Abstract
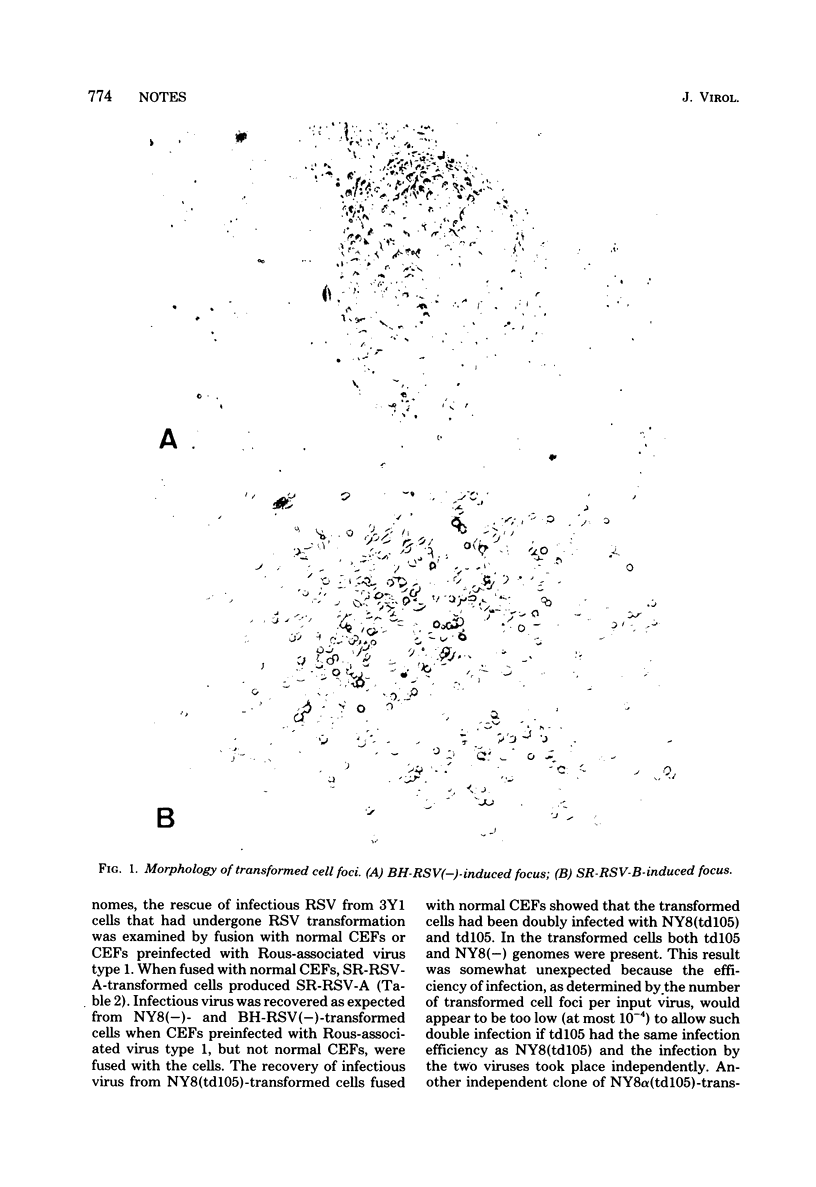
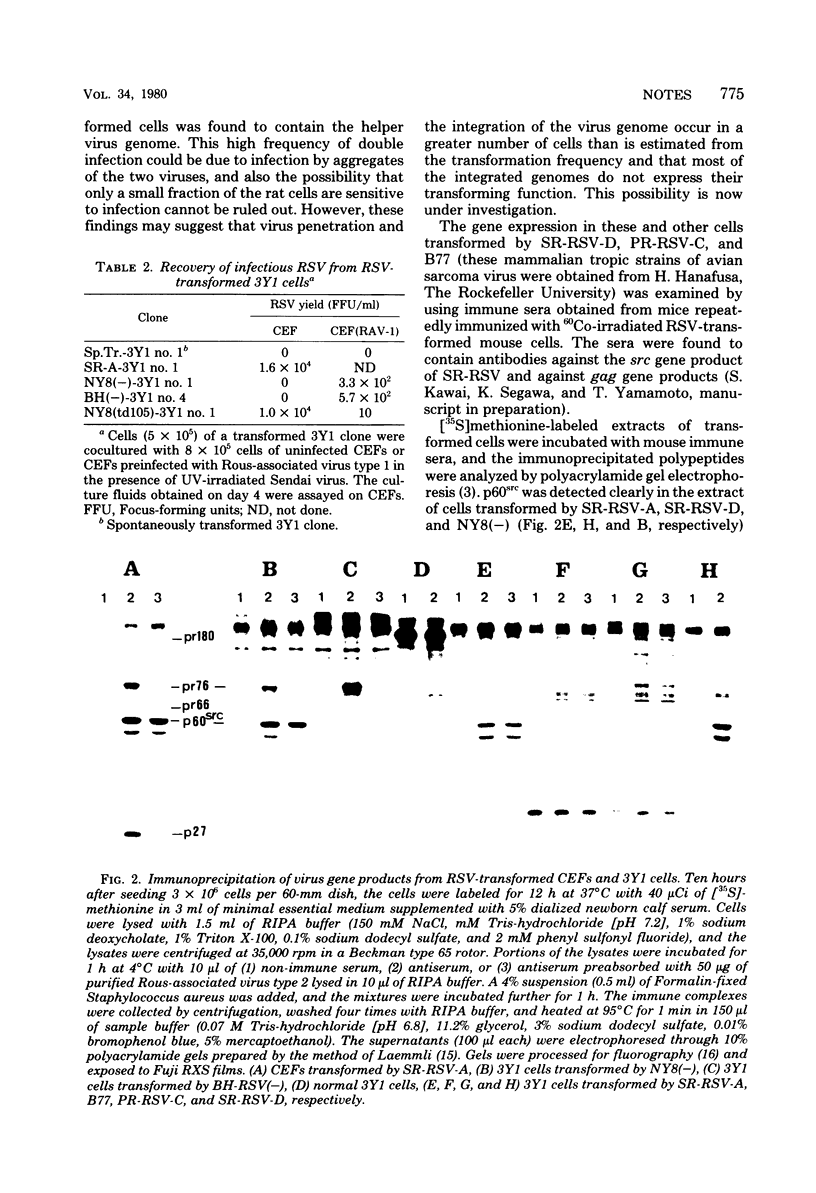
The transformation of a rat cell line, 3Y1, by nonmammalian tropic strains of avian sarcoma virus was tested using cell-virus fusion mediated by Sendai virus or polyethylene glycol. Furthermore, the establishment of several transformed 3Y1 cell clones induced by the Schmidt-Ruppin strain of Rous sarcoma virus (RSV), its derivative mutants, and the Bryan high-titer strain of RSV is reported. The presence and expression of the viral genomes in these cells were examined, and all transformed cell clones tested were found to contain rescuable RSV genomes when they had been fused with normal chicken embryo fibroblast cells or those preinfected with Rous-associated virus type 1. However, the gag gene product, pr76, was barely detectable in wild-type RSV-transformed cells, whereas it was produced in considerable amounts in cells transformed by env-deleted mutants, the Bryan high-titer strain of RSV and NY8 derived from the Schmidt-Ruppin strain of RSV.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson E., Erikson R. L. Antibody to virion structural proteins in mammals bearing avian sarcoma virus-induced tumors. Virology. 1978 Feb;84(2):429–433. doi: 10.1016/0042-6822(78)90259-3. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Hayman M. J., Vogt P. K. Properties of mammalian cells transformed by temperature-sensitive mutants of avian sarcoma virus. Cell. 1977 Jul;11(3):513–521. doi: 10.1016/0092-8674(77)90069-1. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Eisenman R., Vogt V. M., Diggelmann H. Synthesis of avian RNA tumor virus structural proteins. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1067–1075. doi: 10.1101/sqb.1974.039.01.122. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hanafusa T. Determining factor in the capacity of Rous sarcoma virus to induce tumors in mammals. Proc Natl Acad Sci U S A. 1966 Mar;55(3):532–538. doi: 10.1073/pnas.55.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Genetic recombination with avian tumor virus. Virology. 