Abstract
The genioglossus (GG) is considered the principle protrudor muscle of the human tongue. Unlike most skeletal muscles, GG electromyographic (EMG) activities are robustly preserved in sleep and thus may fulfill a critical role in preserving airway patency. Previous studies in human subjects also confirm that the GG EMG increases in response to chemoreceptor and mechanoreceptor stimulation. This increase occurs secondary to the recruitment of previously inactive motor units (MUs) and/or an increase in firing rate of already active MUs. Which strategy the nervous system uses when the synaptic drive onto GG motoneurons increases is not known. Here we report on GG whole muscle and tonic MU activities under conditions that mimic sleep, i.e., mild-moderate elevations in CO2 (3% inspired CO2 or the addition of a 1.0 l dead space) and elevated airway resistance. Based on previous work in rat, we hypothesized that mild hypercapnia would increase the firing rates of tonic MUs and that these effects would be further potentiated by a modest increase in airway resistance. Fine wire and tungsten microelectrodes were inserted into the GG to record whole muscle and single MU activities in 21 subjects (13 women, 8 men; 20–55 yr). Either 3% inspired CO2 or added dead space resulted in a 200–300% increase in the amplitude of both tonic and phasic components of the whole muscle GG EMG and a doubling of minute ventilation. Despite these changes, recordings obtained from a total of 84 tonically discharging GG single MUs provide no evidence of a change in firing rate under any of the conditions. On this basis we conclude that in healthy adults, the increase in the tonic component of the whole muscle GG EMG secondary to mild hypercapnia is due almost exclusively to the recruitment of previously inactive MUs.
INTRODUCTION
In humans, upper airway dilator muscles play an important role in preserving airway patency during sleep (Brouillette and Thach 1979, 1980; Cheng et al. 2008; Hwang et al. 1983; Kuna 2001; Shea et al. 2000). One such upper airway muscle is the extrinsic tongue protrudor muscle, the genioglossus (GG) that originates on the jaw and inserts into the tongue base. This muscle has been well studied in human subjects because it is readily accessible for purposes of electromyography (Sauerland and Harper 1976), electrical stimulation (Oliven et al. 2007), and magnetic resonance imaging (Cheng et al. 2008; Fregosi and Quan 2005).
Historically, upper airway muscle electromyographic (EMG) activities have been considered in terms of phasic (i.e., activity confined largely to inspiration) and tonic (i.e., activity that persists throughout the cycle with or without respiratory modulation) components (Onal et al. 1981; Pillar et al. 2001; Remmers et al. 1978; Sauerland and Mitchell 1975; Shea et al. 2000; Tangel et al. 1992; Weiner et al. 1982). Whereas both components are evident in whole muscle GG EMG recordings obtained in human subjects, research and clinical interests have emphasized primarily the inspiratory-related phasic activities (Akahoshi et al. 2001; Remmers et al. 1978; Sauerland and Harper 1976).
By comparison, much less is known about tonic GG EMG activities. We and others have shown that tonically discharging single motor units (SMUs) comprise the majority of the GG EMG activities in quiet wakefulness (Bailey et al. 2007a; Saboisky et al. 2006) and that these activities are robustly preserved in healthy adults in non-rapid-eye-movement (REM) sleep (Bailey et al. 2007a). Several studies have shown that the amplitude of the tonic component of whole muscle GG EMG increases when central chemoreceptors are stimulated with hypercapnic acidosis (Hudgel et al. 1988; Mateika et al. 1999), inspiratory resistance loading (Tangel et al. 1992), and exposure to episodic hypoxia (Harris et al. 2006). However, because no previous study has characterized both GG whole muscle EMG and SMU activities in human subjects during hypercapnia or under conditions of increased airway resistance, we do not know if the increased tonic drive to GG is associated with recruitment of previously inactive MUs, modulation of the firing rate of active MUs, or both.
Accordingly, in the present study, we characterized whole muscle and GG SMU activities during steady state and progressively increasing hypercapnia in combination with modest increases in airway resistance and tested the hypothesis that respiratory stimulation is associated with recruitment and increased firing rates of already active tonic motor units.
METHODS
We studied 21 volunteers, 8 men and 13 women ages 20–55 yr: mean age: 28.7 ± 13.7 (SD) yr; height: 68.0 ± 4.6 in; weight: 148 ± 31.3 lb; BMI: 22.2 ± 2.3. All subjects were healthy, i.e., free of skeletal abnormalities and no history of major surgery or neural or respiratory pathologies and were not taking medication that could affect nervous system function. Subjects refrained from caffeine consumption for the 12 h prior to the experiment. All experimental procedures were approved by Human Subjects Committee at the University of Arizona. Subjects gave their informed consent prior to participation in the study. Subjects were fitted with a nasal and mouth breathing facemask (Hans Rudolph Model series 8900) with a head cap. We applied dental impression material (Exaflex) around the edges of the mask to ensure a tight seal. Subjects were studied in the supine position, awake and with their eyes open in one or two separate protocols (see following text).
