Abstract
Cancer stem cells (CSCs) are a subpopulation of tumor cells with preferential tumor-initiating capacity and have been purported to be resistant to chemotherapy. It has been shown that breast CSC are, on average, enriched in patient tumors after combination neoadjuvant chemotherapy including docetaxel, doxorubicin, and cyclophosphamide (CPA). Here, we investigate the resistance of breast CSC to CPA alone in a xenograft model. CPA treatment led to a 48% reduction in tumor volume during a 2-week period. Cells bearing the CD44+ CD24- phenotype were reduced by 90% (2.5% to 0.24%) in CPA-treated tumors, whereas cells with aldehyde dehydrogenase activity were reduced by 64% (4.7% to 1.7%). A subsequent functional analysis showed that CPA-treated tumors were impaired in their ability to form tumors, indicating loss of functional tumor-initiating activity. These results are consistent with a CSC phenotype that is sensitive to CPA and indicate that some patient CSC may not display the expected resistance to therapy. Deciphering the mechanism for this difference may lead to therapies to counteract resistance.
Introduction
Tumor resistance to chemotherapeutics is an important problem facing oncology, which ultimately leads to tumor progression and mortality. The mechanisms of resistance may take many forms, including multidrug transporter activity, altered signaling networks, enhanced DNA repair, detoxification, and others. One proposed cause of resistance is derived from the cancer stem cell (CSC) hypothesis [1].
The CSC hypothesis maintains that a small subset of cells in a tumor are solely endowed with the capacity to propagate the tumor and maintain its growth [2]. In parallel to normal stem cells, CSCs are thought to have self-renewal activity and produce daughter cells that differentiate to be incorporated into the growing tumor. Likewise, because normal stem cells often exhibit relative resistance to a variety of therapeutic agents, it has been hypothesized that CSCs also exhibit this phenomenon [1,2]. In support of this hypothesis, the literature shows enrichment of CD44+ CD24- CSCs in breast cancer patients undergoing neoadjuvant therapy [3].
In this work, we test the hypothesis that breast CSC are resistant to cyclophosphamide (CPA). Our results show that CPA treatment of xenografts derived from a breast cancer patient results in a reduction in tumor volume and loss of cells containing CSC markers. These tumors were impaired in their ability to form tumors in secondary nonobese diabetic-severe combined immunodeficient (NOD/SCID) mice, proving a functional loss of stem cell activity. Thus, we propose that resistance to chemotherapeutics is not a global property of breast CSC but is a continuum that is determined by individual characteristics.
Materials and Methods
Tumors and Mice
MC1 cells have been previously described and are derived from metastatic breast tumor tissue [4]. They have been passaged as xenografts (<10 passages since derivation) and have been described as ER-, PR-, and HER2- [5].
To produce tumors, NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME) were injected with 106 cells (unless otherwise specified) into the mammary fat pad in a 1:1 mixture of Matrigel (BD Biosciences, San Jose, CA) and serum-free Dulbecco's modified Eagle medium.
Single-cell suspensions were made by chopping tumor with a razor blade followed by incubation at 37°C for 15 minutes in a collagenase/hyaluronidase solution (Stem Cell Technologies, Vancouver, Canada) in Medium 199 (Gibco, Carlsbad, CA). The suspension was then triturated through a 16-gauge needle, and the reaction was stopped by the addition of Hank's balanced salt solution and 5% FBS and then filtered through a 100-µm strainer. Cells were washed with Hank's balanced salt solution and 5% FBS and were filtered a second time through a 100-µm strainer. Cells were frozen in 85% FBS and 15% DMSO.
Flow Cytometry
Flow cytometry for CD44+ CD24- lin- cells was done as described [4]. All antibodies were from BD Pharmingen (San Jose, CA). Lineage cocktail consisted of anti-CD3, CD10, CD16, CD140b, and CD18. Only live, human cells were counted based on propidium iodide exclusion and lack of H2-kd expression, respectively.
Flow cytometry for aldehyde dehydrogenase (ALDH) activity was done according to the instructions included in the ALDEFLUOR Kit (Stem Cell Technologies). Positive activity was confirmed by inhibition with diethyl amino-benzaldehyde.
Immunofluorescence
ALDH immunofluorescence was performed on paraffin-embedded tumor sections after antigen retrieval for 30 minutes in sodium citrate during a 100°C water bath. Sections were blocked in TBS, 1% BSA, and 10% normal goat serum for 2 hours and were incubated at 4°C overnight in 1:100 anti-ALDH antibody (no. 611195; BD Pharmingen). Sections were then incubated in 1:1000 donkey antimouse Alexa Fluor 488 secondary antibody (no. A21202; Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Sections were mounted with Prolong Gold (Invitrogen) containing 4′-6-diamidino-2-phenylindole for staining of nuclei.
