Abstract
Regulation of the putative amiloride and benzamil (Bz)-insensitive TRPV1t salt taste receptor by phosphatidylinositol 4,5-bisphosphate (PIP2) was studied by monitoring chorda tympani (CT) taste nerve responses to 0.1 M NaCl solutions containing Bz (5 × 10−6 M; a specific ENaC blocker) and resiniferatoxin (RTX; 0–10 × 10−6 M; a specific TRPV1 agonist) in Sprague-Dawley rats and in wildtype (WT) and TRPV1 knockout (KO) mice. In rats and WT mice, RTX elicited a biphasic effect on the NaCl + Bz CT response, increasing the CT response between 0.25 × 10−6 and 1 × 10−6 M. At concentrations >1 × 10−6 M, RTX inhibited the CT response. An increase in PIP2 by topical lingual application of U73122 (a phospholipase C blocker) or diC8-PIP2 (a short chain synthetic PIP2) inhibited the control NaCl + Bz CT response and decreased its sensitivity to RTX. A decrease in PIP2 by topical lingual application of phenylarsine oxide (a phosphoinositide 4 kinase blocker) enhanced the control NaCl + Bz CT response, increased its sensitivity to RTX stimulation, and inhibited the desensitization of the CT response at RTX concentrations >1 × 10−6 M. The ENaC-dependent NaCl CT responses were not altered by changes in PIP2. An increase in PIP2 enhanced CT responses to sweet (0.3 M sucrose) and bitter (0.01 M quinine) stimuli. RTX produced the same increase in the Bz-insensitive Na+response when present in salt solutions containing 0.1 M NaCl + Bz, 0.1 M monosodium glutamate + Bz, 0.1 M NaCl + Bz + 0.005 M SC45647, or 0.1 M NaCl + Bz + 0.01 M quinine. No effect of RTX was observed on CT responses in WT mice and rats in the presence of the TRPV1 blocker N-(3-methoxyphenyl)-4-chlorocinnamide (1 × 10−6 M) or in TRPV1 KO mice. We conclude that PIP2 is a common intracellular effector for sweet, bitter, umami, and TRPV1t-dependent salt taste, although in the last case, PIP2 seems to directly regulate the taste receptor protein itself, i.e., the TRPV1 ion channel or its taste receptor variant, TRPV1t.
INTRODUCTION
In rodents, chorda tympani (CT) taste nerve responses to NaCl are derived from at least two salt taste receptor/ion channels in fungiform taste receptor cells (TRCs). Apical Na+influx through the Na+-specific benzamil (Bz)-sensitive epithelial Na+channel (ENaC) accounts for 60–70% of the CT response to NaCl. The second receptor/ion channel is Bz-insensitive. It does not discriminate between Na+, K+, NH4+, and Ca2+. Several studies suggest that a nonspecific cation channel TRPV1t, a variant of the pain receptor (transient receptor potential vanilloid-1; TRPV1) accounts for the remaining 30–40% of the Bz-insensitive NaCl CT response in rats and wildtype (WT) mice (Lyall et al. 2004, 2005a, 2009). The TRPV1t contribution to the NaCl CT response is increased by 100% or more by TRPV1t agonists: resiniferatoxin (RTX), capsaicin, alcohol, nicotine, cetylpyridinium chloride, naturally occurring Maillard peptides, Maillard reacted peptides conjugated with various sugar moieties, and by elevated temperature (DeSimone and Lyall 2006, 2008; Lyall et al. 2009). Some of the agonists that modulate the TRPV1t-dependent NaCl CT response in rodents also modulate human salt taste (Dewis et al. 2006; Katsumata et al. 2008; Rhyu et al. 2006). In humans, salt taste is predominantly amiloride-insensitive (Feldman et al. 2003; Ossebaard and Smith 1995). This suggests that TRPV1t may also play a role in human salt taste perception.
All of the TRPV1t modulators tested to date produce enhancement of the TRPV1t-dependent NaCl CT response at low concentrations but inhibit it at higher concentrations. The biphasic CT response profiles for the above modulators are observed over a wide range of agonist concentrations (Katsumata et al. 2008; Lyall et al. 2004, 2005a,b, 2007, 2009). At present, the intracellular effectors through which the above agonists modulate the sensitization and desensitization of TRPV1t and produce changes in the TRPV1t-dependent NaCl CT response are not well understood. We have recently shown that the sensitization and desensitization of the TRPV1t-dependent NaCl CT response to RTX is modulated by changes in TRC Ca2+([Ca2+]i) and by altering the phosphorylation state of TRPV1t by protein kinase C epsilon (PKCε), a Ca2+-insensitive isoform of PKC or calcineurin, a Ca2+-dependent serine-threonine phosphatase (PP2B) (Lyall et al. 2009). Changes in [Ca2+]i, PKCε and calcineurin also modulate the activity of native TRPV1 in dorsal root ganglion neurons and the cloned TRPV1 expressed in heterologous cells (Petrocellis and Marzo 2005). In addition to the above modulators, changes in membrane phosphatidylinositol 4, 5-bisphosphate (PIP2) levels modulate TRPV1 activity (Chuang et al. 2001; Nilius et al. 2007; Prescott and Julius 2003). Accordingly, we hypothesize that PIP2 also modulates the TRPV1t-dependent NaCl CT responses by regulating TRPV1t activity in TRCs.
In a mammalian cell, PIP2 represents ∼1% of the total acidic membrane lipids and >99% of the doubly phosphorylated phosphoinositides (Golebiewska et al. 2006). PIP2 has been shown to regulate a variety of ion channels. These include inwardly rectifying and voltage-gated K+channels, the two-pore (2-P) domain K+channels, voltage-gated Ca2+channels, cyclic nucleotide-gated channels, intracellular Ca2+release channels, Cl− channels, TRP channels, and ENaC (Delmas et al. 2005; McLaughlin and Murray 2005; Nilius et al. 2007; Suh and Hille 2005; Yue et al. 2002). Among TRP channels, PIP2 tonically inhibits only TRPV1, and the channel is released from inhibition by PIP2 hydrolysis (Chuang et al. 2001; Nilius et al. 2007; Prescott and Julius 2003). All of the other TRP channels, namely TRPM4, TRPM5, TRPM7, TRPM8, and TRPV5, are activated by PIP2 (Nilius et al. 2007).
Here, we studied the regulation of the native TRPV1t in TRCs by PIP2. We monitored NaCl CT responses in the presence of Bz in anesthetized Sprague-Dawley rats and in wild-type (WT) and TRPV1 knockout (KO) mice in vivo. CT responses were monitored in the absence and presence of RTX, a specific TRPV1t modulator (Lyall et al. 2004, 2005a, 2009), whereas PIP2 levels were either enhanced or diminished in TRC membranes in vivo. Changes in PIP2 were induced by the topical lingual application of U73122, a nonspecific blocker of phospholipase Cs (PLCs) or phenylarsine oxide (PAO), a blocker of phosphoinositide (PI) 4 kinases. In addition, we increased PIP2 in TRC membranes directly by the topical lingual application of diC8-PIP2, a short chain synthetic PIP2. We also studied the effects of PAO, U73122, and diC8-PIP2 on CT responses to representative sweet (sucrose), bitter (quinine), and umami [monosodium glutamate (MSG) and MSG + IMP (inosine 5′-monophosphate)] stimuli and on the ENaC-dependent component of the NaCl CT response. The results summarized here suggest that both the open-state activity and the RTX-induced sensitization and desensitization of TRPV1t are modulated by PIP2. An increase in PIP2 inhibited TRPV1t activity and desensitized the channel toward activation by RTX. In contrast, a decrease in PIP2 enhanced TRPV1t activity and sensitized the channel to further stimulation by RTX. Unlike TRPV1t, ENaC-dependent NaCl CT responses showed no sensitivity to changes in PIP2. On the other hand, an increase in PIP2 enhanced CT responses to sweet and bitter stimuli. We conclude that PIP2 is a common intracellular effector that differentially regulates different taste qualities.
METHODS
CT taste nerve recordings
We generated concentration–response relationships between RTX concentration and the magnitude of the TRPV1t-dependent NaCl CT response in Sprague-Dawley rats, WT mice, and TRPV1 KO mice under various experimental conditions that are expected to alter membrane PIP2 levels in TRCs. Animals were housed in the Virginia Commonwealth University animal facility in accordance with institutional guidelines. All animal protocols were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. Female Sprague-Dawley rats (150–200 g) were anesthetized by intraperitoneal injection of pentobarbital sodium (60 mg/kg), and supplemental pentobarbital sodium (20 mg/kg) was administered as necessary to maintain surgical anesthesia. The animal's corneal reflex and toe-pinch reflex were used to monitor the depth of surgical anesthesia. Body temperatures were maintained at 37°C with a Deltaphase Isothermal PAD (Model 39 DP, Braintree Scientific, Braintree, MA). The left CT taste nerve was exposed laterally as it exited the tympanic bulla, severed, desheathed, and wrapped around a 32-gauge platinum/iridium wire electrode. Stimulus solutions maintained at room temperature were injected into a Lucite chamber affixed by vacuum to a 28-mm2 patch of anterior dorsal lingual surface (Ye et al. 1993). For stimulation or rinsing, 3-ml aliquots were injected at a rate of 1 ml/s into the perfusion chamber. The CT responses were recorded under zero lingual current-clamp and analyzed as described previously (Katsumata et al. 2008; Lyall et al. 2009).