Fractional concentration of end-tidal CO2 and O2 were measured at the mask with rapidly responding analyzers (Models 17515A and 17518A, Vacumed, CA) and the values converted to partial pressures (PETCO2 and PETO2) using the barometric pressure recorded at the beginning of the experiment. The gas analyzers were calibrated before each study with known gases that had been chemically analyzed. Mask pressure (Pmask) was measured by connecting Tygon tubing from a mask port to a second differential pressure transducer (Model PT5, Grass Instruments). The rate of inspiratory airflow was integrated digitally to derive the inspired breath volume. All signals were monitored and recorded on the Spike2 data-acquisition and -analysis system (CED, Cambridge, UK).
EMG recordings
Whole muscle and single motor-unit EMG activities were recorded from the GG muscle. The GG is an extrinsic tongue muscle that arises from the medial aspect of the mandibular symphysis and fans dorsoventrally to insert into the central mass of the tongue (Takemoto 2001). Whole muscle GG EMG activities were recorded via bipolar intramuscular fine-wire electrodes (50 μm, California Finewire, Grover Beach, CA) placed per-orally in the anterior floor of mouth. The protocol for electrode insertion has been reported previously (Bailey et al. 2007a; Pittman and Bailey 2009). Briefly, we determined the distance from the submental skin surface to the inferior border of the GG muscle via ultrasonography (Pro Sound 3500, Aloka, Tokyo, Japan) (Eastwood et al. 2003). Each wire was threaded through a 27-gauge needle and inserted bilaterally, posterior to the lower teeth at points equidistant from the lingual frenulum to a depth of ∼2.5 cm from the mucosal surface. The needle subsequently was removed, and the wire remained in the muscle belly. The electrode wires were then taped to the chin and anchored by the seal of the breathing mask that the subject wore (see following text). Correct electrode location was confirmed by monitoring of EMG activities on tongue protrusion and swallow.
To record GG SMU action potentials, a tungsten needle electrode (10 MΩ at 1 kHz, 1–5 μm tip diameter, 250-μm shaft diameter, Frederick Haer, Bowdoinham, ME) was inserted transcutaneously into the floor of mouth with the entry point ∼1.5 cm from the midline and 1–3 cm posterior to the mandible. Surface electrodes (4 mm diam Ag-AgCl) attached to the skin overlying each mastoid process served as indifferent electrodes for the tungsten EMG recording. A ground strap was placed around the upper arm. Whole muscle and single MU EMG signals were amplified (1,000 times, Model 15, Grass Instruments, West Warwick, RI) and band-pass filtered (0.3–3 kHz). Whole muscle GG activities were sampled at 10 kHz and single MUs were sampled at 20 kHz.
PROTOCOL 1. TO DETERMINE THE EFFECT OF A FIXED LEVEL OF RESPIRATORY ACIDOSIS ON GG WHOLE MUSCLE AND SINGLE MOTOR-UNIT ACTIVITIES.
Subjects (n = 15) sequentially breathed room air (∼4 min), medical grade tank air (∼4 min) and a gas mixture comprising 3% CO2 /balance O2 delivered from neoprene bags (∼4 min). Tank gases were warmed and humidified by addition of several liters of warm water to the bags. The bags were connected via a short length of tubing to a two-way non-rebreathing valve (Series 2700C, Hans Rudolph) in series with a pneumotachometer (Model 4813 PNT, Hans Rudolph) attached to the nasal mask for inspiratory and expiratory airflow measurements. The pressure drop across the pneumotachometer was detected with a differential pressure transducer (Model PT5, Grass Instruments). The resistance of this breathing circuit was 1.15 cm H2O·l−1·s.
PROTOCOL 2. TO DETERMINE THE INTERACTIVE EFFECTS OF A FIXED LEVEL OF RESPIRATORY ACIDOSIS AND A MODEST INCREASE IN AIRWAY RESISTANCE ON GG WHOLE MUSCLE ACTIVITIES AND SINGLE MOTOR-UNIT ACTIVITIES.