Results
Decreased Tumor Volume in Response to CPA Treatment
We began by determining the effect of CPA, a standard part of neoadjuvant therapy for breast cancer, on the growth rate and histological appearance of breast xenografts derived from a patient tumor (MC1), which has been reported to reflect the morphologic and phenotypic characteristics of the original tumor [4].
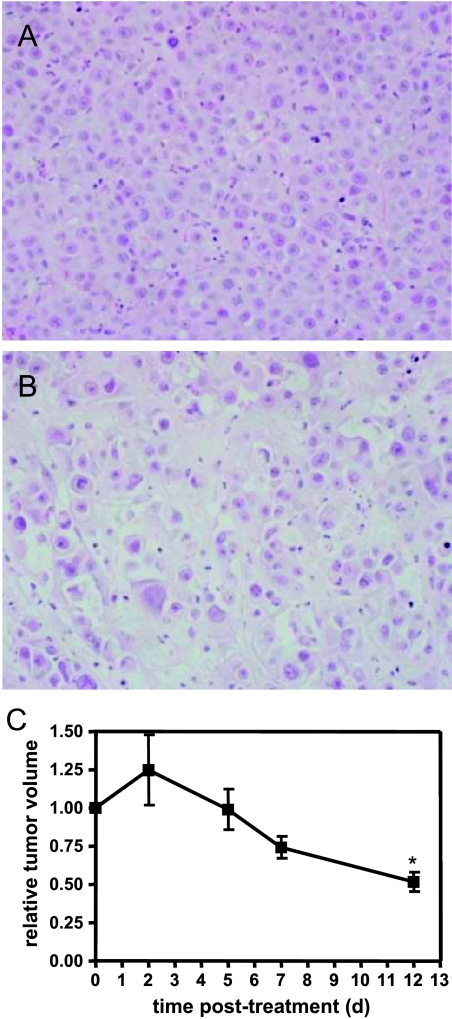
MC1 xenografts were grown in the mammary fat pads of NOD/SCID mice and allowed to reach 1200 to 2200 mm3 before initiation of treatment. Mice were treated intraperitoneally with 100 mg/kg CPA every other day for a total of four treatments. Tumors continued to increase in size 2 days after the initiation of CPA treatment (Figure 1B). Subsequently, a progressive reduction in tumor volume was noted through a 12-day period, ending with tumor harvesting at 14 days. At the 12-day time point, a 48% reduction in tumor volume was observed. Tumors removed 2 weeks after the start of treatment were assessed histologically by staining with hematoxylin/eosin and were found to display gross breakdown of tumor tissue architecture and indications of cell death throughout the tumor in all histological sections analyzed, in contrast to control tumors (Figure 1A). These data show that MC1 tumors respond to CPA monotherapy, which leads to a substantial disruption of tumor organization and decreased tumor volume.
Figure 1.
Characterization of tumor response to CPA. Hematoxylin/eosin staining of a section from a control tumor (A) and a CPA-treated tumor (B). (C) Progression of tumors treated at days 0 to 6 with CPA, relative to tumor volume. Control tumors were of similar size to treated tumors at the initiation of treatment. *P < .05, compared with day 0, t test, n = 4.
CPA Treatment Causes a Loss of Cells Bearing CSC Phenotypic Markers
Breast CSCs were originally defined as being in a population that are CD44+ CD24- lin- [4], whereas more recently, breast cancer cells with ALDH1 activity were reported to be enriched for CSCs [5]. We used flow cytometry to measure the proportion of cells displaying these phenotypes in untreated or CPA-treated xenografts as an indication of the effect of CPA on CSC.
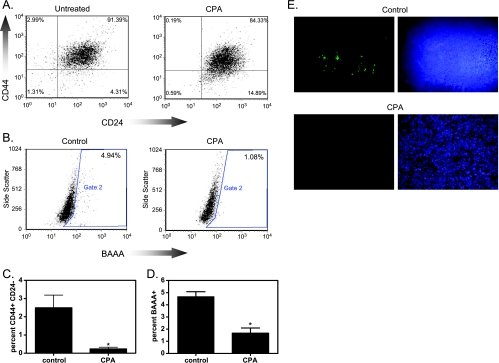
CPA treatment caused a 91% loss of CD44+ CD24- lin- cells in CPA-treated xenografts compared with controls (Figure 2, A and C). In controls, 2.5 ± 0.2% of cells were CD44+ CD24- lin-, whereas xenografts from CPA-treated mice contained 0.24 ± 0.09% CD44+ CD24- lin- cells. Further analysis of ALDH1 activity as a CSC marker was performed using the ALDEFLUOR Kit. There was a 64% decrease in BAAA+ cells in CPA-treated xenografts compared with controls (Figure 2, B and D). ALDH1 activity was detected in 4.7 ± 0.4% of cells from untreated xenografts but in only 1.7 ± 0.4% of cells from CPA-treated xenografts.