WT mice (C57BL/6J) and homozygous TRPV1 KO mice (30–40 g; B6. 129S4-Trpv1tmijul, The Jackson Laboratory, Bar Harbor, ME) were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg), and supplemental pentobarbital sodium (10 mg/kg) was administered as necessary to maintain surgical anesthesia. The rest of the procedure was the same as in rats. At the end of each experiment, animals were killed by an intraperitoneal overdose of pentobarbital sodium (∼195 mg/kg body weight for rats and 150 mg/kg weight for mice).
The composition of the various stimulating solutions used in the CT experiments is shown in Table 1. The various drugs and their concentrations used in this study and their specific targets are listed in Table 2. The anterior lingual surface was stimulated with a rinse solution (R; pH 6) and with a salt solution (pH 6) containing 0 to 10 × 10−6 M RTX. RTX specifically modulates the TRPV1t-dependent NaCl CT response. In previous studies, the RTX-induced increase in the TRPV1t-dependent NaCl CT response varied with pH. The relationship between pH and the magnitude of the CT response was bell shaped. The maximum increase in the CT response was observed around pH 6 (Lyall et al. 2004). RTX versus TRPV1t-dependent NaCl CT concentration–response curves were obtained before and after treating the anterior tongue with various drugs listed in Table 2. In our previous studies (DeSimone et al. 2001), in the presence of the cetylpyridinium chloride (a TRPV1t agonist weaker than RTX) concentration that produced a maximum enhancement in the TRPV1t-dependent NaCl CT response, the CT response was a saturating function of NaCl concentration with a Km of 0.185 ± 0.035 M. In this study, all experiments were done at 0.1 M NaCl to enable us to observe both maximum enhancement and inhibition of the TRPV1t-dependent NaCl CT response in the presence of agonists and antagonists, respectively.
Table 1.
Composition of stimulating solutions for CT experiments
| Solution | Composition | pH |
|---|---|---|
| Rinse (R) | 0.01 M KCI + 0.2 M mannitol + 0.01 M HEPES | 6 |
| Salt stimuli (N) | 0.01 M KCl + 0.1 M NaCl + 0.01 M HEPES | 6 |
| R + RTX | R + 0.1 × 10−6 M to 10 × 10−6 M RTX | 6 |
| N + RTX | N + 0.1 × 10−6 M to 10 × 10−6 M RTX | 6 |
| Control-1 | 0.3 M NH4Cl | |
| Control-2 | 0.3 M NaCl | |
| Sucrose | 0.3 or 0.5 M | |
| KCl | 0–0.5 M | |
| SC45647 | 0.003 or 0.005 M | |
| Quinine | 0.01 M | |
| HCl | 0.03 M | |
| R + mannitol | R + 0.210 M | |
| R + mannitol + SC45647 | R + 0.205 M + 0.005 | |
| N + SC45647 | N + 0.005 + 0.005 M | |
| N + SC45647 + RTX | N + 0.005 M + 1 × 10−6 M | |
| N + quinine | N + 0.01 M | |
| N + quinine + RTX | N + 0.01 M + 1 × 10−6 M | |
| MSG + mannitol | 0.1 M + 0.01 M | |
| MSG + mannitol + RTX | 0.1 M + 0.01 M + 1 × 10−6 M |
CT, chorda tympani; RTX, resiniferatoxin.
Table 2.
Summary of the effect of various drugs that modulate PIP2 levels in TRCs and alter the Bz-insensitive NaCl CT response
| Drug | M | Intracellular Target | Number of Animals (N) |
|---|---|---|---|
| Bz | 5 × 10−6 | ↓ ENaC | 12 |
| SB-366791 | 1 × 10−6 | ↓ TRPV1t | 6 |
| U73122 | 10 × 10−6 to 250 × 10−6 | ↓ PLC and ↑ PIP2 | 3 |
| U73343 | 250 × 10−6 | ↔ PLC | 3 |
| PAO | 5 × 10−6 to 50 × 10−6 | ↓ PI4 kinases and ↓ PIP2 | 8 |
| diC8-PIP2 | 115 × 10−6 or 250 × 10−6 | ↑ PIP2 | 12 |
| MSG + IMP | 0.1 M + 1 × 10−3 | ↑ T1R1 + T1R3 | 3 |
| ↑ GPCRs (T2Rs, T1R1 + T1R3, T1R2 + T1R3) | ↑ PLCβ2 and ↑ TRPM5 | 3 |
The GPCR associated with sweet taste (T1R2 + T1R3) was activated by adding sucrose or SC45647 to the salt mixture, the GPCR associated with umami taste (T1R1 + T1R3) was activated measuring responses to MSG and MSG + IMP, and GPCR associated with bitter taste (T2R) was activated by adding quinine to the salt mixture. Benzamil and SB were added to the salt stimuli and produced their effects on the Bz-insensitive NaCl CT responses immediately. However, all other drugs were dissolved directly in 3 ml of dimethyl sulfoxide (DMSO) and were topically applied to the tongue for at least 30 min. In some experiments, a stock solution of diC8-PIP2 in DMSO (0.584 × 10−3 M) was added to 0.525 M sucrose or 0.00525 M SC45647 solution to yield a stimulating solution containing 250 × 10−6 M diC8-PIP2 + 0.3 M sucrose or 250 × 10−6 M diC8-PIP2 + 0.003M SC45647. Equivalent amount of DMSO was added to the rinse solution. DMSO by itself has no effect on the CT responses to salt stimuli (Lyall et al. 1999). Unless stated otherwise, all drugs were purchased from Sigma. ↑, increase; ↓, decrease; ↔, no change; Bz, benzamil (blocks Na+ entry through apical epithelial Na+ channel; ENaC); SB-366791, N-(3-methoxyphenyl)-4-chlorocinnamide (blocks Na+ entry through TRPV1t); U73122, 1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-y)amino]hexyl]-1H-pyrrole-2,5-dione (a nonspecific blocker of phospholipase Cs (PLCs)) and blocks the hydrolysis of PIP2 to inositol-1,4,5-trisphosphate (IP3) + diacylglycerol (DAG) by PLC (Chuang et al. 2001; Liu et al. 2005; Prescott and Julius 2003); U73343, 1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-2,5-pyrrolidine-dione (an inactive analog of U73122); PAO, phenylarsine oxide (a trivalent arsenic, reacts with vicinal thiol groups in proteins inhibits all isoforms of PI4 kinases and blocks the resynthesis of PIP2 from PI (Liu and Quin 2005)); diC8-PIP2, dioctanoyl-PIP2 (Echelon, Salt Lake City, UT); a short-chain synthetic PIP2 that increases PIP2 levels in cell membranes (Liu et al. 2005); IMP, inosine-5′-monophosphate (specifically enhances CT responses to MSG); GPCRs, G-protein–coupled receptors.
Typically, stimulus solutions remained on the tongue for 1–2 min. The volume of fluid in the chamber at any time is ∼0.03 ml (Ye et al. 1993). With a 3-ml rinse solution, this volume is displaced in <1 s.
Control stimuli consisting of 0.3 M NH4Cl and 0.3 M NaCl (Table 1) applied at the beginning and at the end of experiment were used to assess preparation stability (Fig. 4B and Supplementary Fig. S2).1 The preparation was considered stable only if the difference between the magnitude of the responses to control stimuli at the beginning and at the end of the experiment was <10% (Lyall et al. 2009). The following stimulus series were used in the CT experiments: R → 0.3 M NH4Cl → R → 0.3 M NaCl → R → N → R → (N + Bz) → R → (N + Bz + RTX) → R. The R → (N + Bz + RTX) → R step was repeated for each RTX concentration between 0.1 × 10−6 M and 10 × 10−6 M (Fig. 1 and Supplementary Fig. S1). At the end of the RTX concentration series, the control stimuli were again applied (R → 0.3 M NH4Cl → R→ 0.3 M NaCl → R).
Fig. 4.
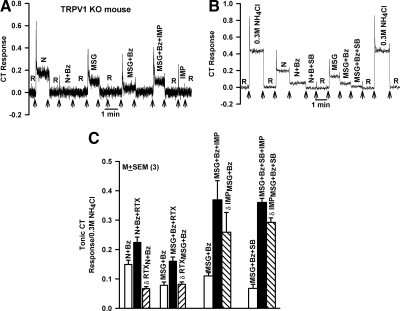
Effect of inhibiting TRPV1t activity on CT responses to umami taste stimuli. A: representative CT recording in a TRPV1 KO mouse in which the tongue was 1st rinsed with a rinse solution (R) and then with N, N + Bz, monosodium glutamate (MSG), MSG + Bz, MSG + Bz + inosine 5′-monophosphate (IMP), and R + IMP. The arrows indicate the time period when the tongue was stimulated with different solutions. B: representative CT recording in which the rat tongue was 1st rinsed with a rinse solution, R, and then with N, N + Bz, N + Bz + SB, MSG, MSG + Bz, and MSG + Bz + SB (Table 1). The control responses to 0.3 M NH4Cl are shown at the beginning and end of the experiment and were nearly identical. The arrows indicate the time period when the tongue was stimulated with different solutions. C: the 1st part of the figure shows the summary of the effects of RTX on the CT responses in the presence of N + Bz and MSG + Bz. The 2nd part of the figure shows the summary of the effects of IMP on the CT responses in the presence of MSG + Bz and MSG + Bz + SB. No statistical difference (P > 0.05; paired) was observed between the RTX-induced increase in the CT response to N + Bz [δ RTXN + Bz = (N + Bz + RTX) − (N + Bz)] or MSG + Bz [δ RTXMSG + Bz = (MSG + Bz + RTX) − (MSG + Bz)]. No statistical difference (P > 0.05; paired) was observed between IMP-induced increase in the CT response to MSG + Bz [δ IMPMSG + Bz = (MSG + Bz + IMP) − (MSG + Bz)] or MSG + Bz + SB [δ IMPMSG + Bz + SB = (MSG + Bz + SB + IMP) − (MSG + Bz + SB)].