This protocol (n = 6) was the same as for protocol 1 except that the non-rebreathing valve was connected to a pneumotachometer (Model 4700 PNT, Hans Rudolph) with a relatively narrow bore. The resistance of this circuit was 2.8 cm H2O·l−1·s and thus below the level of conscious perception in an awake subject (Harver and Daubenspeck 1989). The system dead space for protocol 1 and 2, from mask opening to tubing, was ∼182.0 ml.
PROTOCOL 3. TO DETERMINE THE EFFECT OF PROGRESSIVELY INCREASING RESPIRATORY ACIDOSIS ON GG WHOLE MUSCLE ACTIVITIES AND SINGLE MOTOR-UNIT ACTIVITIES.
Of the 15 subjects that completed protocol 1, 10 also completed this protocol. Subjects initially breathed room air (∼4 min) and then breathed through a length of tubing with a volume of 1.0 l that was connected to a pneumotachometer (Model 4813 PNT, Hans Rudolph; ∼3 min). To maintain inspired O2 between 150 and 160 mmHg medical grade oxygen was bled into the tubing. The resistance of this circuit from the tubing to the mask interior was 0.55 cm H2O·l−1·s.
Data analysis
All data were acquired and analyzed using Spike2 and custom-designed software (Cambridge Electronic Design). The amplified and filtered whole muscle EMG signals were rectified and moving time averaged (MTA) off-line with a time constant of 200 ms using customized software (Spike2, CED). We quantified both tonic and phasic components of the whole muscle GG EMG from the MTA signal obtained over 10–15 consecutive breaths in each condition for all protocols. Tonic activity was defined as the lowest level in whole muscle EMG activity at end-expiration, and phasic activity was defined as the difference between the peak and tonic activities. Tonic and phasic components recorded under each protocol subsequently were expressed as percentages of the values obtained in normocapnia (% baseline) for purposes of comparison.
Single motor-unit action potentials initially were discriminated using a template-matching algorithm based on waveform shape and amplitude as described previously (Bailey et al. 2007a). Subsequently, each waveform was checked by visual inspection against the template unit waveform. Initially, we characterized single motor-unit discharge on the basis of the temporal relationship between the spike train and respiratory airflow. Motor units were considered tonic if discharge persisted throughout the respiratory cycle. Estimates of firing rate and firing rate variability were derived from 10–15 consecutive breaths in each of the condition. Mean values for firing rate were calculated as the reciprocal of the average interspike interval (ISI) over 10–15 consecutive breaths in each condition. Variability in ISI was estimated as the coefficient of variation (CV) of the ISIs, expressed as a percentage [CV = (SD ISI/mean ISI) × 100].
Individual subject means, group means and SDs were obtained for whole muscle GG EMG activity (% baseline) as well as GG MU firing frequency (Hz), coefficient of variation (CV) of the ISI, the partial pressure of end-tidal O2 (PETO2) and CO2 (PETCO2), mask pressure (Pmask), tidal volume (VT), breathing frequency (fR), and minute ventilation (VE) under all conditions. A repeated-measures mixed model ANOVA (PASW Statistics 17) was used to test for significant changes in each of the following dependent variables; VE, PETCO2, whole muscle GG EMG, mean MU firing rate, and MU firing variability as a function of each protocol (protocols 1–3) and conditions (i.e., room air, tank air, 3% CO2, and progressively increasing CO2). If the ANOVA revealed significance, planned post hoc contrasts were analyzed with significance adjusted according to the Bonferroni procedure. A significance level of P < 0.05 was used for all comparisons.
RESULTS
Mean (SD) data describing ventilatory parameters obtained under each protocol are reported in Table 1. Baseline ventilatory parameters in protocols 1 and 2 were the same whether subjects breathed room air or humidified tank air nor were there any differences as a function of the resistance of the circuit thus results obtained in protocols 1 and 2 were collapsed for purposes of subsequent analysis. For protocols 1 and 2, breathing 3% CO2 resulted in a group mean PETCO2 that exceeded 40 mmHg and was associated with a significant increase in minute ventilation (VE) from 5.8 ± 0.9 to 10.8 ± 1.7 liter/min (r2 = 0.61; Table 1; P < 0.01). The mean data show that the increase in VE was due to increases in both tidal volume and breathing frequency (Table 1). By comparison, protocol 3 evoked higher PETCO2 levels than either protocol 1 or 2 and elicited a mean minute ventilation of 15.4 LPM (range: 13.0–22.0 l/min). The increase in VE was attributable to a ∼2.5-fold increase in VT accompanied by modest increases in frequency.
Table 1.