Figure 2.
Effect of CPA on CSC markers. (A) Flow cytometric detection of CD44+ CD24- lin- cells in control and treated xenografts. (B) Flow cytometric detection of ALDH activity in control and treated xenografts. BAAA is the fluorescent product indicating ALDH activity. (C) Compiled data of CD44+ CD24- flow cytometry. (D) Compiled data of ALDEFLUOR flow cytometry. *P < .05 compared with control, t test, n = 6–12. (E) Control and treated tumor sections were stained for ALDH1. Green indicates ALDH1 staining; blue, nuclear DAPI staining.
We further investigated ALDH1-expressing cells in xenografts using immunofluorescence. In controls, ALDH1+ cells were distributed throughout the xenograft (Figure 2E). Two weeks after CPA treatment, xenografts showed substantially reduced numbers of ALDH1 immunofluorescent cells, in agreement with the flow cytometry data.
Thus, CPA treatment of MC1 breast xenografts results in a loss of cells bearing CSC markers or in a loss in the markers themselves. This is consistent with the conclusion that MC1 breast CSCs are sensitive to CPA compared with the non-CSC population. However, loss of CSC phenotypic markers could also indicate a change in the expression without alteration of stem cell activity.
CPA-Treated Xenografts Lose Functional CSC Activity
A functional stem cell assay was performed to determine whether changes in phenotypic markers as measured by flow cytometry and immunofluorescence represent actual changes in CSC activity. This was done by harvesting control and CPA-treated xenografts and injecting 105 or 3 x 104 cells into the mammary fat pad of secondary mice. The time required to produce a tumor was recorded. If ablation of CSC activity occurred after CPA treatment of primary mice, then the time required to produce a tumor should be delayed, relative to controls, and the proportion of animals generating tumors should be reduced. If CPA treatment resulted in the enrichment of CSC, then the time to tumor formation should be accelerated compared with controls, and a greater or equal proportion of animals should produce tumors.
Injection of cells from control tumors produced tumors in secondary mice with 100% efficiency (10/10). The median time to tumor formation was determined to be 31 and 38 days for injection of 105 and 3 x 104 cells, respectively (Figure 3A). In contrast, when cells from CPA-treated tumors were injected into secondary mice, only one of six formed a tumor at the 105 cell dose at 56 days, and none of five formed a tumor at the 3 x 104 dose, when followed for up to 100 days. Thus, CSC activity was reduced in CPA-treated tumors compared with controls (P < .05), consistent with loss of the marker phenotype by flow cytometry and immunofluorescence, and supporting the hypothesis that MC1 CSCs are sensitive to CPA.
Figure 3.
Functional assay for CSC activity. Cells from control and CPA-treated tumors were injected into the mammary fat pad of mice at the indicated cell quantities, and the number of days required for tumor formation was recorded. No tumors formed after injection of 3 x 104 cells from CPA-treated tumors after 100 days of observation.
Discussion
Tumor resistance to therapy has recently been attributed to intrinsic resistance of CSC [3]. It has been proposed that the reduction in tumor size during chemotherapy is due to killing of more sensitive tumor cells without CSC activity, whereas the CSCs are spared and drive regrowth of the tumor. We therefore tested the hypothesis that breast CSCs are resistant to CPA and performed in vivo functional analysis to determine CSC activity. In contrast to a previous report that beast CSCs are resistant to neoadjuvant therapy with docetaxel or doxorubicin and CPA [3], we find a case in which breast CSCs are sensitive to CPA alone. The data show a reduction in the proportion of cells bearing the breast CSC phenotype using two distinct definitions [4,5]. CD44+ CD24- lin- cells are reduced as well as BAAA+ cells in the ALDEFLUOR enzymatic assay. In addition, ALDH1 immunofluorescence of sections from treated tumors show reduced ALDH1+ cells, in agreement with the ALDEFLUOR assay results. Importantly, we show reduced functional stem cell activity in CPA-treated xenografts.
A functional assay is important for stem cell studies because phenotypic markers define a population that is enriched for stem cells but that also contains non-stem cells. This was demonstrated by the study of Ginestier et al. [5], in which the overlap of breast tumor CD44+ CD24- cells and BAAA+ cells (containing ALDH activity) was found to be more enriched for functional stem cell activity than either population alone. In addition, the BAAA- component of CD44+ CD24- cells had little to no functional stem cell activity. Hence, a simple analysis of marker-positive populations by flow cytometry is not sufficiently robust to prove that an intervention affected CSC.