Fig. 1.
Effects of phenylarsine oxide (PAO) and phosphatidylinositol 4,5-bisphosphate (PIP2) on N + benzamil (Bz) chorda tympani (CT) responses in the absence and presence of resiniferatoxin (RTX). Figure shows 2 representative CT recordings in which the rat tongue was 1st rinsed with a rinse solution, R (Table 1), and then with N + Bz solutions containing 0–10 × 10−6 M RTX (Table 1) before (Control) and after topical lingual application of (A) 15 × 10−6 M PAO (Post-PAO) or (B) 250 × 10−6 M diC8-PIP2 (Post-PIP2). The arrows indicate the time period when the tongue was stimulated with different solutions.
The data were digitized and analyzed off-line. In CT experiments, both transient (phasic) and tonic (steady-state) parts of the NaCl CT response were quantified. We also quantified the transient (phasic) response to the application of rinse (R) to a tongue already superfused with R. To quantify the phasic part of the CT response, the height of the stimulus-induced maximum CT response relative to baseline response was divided by the mean steady-state (tonic) response to 0.3 M NH4Cl. To quantify the tonic (steady-state) part of a response, the area under the response versus time curve was taken over the final 30 s of the response. To normalize, this area was divided by the area under the 0.3 M NH4Cl response curve over the final 30 s of the tonic response period. The normalized data were reported as means ± SE of the number of animals. In some figures, raw CT recordings are shown in the same animal before and after topical lingual application of a drug with an arbitrary Y scale that reflects the output of the integrator.
For clarity, the points on the graphs of the mean normalized tonic responses versus the logarithm of the RTX concentration were connected by smooth curves. The curves were generated using a fitting function that models the characteristic biphasic property of the RTX concentration versus the magnitude of the CT response. The biphasic property has been observed with every agonist of the TRPV1t-dependent NaCl CT response thus far examined (Katsumata et al. 2008; Lyall et al. 2004, 2005b, 2007, 2009). The fitting function used was
| (1) |
where
| (2) |
and
| (3) |
Here R is the response, x is the logarithm of the RTX concentration expressed in moles per liter, and a, b, d, m, n, and r are parameters chosen by least squares criteria (Katsumata et al. 2008; Lyall et al. 2009).
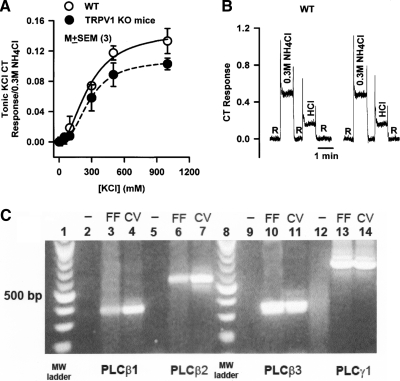
Detection of PLCβ1, PLCβ2, PLCβ3, and PLCγ1 in anterior and posterior taste fields
To confirm that the effects of U73122 on TRPV1t-dependent NaCl CT responses are caused by its effect on PLCs, the PCR technique was used to detect the presence of several PLC isoforms (PLCβ1,PLCβ2, PLCβ3, and PLCγ1) in TRCs. Anterior lingual epithelium containing fungiform papillae and lingual epithelium from circumvallate papilla were obtained after collagenase treatment (Lyall et al. 2004). RNA was prepared using the RNeasy Protect Kit (Qiagen, Valencia, CA), and the cDNA was generated using the M-MLV Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA) according to the manufacturers' protocols. PCR screening of the fungiform and circumvallate cDNAs for the presence of rat PLCβ1,PLCβ2, PLCβ3, and PLCγ1 was performed with Taq DNA Poymerase (Roche, Indianapolis, IN) using the primer pairs shown in Table 3. The reaction annealing temperatures and PCR product sizes are also listed in Table 3. As negative controls, the reactions were run with water replacing the cDNA template.
Table 3.
Primer pairs
| Intracellular Effector (GenBank No.) | Direction | Sequence | Product Size (Annealing Temperature) |
|---|---|---|---|
| PLCβ1 (NM_001077641) | Forward | CACCTCTGAAGCAAGAGCAGGTC | 360 bp (55.5°C) |
| Reverse | GTTGTCATGGTGAATCCATGGG | ||
| PLCβ2 (NM_053478) | Forward | CTCGCTTTGGGAAGTTTGC | 526 bp (55.5°C) |
| Reverse | GCATTGACTGTCATCGGGT | ||
| PLCβ3 (NM_033350) | Forward | GACATGTTTGGTCTCCCAGTTGAC | 306 bp (55.5°C) |
| Reverse | GGTCATCTGGGATGTAGTCAGAAGC | ||
| PLCγ1 (NM_013187) | Forward | CCAAGGACCTGAAGAACATGCTG | 627 bp (57.5°C) |
| Reverse | GTGTGCCCATGGTAAATGACTGG |
PCR reaction products were separated using 1.2% agarose gel at 100 V applied potential for 2.5 h.
Measurement of PIP2 in isolated anterior lingual epithelium using thin-layer chromatography
Rat lingual epithelium containing fungiform papillae was isolated as described before (Lyall et al. 2004). Isolated lingual epithelia from eight rats were pooled and labeled for 4 h with 32P. After chemical treatment with PAO (10 × 10−6 M or 20 × 10−6 M), U73122 (50 × 10−6 M or 100 × 10−6 M), or U73343 (100 × 10−6 M) for 10 min, the reaction was stopped by the addition of 1.8 ml chloroform-methanol-HCl (100:200:2, vol/vol/vol). The phases were separated by addition of 0.6 ml of chloroform and 0.6 ml of 2 M KC1. The lower organic phase was collected, and the aqueous phase was washed again with 2 ml of chloroform to yield a residual organic phase. The combined organic phases were dried under nitrogen; resuspended in 50 μl of chloroform-methanol (9:l) containing 15 μg of unlabeled PIP2; and applied to silica gel-H plates impregnated with 1% potassium oxalate for thin layer chromatography. The lipids were separated using chloroform-methanol-4 N NH4OH (45:35:10, vol/vol/vol), air-dried, and sprayed with 1,2-dichlorofluorescein (0.1%) in isopropyl alcohol. The lipids were visualized under ultraviolet light at 357 nm. The spots corresponding to PIP2 were scraped and counted in a liquid scintillation counter (Murthy and Makhlouf 1991).
Statistical methods
In CT studies, Student's t-test was used to analyze the differences between sets of data. Because we are comparing the normalized CT responses before and after the topical application of TRPV1t modulators in the same CT preparation, a paired t-test was used to evaluate statistical significance. In experiments in which repeated measurements to sequential drug applications were obtained, the data were analyzed using two-way ANOVA and all statistical significant values were Bonferroni-adjusted (Lyall et al. 2009). For all other comparisons, Student's t-test was used to analyze the differences between sets of data.
RESULTS
Regulation of TRPV1t activity by PIP2
To study whether TRPV1t activity is modulated by PIP2, RTX versus N + Bz CT concentration–response relations were obtained in separate groups of rats before and after topical lingual application of PAO, U73122, U73343, or diC8-PIP2, drugs that are expected to alter PIP2 levels in cell membranes (Table 2).
Effects of PAO and RTX on TRPV1t activity
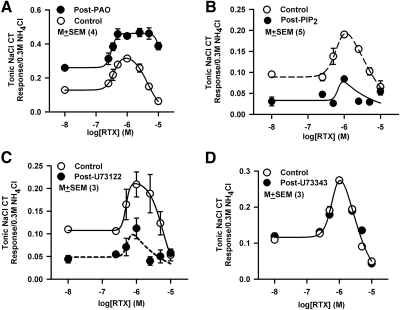
Consistent with previous studies (Lyall et al. 2004, 2005a, 2009), RTX initially produced an increase in the N + Bz CT response between 0.25 × 10−6 M and 1 × 10−6 M (Fig. 1A, Control). Above 1 × 10−6 M RTX, the CT response was diminished. In four rats, a bell-shaped concentration–response relationship was observed between RTX concentration and the magnitude of the tonic N + Bz CT response (Fig. 2A, ○). The maximum increase in the CT response occurred at 1 × 10−6 M RTX. At 10 × 10−6 M RTX, the CT response was inhibited close to the rinse baseline. RTX also enhanced the phasic N + Bz CT response in a biphasic manner. Maximum enhancement and inhibition in the phasic response was also observed at 1 × 10−6 M and 10 × 10−6 M RTX, respectively (Supplementary Fig. S1D). These results show that both phasic and tonic components produce similar RTX concentration versus N + Bz CT response relations. The phasic CT response has two components. One component is derived from the transient mechanical rinse artifact that is observed every time the lingual surface is superfused with a rinse or test solution (Supplementary Fig. S1B). The second component constitutes the chemical response of the nerve to a taste stimuli applied to the tongue. The phasic response is sensitive to the rate at which the taste stimulus is superfused in the lingual chamber (Lyall et al. 2001). In contrast, tonic component of the CT response is stable and is not affected by the rate of superfusion and the mechanical rinse artifact. Accordingly, in this study, most of the data analysis was performed on the tonic component of the CT response.
