 2008 [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Kerr R, Maxwell DJ. Absence of co-localized glutamic acid decarboxylase and neuropeptides in noradrenergic axons of the rat spinal cord. Brain Res 749: 164–169, 1997 [DOI] [PubMed] [Google Scholar]
- Perrier J-F, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Rev 40: 223–229, 2002 [DOI] [PubMed] [Google Scholar]
- Perrier J-F, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol 89: 954–959, 2003 [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001 [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC. Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100: 292–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Powers RK, Cope TC. Amplification and linear summation of synaptic effects on motoneuron firing rate. J Neurophysiol 85: 43–53, 2001 [DOI] [PubMed] [Google Scholar]
- Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol 97: 3166–3180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank MM, Vavrek R, Murray KC, Sanelli L, Fouad K, Gorassini MA, Bennett DJ. Peripheral norepinephrine crosses the blood-brain barrier and contributes to spasms after spinal cord injury. Soc Neurosci Abstr 76.9, 2008 [Google Scholar]
- Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res 371: 324–334, 1986 [DOI] [PubMed] [Google Scholar]
- Walton C, Kalmar JM, Cafarelli E. Effect of caffeine on self-sustained firing in human motor units. J Physiol 545: 671–679, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton C, Kalmar JM, Cafarelli E. Caffeine increases spinal excitability in humans. Muscle and Nerve 28: 359–364, 2003 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci 101: 107–117, 2006 [DOI] [PubMed] [Google Scholar]
- Zeng J, Powers RK, Newkirk G, Yonkers M, Binder MD. Contribution of persistent sodium currents to spike-frequency adaptation in rat hypoglossal motoneurons. J Neurophysiol 93: 1035–1041, 2005 [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Seebach BS, Gao BX. Changes in serotonin-induced potentials during spinal cord development. J Neurophysiol 69: 1338–1349, 1993 [DOI] [PubMed] [Google Scholar]