Abstract
A series of 1-aryl-5-(3′,4′,5′-trimethoxyphenyl) derivatives and their related 1-(3′,4′,5′-trimethoxyphenyl)-5-aryl-1,2,4-triazoles, designed as cis-restricted combretastatin analogues, were synthesized and evaluated for antiproliferative activity, inhibitory effects on tubulin polymerization, cell cycle effects, and apoptosis induction. Their activity was greater than, or comparable with, that of the reference compound CA-4. Flow cytometry studies showed that HeLa and Jurkat cells treated with the most active compounds 4l and 4o were arrested in the G2/M phase of the cell cycle in a concentration dependent manner. This effect was accompanied by apoptosis of the cells, mitochondrial depolarization, generation of reactive oxygen species, activation of caspase-3, and PARP cleavage. Compound 4l was also shown to have potential antivascular activity, since it induced endothelial cell shape change in vitro and disrupted the sprouting of endothelial cells in the chick aortic ring assay.
Introduction
The microtubule system of eukaryotic cells is a critical element in a variety of fundamental cellular processes, such as cell division, formation and maintenance of cell shape, regulation of motility, cell signaling, secretion, and intracellular transport.1 Inhibition of microtubule function using tubulin targeting agents, many of which are natural products, is a validated approach for anticancer therapy.2 One of the most important antimitotic agents is combretastatin A-4 (CA-4,a 1; Chart 1). CA-4, isolated from the bark of the South African tree Combretum caffrum,3 is one of the well-known natural tubulin-binding molecules affecting microtu-bule dynamics by binding to the colchicine site.4 CA-4 shows potent cytotoxicity against a wide variety of human cancer cell lines, including those that are multidrug resistant.5 A water-soluble disodium phosphate derivative of CA-4 (named CA-4P) has shown promising results in human cancer clinical trials,6 thus stimulating significant interest in a variety of CA-4 analogues.7
Chart 1.
Inhibitors and Potential Inhibitors of Tubulin Polymerization
It has been established by previous SAR studies that both the 3′,4′,5′-trimethoxy substitution pattern on the A-ring and the cis-olefin configuration at the bridge were essential for optimal activity, while B-ring structural modifications were tolerated by the target.7 However, the cis configuration of CA-4 is prone to isomerize to the thermodynamically more stable trans form during storage and administration, producing a dramatic reduction in both antitubulin and antiproliferative activities. Thus, to retain the cis-olefin configuration of CA-4 required for bioactivity, several groups have reported that the isomerization from cis- to trans-olefin can be avoided by incorporating the double bond in five-member aromatic heterocyclic rings, such as pyrazole,8 imidazole,9 thiazole,8 isoxazole,10 1,2,3-thiadiazole,11 isomeric triazoles,8,12,13 and 1,2,3,4-tetrazole.8
Welsh and co-workers reported a series of 3,4-diaryl-1,2,4-triazoles (compounds 2a–c) with antiproliferative activity, but this was 10-fold reduced relative to the activity of CA-4. Nevertheless, these compounds had activity similar to that of CA-4 as inhibitors of tubulin polymerization.13
In this article we reconfigured the substitution pattern around the triazole ring by the preparation of two different regioisomeric series of 1,5-diaryl-substituted 1,2,4-triazole derivatives with general structures 3 and 4. In these two series of designed analogues, obtained by interchanging the substitution pattern of rings A and B, we fixed one of the aryl groups as a 3′,4′,5′-trimethoxyphenyl moiety, identical with the A-ring of CA-4, and examined several substitutions with electron-withdrawing (F, Cl, and NO2) or electron-releasing (Me, MeO, and EtO) groups (EWG and ERG, respectively) on the other aryl moiety, corresponding to the B-ring of CA-4.
To compare the effect of para-, meta-, and ortho-substitution on compounds with general formula 4, the methoxy and ethoxy groups were introduced at different positions of the B-phenyl ring to furnish derivatives 4h–j and 4l–n, respectively.
Chemistry
The target 1,5-diaryl-1,2,4-triazoles 3a–k and 4a–r were synthesized as outlined in Scheme 1. 1-Aryl-1,2,4-triazoles 6a–i were prepared by the condensation of the substituted arylhydrazine hydrochlorides 5a–i in formamide at 120 °C. The 1-(3′,4′,5′-trimethoxyphenyl)-1,2,4-triazole 8 was obtained by an efficient N-arylation procedure of 1,2,4-triazole with 1-bromo-3,4,5-trimethoxybenzene catalyzed by CuI and CsCO3.14 The intermediates 6a–i and 8 were chemoselectively brominated at the 5-position of the 1,2,4-triazole ring by NBS in refluxing CCl4 to yield derivatives 7a–i and 9, respectively. The Suzuki cross-coupling reaction with the appropriate arylboronic acid under heterogeneous conditions [Pd(PPh3)4, K2CO3] in refluxing toluene furnished the 1-aryl-5-(3′,4′,5′-trimethoxyphenyl)- and 1-(3′,4′,5′-trimethoxyphenyl)-5-aryl-1,2,4-triazoles 3a–i and 4a–r, respectively. Starting from 3h,i, the nitro group was reduced with hydrogen in the presence of 10% Pd/C in DMF, affording amino derivatives 3j,k, respectively.
Scheme 1.
a Reagents and conditions: (a) HCONH2, 120 °C, 18 h; (b, e) NBS, benzoylperoxide (cat.), CCl4, reflux; (c, f) Pd(PPh3)4,K2CO3, PhMe, reflux, 18 h; (d) 1-bromo-3,4,5-trimethoxybenzene, CsCO3, CuI, DMF, 120 °C, 18 h; (g) H2, 10% Pd/C, DMF.
The chemoselective bromination at the 5-position of the 1,2,4-triazole ring was unambiguously attributed by 1H–1H NOE experiments on the representative compound 8. These experiments permit assignment of the signals at 8.489 and 8.085 to the H-5 and H-3 protons, respectively, of the 1,2,4-triazole nucleus. A positive NOE correlation was observed between the signals at 8.489 (H-5) and 6.876 ppm, in which this latter signal corresponds to the aromatic orthoprotons of the 3′,4′,5′-trimethoxyphenyl moiety, and at the same time signals at 8.489 and 6.876 ppm did not show any NOE with the signal at 8.085 ppm.
Results and Discussion
In Vitro Antiproliferative Activities
The series of 1-aryl-5-(3′,4′,5′-trimethoxyphenyl) and their related 1-(3′,4′,5′-trimethoxyphenyl)-5-aryl-1,2,4-triazoles, corresponding to compounds 3a–k and 4a–r, respectively, were evaluated for their antiproliferative activity against a panel of six different human tumor cell lines and compared with the reference compound CA-4 (1). Data for inactive compounds (IC50 > 10 µM) are not shown in Table 1. Two of the synthesized compounds, 4l and 4o, had the best antiproliferative activities against these cell lines and, overall, were as active as CA-4. In particular, the 3′-chloro-4′-ethoxyphenyl derivative 4o exhibited IC50 values ranging from 3 to 20 nM against the cell lines, compared with the range of 4–370 nM obtained with CA-4. It was less active than CA-4 only against the K-562 cells.
Table 1.
In Vitro Cell Growth Inhibitory Effects of Compounds 3g, 4g,h,k,l,o,p, and CA-4 (1)
| compd | IC50a(nM) |
|||||
|---|---|---|---|---|---|---|
| HeLa | A549 | HL-60 | Jurkat | K562 | MCF-7 | |
| 3g | >10000 | >10000 | 6200 ± 1200 | 93 ± 30 | 3600 ± 900 | 7400 ± 1200 |
| 4g | 250 ± 50 | 1100 ± 100 | 90 ± 6 | 300 ± 80 | > 10000 | 540 ± 20 |
| 4h | 280 ± 60 | 520 ± 90 | 120 ± 10 | 50 ± 10 | 950 ± 40 | 360 ± 50 |
| 4k | 150 ± 20 | 800 ± 50 | 500 ± 20 | 50 ± 10 | 340 ± 50 | 390 ± 90 |
| 4l | 15 ± 4 | 100 ± 20 | 20 ± 3 | 5 ± 0.2 | 20 ± 8 | 50 ± 9 |
| 4o | 6 ± 2 | 10 ± 5 | 3 ± 0.2 | 3 ± 0.6 | 20 ± 10 | 17 ± 1 |
| 4p | 600 ± 20 | >10000 | 700 ± 200 | 650 ± 60 | 800 ± 100 | 610 ± 10 |
| CA-4 | 4 ± 1 | 180 ± 50 | 1 ± 0.2 | 5 ± 0.6 | 5 ± 0.1 | 370 ± 100 |
IC50: compound concentration required to inhibit tumor cell proliferation by 50%. Data are expressed as the mean ± SE from the dose–response curves of at least three independent experiments.
Apparently, the relative positions of the two aromatic rings on the 1,2,4-triazole moiety did not seem to be critical for antiproliferative activity. With the exception of the weak activity observed with the p-methoxyphenyl derivative 3g, all the 1-aryl-1,2,4-triazole derivatives 3a–k were inactive (IC50 > 10 µM). Switching the position of the two aryls on the 1,2,4-triazole ring (3a vs 4a, 3b vs 4b, 3c vs 4c, 3d vs 4d, 3f vs 4f, and 3i vs 4r) did not yield active compounds except that there was a considerable difference in potency observed between the regioisomeric 4′-methoxyphenyl derivatives 3g and 4h (the latter was considerably more active than the former in four out of the six cell lines).
In the series of 1-(3′,4′,5′-trimethoxyphenyl)-1,2,4-triazole analogues 4h–j and 4l–n, the position of methoxy or ethoxy substituent on the 5-phenyl ring had a profound influence on antiproliferative activity. Starting from compound 4h, moving the methoxy group from the para- to the meta- and ortho-positions (compounds 4i and 4j, respectively) led to a dramatic drop of potency. The same effect was observed for the ethoxy substituent (4l vs 4m and 4n). The p-ethoxy derivative 4l was 4- to 40-fold more potent than its methoxy counterpart 4h. The enhanced effect on activity resulting from replacement of the methoxy group with an ethoxy moiety in colchicine site compounds was previously observed by us and by others.15
Replacement of the p-methoxy group with a weak electron-releasing thiomethyl group resulted in derivative 4k, which overall had activity similar to that of 4h against Jurkat and MCF-7 cells. Similarly, except with the K562 cells, replacing the 4′-methoxy of 4h with a 4′-methyl group (4g) overall had only minor effects on antiproliferative activities.