Fig. 2.
Summary of the effects of PAO, PIP2, U73122, and U73343 on N + Bz CT responses in the absence and presence of RTX. A: in 4 animals, normalized tonic N + Bz + RTX (0–10 × 10−6 M) CT responses were compared before and after PAO treatment. Significant difference was found between RTX interactions with the N + Bz CT response post-PAO treatment relative to control (P < 0.0002 for both; 2-way ANOVA). B: in 5 animals, normalized tonic N + Bz + RTX (0–10 × 10−6 M) CT responses to RTX were compared before and after diC8-PIP2 treatment. Significant difference was found between RTX interactions with the N + Bz CT response post-PIP2 treatment relative to control (P = 0.0025 for both; 2-way ANOVA). C: in 3 animals, normalized tonic N + Bz + RTX (0–10 × 10−6 M) CT responses were compared before and after U73122 treatment. Significant difference was found between RTX interactions with the N + Bz CT response post-U73122 treatment relative to control (P = 0.0054 for both; 2-way ANOVA). D: in 3 animals, normalized tonic N + Bz + RTX (0–10 × 10−6 M) CT responses were compared before and after U73343 treatment. No significant differences were found for RTX interactions with the N + Bz CT response post-U73343 treatment relative to control concentration (P > 0.05 for both, 2-way ANOVA).
CT responses were also monitored after topical lingual application of PAO at 5 × 10−6, 15 × 10−6, or 50 × 10−6 M. At 15 × 10−6 M, PAO enhanced the magnitude of the control N + Bz CT response and the CT response in the presence of RTX (Fig. 1A, Post-PAO). In a separate set of rats, 5 × 10−6 M PAO induced no change in the N + Bz CT responses in the absence or presence of RTX relative to control (data not shown). Increasing the PAO concentration to 50 × 10−6 M produced qualitatively similar effects on the CT response as observed with 15 × 10−6 M PAO (data not shown). Because the magnitudes of the CT responses were not different at 15 × 10−6 M and 50 × 10−6 M PAO, the data were pooled for analysis. In four animals, PAO enhanced the magnitude of the tonic N + Bz CT response in the absence and presence of RTX (Fig. 2A, ●). No attenuation in the CT response was observed between 1 × 10−6 and 10 × 10−6 M RTX relative to control. These results suggest that blocking the resynthesis of PIP2 from PI by PAO increased the open-state activity of native TRPV1t and sensitized it to further stimulation with RTX. It also eliminated the desensitization of the channel at RTX concentrations >1 × 10−6 M.
Effect of PIP2 and RTX on TRPV1t activity
PIP2 levels in TRC membranes were increased by the topical lingual application of either 115 × 10−6 M or 250 × 10−6 M diC8-PIP2. Under control conditions (Fig. 1B, Control), RTX produced a biphasic effect on the N + Bz CT response as before. No significant effects were observed on the CT responses to N, n + Bz, or n + Bz + RTX relative to control with 115 × 10−6 M diC8-PIP2 treatment (data not shown). After 250 × 10−6 M diC8-PIP2 treatment, the N + Bz CT response was decreased in the absence of RTX to near baseline. In addition, diC8-PIP2 attenuated the effect of RTX on the N + Bz CT response (Fig. 1B, Post-PIP2) relative to control. In five rats, diC8-PIP2 inhibited the basal level of the tonic N + Bz CT response and attenuated the magnitude of the neural response in the presence of RTX (Fig. 2B).
Effects of U73122, U73343, and RTX on TRPV1t activity
U73122, a nonspecific inhibitor of PLCs, blocks the hydrolysis of PIP2 to IP3 + DAG and is expected to increase PIP2 levels in cell membrane (Pochynyuk et al. 2008). In three animals, under control conditions (Fig. 2C, ○) RTX produced a biphasic effect on the tonic N + Bz CT responses as before. CT responses were also monitored after topical lingual application of U73122 at 10 × 10−6, 50 × 10−6, 150 × 10−6, or 250 × 10−6 M. U73122 at 10 × 10−6 and 50 × 10−6 M produced no changes in the CT response to N + Bz in the absence or presence of RTX relative to control (data not shown). U73122 at 150 × 10−6 or 250 × 10−6 M produced similar changes in N + Bz and N + Bz + RTX CT responses as shown in Fig. 1B. Because the magnitudes of the CT responses were not different at 150 × 10−6 or 250 × 10−6 M U73122, the data were pooled for analysis. In three animals, after exposure to U73122, the N + Bz CT response was decreased in the absence of RTX to near baseline. In addition, U73122 attenuated the effect of RTX on the N + Bz CT response relative to control (Fig. 2C, ●). In three additional rats, the inactive analog, U73343, at 250 × 10−6 M produced no effect on the N + Bz CT response in the absence or presence of RTX (Fig. 2D). These results show that the open-state TRPV1t activity and its sensitivity to further stimulation by RTX are enhanced at lower cell membrane PIP2 levels. However, increasing cell membrane PIP2 levels by inhibiting PLCs or by direct application of a synthetic PIP2, inhibited open-state TRPV1t activity and its sensitivity to further stimulation by RTX.
RTX and SB-366791 specifically modulate TRPV1t activity without altering CT responses to sweet, bitter, and umami taste stimuli
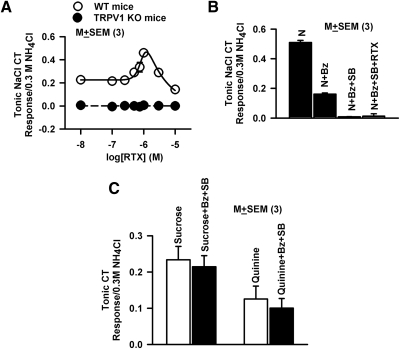
To study whether TRPV1t inhibition or activation specifically modulates the N + Bz CT response or has nonspecific effects on other taste qualities, further experiments were performed to investigate the relationship between TRPV1t activity and CT responses to sweet, bitter, and umami stimuli. RTX induced a biphasic response in both tonic (Fig. 3A, ○) and phasic (Supplementary Fig. S1, A and C) N + Bz CT responses in WT mice. The maximum increase in the tonic and phasic responses was obtained at 1 × 10−6 M RTX. The responses were reduced to the rinse artifact level at 10 × 10−6 M RTX. In TRPV1 KO mice, both the tonic (Fig. 3A, ●) and phasic (Supplementary Fig. S1, B and C) NaCl CT responses were inhibited to baseline in the presence of Bz. This indicates that TRPV1 KO mice elicit no CT response to N + Bz. The tonic and phasic responses to n + Bz remained at baseline in the presence of 1 × 10−6 and 10 × 10−6 M RTX. Taken together, the above results indicate that both phasic and tonic components of the Bz-insensitive NaCl CT response are derived from Na+influx through TRPV1t channels.
Fig. 3.
Effects of RTX and SB-366791 on CT responses to N + Bz, sucrose, and quinine. A: effects of RTX on the normalized tonic N + Bz CT responses from 3 wild-type (WT) and 3 knock-out (KO) mice. In KO mice, RTX did not elicit a CT response above baseline between 0 and 10 × 10−6 M (P > 0.05 with respect to 0; paired 2-sample t-test). B: effects of Bz, RTX, and SB-366791 (SB) on the normalized tonic N + Bz CT responses from 3 rats. In the presence of N + Bz, the CT response was significantly decreased from N (P = 0.0001). In the presence of N + Bz + SB-366791, it further decreased to baseline (P > 0.05 with respect to 0; paired 2-sample t-test). RTX elicited no tonic CT response above baseline in the presence of Bz + SB. C: effects of Bz + SB-366791 (1 × 10−6 M) on the tonic CT responses to 0.5 M sucrose and 0.01 M quinine. No statistical difference was observed between the tonic CT response to sucrose and quinine in the absence and presence of Bz + SB (P > 0.05; paired; n = 3).
SB-366791 is a specific blocker of TRPV1 (Gunthorpe et al. 2004) and TRPV1t (Lyall et al. 2004). SB-366791 (1 × 10−6 M) inhibited the rat CT response to N + Bz to rinse baseline (Fig. 3B). In the continuous presence of SB-366791, RTX at 1 × 10−6 M induced no increase in the neural response above baseline (Fig. 3B). SB-366791 produced similar results in WT mice as shown in Fig. 3B (data not shown). These results suggest that RTX failed to modulate the N + Bz CT response in rats and WT mice in the presence of SB-366791, a TRPV1/TRPV1t inhibitor, or in KO mice in which the native TRPV1/TRPV1t activity is silenced genetically.