Mean values for end-tidal CO2 (PETCO2), minute inspired ventilation (VE), tidal volume (VT), breathing frequency (fR), and motor unit firing frequency (Hz) for each protocol
| Protocols 1 and 2 (Steady State) |
Protocol 3 (Dead Space) |
||||
|---|---|---|---|---|---|
| Room Air | Tank Air | 3% CO2 | Start | End | |
| PETCO2, mmHg | 37.3 ± 1.4 | 37.3 ± 1.5 | 40.7* ± 1.8 | 37.1 ± 1.0 | 44.0* ± 1.5 |
| VE, l/m | 5.8 ± 0.9 | 6.2 ± 1.1 | 10.8* ± 1.7 | 6.3 ± 1.0 | 15.4* ± 3.3 |
| VT, l | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.6* ± 0.1 | 0.4 ± 0.07 | 1.0* ± 0.1 |
| fR, bpm | 12.9 ± 2.0 | 13.2 ± 2.0 | 16.6* ± 2.6 | 13.4 ± 1.9 | 15.4 ± 1.9 |
| Firing rate, Hz | 18.9 ± 3.2 | 19.9 ± 3.5 | 18.2 ± 2.7 | 19.2 ± 2.1 | 17.7 ± 2.4 |
, value that is significantly different relative to the normocapnic condition in each protocol.
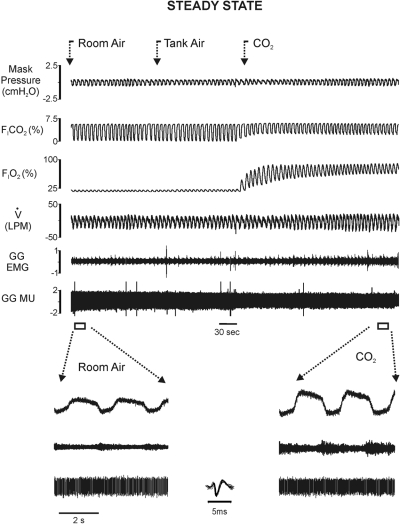
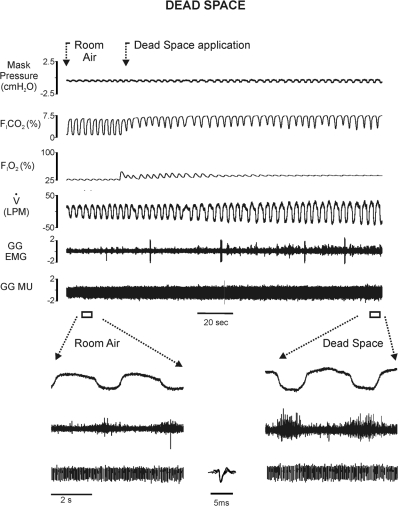
Figures 1 and 2 are representative raw records illustrating one subject's responses in each protocol. Figure 1 shows whole muscle GG EMG and single MU activities, flow, PETCO2, PETO2, and Pmask in steady-state room air, tank air, and 3% inspired CO2. Note that the magnitude of whole muscle GG EMG activities exhibited by this subject during eupneic breathing in normocapnia are representative of the activities sampled in the majority of subjects. The steady-state increase in FICO2 in this subject resulted in increased inspiratory airflow and whole muscle GG EMG amplitude (shown on a faster time base in the following text), consistent with the mean data. Representative raw recordings obtained from the same subject under the dead space protocol are shown in Fig. 2. Relative to the steady-state condition, dead space breathing was associated with larger amplitude VT, somewhat slower breathing frequency, and larger whole muscle GG EMG amplitude (shown on a faster time base in the following text).
Fig. 1.
Original recordings of (from topmost trace down) mask pressure, fractional inspired CO2, fractional inspired O2, airflow (inspiration represented by downward deflection), unprocessed genioglossus whole muscle, and single genioglossus (GG) motor-unit electromyographic (EMG) recordings obtained during a steady-state protocol. Segments of the record in normocapnia and steady-state hypercapnia (FICO2 = 3%) are displayed on a faster time base below. Note steady-state hypercapnia was associated with an increase in inspiratory airflow and GG EMG activities. Inset: superimposition of 10 single motor-unit action potentials on a faster time base. MU, motor unit.
Fig. 2.
Original recordings of (topmost trace down) mask pressure, fractional inspired CO2, fractional inspired O2, airflow, unprocessed GG whole muscle and single MU recordings obtained in normocapnia and dead space breathing protocol. Segments of the record from both conditions are displayed on a faster time base below. Note that dead space breathing resulted in additional increases in airflow and GG EMG activities over and above that seen under the steady-state protocol. Inset: superimposition of 10 single motor-unit action potentials on a faster time base.