Since the 4′-ethoxy group of 4l was favorable for potency, it is important to point out that introduction ofan additional EWG chlorine group at the 3′-position of 4′-ethoxyphenyl ring, resulting in compound 4o, produced a 2- to 15-fold increase in antiproliferative activity against four of the six cell lines, while 4l and 4o were equipotent against the Jurkat and K562 cells.
Finally, among the antiproliferative compounds, replacing the 4′-ethoxy group of 4l with the bulky isopropoxy moiety (4p) caused a sharp drop in antiproliferative activity in all cell lines, suggesting that an increase in steric bulk at this position causes a decrease in potency.
Inhibition of Tubulin Polymerization and Colchicine Binding
To investigate whether the antiproliferative activities of compounds 3g, 4g,h, 4k,l, and 4o,p derived from an interaction with tubulin, they were evaluated for their inhibition of tubulin polymerization and for effects on the binding of [3H]colchicine to tubulin (Table 2).16 For comparison, CA-4 was examined in contemporaneous experiments. In the assembly assay, compound 4l was found to be the most active (IC50 = 0.76 µM), and it was almost twice as potent as CA-4 (IC50 = 1.2 µM). While 4l was generally less potent than 4o as an antiproliferative agent, 4l was about twice as active as 4o as an inhibitor of tubulin assembly. Derivative 4o was also slightly less active than CA-4 as an inhibitor of tubulin assembly. Compound 4h was the next most active agent as an inhibitor of tubulin assembly (about half as potent as CA-4). The remaining compounds with lower antiproliferative effects on cancer cells were still less active as inhibitors of tubulin assembly. For these seven compounds and CA-4, the order of activity was 4l > CA-4 > 4o > 4h > 4k > 4g > 4p ≫ 3g.
Table 2.
Inhibition of Tubulin Polymerization and Colchicine Binding by Compounds 3g, 4g,h,k,l,o,p, and CA-4
| compd | tubulin assembly,a IC50 ± SD (µM) |
colchicine binding,b % ± SD |
|---|---|---|
| 3g | 16 ± 1 | nd |
| 4g | 3.9 ± 0.4 | 33 ± 6 |
| 4h | 2.3 ± 0.0 | 55 ± 1 |
| 4k | 3.6 ± 0.1 | 38 ± 3 |
| 4l | 0.76 ± 0.1 | 86 ± 2 |
| 4o | 1.5 ± 0.2 | 75 ± 0.1 |
| 4p | 5.1 ± 0.8 | 37 ± 3 |
| CA-4 (1) | 1.2 ± 0.1 | 87 ± 3 |
Inhibition of tubulin polymerization. Tubulin was at 10 µM.
Inhibition of [3H]colchicine binding. Tubulin, colchicine, and tested compound were at 1, 5, and 1 µM, respectively. nd: not determined.
In the colchicine binding studies, derivative 4l was as potent as CA-4, which in these experiments inhibited colchicine binding by 87%, while 4o was slightly less potent (75% inhibition). The potent inhibition observed with the two compounds indicates that 4l and 4o bind to tubulin at a site overlapping the colchicine site. Inhibition of colchicine binding by compounds 4g,h, 4k, and 4p fell into the 33–55% range. In this series of seven compounds, inhibition of [3H]colchicine binding correlated more closely with inhibition of tubulin assembly than with antiproliferative activity. In conclusion, we note that CA-4 is one of the most potent colchicine compounds yet described. It is thus significant that two agents in the present series have activities comparable to that CA-4 as inhibitors of tubulin assembly and, less frequently observed, as inhibitors of colchicine binding to tubulin.
Analysis of Cell Cycle
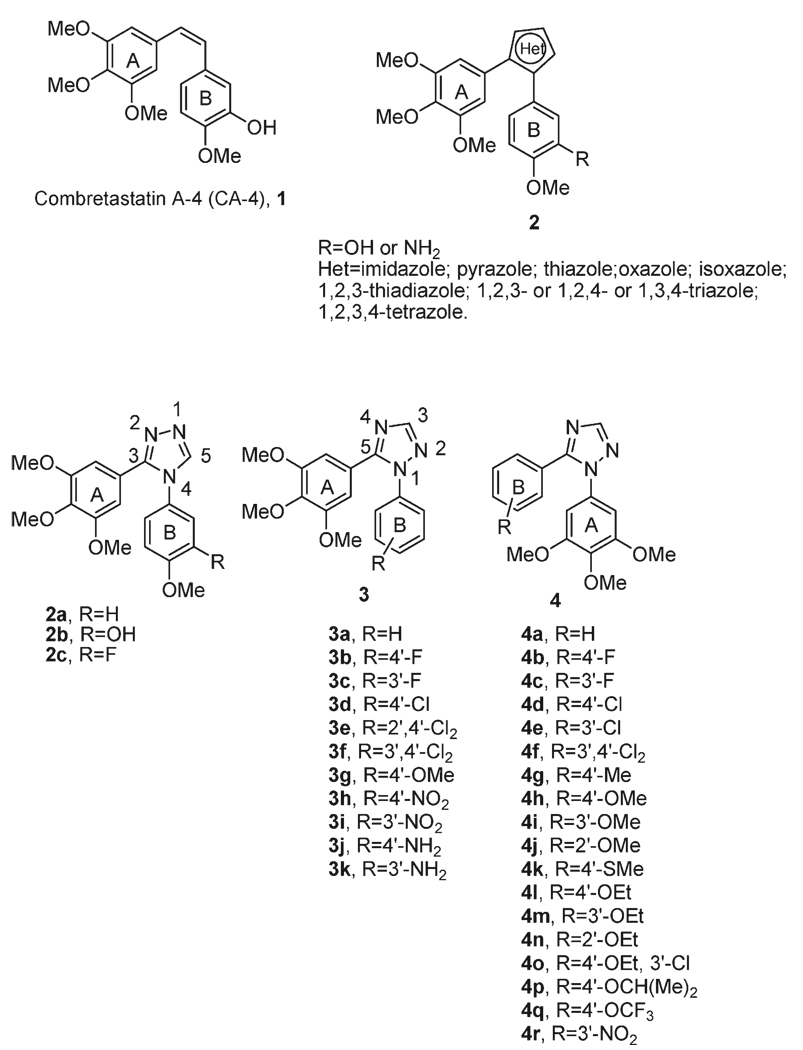
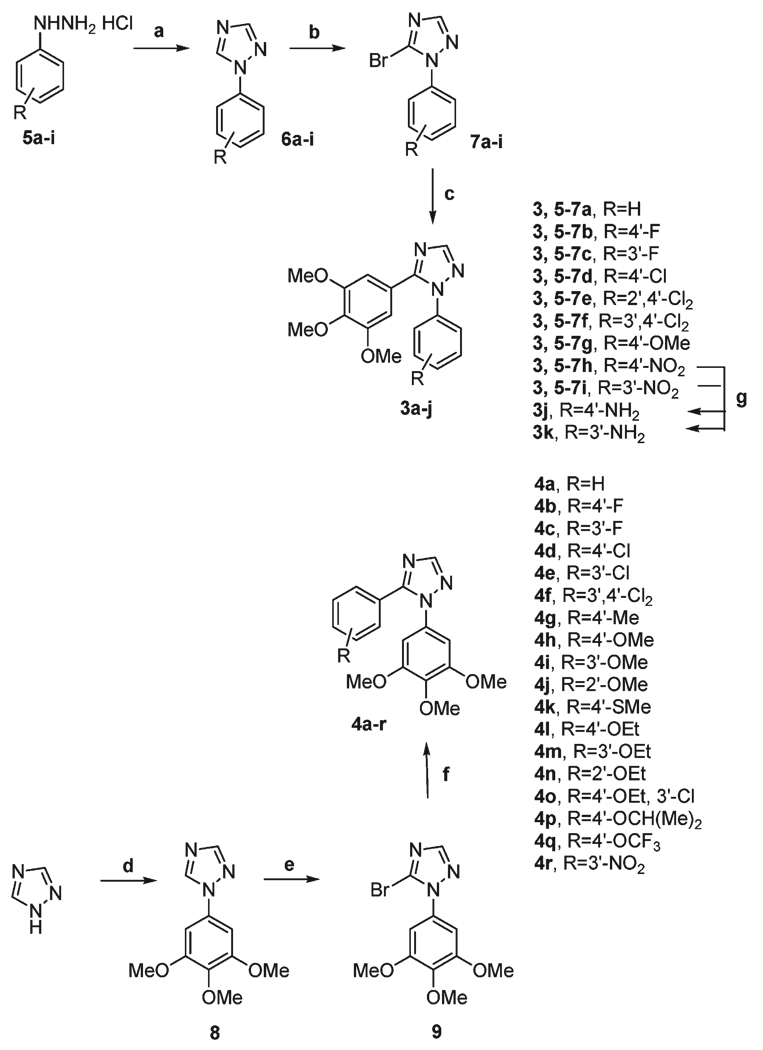
The effects of different concentrations of compounds 4l and 4o on cell cycle progression were examined with HeLa and Jurkat cells (Figure 1). Untreated HeLa cells showed a classical pattern of proliferating cells distributed in the G1 (55.2%), S (32.4%), and G2/M (12.4%) phases. Both 4l and 4o caused a clear G2/M arrest pattern in a concentration-dependent manner, with a concomitant decrease of cells in other phases of the cell cycle (Figure 1). In particular, as shown in Figure 2 (upper panels), the G2/M cell population increased from 12% in the control to over 70% with 30 nM compounds 4l and 4o at 48 h.
Figure 1.
Effect of 4l and 4o induced G2/M phase arrest in HeLa (upper panels) and Jurkat cells (lower panels). Cells were treated with different concentrations ranging from 7 to 250 nM for 24 h. Then the cells were fixed and stained with PI to analyze DNA content by flow cytometry. Data are presented as the mean ± SEM of three independent experiments.
Figure 2.
Effect of 4l and 4o induced G2/M phase arrest in HeLa (upper panels) and Jurkat cells (lower panels). Cells were treated with a concentration of 30 nM for 24 and 48 h. Then the cells were fixed and stained with PI to analyze DNA content by flow cytometry. Data are presented as the mean ± SEM of three independent experiments.