Consistent with previous studies (Lyall et al. 2004), RTX (1 × 10−6 or 10 × 10−6 M) (data not shown) or Bz + SB-366791 (1 × 10−6 M) (Fig. 3C) had no effect on the tonic CT responses to 0.3 M sucrose or 0.01 M quinine. In an earlier study (Lyall et al. 2004), steady-state tonic CT responses to 0.02 M HCl were also not altered in the presence of 1 × 10−6 M or 10 × 10−6 M RTX. RTX also does not alter the ENaC-dependent NaCl CT response (Lyall et al. 2004).
TRPV1 KO mice elicited CT responses to MSG + Bz (Fig. 4A). Consistent with this, rats elicited CT responses to MSG + Bz + SB-366791 (Fig. 4B). In rats and WT mice, in the presence of Bz + SB-366791, there is no contribution of Na+to glutamate CT response. An equivalent concentration of MSG elicited a smaller CT response relative to NaCl in TRPV1 KO mice (Fig. 4A), WT mice (data not shown), and rats (Fig. 4B). This is because Na+and Cl− are relatively permeable across the paracellular shunt (tight junctions) between adjacent TRCs in the taste bud. In contrast, glutamate, a larger anion than Cl−, has a lower permeability across the paracellular shunt. Thus, in MSG solutions, Na+has a greater permeability across the paracellular shunt relative to glutamate. This generates a diffusion potential across the lingual epithelium that is positive in the basolateral compartment. This, in turn, hyperpolarizes the receptor potential and results in a lower CT response to MSG relative to NaCl (Ye et al. 1991). CT responses to N + Bz and MSG + Bz were monitored in the absence or presence of 0 and 1 × 10−6 M RTX (Supplementary Fig. S2A). In three rats, RTX increased the tonic CT response in the presence of MSG + Bz by the same magnitude (δ RTX) as in N + Bz (Fig. 4C; P > 0.05, paired). These results suggest that, although the TRPV1t-dependent NaCl CT response is anion dependent, the RTX-induced increase in Na+ flux through TRPV1t does not depend on the accompanying anion.
IMP increased the CT response to MSG in the presence of Bz or Bz + SB by the same magnitude (Fig. 4C; P > 0.05, paired). These results suggest that genetically silencing TRPV1t in TRPV1 KO mice or inhibiting its activity pharmacologically with SB-366791 does not affect CT responses to glutamate and glutamate + IMP. Taken together, the above results suggest that RTX is a specific modulator of TRPV1t. At the concentrations used in this study, RTX had no effect on sweet, bitter, or umami stimuli.
Changes in membrane PIP2 levels do not alter ENaC activity in TRCs
Topical lingual application of PAO (15 × 10−6 M) enhanced the tonic CT response to 0.1 and 0.3 M NaCl (Fig. 5A, filled bars) relative to control (Fig. 5A, open bars). All of the increase in the 0.1 M NaCl CT response could be accounted for by the increase in the NaCl + Bz CT response (P < 0.0001; paired). PAO did not induce a significant change in the ENaC-dependent NaCl CT response relative to control (Fig. 5A, Bz-sensitive; P > 0.05, paired).
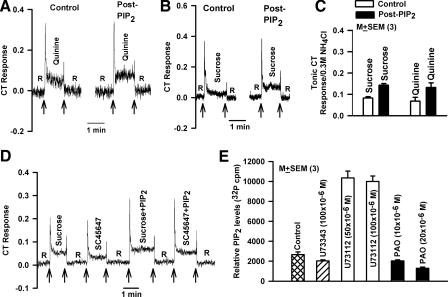
Fig. 5.
A and B: effect of PAO and diC8-PIP2 on TRPV1t-dependent and ENaC-dependent NaCl CT responses. A: in 4 rats, the post-PAO values for 0.1 M NaCl (P = 0.0068; paired), 0.3 M NaCl (P = 0.0001), and 0.1 M NaCl + Bz (P = 0.0001; paired) were significantly enhanced relative to control. No effect of PAO was observed on the ENaC-dependent NaCl CT response (Bz-sensitive; P > 0.05; paired). B: in 6 rats, the post-PIP2 values for 0.1 M NaCl, 0.3 M NaCl, and 0.1 M NaCl + Bz were significantly attenuated relative to control (*P < 0.0001; paired). No effect of diC8-PIP2 was observed on the ENaC-dependent NaCl CT response (Bz-sensitive; P > 0.05; paired). C and D: effect of topical lingual application of U73122 on CT responses to sweet and bitter stimuli. Two representative CT responses are shown in which the rat tongue was 1st rinsed with a rinse solution, R (Table 1), and then with (C) 0.01 M quinine or (D) 0.3 M sucrose (Table 1) before (Control) and after topical lingual application of 250 × 10−6 M U73122 (Post-U73122). The arrows indicate the time period when the tongue was stimulated with different solutions. In 3 rats, topical lingual application of U73122 inhibited the CT response to sucrose and quinine to rinse baseline relative to control (P > 0.05 with respect to 0; paired).
Topical lingual application of diC8-PIP2 (250 × 10−6 M) decreased the magnitude of the tonic CT response to 0.1 and 0.3 M NaCl (Figs. 5B, filled bars) relative to control (Figs. 5B, open bars). All of the decrease in the 0.1 M NaCl CT response could be accounted for by the decrease in the NaCl + Bz CT response (P < 0.0001, paired). diC8-PIP2 did not induce a significant change in the ENaC-dependent NaCl CT response relative to control (Figs. 5B, Bz-sensitive; P > 0.05, paired). Topical lingual application of U73122 (150 × 10−6 M) produced qualitatively similar changes in NaCl and NaCl + Bz as shown in Fig. 5B (data not shown). The inactive analog, U73343, had no effect on the CT responses to NaCl and NaCl + Bz relative to control (data not shown). These results suggest that in fungiform TRCs, ENaC activity is not sensitive to changes in PIP2.
Changes in membrane PIP2 levels modulate CT responses to sweet and bitter stimuli
We further studied whether the concentration of U73122 (a nonspecific blocker of PLCs) that blocks the N + Bz CT response also blocks CT responses to 0.01 M quinine and 0.5 M sucrose. U73122 at 150 × 10−6 M inhibited the tonic CT response to quinine (Fig. 5C) and sucrose (Fig. 5D) to near baseline relative to control. In three rats, after U73122 treatment, the tonic CT responses to sucrose and quinine were not statistically different from baseline (P > 0.05; paired; data not shown). In a separate set of rats, topical lingual application of 50 × 10−6 M U73122 did not produce any effect on the CT responses to sucrose or quinine relative to control (data not shown). These results show that the U73122 concentration that inhibited the N + Bz CT response is also an effective blocker of CT responses to sweet and bitter taste stimuli.
In type II TRCs, PLCβ2 is involved in the transduction of sweet, bitter, and umami taste (Chandrashekar et al. 2006). PIP2 is a substrate for PLCβ2. We hypothesize that an increase in exogenous PIP2 levels will augment endogenous membrane PIP2. This will result in an increase in the concentration of substrate for PLCβ2, which becomes activated in the presence of sweet and bitter stimuli. At 250 × 10−6 M, diC8-PIP2 enhanced the CT response to quinine (Fig. 6A) and sucrose (Fig. 6B) relative to control. The normalized tonic CT responses from three rats are summarized in Fig. 6C. The results show that the concentration of diC8-PIP2 that blocks the N + Bz CT response also modulates the neural response to sweet and bitter stimuli.
Fig. 6.
Effect of diC8-PIP2 on CT responses to sweet and bitter stimuli. Two representative rat CT responses are shown in which the rat tongue was 1st rinsed with a rinse solution, R (Table 1), and then with 0.01 M quinine (A) or 0.5 M sucrose (B) (Table 1) before (Control) and after topical lingual application of 250 × 10−6 M diC8-PIP2 (Post-PIP2). The arrows indicate the time period when the tongue was stimulated with different solutions. C: summary of the effects of diC8-PIP2 on the CT responses to sucrose and quinine. Topical lingual application of diC8-PIP2 significantly enhanced the CT response to sucrose (P < 0.0001) and quinine (P < 0.05) relative to control (paired). D: a representative CT response is shown in which rat tongue was 1st stimulated with a rinse solution, R (Table 1), and then with 0.3 M sucrose, 0.003 M SC45647, 0.3 M sucrose + 250 × 10−6 M diC8-PIP2, and 0.003 M SC45647 + 250 × 10−6 M diC8-PIP2. The arrows indicate the time period when the tongue was stimulated with different solutions. E: changes in 32P-PIP2 induced by PAO and U73122. Treating the anterior lingual epithelium with 50 × 10−6 M (P = 0.0005) or 100 × 10−6 M (P = 0.003) U73122 increased 32P-PIP2 relative to control. Treating the anterior lingual epithelium with 10 × 10−6 M PAO did not alter 32P-PIP2 relative to control (P > 0.05). However, at 20 × 10−6 M, PAO decreased 32P-PIP2 relative to control (P = 0.01). At 100 × 10−6, M U73343 had no effect on 32P-PIP2 (P > 0.05; paired).
In mixtures containing 0.3 M sucrose + 250 × 10−6 M diC8-PIP2 or 0.003 M SC45647 + 250 × 10−6 M diC8-PIP2, PIP2 produced an increase in the sweet response when applied along with the sweet stimulus (Fig. 6D). This is consistent with the fact that PIP2 is lipid soluble and is incorporated into cell membranes very rapidly. However, in most of our studies, we topically applied synthetic PIP2 and other drugs (PAO and U73122) for 30 min to ensure that the drugs reach their intracellular targets and produce maximum effects at the concentrations used.