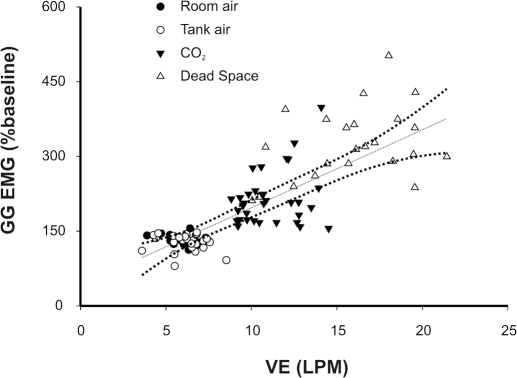
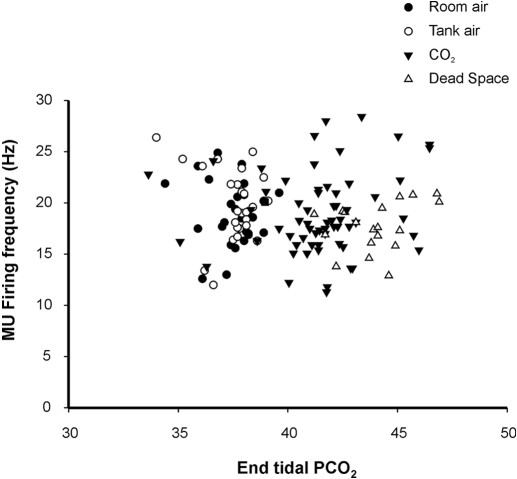
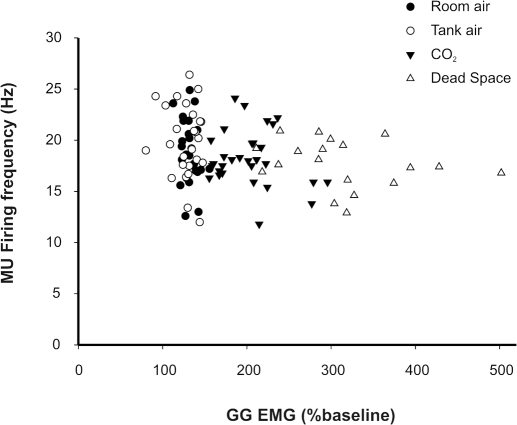
As stated, whole muscle GG EMG activities were evident in all subjects under resting conditions, and the magnitude of these activities consistently increased with 3% inspired CO2 and dead space breathing. Figure 3 illustrates the VE-whole muscle GG EMG relationship recorded in steady state and progressively increasing PETCO2 for each subject. Note that whole muscle GG EMG activities paralleled the increase in ventilation (beta = 0.80; r2 = 0.64). Figure 4 depicts the mean discharge rate for all tonic GG SMUs in each protocol and condition. MUs exhibited a broad range of firing rates (10.4–32.4 Hz); however, there was no significant difference in mean MU firing rate as a function of gas condition. Although some MUs showed modulation of firing rate with 3% inspired CO2 and dead space breathing, both increases and decreases in firing frequency were observed. Thus no systematic relationship between MU firing rate and increased breathing effort was detected (P < 0.116).
Fig. 3.
GG EMG and VE relationship. Steady-state and dead space breathing associated increases in ventilation are paralleled by increases in whole muscle GG EMG. - - -, result of a least-squares regression analysis (beta coefficient = 0.80; r2 = 0.64) shown with 95% confidence intervals.
Fig. 4.
MU firing frequency as a function of increments in PETCO2. Steady-state (protocols 1 and 2) and dead space breathing associated GG single MU firing rates do not correspond with increments in PETCO2.
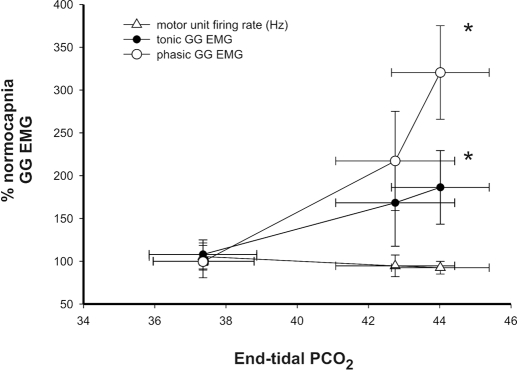
To ascertain the relationship between MU firing rate and drive to the whole muscle, we performed a correlation analysis of firing rate (Hz) and whole muscle GG EMG however, the relationship was not significant and, if anything, the mean MU firing rate tended to decline as drive to the whole muscle increased (Fig. 5). Importantly, however, both the tonic and phasic components of the whole muscle EMG clearly increased as respiratory drive was increased secondary to elevations in PETCO2 (see Fig. 6). When coupled with the absence of change in MU firing rates, this observation suggests that the increase in both the phasic and tonic components of the whole muscle GG EMG occurred via the recruitment of previously silent MUs rather than by increases in the firing rate of already active MUs.