In the leukemia T-cell Jurkat line, both compounds also induced a significant block in the G2/M phase. With both cell lines, the accumulation of G2/M cells increased to varying extents in a time-dependent manner (Figure 2). In addition, treatment of cells with 4l or 4o caused the appearance of a hypodiploid peak (sub-G1) indicative of apoptosis (data not shown).
Loss of Plasma Membrane Asymmetry during Apoptosis
To better characterize drug-induced apoptosis, we performed a biparametric cytofluorimetric analysis using propidium iodide (PI) and annexin-V-FITC, which stain DNA and phosphatidylserine (PS) residues, respectively.17 Annexin-V is a Ca2+-dependent phospholipid binding protein with high affinity for PS. Annexin-V staining precedes the loss of membrane integrity that accompanies the final stages of cell death resulting from either apoptotic or necrotic processes. Because the externalization of PS occurs in the earlier stages of apoptosis, annexin-V staining identifies apoptosis at an earlier stage than the appearance of sub-G1 cells. These cells appear at a later stage of cell death and indicate the occurrence of nuclear changes such as DNA fragmentation.
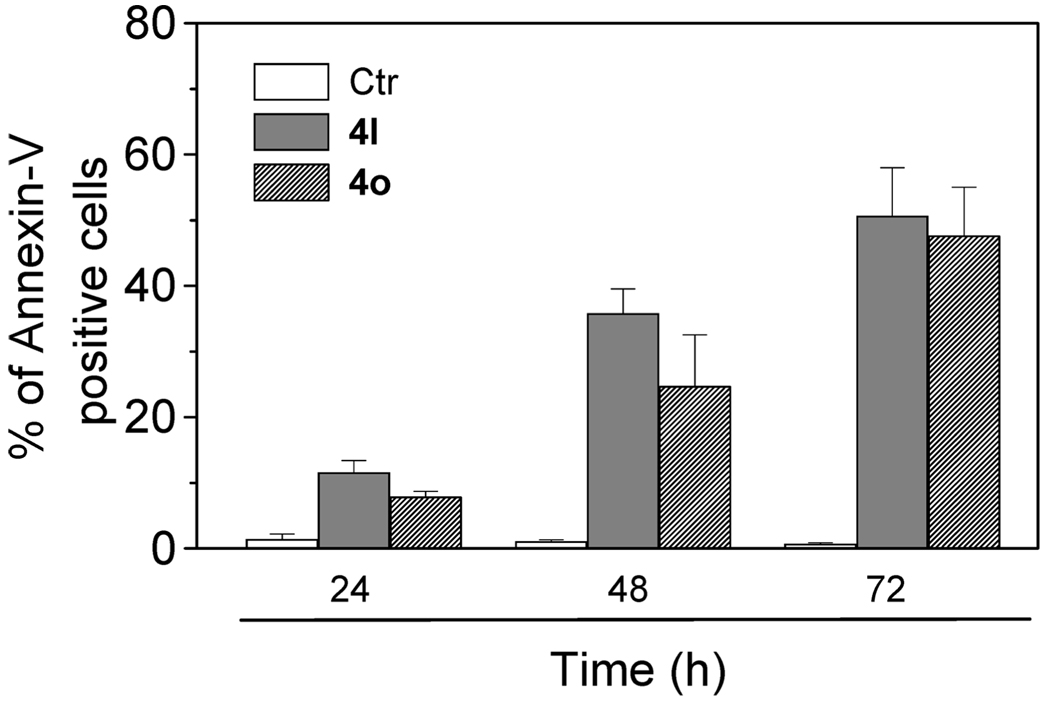
After treatment with 4l and 4o at 50 nM for different times, Jurkat cells were labeled with the two dyes, and the resulting red (PI) and green (FITC) fluorescence was monitored by flow cytometry. It can be observed from Figure 3 that 4l and 4o provoked a significant induction of apoptotic cells after 24 h of treatment. The percentage of annexin-V positive cells then further increased at 48 and 72 h. These findings prompted us to further investigate the apoptotic process after treatment with the two compounds.
Figure 3.
Flow cytometric analysis of apoptotic cells after treatment of Jurkat cells with 4l and 4o. After different times of treatment, cells were harvested and labeled with annexin-V-FITC and PI and then analyzed by flow cytometry. The data are expressed as the mean of percentage of annexin-V positive cells ± SEM for four independent experiments.
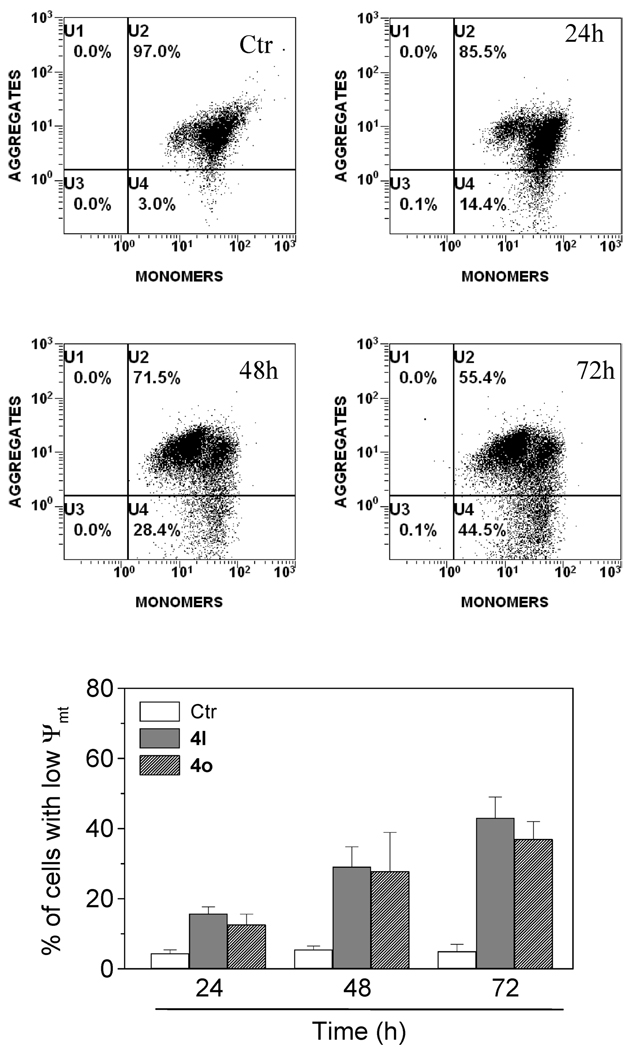
Induction of Mitochondrial Depolarization
Mitochondria play an essential role in the propagation of apoptosis.18 It is well established that at an early stage apoptotic stimuli alter the mitochondrial transmembrane potential (Δψmt). Δψmt was monitored by the fluorescence of the dye JC-1.19 With normal cells (high Δψmt), JC-1 displays a red fluorescence (590 nm). This is caused by spontaneous and local formation of aggregates that is associated with a large shift in the emission. In contrast, when the mitochondrial membrane is depolarized (low Δψmt), JC-1 forms monomers that emit at 530 nm. Treated Jurkat cells in the presence of derivatives 4l and 4o (50 nM) exhibited a remarkable shift in fluorescence compared with control cells, indicating depolarization of the mitochondrial membrane potential (Figure 4, upper panels). The percentage of cells with low Δψmt following treatment increased in a time-dependent fashion (Figure 4, lower panel). The disruption of Δψmt is associated with the appearance of annexin-V positivity in the treated cells when they are in an early apoptotic stage. In fact, the dissipation of Δψmt is characteristic of apoptosis and has been observed with both microtubule stabilizing and destabilizing agents, including CA-4, in different cell types.20
Figure 4.
Assessment of mitochondrial dysfunction after treatment with compound 4l or 4o. Upper and middle panels show representative histograms of Jurkat cells treated with 50 nM 4l for the indicated times and stained with PI and annexin-V-FITC. Lower panel shows induction of loss of mitochondrial membrane potential after 24, 48, and 72 h of incubation of Jurkat cells with 50 nM 4l or 4o. Cells were stained with the fluorescent probe JC-1 and analyzed by flow cytometry. Data are expressed as the mean ± SEM for three independent experiments.
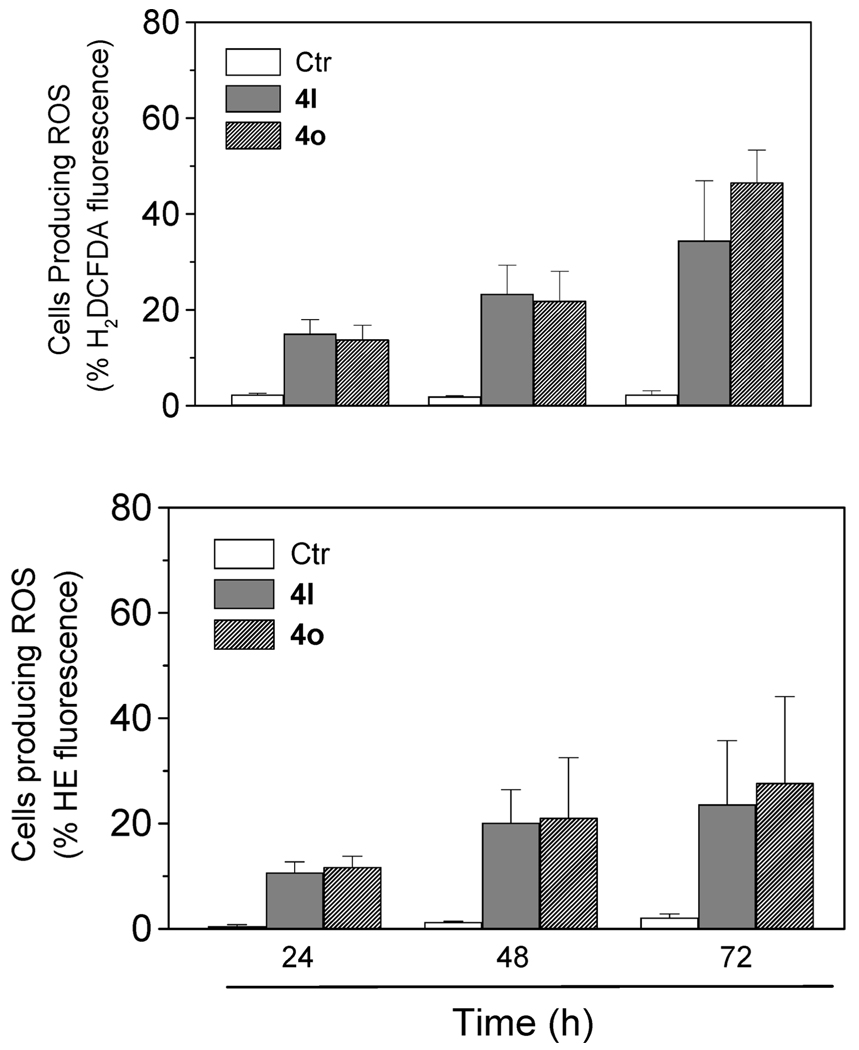
Mitochondrial Generation of Reactive Oxygen Species (ROS)
Mitochondrial membrane depolarization is associated with mitochondrial production of ROS.21 Therefore, we investigated whether ROS production increased after treatment with the test compounds. We utilized the fluorescence indicator hydroethidine (HE), whose fluorescence appears if ROS are generated.22 HE is oxidized by superoxide anion into the ethidium ion, which fluoresces red. Superoxide is produced by mitochondria because of a shift from the normal four-electron reduction of O2 to a one-electron reduction when cytochrome c is released from mitochondria. ROS generation was also measured with the dye 2,7-dichlorodihydrofluorescein diacetate (H2-DCFDA), which is oxidized to the fluorescent compound dichlorofluorescein (DCF) by a variety of peroxides, including hydrogen peroxide.22
The results are presented in Figure 5, where it can be observed that 4l and 4o induced the production of large amounts of ROS in comparison with control cells, which agrees with the previously described dissipation of Δψmt. The amount of ROS produced increased over the entire 72 h treatment time.