Effect of PAO and U73112 on PIP2 levels in the isolated anterior lingual epithelium
Treating the isolated anterior lingual epithelium containing fungiform taste papillae in vitro with 50 × 10−6 or 100 × 10−6 M U73122 increased the 32P-PIP2 counts per minute (cpm) by fourfold relative to untreated tissue (Fig. 6E; P = 0.0005 and 0.0003, respectively, paired). No increase in 32P was observed with U73343 (P > 0.05, paired). In contrast, PAO produced a dose-dependent decrease in PIP2 relative to control. At 20 × 10−6 M, PAO decrease PIP2 by 51.5% relative to control (P = 0.01, paired). These results suggest that PAO and U73122 produce their effects by directly altering PIP2 levels in taste cells.
Effect of G protein–coupled receptor and PLCβ2 activation on TRPV1t activity
U73122 not only inhibited the N + Bz CT response (Fig. 2C) but also blocked CT responses to bitter and sweet taste stimuli (Fig. 5, C and D). Therefore in the next series of experiments, we studied whether RTX-sensitivity of TRPV1t is altered by the activation of GPCR-induced decrease in PIP2 via PLCβ2 in sweet and bitter sensing type II TRCs. RTX (1 × 10−6 M) produced the same increase in the CT response in N + Bz, N + Bz + 0.005 M SC45647 (Supplementary Fig. S2B), or N + Bz + 0.01 M quinine (Supplementary Fig. S2C). In three rats, no statistical difference was observed between the RTX-induced increase in the TRPV1t-dependent NaCl CT response in the absence or presence of SC45647 or quinine (P > 0.05, paired; data not shown). Taken together, the results shown in Supplementary Fig. S2, A–C, suggest that in sweet, bitter, and umami-sensing TRCs, GPCR-induced activation of PLCβ2, and the resulting decrease in PIP2 did not alter the RTX sensitivity of the N + Bz CT response. These studies suggest, but do not prove, that TRPV1t activity is independent of TRCs expressing GPCRs for bitter, sweet and umami taste.
Relationship between TRPV1t activity and CT responses to non-Na+salts
TRPV1t is a nonspecific cation channel (Lyall et al. 2004, 2005b, 2009). We hypothesize that inhibition or activation of TRPV1t would attenuate or enhance, respectively, CT responses to cations other than Na+. Consistent with this, in TRPV1 KO mice, the tonic CT response (Rm) to increasing KCl concentrations (Fig. 7A) was significantly smaller relative to its value in WT mice (Table 4). The concentration of KCl at which the CT response was half-maximum (K) was not different between WT and KO mice. These results suggest that in fungiform TRCs, part of the CT response to KCl is derived via K+entry through TRPV1t. We have previously shown that TRPV1t agonists and antagonists modulate a significant part of the CT responses to NH4Cl (DeSimone et al. 2001) and CaCl2 (Lyall et al. 2009). However, TRPV1t is not permeable to all cations. In TRPV1 KO mice, CT responses to normalized 0.03 M HCl were not different from WT mice (Fig. 7B). These results suggest that CT responses to HCl do not depend on H+entry through TRPV1t or TRPV1 (Liu and Simon 2001; Lyall et al. 2004). Taken together, these results suggest that TRPV1t plays a significant role in the elicitation of CT responses to Na+, K+, NH4+, and Ca2+.
Fig. 7.
CT responses to KCl and HCl in WT and TRPV1 KO mice. A: the tonic KCl + Bz CT responses from 3 WT and 3 KO are summarized. B: representative CT responses to 0.3 M NH4Cl and 0.03 M HCl in WT and TRPV1 KO mice. The arrows indicate the time period when the tongue was stimulated with different solutions. In 3 WT and 3 KO mice, the mean ratio between tonic HCl CT and tonic NH4Cl CT response was 3.32 and 3.12, respectively. C: detection of phospholipase Cs (PLC)β1, PLCβ2, PLCβ3, and PLCγ1 in anterior and posterior taste fields. Using specific primer pairs (Table 3) in cDNAs made from rat anterior lingual epithelium containing fungiform taste papillae (FF) and lingual epithelium containing circumvallate papilla (CV), a single band of appropriate size was detected for PLCβ1, PLCβ2, PLCβ3, and PLCγ1. The negative controls (−) were run without cDNA template. Lanes 1 and 8 shows the molecular weight (MW) markers.
Table 4.
Fitted parameters for KCI CT responses in WT and TRPV1 KO mice
| Mice | Rm | K | n |
|---|---|---|---|
| Wildtype | 0.146 ± 0.010 | 0.278 ± 25 | 2.0 ± 0.3 |
| TRPV1 knockout | 0.108 ± 0.003 | 0.279 ± 9 | 2.5 ± 0.2 |
| P | <0.0001 | 0.93 | 0.012 |
| (4) |
See Table 2 for abbreviations.
Detection of PLCβ1, PLCβ2, PLCβ3, and PLCγ1 in anterior and posterior taste fields
In cDNA made from anterior lingual epithelium containing fungiform papillae and lingual epithelium containing circumvallate papilla, using specific primer pairs shown in Table 3, we were able to detect the message for several PLC isoforms (PLCβ1, PLCβ2, PLCβ3, and PLCγ1; Fig. 7C). Most of the above PLC isoforms have previously been shown to be localized only in TRCs (Chandrashekar et al. 2006; Hacker et al. 2008; Toyono et al. 2005). These results suggest that U73122 increases membrane PIP2 levels by inhibiting one or more PLC isoforms expressed in TRCs.
DISCUSSION
The results presented in this paper show that PIP2 modulates TRPV1t activity in a manner similar to that of native TRPV1 in dorsal root ganglion neurons or the cloned TRPV1 channel expressed in heterologous cells. Below we will discuss the physiological significance of PIP2 regulation of the putative TRPV1t salt taste receptor and the potential role of PIP2 in other taste qualities.
An increase in PIP2 inhibits and a decrease in PIP2 enhances TRPV1t activity
Direct application of diC8-PIP2 (Figs. 1B and 2B) or blocking PLC activity with U73122 (Fig. 2C) not only inhibited the control N + Bz CT response but also attenuated the RTX-induced increase in the neural response. Because both treatments produced equivalent results, it suggests that open-state TRPV1t activity and its further activation by RTX are inhibited by an increase in cell membrane PIP2 levels. Consistent with this, inhibiting PLCs with U73122 increased PIP2 levels in isolated lingual epithelium containing fungiform taste buds above control values (Fig. 6E). A decrease in PIP2 levels induced by blocking its resynthesis by inhibiting PI4 kinases with PAO (Fig. 6E) significantly enhanced the open-state TRPV1t activity and its further activation by RTX (Figs. 1A and 2A). These results are consistent with observations that lowering of plasma membrane PIP2 levels through antibody sequestration or PLC-mediated hydrolysis mimic the potentiating effects of nona-peptide bradykinin or nerve growth factor on cells expressing TRPV1 (Chuang et al. 2001). It is suggested that endogenous PIP2 inhibits TRPV1 and PLC catalyzes the hydrolysis of PIP2 to yield IP3 and DAG and releases TRPV1 from PIP2-mediated inhibition (Chuang et al. 2001). DAG activates PKC, and sensitizes TRPV1 to further activation by agonists (Petrocellis and Marzo 2005). Consistent with this, phorbol 12-myristate 13-acetate (PMA), a specific activator of PKC, increased the sensitivity of TRPV1t-dependent NaCl CT responses to RTX. Inhibiting PKC activity by RO31-8220 or topical lingual application of pseudo-substrate inhibitor peptide, a specific blocker of PKCε, abolished the PMA-induced sensitization of the TRPV1t-dependent NaCl CT responses to RTX (Lyall et al. 2009).
In dorsal root ganglion neurons that express native TRPV1 or cloned TRPV1 expressed in HEK293 cells, no inward currents are observed in the absence of TRPV1 agonists at normal physiological pH and temperature. It is suggested that interactions between several signaling effectors (PIP2, [Ca2+]i, ATP) and the phosphorylation state of TRPV1 determine whether TRPV1 channel is tonically active or inactive. ATP and calmodulin interact with the TRPV1 protein at its N-terminal ankyrin repeat domains. These domains contain long finger like folds between ankyrin repeats that can bind both ATP and calmodulin. PIP2 binds to the C terminus of TRPV1 protein. The binding of ATP to the N terminus or PIP2 binding to the C terminus of the TRPV1 protein prevents desensitization during repeated applications of capsaicin. Calmodulin binding to the N terminus seems to play an opposing role and is necessary for desensitization (Vennekens et al. 2008).
Our results further suggest that PIP2 and RTX interact with TRPV1t at two different sites. Consistent with this, in TRPV1, deletion of a very short region in the C terminus (residues 777–792, including 4 basic residues, in rats) eliminated PIP2 inhibition (Prescott and Julius 2003). At present, it is not clear if this region forms a PIP2-binding pocket. In contrast, residues Y511 and T550 in the third and fourth transmembrane domain are responsible for binding to capsaicin. RTX binding requires the presence of methionine in position 547 (Petrocellis and Marzo 2005).