Fig. 5.
MU firing frequency as a function of whole muscle GG EMG in steady-state and dead space protocols. Average single MU firing frequency declines despite clear increases in the magnitude of whole muscle GG EMG (%baseline).
Fig. 6.
Effect of steady state and progressive increases in PETCO2 on single motor-unit discharge rate (bottom), tonic (middle), and phasic components of the whole muscle GG EMG. Average single MU firing rates decline whereas drive to the whole muscle as reflected in phasic and tonic components (% baseline) rise. *, significantly different relative to the normocapnic condition (P < 0.05).
Last, in view of Matthews'(1996) model of motoneuronal variability, we anticipated that an increase in facilitatory input onto the hypoglossal pool with hypercapnia would increase discharge rate variability. Accordingly, we examined the variability in the firing rates of tonic MUs as a function of the hypercapnic stimulus by computing the CV of the ISI. Contrary to expectation, there was no evidence that successive increases in chemoreceptor stimulation or alteration in airway resistance exerted any effect on the mean firing rate variability of tonic MUs (results not shown).
DISCUSSION
Neural drive to the GG muscle in humans typically has been assessed in terms of the phasic and tonic components of the whole muscle EMG as a function of sleep state (Fogel et al. 2003; Mezzanotte et al. 1992; Tangel et al. 1992; Worsnop et al. 1998), negative and positive airway pressure (Akahoshi et al. 2001; Alex et al. 1987; Fogel et al. 2001; Pillar et al. 2001; Shea et al. 2000), anesthesia (Pillar et al. 2001), hypercapnia (Akahoshi et al. 2001; Shea et al. 2000; Stanchina et al. 2002), and exercise (Williams et al. 2000). Yet none has simultaneously studied whole muscle and SMU activities in hypercapnia.
In light of recent work showing tonic GG activity contributes to pharyngeal airway patency (McGinley et al. 2008) and that ∼90% of tonic GG MUs active in wakefulness also discharge during NREM sleep (Bailey et al. 2007a), the focus in this study was on tonic MU activities and the potential to modulate them during hypercapnia. Remarkably we did not detect significant alterations in firing rate in response to either steady state or progressive hypercapnia despite a significant increase in the tonic component of the whole muscle EMG. Taken together, these data suggest that in healthy human subjects, the increase in whole muscle GG EMG in mild hypercapnia is due almost exclusively to the recruitment of previously inactive motor units.
There are several possibilities that could account for the absence of rate coding observed here. First the intensity of muscle contraction can be increased via two mechanisms, an increase in the number of active motor units (recruitment) and/or an increase in the firing rates of already active motor units (rate coding). Rate coding is thought to be important in precise gradation of force, e.g., in small muscles of the human hand (De Luca et al. 1982). In contrast, recruitment of inactive motor units plays a much greater role in modulating force during powerful but less precise contractions in human hindlimb muscles (Adrian and Bronk 1929; Kukulka and Clamann 1981).
Previous studies of respiratory muscles in human subjects, however, indicate that the relative roles of recruitment and rate coding may vary for different muscles. For example, as neural drive increases there is a bias toward recruitment of scalene and intercostal muscle MUs, whereas diaphragm MUs exhibit more prominent frequency modulation (Adrian and Bronk 1928; Gandevia et al. 1999). A bias toward recruitment might be anticipated at low levels of force wherein the number of active MUs at baseline is small relative to the total number of MUs in the pool (Milner-Brown et al. 1973). Conversely if a large proportion of the MU pool is already active under baseline conditions, increased firing rate may become the principle means of achieving higher forces (Fuglevand et al. 1993; Milner-Brown et al. 1973). Thus whether rate coding or recruitment is favored may in large part depend on how many MUs are active at baseline.
The hypoglossal motoneuron pool is excited by a number of different systems serving respiration, posture, and voluntary movements of the tongue. Breathing is largely an unconscious act, and therefore GG MUs are under the control of the brain stem respiratory control centers that are stimulated by input from mechanoreceptors and chemoreceptors. The tonic MUs examined here were already active at baseline and discharged at a mean firing rate of ∼19.0 Hz. Although this firing rate is high relative to diaphragm (12.6 Hz) and intercostal (10.1 Hz) MUs (Gandevia et al. 1999), it is within the mid-range of firing rates observed during volitional tongue movements (Bailey et al. 2007b). Thus in voluntary tongue protrusion tasks, GG MU firing rates at recruitment averaged ∼13.0 Hz and increased steeply (24–33 Hz) as the magnitude of the protrusion increased (Bailey et al. 2007b; Pittman and Bailey 2009). On the basis of these observations, there would appear to be considerable potential for additional increases in GG MU firing rate given sufficient activation.