Figure 5.
Mitochondrial production of ROS in Jurkat cells. After 24, 48, and 72 h of incubation with 4l or 4o, cells were stained with H2DCFDA (upper panel) or HE (lower panel) and analyzed by flow cytometry. Data are expressed as the mean ± SEM of three independent experiments.
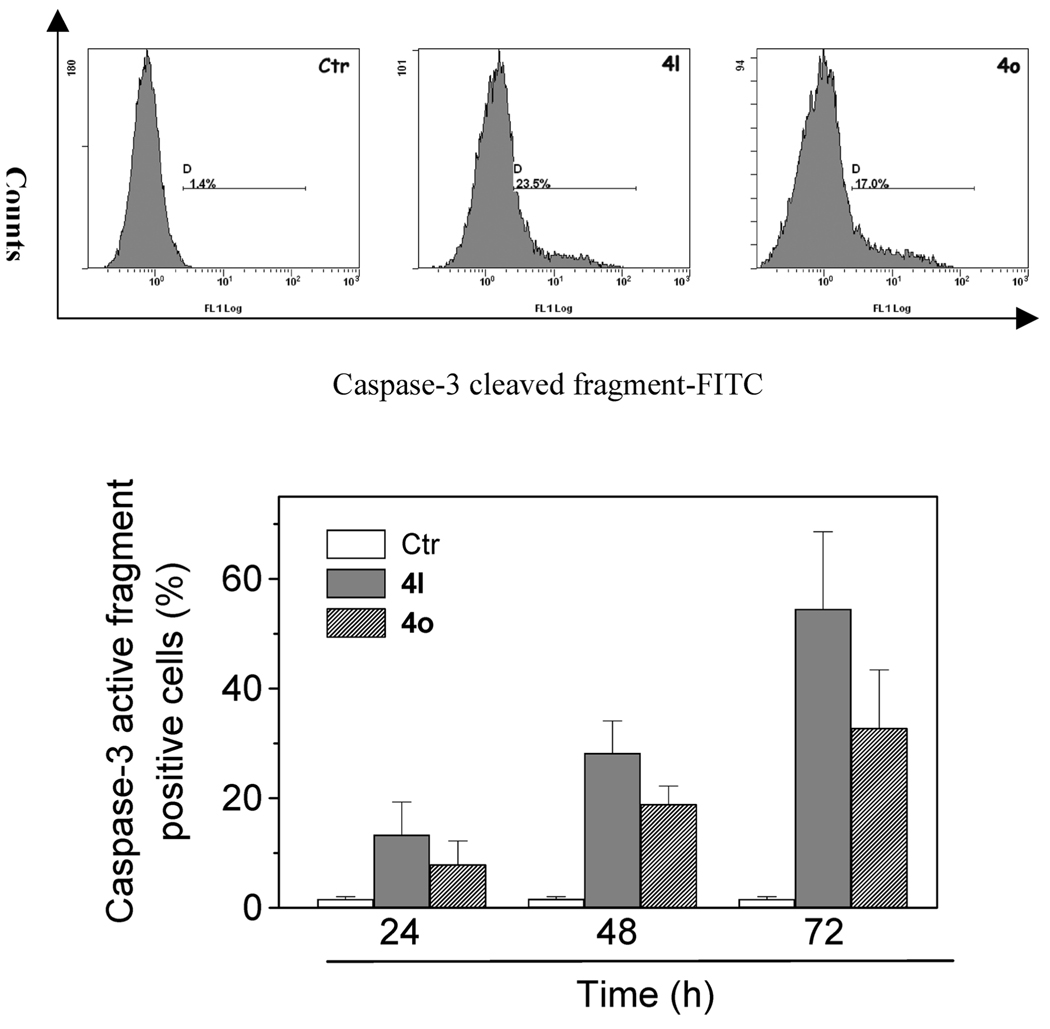
Caspase-3 Activation, Poly ADP-Ribose Polymerase (PARP) Cleavage, and Bcl-2 Down-Regulation
Caspases are the central executioners of apoptosis mediated by various inducers.23 Caspases are synthesized as proenzymes that are activated by cleavage. Caspases-2, -8, -9, and -10 are termed apical caspases and are usually the first to be stimulated in the apoptotic process. Their activation in turn leads to their activation of effector caspases, in particular caspase-3.24 Exposure of Jurkat cells to compound 4l or 4o resulted in the activation of caspase-3 in a time-dependent manner, as shown in Figure 6.
Figure 6.
Caspase-3 induced activity by compound 4l and 4o. Jurkat cells were incubated in the presence of 4l and 4o at 50 nM. After 24, 48, and 72 h of treatment, cells were harvested and stained with an antihuman active caspase-3 fragment monoclonal antibody conjugated with FITC: (upper panels) representative histograms of Jurkat cells incubated in the presence of 50 nM 4l and 4o for 48 h; (lower panel) percentage of caspase-3 active fragment positive cells after 24,48, and 72 h of treatment. Data are expressed as the mean ± SEM of three independent experiments.
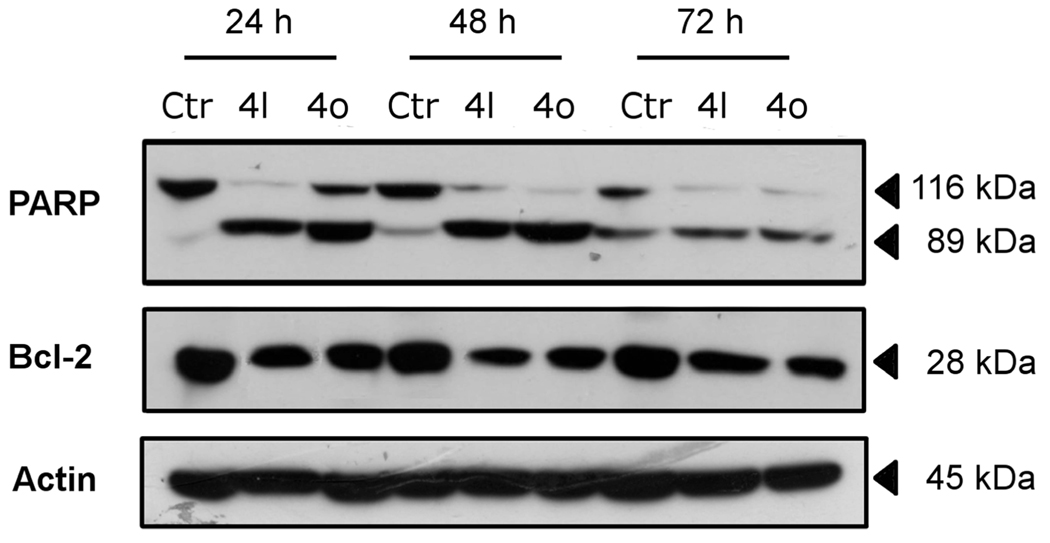
PARP is a 116 kDa nuclear protein that appears to be involved in apoptosis.25 This protein is one of the main cleavage targets of caspase-3 both in vitro and in vivo.25 As shown in Figure 7 (top panel), immunoblot analysis demonstrated extensive formation of the typical 89 kDa fragment of PARP following a 24 h treatment with either 50 nM 4l or 50 nM 4o. There was a further increase, especially with 4o, after treatment for another 24 h, most noticeable through disappearance of uncleaved PARP. Similar results were obtained with CA-4 in HeLa cells.20b
Figure 7.
Western blot analysis for the cleavage of PARP and the expression of Bcl-2 in Jurkat cells. Control lanes (Ctr) refer to untreated cells. In the other lanes the cells were treated with 50 nM 4l or 4o for the indicated times. Whole cell lysates were subjected to SDS–PAGE, followed by blotting with an anti-PARP, anti-Bcl-2, or anti-actin antibody.
Bcl-2 is a protein that has been extensively investigated as a modulating agent of apoptosis and plays a major role as an inhibitor of apoptosis. It does this by regulating the mitochondrial membrane potential, thus avoiding release of cytochrome c and caspase activation.26 Therefore, we examined whether the induction of apoptosis by 4o and 4l is associated with changes in the expression of this protein. As depicted in Figure 7 (middle panel), immunoblot analysis showed that treatment with either compound resulted in decreased expression of Bcl-2. Altogether these data indicate that the induction of apoptosis by these new derivatives is associated with Bcl-2 down-regulation and caspase-3 activation that in turn stimulated PARP cleavage.