1972 Jul;49(1):37–44. doi: 10.1016/s0042-6822(72)80005-9. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Recombination between a temperature-sensitive mutant and a deletion mutant of Rous sarcoma virus. J Virol. 1976 Aug;19(2):389–397. doi: 10.1128/jvi.19.2.389-397.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A., Summers J. Rat cell line 3y1 and its virogenic polyoma- and sv40- transformed derivatives. Int J Cancer. 1975 Apr 15;15(4):694–706. doi: 10.1002/ijc.2910150419. [DOI] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J. Nature of Rous sarcoma virus-specific RNA in transformed and revertant field vole cells. J Virol. 1979 Feb;29(2):507–515. doi: 10.1128/jvi.29.2.507-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Lau A. F., Faras A. J., Spector D. H. Post-transcriptional control of avian oncornavirus transforming gene sequences in mammalian cells. Nature. 1977 Sep 8;269(5624):175–179. doi: 10.1038/269175a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Murine xenotropic type C viruses. III. Phenotypic mixing with avian leukosis and sarcoma viruses. Virology. 1977 Apr;77(2):811–825. doi: 10.1016/0042-6822(77)90501-3. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genet. 1975 Oct;1(4):397–400. doi: 10.1007/BF01538671. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Isolation of noninfectious particles containing Rous sarcoma virus RNA from the medium of Rous sarcoma virus-transformed nonproducer cells. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1655–1662. doi: 10.1073/pnas.57.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Boettiger D. Complementation rescue of Rous sarcoma virus from transformed mammalian cells by polyethylene glycol-mediated cell fusion. J Virol. 1977 Jul;23(1):133–141. doi: 10.1128/jvi.23.1.133-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Wong A. L. Phenotypic mixing between avian and mammalian RNA tumor viruses: I. Envelope pseudotypes of Rous sarcoma virus. Virology. 1977 Feb;76(2):826–834. doi: 10.1016/0042-6822(77)90262-8. [DOI] [PubMed] [Google Scholar]