Previous studies conducted in non-human mammals also show significant rate coding of GG MUs or hypoglossal motoneurons in high drive. For example, in rat (John et al. 2005), ∼75% of phasic GG MUs exhibit rate coding with increasing blood CO2, and the magnitude of rate coding increases as a function of respiratory drive, contributing ∼15% of the hypercapnia induced change in the GG whole muscle EMG. Similarly, hypoglossal motoneurons exhibit rate coding during moderate-severe hypercapnia in anesthetized cat (Mitra and Cherniack 1983). Thus in both preparations, there is a bias that favors rate coding when drive is sufficiently enhanced.
In the current study, we recorded the activities of 84 tonic motor units in normocapnia and in steady state and progressively increasing hypercapnia. Hypercapnia doubled ventilatory output and evoked a 200–300% increase in the whole muscle EMG without disrupting the stability of single motor-unit recordings. Although this increase is significant, it is unclear what fraction of the potential GG activation this represents. In an effort to arrive at such an estimate, we subsequently examined the raw records for swallows, which represent maximal or near maximal activation (Lowe et al. 1977) of the GG, and normalized the respiratory-related activities against such events. We found that the respiratory-related activity measured during hypercpania evoked only ∼20% of the output measured during swallowing. Importantly, this estimate for respiratory-related GG EMG activities in hypercapnia is consistent with previous estimates in young adults performing heavy supine exercise (Williams et al. 2000) or during upper airway obstruction (McGinley et al. 2008), wherein the whole muscle GG EMG approached 20–25% of maximum. Given that output from the genioglossus motoneuron pool may have approached only one-fifth of the maximum potential output in the present study, it seems reasonable to assume that additional increments in force output would be achieved initially via recruitment of previously inactive motor units as described in the preceding text. However, once all the MUs in the pool have been recruited, then the bias presumably would shift in favor of rate coding.
In view of the preceding assessment and too the results of studies conducted in rat and cat (see preceding text), it may be necessary to establish considerably higher levels of neural drive before rate coding of GG MUs emerges. Although in preliminary tests we utilized a more robust stimulus i.e., 5% inspired CO2 and obtained larger ventilatory responses and correspondingly larger-amplitude whole muscle EMG activities, our ability to reliably follow the activities of identified single MUs was hampered by recruitment of previously inactive MUs.
Last, because in healthy adults, both PaCO2 and airway resistance increase at sleep onset (Fogel et al. 2000; Horner et al. 1991; White et al. 1998), we were interested in the effect of increasing airway resistance on tonic GG MU activities. Despite a doubling of inspiratory flow resistance, there was no significant increase in the firing rate of GG MUs. Although the resistance we applied was modest (2.8 cm H2O·l−1·s) relative to average airway resistances at sleep onset (∼6.0 cm H2O·l−1·s), it fell beneath the threshold of conscious perception for awake subjects (Harver and Daubenspeck 1989) and thus ensured that the muscle and motor-unit activities were uncontaminated by behavioral responses.
GRANTS
The project described was supported by National Institute of Deafness and Other Communication Disorders Grant K23DC-007597 to E. F. Bailey.
ACKNOWLEDGMENTS
The authors thank R. F. Fregosi for technical advice and many helpful comments on the manuscript and M. Borgstrom for assistance with the statistical analyses. We are grateful to J. Allen for assisting with experiments and motor-unit discrimination.