Antivascular Activity
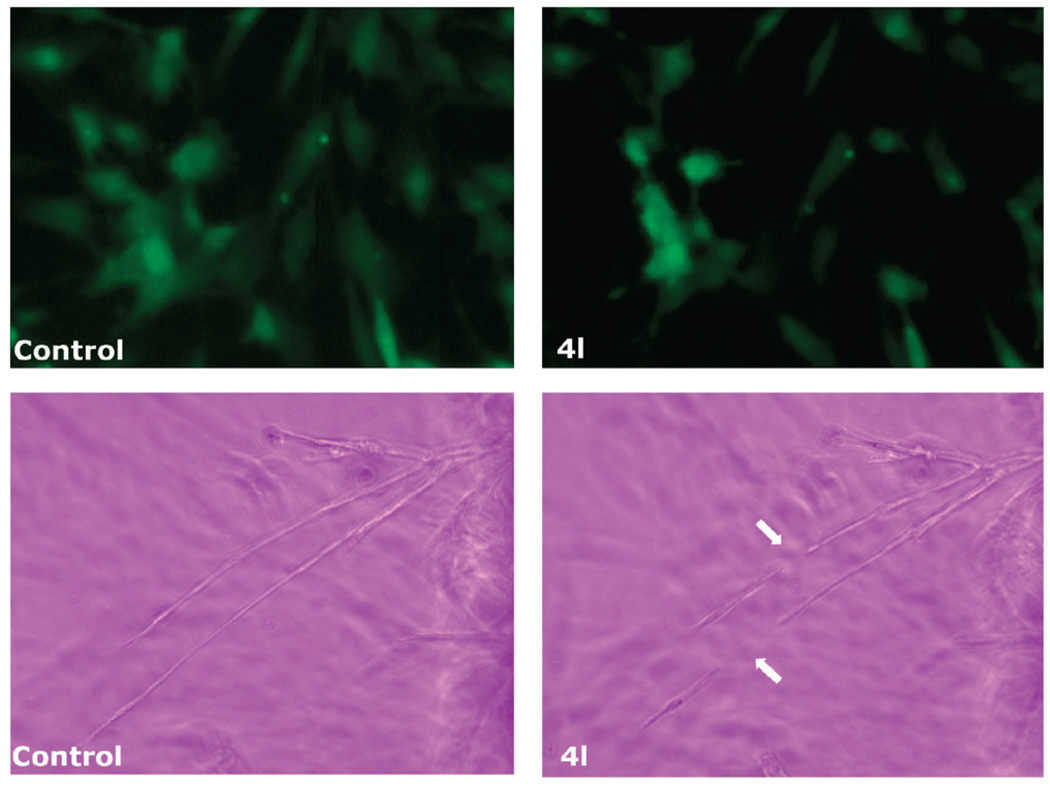
CA-4 and its analogues in clinical development have been shown to quickly and selectively shut down the blood flow of tumors.6a,b This family of drugs is therefore called antivascular or vascular disrupting agents. The effect is thought to be mediated by inducing endothelial cell shape change, possibly through disrupting microtubule dynamics.27 We tested the derivative 4l for its ability to induce rapid endothelial cell shape changes using a human umbilical vein endothelial cell (HUVEC) culture assay and a chick aortic ring assay.28 The HUVEC line expressed green fluorescent protein (GFP). In these model systems, we found that changes occurred rapidly and were extensive 30 min after drug addition, corresponding to the quick in vivo effect of shutting down tumor circulation. Like CA-4, 4l caused spreading HUVECs to retract and form blebs on their membranes at a concentration as low as 0.5 µM, and the effect was prominent at 10 µM. The area of GFP positive HUVECs was reduced to 30% of the area measured before treatment (Figure 8). Compound 4l also disrupted formation of vascular sprouts from chick aortic ring after only a 30 min incubation. These observations suggest that 4l, like CA-4, would most likely cause severe vascular disruption in vitro and it could be considered as a new vascular disrupting agent.
Figure 8.
Effect of compound 4l on endothelial cell shape in cultured HUVECs (top panels) and chick aortic vascular sprouts (lower panels). Cells or aortic arches from 14-day chick embryos were treated with 4l and analyzed by confocal microscopy. The left-hand panels are cells prior to compound addition, and the right-hand panels show cells before and after 30 min of treatment with 10 µM 4l. In the lower panels the arrows indicate the disruption of the aortic vascular sprout induced by drug treatment.
Molecular Modeling Studies
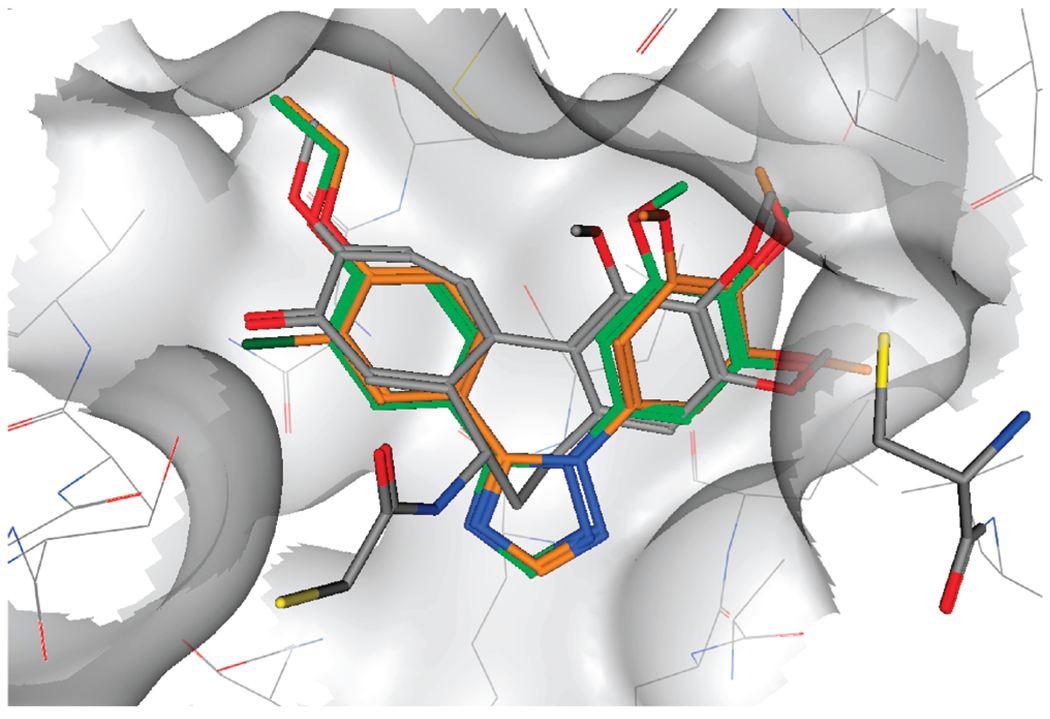
To rationalize the experimental data obtained, molecular docking studies were performed on this series of compounds. The docking pose observed for 4l showed a very similar binding mode to the cocrystallized DAMA-colchicine29 with the trimethoxyphe-nyl ring in close contact to Cys 241 (residue numbering derived from the crystal structure used) and the other aromatic moiety placed deep in the binding pocket (Figure 9). These results are in accordance with the observed experimental results; in particular, the ethoxy group is placed in a tight hydrophobic region, which does not seem able to accommodate a bulkier group, explaining the loss in activity of compound 4p. It should also be noted that the methoxy analogue 4h does not occupy this hydrophobic area as efficiently as 4l, possibly explaining the reduced biological activity observed for 4h. Furthermore, in the case of 4o, the chlorine atom in position 3 of the aromatic ring is placed in the same position as the carbonyl group of DAMA-colchi-cine, and also in this case, only relatively small groups should be tolerated in this position. In further support of this model is the inactivity of compounds 4i and 4m, which have a methoxy group and an ethoxy group, respectively, at position 3 of the aromatic ring. These compounds could not be docked successfully in the colchicine binding site.
Figure 9.
Presumptive binding mode of compound 4l (green) and compound 4o (orange). DAMA-colchicine is shown in gray, and Cys 241 is shown on the right. All compounds and Cys 241 are in stick representation.
Conclusions
In conclusion, we proposed that the 1,5-diarylsubstituted 1,2,4-triazole ring could serve as a suitable mimic to retain the bioactive configuration afforded by the cis-double bond present in CA-4. In the present study, we fixed one of the aryl groups as a 3′,4′,5′-trimethoxyphenyl moiety, and the modifications were mainly focused on variation of the substituents on the second phenyl ring. It is clear that the substitution pattern on the phenyl at the 5-position of the 1,2,4-triazole ring plays an important role for antitubulin and antiprolifera-tive activities, and this was supported by the molecular docking studies. The results demonstrated that either the 4′-ethoxy (4l) substituent or the 4′-ethoxy and 3′-chloro (4o) substitu-ents on the second phenyl ring could replace the B-ring of CA-4, at least with a 1,2,4-triazole ring as the bridge. Both these derivatives exhibited potent tubulin polymerization inhibitory activity as well as antiproliferative activity, comparable with that of CA-4. Compound 4l was the most potent inhibitor of tubulin polymerization and one of the most potent inhibitors of colchicine binding (IC50 = 0.76 µM for assembly, 86% inhibition of the binding of [3H]colchicine). We also showed by flow cytometry that 4l and 4o had cellular effects typical for microtubule-interacting agents, causing accumulation of cells in the G2/M phase of the cell cycle. Further studies showed that 4l and 4o are potent inducers of apoptosis in the Jurkat cell line. Apoptosis induced by antimitotic agents has been associated with alteration in a variety of cellular signaling pathway. As with many antimitotic drugs, compounds 4l and 4o are able to induce Bcl-2 down-regulation just after 24 h of treatment. Bcl-2 prevents the initiation of the cellular apoptotic program by stabilizing mitochondrial permeability. The loss of Δψmt results in an uncoupling of the respiratory chain and the efflux of small molecules such as caspase-9 and the apoptosis-inducing factor (AIF), which in turn can stimulate proteolytic activation of caspase-3. Our results confirm that the induction of apoptosis by 4l and 4o is associated with down-regulation of Bcl-2, dissipation of the mitochondrial transmembrane potential, and activation of caspase-3, which is coupled with terminal events of apoptosis such as PARP cleavage. Finally, preliminary experiments have assessed the potential antivascular activity of compound 4l. The ability of this compound to inhibit vascular sprouting is consistent with antivascular agent utility and warrants further testing in preclinical in vivo cancer models.