One weakness of the current study is that it is limited to one tumor type. However, it adequately proves the hypothesis that all breast CSCs are resistant to therapy is false. Given the cases of glioblastoma displaying sensitivity to specific therapies [6,7], it is likely that more CSCs will be found to be sensitive. Li et al. [3] showed that breast CSCs have the general property of chemotherapy resistance at the population level, but it is not known if some patient tumors in their sample behaved like this one but are obscured by most resistant CSC samples.
The mechanism responsible for CSC sensitivity in this sample is currently an area of investigation. ALDH expression can detoxify CPA active metabolites and has been thought to mediate stem cell resistance to this agent [8]. Whereas theoretically true, much direct data demonstrating this are in the context of ALDH gene transfer, leading to overexpression [9,10]. Endogenous levels of ALDH may not be sufficient to confer resistance at the cellular level, as has been demonstrated by Levi et al. [11]. In hepatocellular CSCs, CPA treatment resulted in the enrichment of CSCs [12]. The liver is a major site for CPA detoxification, which may explain this observation [11]. In our study, endogenous levels of ALDH do not seem to be sufficient to confer CPA resistance in CSCs at the clinically relevant dose given. Therefore, other mechanisms must be responsible for conferring sensitivity, possibly related to the DNA damage response associated with CPA.
The hypothesis that all CSCs are resistant to chemotherapy may be an oversimplification that fails to take into account potentially important genetic and epigenetic differences between human patients, which may contribute to individual tumor response. We demonstrate here a case in which a patient-derived tumor exhibits sensitivity to CPA. Thus, the assumption that breast CSCs are globally resistant to CPA is false, and further investigation of the extent and frequency of this phenomenon is an important extension of this work. It will be important to take individual responses into account when designing clinical trials targeting CSCs, just as with any other targeted therapy.
Acknowledgments
The authors thank Max Wicha, Gabriela Dontu, and Christoph Ginestier for cells, helpful discussion, and expertise.
Footnotes
This work was supported by the Elsa U. Pardee Foundation (S.P.Z.) and a LUNGevity Foundation-American Cancer Society fellowship (S.P.Z.).
References
- 1.Ishii H, Iwatsuki M, Ieta K, Ohta D, Haraguchi N, Mimori K, Mori M. Cancer stem cells and chemoradiation resistance. Cancer Sci. 2008;99:1871–1877. doi: 10.1111/j.1349-7006.2008.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dontu G, Liu S, Wicha MS. Stem cells in mammary development and carcinogenesis: implications for prevention and treatment. Stem Cell Rev. 2005;1:207–213. doi: 10.1385/SCR:1:3:207. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beier D, Rohrl S, Pillai DR, Schwarz S, Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U, Trampe-Kieslich A, et al. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 2008;68:5706–5715. doi: 10.1158/0008-5472.CAN-07-6878. [DOI] [PubMed] [Google Scholar]
- 7.McCord AM, Jamal M, Williams ES, Camphausen K, Tofilon PJ. CD133+ glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin Cancer Res. 2009;15:5145–5153. doi: 10.1158/1078-0432.CCR-09-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russo JE, Hilton J. Characterization of cytosolic aldehyde dehydrogenase from cyclophosphamide resistant L1210 cells. Cancer Res. 1988;48:2963–2968. [PubMed] [Google Scholar]
- 9.Takebe N, Zhao SC, Adhikari D, Mineishi S, Sadelain M, Hilton J, Colvin M, Banerjee D, Bertino JR. Generation of dual resistance to 4-hydroperoxycyclophosphamide and methotrexate by retroviral transfer of the human aldehyde dehydrogenase class 1 gene and a mutated dihydrofolate reductase gene. Mol Ther. 2001;3:88–96. doi: 10.1006/mthe.2000.0236. [DOI] [PubMed] [Google Scholar]
- 10.Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levi BP, Yilmaz OH, Duester G, Morrison SJ. Aldehyde dehydrogenase 1a1 is dispensable for stem cell function in the mouse hematopoietic and nervous systems. Blood. 2009;113:1670–1680. doi: 10.1182/blood-2008-05-156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan S, Chen JS, Sun LJ, Yao HR. Selective enrichment of hepatocellular cancer stem cells by chemotherapy. J Int Med Res. 2009;37:1046–1056. doi: 10.1177/147323000903700409. [DOI] [PubMed] [Google Scholar]