REFERENCES
- Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibers. I. Impulses in single fibers of the phrenic nerve. J Physiol 66: 81–101, 1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibers. II. The frequency of discharge in reflex and voluntary contractions. J Physiol 67: 120–151, 1929 [PMC free article] [PubMed] [Google Scholar]
- Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol 531: 677–691, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex CG, Aronson RM, Onal E, Lopata M. Effects of continuous positive airway pressure on upper airway and respiratory muscle activity. J Appl Physiol 62: 2026–2030, 1987 [DOI] [PubMed] [Google Scholar]
- Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol 98: 3284–3291, 2007a [DOI] [PubMed] [Google Scholar]
- Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 97: 933–936, 2007b [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol 46: 772–779, 1979 [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. Control of genioglossus muscle inspiratory activity. J Appl Physiol 49: 801–808, 1980 [DOI] [PubMed] [Google Scholar]
- Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol 586: 4283–4294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behavior of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol 94: 1849–1858, 2003 [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med 164: 2025–2030, 2001 [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Shea SA, Edwards JK, White DP. Reduced genioglossal activity with upper airway anesthesia in awake patients with OSA. J Appl Physiol 88: 1346–1354, 2000 [DOI] [PubMed] [Google Scholar]
- Fogel RB, White DP, Pierce RJ, Malhotra A, Edwards JK, Dunai J, Kleverlaan D, Trinder J. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol 553: 533–544, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Quan SF. MRI of pharyngeal airway in children with sleep-disordered breathing. J Appl Physiol 99: 2470; author reply 2470, 2005 [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AE. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70: 2470–2488, 1993 [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Gorman RB, McKenzie DK, De Troyer A. Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Respir Crit Care Med 160: 1598–1603, 1999 [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–1119, 2006 [DOI] [PubMed] [Google Scholar]
- Harver A, Daubenspeck JA. Human breathing pattern responses to loading with increased background impedance. J Appl Physiol 66: 680–686, 1989 [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol 436: 31–44, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgel DW, Hendricks C, Dadley A. Alteration in obstructive apnea pattern induced by changes in oxygen- and carbon-dioxide-inspired concentrations. Am Rev Respir Dis 138: 16–19, 1988 [DOI] [PubMed] [Google Scholar]
- Hwang JC, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. J Appl Physiol 55: 793–798, 1983 [DOI] [PubMed] [Google Scholar]
- John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med 172: 1331–1337, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res 219: 45–55, 1981 [DOI] [PubMed] [Google Scholar]
- Kuna ST. Effects of pharyngeal muscle activation on airway size and configuration. Am J Respir Crit Care Med 164: 1236–1241, 2001 [DOI] [PubMed] [Google Scholar]
- Lowe AA, Gurza SC, Sessle BJ. Regulation of genioglossus and masseter muscle activity in man. Arch Oral Biol 22: 579–584, 1977 [DOI] [PubMed] [Google Scholar]
- Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976–1982, 1999 [DOI] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol 492: 597–628, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105: 197–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89: 1571–1579, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol 230: 371–390, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra J, Cherniack NS. The effects of hypercapnia and hypoxia on single hypoglossal nerve fiber activity. Respir Physiol 54: 55–66, 1983 [DOI] [PubMed] [Google Scholar]
- Oliven A, Odeh M, Geitini L, Oliven R, Steinfeld U, Schwartz AR, Tov N. Effect of coactivation of tongue protrusor and retractor muscles on pharyngeal lumen and airflow in sleep apnea patients. J Appl Physiol 103: 1662–1668, 2007 [DOI] [PubMed] [Google Scholar]
- Onal E, Lopata M, Ginzburg AS, O'Connor TD. Diaphragmatic EMG and transdiaphragmatic pressure measurements with a single catheter. Am Rev Respir Dis 124: 563–565, 1981 [DOI] [PubMed] [Google Scholar]
- Pillar G, Fogel RB, Malhotra A, Beauregard J, Edwards JK, Shea SA, White DP. Genioglossal inspiratory activation: central respiratory vs mechanoreceptive influences. Respir Physiol 127: 23–38, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman LJ, Bailey EF. Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol 101: 276–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 44: 931–938, 1978 [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95: 2213–2221, 2006 [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol 51: 160–170, 1976 [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med 33: 444–455, 1975 [PubMed] [Google Scholar]
- Shea SA, Akahoshi T, Edwards JK, White DP. Influence of chemoreceptor stimuli on genioglossal response to negative pressure in humans. Am J Respir Crit Care Med 162: 559–565, 2000 [DOI] [PubMed] [Google Scholar]
- Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med 165: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- Takemoto H. Morphological analyses of the human tongue musculature for three-dimensional modeling. J Speech Lang Hear Res 44: 95–107, 2001 [DOI] [PubMed] [Google Scholar]
- Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol 73: 1058–1066, 1992 [DOI] [PubMed] [Google Scholar]
- Weiner D, Mitra J, Salamone J, Cherniack NS. Effect of chemical stimuli on nerves supplying upper airway muscles. J Appl Physiol 52: 530–536, 1982 [DOI] [PubMed] [Google Scholar]
- White DP, Edwards JK, Shea SA. Local reflex mechanisms: influence on basal genioglossal muscle activation in normal subjects. Sleep 21: 719–728, 1998 [DOI] [PubMed] [Google Scholar]
- Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol 89: 590–598, 2000 [DOI] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol 85: 908–920, 1998 [DOI] [PubMed] [Google Scholar]