Experimental Section
Chemistry
Materials and Methods
1H NMR spectra were recorded on a Bruker AC 200 spectrometer. Chemical shifts (δ) are given in ppm upfield from tetramethylsilane as internal standard, and the spectra were recorded in appropriate deuterated solvents, as indicated. Positive-ion electrospray ionization (ESI) mass spectra were recorded on a double-focusing Finnigan MAT 95 instrument with BE geometry. Melting points (mp) were determined on a Buchi-Tottoli apparatus and are uncorrected. All products reported showed 1H NMR spectra in agreement with the assigned structures. The purity of tested compounds was determined by combustion elemental analyses conducted by the Microanalytical Laboratory of the Department of Chemistry of the University of Ferrara with a Yanagimoto MT-5 CHN recorder elemental analyzer. All tested compounds yielded data consistent with a purity of at least 95% compared with the theoretical values. All reactions were carried out under an inert atmosphere of dry nitrogen, unless otherwise indicated. Standard syringe techniques were used for transferring dry solvents. Reaction courses and product mixtures were routinely monitored by TLC on silica gel (precoated F254 Merck plates), and compounds were visualized with aqueous KMnO4. Flash chromatography was performed using 230–400 mesh silica gel and the indicated solvent system. Organic solutions were dried over anhydrous Na2SO4. Arylhydrazine hydrochlorides 5a–i and arylboronic acids are commercially available and used as received.
General Procedure A for the Synthesis of 1-Aryl-1H-[1,2,4]-triazoles 6a–i
A suspension of the appropriate arylhydrazine hydrochloride (10 mmol) in 10 mL of formamide was heated at 120 °C for 12 h. The mixture was cooled and dissolved in a mixture of ethyl acetate (20 mL) and water (10 mL). The organic layer was washed with water (3 × 10 mL), brine (10 mL), dried (Na2SO4), filtered, and concentrated to give a residue purified by column chromatography on silica gel.
1-(4-Methoxyphenyl)-1H-1,2,4-triazole (6g)
Following general procedure A, compound 6g was purified by chromatogra-phy, eluting with petroleum ether–EtOAc (1:1). Brown solid, yield 71%, mp 88–89 °C. 1H NMR (CDCl3) δ: 3.88 (s, 3H), 7.03 (d, J = 9.0 Hz, 2H), 7.58 (d, J = 9.0 Hz, 2H), 8.10 (s, 1H), 8.47 (s, 1H).
Synthesis of 1-(3,4,5-Trimethoxyphenyl)-1 H-1,2,4-triazole (8)
To a round-bottom flask charged with CuI (382 mg, 2 mmol) were added CsCO3 (6.52 g., 20 mmol), 1,2,4-triazole (1 g, 14.5 mmol), 3,4,5-trimethoxyphenylboronic acid (2.47 g, 10 mmol), and DMF (20 mL) under nitrogen. The system was then evacuated twice, backfilled with nitrogen, and heated at 120 °C for 24 h. The reaction mixture was then cooled to room temperature and diluted with EtOAc (20 mL) and water (10 mL). The aqueous phase was filtered on a pad of Celite, and the filtrate was washed with EtOAc (2 × 10 mL). The combined organic extracts were washed with brine (10 mL), dried over Na2SO4, and concentrated. The resulting residue was purified by column chromatog-raphy on silica gel (EtOAc) to provide 8 as a white solid. Yield 78%, mp 119–121 °C. 1H NMR (CDCl3) δ: 3.88 (s, 3H), 3.93 (s, 6H), 6.88 (s, 2H), 8.09 (s, 1H), 8.49 (s, 1H). Anal. (C17H17N3O3) C, H, N.
General Procedure B for the Synthesis of 1-Aryl-5-bromo-1H-[1,2,4]triazoles (7a–i) and 1-(3,4,5-Trimethoxyphenyl)-5-bromo-1H-[1,2,4]triazoles (9)
To a suspension of the appropriate 1-aryl-1H-[1,2,4]triazole 6a–i or 1-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole 9 (3 mmol) in 15 mL of CCl4 were added NBS (1.07 g, 6 mmol) and a catalytic amount of benzoyl peroxide (72 mg, 0.3 mmol). The solution was heated to reflux for 12 h, then cooled and filtered through Celite, washing the solids with warm CCl4 (5 mL). The solvent was removed in vacuo and the residue purified by column chromatography on silica gel.
5-Bromo-1-(4-methoxyphenyl)-1H-1,2,4-triazole (7g)
Following general procedure B, compound 7g was purified by chromatography, eluting with petroleum ether–EtOAc (8:2). Gray solid, yield 52%, mp 103–105 °C. 1H NMR (CDCl3) δ: 3.88 (s, 3H), 7.02 (d, J = 6.8 Hz, 2H), 7.44 (d, J = 6.8 Hz, 2H), 8.01 (s, 1H).
5-Bromo-1-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole (9)
Following general procedure B, compound 9 was purified by chromatography, eluting with petroleum ether–EtOAc (3:7). Yellow solid, yield 68%, mp 182–184 °C. 1H NMR (CDCl3) δ: 3.89 (s, 3H), 3.90 (s, 6H), 6.75 (s, 2H), 8.02 (s, 1H).
General Procedure C (Suzuki Coupling) for the Synthesis of Compounds 3a–i and 4a–r
A mixture of 5-bromo-1-aryl-1H-[1,2,4]triazoles 7a–i or 1-(3,4,5-trimethoxyphenyl)-5-bro-mo-1H-1,2,4-triazole 9 (0.5 mmol), potassium carbonate (104 mg, 0.75 mmol, 1.5 equiv), the appropriate arylboronic acid (1 mmol, 2 equiv), and tetrakis(triphenylphosphine)palladium (13.5 mg, 0.012 mmol) in dry toluene (10 mL) was stirred at 100 °C under nitrogen for 18 h, cooled to ambient temperature, filtered through Celite, and evaporated in vacuo. The residue was dissolved with EtOAc (30 mL), and the resultant solution was washed sequentially with 5% NaHCO3 (10 mL), water (10 mL), and brine (10 mL). The organic layer was dried, filtered, and evaporated, and the residue was purified by flash chromatography on silica gel.
1-(4-Methoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-1-(4-methoxyphenyl)-1H-1,2,4-triazole (3g)
Following general procedure C, compound 3g was purified by chromatography, eluting with petroleum ether–EtOAc (6:4). Yellow solid, yield 58%, mp 117–119 °C. 1H NMR (CDCl3) δ: 3.67 (s, 6H), 3.85 (s, 3H), 3.86 (s, 3H), 6.74 (s, 2H), 6.96 (d, J = 9.0 Hz, 2H), 7.31 (d, J = 9.0 Hz, 2H), 8.05 (s, 1H). MS (ESI): [M]+ = 341.8. Anal. (C18H19N3O4) C, H, N.
1-(3,4,5-Trimethoxyphenyl)-5-p-tolyl-1H-1,2,4-triazole (4g)
Following general procedure C, compound 4g was purified by chromatography, eluting with petroleum ether–EtOAc (1:1). White solid, yield 55%, mp 106–108 °C. 1H NMR (CDCl3) δ: 2.36 (s, 3H), 3.73 (s, 6H), 3.88 (s, 3H), 6.56 (s, 2H), 7.22 (d, J = 8.4 Hz, 2H), 7.42 (d, J =8.4 Hz, 2H), 8.06 (s, 1H). **MS (ESI): [M + 1]+ = 326.5. Anal. (C18H19N3O3)C, H, N.
1-(3,4,5-Trimethoxyphenyl)-5-(4-methoxyphenyl)-1H-1,2,4-triazole (4h)
Following general procedure C, compound 4h was purified by chromatography, eluting with petroleum ether–EtOAc (1:1). White solid, yield 56%, mp 72–74 °C. 1H NMR (CDCl3) δ: 3.75 (s, 6H), 3.86 (s, 3H), 3.88 (s, 3H), 6.58 (s, 2H), 6.90 (d, J = 9.0 Hz, 2H), 7.48 (d, J = 9.0 Hz, 2H), 8.04 (s, 1H). MS (ESI): [M]+ = 341.7. Anal. (C18H19N3O4) C, H, N.
1-(3,4,5-Trimethoxyphenyl)-5-(4-(methylthio)phenyl)-1H-1,2,4-triazole (4k)
Following general procedure C, compound 4k was purified by chromatography, eluting with petroleum ether–EtOAc (1:1). White solid, yield 86%, mp 123–125 °C. 1H NMR (CDCl3) δ: 2.48 (s, 3H), 3.74 (s, 6H), 3.88 (s, 3H), 6.56 (s, 2H), 7.18 (d, J = 8.6 Hz, 2H), 7.44 (d, J = 8.6 Hz, 2H), 8.02 (s, 1H). MS (ESI): [M]+=357.3. Anal. (C18H19N3O3S) C, H, N.
1-(3,4,5-Trimethoxyphenyl)-5-(4-ethoxyphenyl)-1H-1,2,4-triazole (4l)
Following general procedure C, compound 4l was purified by chromatography, eluting with petroleum ether–EtOAc (1:1). White solid, yield 55%, mp 107–109 °C. 1H NMR (CDCl3) δ:1.42(t, J = 7.0 Hz, 3H), 3.75 (s, 6H), 3.88 (s, 3H), 4.02 (q, J = 7.0 Hz, 2H), 6.58 (s, 2H), 6.84 (d, J = 8.8 Hz, 2H), 7.46 (d, J = 8.8 Hz, 2H), 8.04 (s, 1H). MS (ESI): [M + H]+ = 356.0. Anal. (C19H21N3O4) C, H, N.
5-(3-Chloro-4-ethoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole (4o)
Following general procedure C, compound 4o was purified by chromatography, eluting with petroleum ether–EtOAc (1:1). Yellow solid, yield 56%, mp 107–109 °C. 1H NMR (CDCl3) δ: 1.47 (t, J = 7.2 Hz, 3H), 3.76 (s, 6H), 3.88 (s, 3H), 4.12 (q, J = 7.2 Hz, 2H), 6.56 (s, 2H), 6.84 (d, J =8.6 Hz, 1H), 7.30 (d, J = 8.6 Hz, 1H), 7.69 (s, 1H), 8.04 (s, 1H). MS (ESI): [M + H]+ = 390.6. Anal. (C19H20ClN3O4)C, H, N.
5-(4-Isopropoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazole (4p)
Following general procedure C, compound 4p was purified by chromatography, eluting with petroleum ether–EtOAc (1:1). White solid, yield 83%, mp 120–122 °C. 1H NMR (CDCl3) δ: 1.31 (d, J = 6.2 Hz, 6H), 3.74 (s, 6H), 3.88 (s, 3H), 4.57 (m, 1H), 6.58 (s, 2H), 6.85 (d, J = 9.2 Hz, 2H), 7.45 (d, J = 9.2 Hz, 2H), 8.03 (s, 1H). MS (ESI): [M + 1]+ = 370.2. Anal. (C20H23N3O4)C, H, N.