It has been suggested that, besides PIP2, phosphatidyl inositol 4 phosphate may also have a role in maintaining TRPV1 activity (Lukacs et al. 2007). Depending on the experimental conditions, both inhibitory and activating effects of PIP2 have been observed on TRPV1 (Vennekens et al. 2008). In excised patches, capsaicin-activated currents were inhibited by l-polylysine, a PIP2 scavenger, and potentiated by PIP2 (Stein et al. 2006). In the presence of extracellular Ca2+, high capsaicin concentrations desensitize TRPV1 by activating PLC, inducing depletion of both PIP2 and its precursor phosphoinositol 4-phosphate. In these studies, U73122 and dialysis of PIP2 and phosphoinositol 4-phosphate inhibited the desensitization of TRPV1. In addition, the conversion of PIP2 to phosphoinositol 4-phosphate by PIP2-5 phosphatase did not inhibit TRPV1 at high capsaicin concentrations. These studies suggest that phosphoinositol 4-phosphate plays a significant role in maintaining channel activity. In contrast, currents induced by low capsaicin concentrations and moderate heat were potentiated by a decrease in PIP2 and inhibited by an increase in PIP2 (Lukacs et al. 2007). It is likely that the sensitization and desensitization of TRPV1 may be regulated by interactions of several intracellular effectors. This includes Ca2+, calmodulin and ATP interactions with the N terminus and PIP2 binding to the C terminus of the channel protein (Lishko et al. 2007). Further studies are needed to determine whether, along with PIP2, phosphoinositol 4-phosphate also plays a role in the regulation of TRPV1t in the absence and presence of agonists.
In contrast to TRPV1t, ENaC activity was insensitive to changes in PIP2 (Fig. 5, A and B). In A6 cells, an increase in PIP2 was shown to increase ENaC activity by direct interaction with β- or γ-ENaC subunits (Yue et al. 2002). In another study, inhibiting PLC with U73122 in A6 cells enhanced ENaC activity by increasing the mean open time of the channel (Pochynyuk et al. 2008). These results suggest that PIP2 regulation of ENaC may be tissue and cell specific.
An increase in PIP2 enhanced the responses to representative sweet and bitter stimuli (Fig. 6, A–C). PIP2 is a substrate of PLC. An increase in substrate concentration is expected to increase PLC activity. Sweet and bitter sensing TRCs express PLCβ2. Inhibiting PLCβ2 activity by U73122 inhibited the tonic CT responses to representative sweet and bitter stimuli (Fig. 5, C and D). Thus overall, our data suggest that, in TRCs, an increase in PIP2 specifically inhibits TRPV1t. It increases PLCβ2 activity and does not alter ENaC activity in TRCs.
Relationship between TRPV1t activity and CT responses to salty, sweet, sour, bitter, and umami stimuli
In TRPV1 KO mice, KCl concentration versus the magnitude of the CT response profiles gave significantly smaller Rm values relative to WT mice (Fig. 7A; Table 3). In an earlier study (DeSimone et al. 2001), cetylpridinium chloride, a compound that modulated the TRPV1t-dependent NaCl CT response, also altered responses to KCl and NH4Cl in a biphasic manner. The concentration of cetylpridinium chloride that completely inhibited the N + Bz CT response to baseline induced an ∼30% decrease in the CT response to 0.1 M KCl. These results suggest that a significant part of the KCl CT response is derived from K+entry through TRPV1t. RTX also produced a biphasic response on the CT response to CaCl2, and SB-366791, a specific TRPV1t/TRPV1 blocker, inhibited a significant part of the CaCl2 CT response (Lyall et al. 2009). These results suggest that a significant part of the CT response to CaCl2 also depends on Ca2+influx through TRPV1t. These data further strengthen the hypothesis that TRPV1t is a nonselective cation channel that is permeable to Na+, K+, Ca2+, and NH4+ and plays a significant role in eliciting CT responses to the above cations (Lyall et al. 2004).
However, TRPV1t is not permeable to H+(Fig. 7B). This conclusion is supported by the observations that, in the absence of an agonist, TRPV1t-dependent NaCl CT responses show no pH sensitivity (Lyall et al. 2002). In addition, HCl CT responses are not altered in the presence of 1 × 10−6 or 10 × 10−6 M RTX (Lyall et al. 2004). Thus, although a decrease in pH is a sufficient signal to activate TRPV1, a decrease in pH by itself does not modulate the activity of TRPV1t. However, the agonist-induced increase in the TRPV1t-dependent NaCl CT responses showed pH sensitivity, indicating that agonist-induced activation of the channel is pH dependent (Katsumata et al. 2008; Lyall et al. 2004, 2005a).
Genetically silencing TRPV1 (Fig. 4A) or inhibiting its activity pharmacological (Fig. 4, B and C) did not alter CT responses to MSG or MSG + IMP. In sweet, bitter, or umami sensing type II TRCs, activation of specific GPCRs is linked to the activation of PLCβ2, hydrolysis of PIP2 into IP3 + DAG, and a decrease in membrane PIP2 levels. However, the RTX-induced increase in the TRPV1t-dependent NaCl CT response was not significantly affected in mixtures containing N + Bz + quinine or N + Bz + SC45647 (Supplementary Fig. S2). In contrast, a decrease in membrane PIP2 levels increased the TRPV1t-dependent NaCl CT response in the presence of RTX (Figs. 1A and 2A). These results suggest that TRPV1t activity is most likely not dependent on TRCs expressing GPCRs and PLCβ2. Consistent with this, CT responses to sucrose and quinine were not altered in the presence of 1 × 10−6 or 10 × 10−6 M RTX (Lyall et al. 2004). Our data suggest that the observed effects of U73122 on the TRPV1t-dependent NaCl CT response (Fig. 2C) are most likely caused by the inhibition of one or more PLC isoforms (Fig. 7B) expressed in a subset of TRCs that are not involved in sweet, bitter, and umami taste transduction.
Relationship between TRPV1t activity and salt taste perception
In WT mice and rats, the TRPV1t-dependent NaCl CT response contributes ∼30% of the total NaCl CT response (Fig. 5, A and B). Using salt solutions containing amiloride, NaCl detection thresholds were found not to differ between TRPV1 KO mice and WT mice (Ruiz et al. 2006; Treesukosol et al. 2007). These results suggested that, in TRPV1 KO mice, additional salt taste receptors, present in taste fields other than the fungiform TRCs, must contribute to salty taste. During stimulation of the tongue with salt solutions containing agonists, the contribution of TRPV1t to the total NaCl CT response can be increase by 100% or more (DeSimone and Lyall 2006, 2008; Lyall et al. 2009). In humans, salt taste is predominantly amiloride insensitive and may contribute as much as 80% to human salt taste perception (Ossebaard and Smith 1995). Recent studies suggest that the activation of TRPV1t, especially with nonpungent agonists, such as Maillard-reacted peptides, produced increased salt taste sensitivity in humans (Katsumata et al. 2008) and in animal models (Rhyu et al. 2009). Human subjects show significant increase in salt taste perception when NaCl was presented in mixtures containing low concentrations of Maillard-reacted peptides. At high peptide concentrations of the peptide, the same human subjects reported a significant decrease in salt taste perception (Katsumata et al. 2008). This suggests that TRPV1t may also play a role in human salt taste perception.
In Dahl salt-sensitive rats, maintained on a 0.32% Na+diet containing a concentration of Maillard-reacted peptide conjugated with galacturonic acid that produces maximum activation of the TRPV1t-dependent NaCl CT response, lowered Na+balance and inhibited the spontaneous rise in blood pressure observed in rats maintained on the salt diet alone (Masilamani et al. 2009). In contrast, Dahl salt-sensitive rats given Maillard-reacted peptide conjugated with xylose that produced maximum inhibition of the TRPV1t-dependent NaCl CT response increased the rate at which the blood pressure rose spontaneously relative to rats maintained on the salt diet without the peptide (Masilamani et al. 2009). Thus under the conditions in which TRPV1t is upregulated and contributes significantly to salt taste perception, modulating its activity affects changes in salt taste sensitivity in a predictable manner.
A major issue with using TRPV1t agonists as salt taste enhancers is that they increase TRPV1t-dependent NaCl CT responses within a narrow range of concentrations. At high concentrations, all of the compounds tested to date behave as antagonists of TRPV1t and decrease NaCl CT responses. The results presented in this paper and in an earlier publication (Lyall et al. 2009) suggest that the TRPV1t agonist concentration range can be greatly extended by inhibiting the desensitization of TRPV1t channel by lowering cell Ca2+, preventing channel dephosphorylation, and lowering cell membrane PIP2 levels. The same experimental manipulations also eliminate the salt taste–suppressing effect of most TRPV1t modulators at high concentrations. Further understanding of how TRPV1t is regulated in TRCs may facilitate the use of TRPV1t agonists as salt taste enhancers and blood pressure–lowering drugs in the future.