General Procedure D for the Synthesis of 3j–k
A solution of the appropriate nitrophenyl derivative 7h,i (0.25 mmol) in DMF (5 mL) was hydrogenated over 35 mg of 10% Pd/C at 60 psi for 2 h. The catalyst was removed by filtration on Celite, and the filtrate was concentrated to give a residue which was purified by column chromatography on silica gel.
Biology
Materials and Methods
Antiproliferative Assays
Human T-leukemia (Jurkat), human promyelocytic leukemia (HL-60), and human chronic myelogenous leukemia (K562) cells were grown in RPMI-1640 medium (Gibco Milano, Italy). Breast adenocarcinoma (MCF7), human nonsmall lung carcinoma (A549) and human cervix carcinoma (HeLa) cells were grown in DMEM medium (Gibco, Milano, Italy), all supplemented with 115 units/mL of penicillin G (Gibco, Milano, Italy), 115 µg/mL streptomycin (Invitrogen, Milano, Italy) and 10% fetal bovine serum (Invitrogen, Milano, Italy). Individual wells of a 96-well tissue culture microtiter plate were inoculated with 100 µL of complete medium containing 8 × 103 cells. The plates were incubated at 37 °C in a humidified 5% CO2 incubator for 18 h prior to the experiments. After medium removal, 100 µL of the drug solution, dissolved in complete medium at different concentrations, was added to each well and incubated at 37 °C for 72 h. Cell viability was assayed by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test as previously described.30 The IC50 was defined as the compound concentration required to inhibit cell proliferation by 50%.
Effects on Tubulin Polymerization and on Colchicine Binding to Tubulin
To evaluate the effect of the compounds on tubulin assembly in vitro,16a varying concentrations of compounds were preincubated with 10 µM bovine brain tubulin in glutamate buffer at 30 °C and then cooled to 0 °C. After addition of 0.4 mM GTP, the mixtures were transferred to 0 °C cuvettes in a recording spectrophotometer and warmed to 30 °C. Tubulin assembly was followed turbidimetrically at 350 nm. The IC50was defined as the compound concentration that inhibited the extent of assembly by 50% after a 20 min incubation. The capacity of the test compounds to inhibit colchicine binding to tubulin was measured as described16b except that the reaction mixtures contained 1 µM tubulin, 5 µM [3H]colchicine, and 1 µM test compound.
Flow Cytometric Analysis of Cell Cycle Distribution and Apoptosis
For flow cytometric analysis of DNA content, 5 × 105 HeLa or Jurkat cells in exponential growth were treated with different concentrations of the test compounds for 24 and 48 h. After an incubation period, the cells were collected, centrifuged, and fixed with ice-cold ethanol (70%). The cells were then treated with lysis buffer containing RNase A and 0.1% Triton X-100 and stained with PI. Samples were analyzed on a Cytomic FC500 flow cytometer (Beckman Coulter). DNA histograms were analyzed using MultiCycle for Windows (Phoenix Flow Systems).
Annexin-V Assay
Surface exposure of PS on apoptotic cells was measured by flow cytometry with a Coulter Cytomics FC500 (Beckman Coulter) by adding annexin-V-FITC to cells according to the manufacturer’s instructions (Annexin-V Fluos, Roche Diagnostic). Simultaneously the cells were stained with PI. Excitation was set at 488 nm, and the emission filters were at 525 and 585 nm, respectively.
Assessment of Mitochondrial Changes
The mitochondrial membrane potential was measured with the lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine (JC-1, Molecular Probes), as described.19,30 Briefly, after different times of treatment, the cells were collected by centri-fugation and resuspended in Hank’s balanced salt solution (HBSS) containing 1 µM JC-1. The cells were then incubated for 10 min at 37 °C, centrifuged, and resuspended in HBSS. The production of ROS was measured by flow cytometry using either HE (Molecular Probes) or H2DCFDA (Molecular Probes).
After different times of treatment, cells were collected by centrifugation and resuspended in HBSS containing the fluorescence probe HE or H2DCFDA at 2.5 or 0.1 µM, respectively. The cells were then incubated for 30 min at 37 °C, centrifuged, and resuspended in HBSS. The fluorescence was directly recorded with the flow cytometer, using an excitation wavelength of 488 nm and emission wavelengths of 585 and 530 nm for HE and H2DCFDA, respectively.
Caspase-3 Assay
Caspase-3 activation in Jurkat cells was evaluated by flow cytometry using a human active caspase-3 fragment antibody conjugated with FITC (BD Pharmingen). Briefly, after different incubation times in the presence of test compounds, the cells were collected by centrifugation and resuspended in Cytofix (BD Pharmingen) buffer for 20 min, washed with Perm/Wash (BD Pharmingen), and then incubated for 30 min with the antibody. After this period, cells were washed and analyzed by flow cytometry. Results are expressed as percentage of caspase-3 active fragment positive cells.
Western Blot Analysis
Jurkat cells were incubated in the presence of test compounds and, after different times, were collected, centrifuged, and washed two times with ice cold phosphate buffered saline (PBS). The pellet was then resuspended in lysis buffer. After the cells were lysed on ice for 30 min, lysates were centrifuged at 15000g at 4 °C for 10 min. The protein concentration in the supernatant was determined using the BCA protein assay reagents (Pierce, Italy). Equal amounts of protein (20 µg) were resolved using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) (7.5–15% acrylamide gels) and transferred to a PVDF Hybond-p membrane (GE Healthcare). Membranes were blocked with I-block (Tropix), and the membrane was gently rotated overnight at 4 °C. Membranes were then incubated with primary antibodies against Bcl-2, PARP (all rabbit, 1:1000, Cell Signaling), or β-actin (mouse, 1:10,000, Sigma) for 2 h at room temperature. Membranes were next incubated with peroxidase-labeled goat antirabbit IgG (1:100000, Sigma) or peroxidase-labeled goat antimouse IgG (1:100000, Sigma) for 60 min. All membranes were visualized using ECL Advance (GE Healthcare) and exposed to Hyperfilm MP (GE Healthcare). To ensure equal protein loading, each membrane was stripped and reprobed with an anti-β-actin antibody.
Endothelial Cell Shape Change Assays
HUVECs expressing GFP (green fluorescence protein) were cultured in EGM-2 media with endothelial cell growth supplement and transferred into 18-well µ-Slide plates coated with collagen 1 day before assay. Cell morphology and GFP fluorescence were recorded in a Nikon confocal microscope with an imaging system before and after drug additions at different time points. The area of HUVECs with GFP was further analyzed by the imaging software MetaMorph (Molecular Devices). The chick aortic ring assay was performed as described in ref 28. Briefly, aortic arches were dissected from day 14 chick embryos, cut into cross-sectional slices, and implanted in 10 µL of Matrigel (Becton Dickinson) in eight-well Lac-Tek chamber slides with complete media. Endothelial cell sprouts or channels were formed in 24–48 h. Various concentrations of 4 L were added, and images of the vascular channels were captured with a digital camera before and 30 min after drug addition.
Molecular Modeling
All molecular modeling studies were performed on a MacPro dual 2.66 GHz Xeon computer running Ubuntu 8. The tubulin structure was downloaded from the PDB data bank (http://www.rcsb.org/; PDB code 1SA0).30 Hydrogen atoms were added to the protein, using Molecular Operating Environment (MOE),31 and minimized keeping all the heavy atoms fixed until a rmsd gradient of 0.05 kcal mol−1 Å−1 was reached. Ligand structures were built with MOE and minimized using the MMFF94x force field until a rmsd gradient of 0.05 kcal mol−1 Å−1 was reached. The docking simulations were performed using PLANTS.32
Supplementary Material
Footnotes
Abbreviations: CA-4, combretastatin A-4; EWG, electron-withdrawing group; ERG, electron-releasing group; SAR, structure–activity relationship; CuI, copper iodide; CsCO3, cesium carbonate; NBS, N-bromo-succinimide; CCl4, carbon tetrachloride; PI, propidium iodide; PS, phosphatidylserine; HE, hydroethidine; H2DCFDA, 2,7-dichlorodi-hydrofluorescein diacetate; DCF, dichlorofluorescein; PARP, poly ADP-ribose polymerase; HUVEC, human umbilical vein endothelial cell; GFP, green fluorescent protein; HBSS, Hank’s balanced salt solution; PBS, phosphate buffered saline; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Supporting Information Available: Additional information for synthesis procedures and spectroscopic data for compounds 6a–f,h,i, 7a–f,h,i, 3a–f,h–k, and 4a–f,h–j,m,n,q,r. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Amos LA. Microtubule structure and its stabilisation. Org. Biomol. Chem. 2004;2:2153–2160. doi: 10.1039/b403634d. [DOI] [PubMed] [Google Scholar]; b Walczak CE. Microtubule dynamics and tubulin interacting proteins. Curr. Opin. Cell. Biol. 2000;12:52–56. doi: 10.1016/s0955-0674(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 2.a Mahindroo N, Liou JP, Chang JY, Hsieh HP. Antitubulin agents for the treatment of cancer. A medicinal chemistry update. Expert Opin. Ther. Pat. 2006;16:647–691. [Google Scholar]; b Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]; c Hadfield JA, Ducki S, Hirst N, McGown AT. Tubulin and microtubules as targets for anticancer drugs. Prog. Cell Cycle Res. 2003;5:309–325. [PubMed] [Google Scholar]; d Honore S, Pasquier E, Braguer D. Understanding microtubule dynamics for improved cancer therapy. Cell. Mol. Life. Sci. 2005;62:3039–3065. doi: 10.1007/s00018-005-5330-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Pellegrini F, Budman DR. Review: tubulin function, action of antitubulin drugs, and new drug development. Cancer Invest. 2005;23:264–273. doi: 10.1081/cnv-200055970. [DOI] [PubMed] [Google Scholar]; f Attard G, Greystoke A, Kaye S, De Bono J. Update on tubulin binding agents. Pathol. Biol. 2006;54:72–84. doi: 10.1016/j.patbio.2005.03.003. [DOI] [PubMed] [Google Scholar]; g Lawrence NJ, McGown AT. The chemistry and biology of antimitotic chalcones and related enone systems. Curr. Pharm. Des. 2005;11:1679–1693. doi: 10.2174/1381612053764733. [DOI] [PubMed] [Google Scholar]