Based on the comparative effects of PIP2 modulators on the CT response to salty, sweet, bitter, and umami stimuli summarized in Table 5, our data suggest that TRPV1t, a nonspecific cation channel, accounts for all of the Bz-insensitive CT response to Na+in WT mice and rats. TRPV1t also contributes significantly to the CT response to K+, Ca2+, and NH4+ salts but is not permeable to H+. RTX is a specific agonist of TRPV1t and produces biphasic effects on the CT responses to Na+, K+, Ca2+, and NH4+. At low concentrations (≤1 × 10−6 M), RTX is a TRPV1t agonist, and at high concentrations (>1 × 10−6 M), it behaves as an antagonist of the channel. TRPV1t activation by RTX does not depend on the anion associated with Na+. TRPV1t activity is most likely not associated with TRCs that express GPCRs for sweet, bitter, or umami taste. We conclude that, similar to the case with TRPV1, PIP2 is a specific blocker of TRPV1t activity. An increase in PIP2 specifically inhibited the TRPV1t-dependent NaCl CT response and desensitized it to further stimulation by RTX. These data strengthen the hypothesis that TRPV1t is a candidate mammalian amiloride- and Bz-insensitive salt taste receptor and is regulated by changes in cell [Ca2+]i, PKCε, calcineurin (Lyall et al. 2009), and PIP2.
Table 5.
Comparative effects of PIP2 modulators on the CT response to salty, sweet, bitter, and umami stimuli
| Drug | Bz-insensitive NaCl CT (−RTX) | Bz-insensitive NaCl CT (+RTX) | Bz-sensitive NaCl CT | Sucrose CT | SC45647 CT | Glycine CT | Quinine CT | MSG CT |
|---|---|---|---|---|---|---|---|---|
| Bz | ↔ | ↔ | ↓ | ↔ | ↔ | ↔ | ↔ | ↓ |
| SB-366791 | ↓ | ↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ |
| RTX | ↑ | biphasic | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| PAO | ↑ | ↑ | ↔ | |||||
| U73122 | ↓ | ↓ | ↔ | ↓ | ↓ | ↓ | ↓ | |
| U73343 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| diC8-PIP2 | ↓ | ↓ | ↔ | ↑ | ↑ | ↑ | ||
| MSG | ↔ | ↔ | ↑ | |||||
| SC45647 | ↔ | ↔ | ↑ | |||||
| Quinine | ↔ | ↔ | ↑ | |||||
| IMP | ↔ | ↔ | ↑ |
Bz and SB-366791 decrease MSG response indirectly by inhibiting Na+ flux via ENaC and TRPV1t, respectively. SB-366791 also decreases part of the CT response to K+, Ca2+, NH4+. See Table 2 for abbreviations.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants DC-000122 and DC-005981 to V. Lyall and a grant from the Campbell Soup Company to J. A. DeSimone.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Gerard L. Heck for technical help with CT recordings.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature 444: 288–294, 2006 [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962, 2001 [DOI] [PubMed] [Google Scholar]
- Delmas P, Coste B, Gamper N, Shapiro MS. Phosphoinositide lipid second messengers: new paradigms for calcium channel modulation. Neuron 47: 179–182, 2005 [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Taste receptors in the gastrointestinal tract III. Salty and sour taste: sensing of sodium and protons by the tongue. Am J Physiol Gastrointest Liver Physiol 291: G1005–G1010, 2006 [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Amiloride-sensitive ion channels. In: The Senses: A Comprehensive Reference, Olfaction and Taste, edited by Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, Firestein S, Beauchamp GK. San Diego, CA: Academic Press, 2008, vol. 4, p. 281–288 [Google Scholar]
- DeSimone JA, Lyall V, Heck GL, Phan TH, Alam RI, Feldman GM, Buch RM. A novel pharmacological probe links the amiloride-insensitive NaCl, KCl, and NH4Cl chorda tympani taste responses. J Neurophysiol 86: 2638–2641, 2001 [DOI] [PubMed] [Google Scholar]
- Dewis M, DeSimone JA, Phan THT, Heck GL, Lyall V. Effect of N-geranyl cyclopropylcarboximide (NGCC) on TRPV1 variant salt taste receptor (TRPV1t). Chem Senses 31: A105, 2006 [Google Scholar]
- Feldman GM, Mogyorósi A, Heck GL, DeSimone JA, Santos CR, Clary RA, Lyall V. Salt-evoked lingual surface potential in humans. J Neurophysiol 90: 2060–2064, 2003 [DOI] [PubMed] [Google Scholar]
- Golebiewska U, Gambhir A, Hangyás-Mihályné G, Zaitseva I, Rädler J, McLaughlin S. Membrane-bound basic peptides sequester multivalent (PIP2), but not monovalent (PS), acidic lipids. Biophys J 91: 588–599, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Luis Hannan S, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 46: 133–149, 2004 [DOI] [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol 99: 1503–1514, 2008 [DOI] [PubMed] [Google Scholar]
- Katsumata T, Nakakuki H, Tokunaga C, Fujii N, Egi M, Phan TH, Mummalaneni S, DeSimone JA, Lyall V. Effect of Maillard reacted peptides on human salt taste and the amiloride-insensitive salt taste receptor (TRPV1t). Chem Senses 33: 665–680, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54: 905–918, 2007 [DOI] [PubMed] [Google Scholar]
- Liu B, Qin F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5 bisphosphate. J Neurosci 25: 1674–1681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4, 5-bisphosphate. J. Neuroscience 25: 4835–4843, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Simon SA. Acidic stimuli activates two distinct pathways in taste receptor cells from rat fungiform papillae. Brain Res 923: 58–70, 2001 [DOI] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci 27: 7070–7080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan DQ, Ereso GL, Phan THT, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, DeSimone JA. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol 281: C1005–C1013, 2001 [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan TH, Russell OF, Malik SA, Heck GL, DeSimone JA. Modulation of rat chorda tympani NaCl responses and intracellular Na+activity in polarized taste receptor cells by pH. J Gen Physiol 120: 793–815, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, DeSimone JA, Feldman GM. Effects of osmolarity on taste receptor cell size and function. Am J Physiol 277: C800–C813, 1999 [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan THT, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. I. Effect on TRC volume and Na+flux. J Gen Physiol 125: 569–585, 2005a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan THT, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J Gen Physiol 125: 587–600, 2005b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan THT, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol 558: 147–159, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Phan TH, Mummalaneni S, Mansouri M, Heck GL, Kobal G, DeSimone JA. Effect of nicotine on chorda tympani responses to salty and sour stimuli. J Neurophysiol 98: 1662–1674, 2007 [DOI] [PubMed] [Google Scholar]
- Lyall V, Phan TH, Mummalaneni S, Melone P, Mahavadi S, Murthy KS, DeSimone JA. Regulation of the benzamil-insensitive salt taste receptor by intracellular Ca2+, protein kinase C and calcineurin. J Neurophysiol 102: 1591–1605, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masilamani S, Coleman J, Melone P, Mummalaneni S, Katsumata T, DeSimone J, Lyall V. Maillard reacted peptides (MRPs) modulate benzamil(Bz)-insensitive NaCl chorda tympani (CT) taste nerve responses and blood pressure (BP) in Dahl salt-sensitive rats. Chem Senses 34: A45–A46, 2009 [Google Scholar]
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438: 605–611, 2005 [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Phosphoinositide metabolism in intestinal smooth muscle: preferential production of Ins(1,4,5)P3 in circular muscle cells. Am J Physiol 261: G945–G951, 1991 [DOI] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Karashima Y, Voets T. Regulation of TRP channels: a voltage-lipid connection. Biochem Soc Trans 35: 105–108, 2007 [DOI] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Effect of amiloride on the taste of NaCl, Na-gluconate and KCl in humans: implications for Na+receptor mechanisms. Chem Senses 20: 37–46, 1995 [DOI] [PubMed] [Google Scholar]
- Petrocellis LD, Marzo VD. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci 77: 1651–1666, 2005 [DOI] [PubMed] [Google Scholar]
- Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol 294: F38–F46, 2008 [DOI] [PubMed] [Google Scholar]
- Prescott ED, Julius D. A modular PIP2 binding site as a determinant of capsaicin receptor sensitivity. Science 300: 1284–1288, 2003 [DOI] [PubMed] [Google Scholar]
- Rhyu M, Ogasawara M, Egi M, Phan THT, DeSimone JA, Heck GL, Lyall V. Effect of Maillard peptides (MPs) on TRPV1 variant salt taste receptor (TRPV1t). Chem Senses 31: A105, 2006 [Google Scholar]
- Rhyu M, Song A, Abe K, Lyall V. Effect of kokumi taste active peptides on amiloride-insensitive salt taste preferences in C57BL/6J mice. Chem Senses 34: A41–A42, 2009 [Google Scholar]
- Ruiz C, Gutknecht S, Delay E, Kinnamon S. Detection of NaCl and KCl in TRPV1 knockout mice. Chem Senses 31: 813–820, 2006 [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128: 509–522, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol 15: 370–378, 2005 [DOI] [PubMed] [Google Scholar]
- Toyono T, Kataoka S, Seta Y, Toyoshima K. Expression of phospholipase C-beta4 in rat circumvallate taste buds. Chem Senses 30: i27–i28, 2005 [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Lyall V, Heck GL, DeSimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Physiol 292: R1799–R1809, 2007 [DOI] [PubMed] [Google Scholar]
- Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des 14: 18–31, 2008 [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. The anion paradox in sodium taste reception: resolution by voltage-clamp studies. Science 254: 724–726, 1991 [DOI] [PubMed] [Google Scholar]
- Ye Q, Heck GL, DeSimone JA. Voltage dependence of the rat chorda tympani response to Na+salts: implications for the functional organization of taste receptor cells. J Neurophysiol 70: 167–178, 1993 [DOI] [PubMed] [Google Scholar]
- Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem 277: 11965–11969, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.