- 3.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experentia. 1989;45:209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 4.Lin CM, Ho HH, Pettit GR, Hamel E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry. 1989;28:6984–6991. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 5.McGown AT, Fox BW. Differential cytotoxicity of combretastatins A1 and A4 in two daunorubucin-resistant P388 cell lines. Cancer Chemother. Pharmacol. 1990;26:79–81. doi: 10.1007/BF02940301. [DOI] [PubMed] [Google Scholar]
- 6.a Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br. J. Cancer. 1999;81:1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, Bohlen P, Chaplin DJ, May C, Rafii S. Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J. Clin. Invest. 2005;115:2992–3006. doi: 10.1172/JCI24586. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Chaplin DJ, Hill SA. The development of combretastatin A4 phosphate as a vascular targetingagent. Int. J. Radiat. Oncol., Biol., Phys. 2002;54:1491–1496. doi: 10.1016/s0360-3016(02)03924-x. [DOI] [PubMed] [Google Scholar]; d Young SL, Chaplin DJ. Combretastatin A-4 phosphate: background and current clinical status. Expert Opin. Invest. Drugs. 2004;13:1171–1182. doi: 10.1517/13543784.13.9.1171. [DOI] [PubMed] [Google Scholar]; e Bilenker JH, Flaherty KT, Rosen M, Davis L, Gallagher M, Stevenson JP, Sun W, Vaughn D, Giantonio B, Zimmer R, Scnall M, O’Dwyer PJ. Phase I trial of combretastatin A-4 phospate with carboplatin. Clin. Cancer Res. 2005;11:1527–1533. doi: 10.1158/1078-0432.CCR-04-1434. [DOI] [PubMed] [Google Scholar]
- 7.a Nam NH. Combretastatin A-4 analogues as antimitotic antitumor agents. Curr. Med. Chem. 2003;10:1697–1722. doi: 10.2174/0929867033457151. [DOI] [PubMed] [Google Scholar]; b Chaudari A, Pandeya SN, Kumar P, Sharma PP, Gupta S, Soni N, Verma KK, Bhardwaj G. Combretastain A-4 analogues as anticancer agents. Mini-Rev. Med. Chem. 2007;12:1186–1205. doi: 10.2174/138955707782795647. [DOI] [PubMed] [Google Scholar]; c Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA. Medicinal chemistry of combretastatin A4: present and future directions. J. Med. Chem. 2006;49:3033–3044. doi: 10.1021/jm0512903. [DOI] [PubMed] [Google Scholar]; d Gaukroger K, Hadfield JA, Lawrence NJ, Nlan S, McGown AT. Structural requirements for the interaction of combretastatins with tubulin: how important is the trimethoxy unit? Org. Biomol. Chem. 2003;1:3033–3037. doi: 10.1039/b306878a. [DOI] [PubMed] [Google Scholar]; e Hatanaka T, Fujita K, Ohsumi K, Nakagawa R, Fukuda Y, Nihei Y, Suga Y, Akiyama Y, Tsuji T. Novel B-ring modified combretastatin analogues: synthesis and antineoplastic activity. Bioorg. Med. Chem. Lett. 1998;8:3371–3374. doi: 10.1016/s0960-894x(98)00622-2. [DOI] [PubMed] [Google Scholar]
- 8.Ohsumi K, Hatanaka T, Fujita K, Nakagawa R, Fukuda Y, Nihai Y, Suga Y, Morinaga Y, Akiyama Y, Tsuji T. Synthesis and antitumor activity of cis-restricted combretastatins 5-membered heterocyclic analogues. Bioorg. Med. Chem. Lett. 1988;8:3153–3158. doi: 10.1016/s0960-894x(98)00579-4. [DOI] [PubMed] [Google Scholar]
- 9.a Bellina F, Cauteruccio S, Monti S, Rossi R. Novel imidazole-based combretastatin A-4 analogues. Evaluation of their in vitro antitumor activity and molecular modeling study of their binding to the colchicine site of tubulin. Bioorg. Med. Chem. Lett. 2000;16:5757–5762. doi: 10.1016/j.bmcl.2006.08.087. [DOI] [PubMed] [Google Scholar]; b Wang L, Woods KW, Li Q, Barr KJ, McCroskey RW, Hannick SM, Gherke L, Credo RB, Hui Y-H, Marsh K, Warner R, Lee JY, Zielinski-Mozng N, Frost D, Rosenberg SH, Sham HL. Potent, orally active heterocycle-based combretastatin A-4 analogues: synthesis, structure–activity relationship, pharmacokinetics, and in vivo antitumor activity evaluation. J. Med. Chem. 2002;45:1697–1711. doi: 10.1021/jm010523x. [DOI] [PubMed] [Google Scholar]
- 10.Kaffy J, Pontikis R, Carrez D, Croisy A, Monneret C, Florent J-C. Isoxazole-type derivatives related to combretastatin A-4, synthesis and biological evaluation. Bioorg. Med. Chem. 2006;14:4067–4077. doi: 10.1016/j.bmc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Li W, Yang C, Chen D, Ding J, Chen Y, Lin L, Xie Y. Synthesis and activity of combretastatin A-4 analogues: 1,2,3-thiadiazoles as potent antitumor agents. Bioorg. Med. Chem. Lett. 2007;17:869–873. doi: 10.1016/j.bmcl.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 12.Odlo K, Hentzen J, Fournier dit Chabert J, Ducki S, Gani OABSM, Sylte I, Skrede M, Florenes VA, Hansen TV. 1,5-Disubstituted 1,2,3-triazoles as cis-restricted analogues of combretastatin A-4: synthesis, molecular modeling and evaluation as cytotoxic agents and inhibitors of tubulin. Bioorg. Med. Chem. 2008;16:4829–4838. doi: 10.1016/j.bmc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Peng Y, Wang XI, Keeman SM, Aurora S, Welsh WJ. Highly potent triazole-based tubulin polymerization inhibitors. J. Med. Chem. 2007;50:749–754. doi: 10.1021/jm061142s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, Guo P, Li G, Lan J, Xie R, You J. Simple copper salt-catalyzed N-arylation of nitrogen-containing heterocycles with aryl and heteroaryl halides. J. Org. Chem. 2007;72:8535–8538. doi: 10.1021/jo0712289. [DOI] [PubMed] [Google Scholar]
- 15.a Romagnoli R, Pier Baraldi PG, Carrion MD, Cruz-Lopez O, Lopez Cara C, Tolomeo M, Grimaudo S, Di Cristina A, Pipitone MR, Balzarini J, Zonta N, Brancale A, Hamel E. Design, synthesis and structure–activity relationship of 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]furan derivatives as a novel class of antitubulin agents. Bioorg. Med. Chem. 2009;17:6862–6871. doi: 10.1016/j.bmc.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cushman M, He H-M, Katzenellenbogen JA, Hamel E. Synthesis, antitubulin and antimitotic activity and cytotoxicity of analogs of 2-meth-oxyestradiol, an endogenous mammalian metabolite of estradiol that inhibits tubulin polymerization by binding to the colchicine. J. Med. Chem. 1995;38:2041. doi: 10.1021/jm00012a003. [DOI] [PubMed] [Google Scholar]; c Kim S, Min SY, Lee SK, Cho W-J. Comparative molecular field analysis study of stilbene derivatives active against A549 lung carcinoma. Chem. Pharm. Bull. 2003;51:516–521. doi: 10.1248/cpb.51.516. [DOI] [PubMed] [Google Scholar]
- 16.a Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem. Biophys. 2003;38:1–21. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]; b Verdier-Pinard P, Lai J-Y, Yoo H-D, Yu J, Marquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E. Structure–activity analysis of the interaction of curacin A, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol. Pharmacol. 1998;53:62–67. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]
- 17.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phos-phatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 18.a Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (Δψm) in apoptosis: an update. Apoptosis. 2003;3:115–128. doi: 10.1023/a:1022945107762. [DOI] [PubMed] [Google Scholar]; b Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2005;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 19.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1 but not DiOC6(3) or rhodamine 123 is a reliable fluorescent probe to assess ΔΨ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 20.a Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;8:413–450. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]; b Vitale I, Antoccia A, Cenciarelli C, Crateri P, Meschini S, Arancia G, Pisano C, Tanzarella C. Combretastatin CA-4 and combretastatin derivative induce mitotic catastrophe dependent on spindle checkpoint and caspase-3 activation in non-small cell lung cancer cells. Apoptosis. 2007;12:155–166. doi: 10.1007/s10495-006-0491-0. [DOI] [PubMed] [Google Scholar]
- 21.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.a Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichloro-fluorescein. J. Leukocyte Biol. 1990;47:440–448. [PubMed] [Google Scholar]; b Cai J, Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998;273:11401–11404. doi: 10.1074/jbc.273.19.11401. [DOI] [PubMed] [Google Scholar]; c Nohl H, Gille L, Staniek K. Intracellular generation of reactive oxygen species by mitochondria. Biochem. Pharmacol. 2005;69:719–723. doi: 10.1016/j.bcp.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.a Thomberry AN, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]; b Earshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates and functions during apoptosis. Annu. Rev. Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]; c Reed JC. Apoptosis-based therapies. Nat. Rev. Drug Discovery. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]; d Denault J-B, Salvesen GS. Caspases: keys in the ignition of cell death. Chem. Rev. 2002;102:4489–4499. doi: 10.1021/cr010183n. [DOI] [PubMed] [Google Scholar]
- 24.Porter AG, Janicke RU. Emerging role of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 25.Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- 26.a Kluck RM, Bossy-Wetzel E, Green DR. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]; b Knudson CM, Korsmeyer SJ. Bcl-2 and Bax function independently to regulate cell death. Nat. Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 27.Satchi-Fainaro R, Puder M, Davies J, Tran H, Greene AK, Corfas G, Folkman J. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat. Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- 28.Galbraith SM, Chaplin DJ, Lee F, Stratford MR, Locke RJ, Vojnovic B, Tozer G. M Effects of combretastatin A4 phosphate on endothelial cell morphology in vitro and relationship to tumour vascular targeting activity in vivo. Anticancer Res. 2001;21:93–102. [PubMed] [Google Scholar]
- 29.Ravelli RBG, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 30.Viola G, Fortunato E, Cecconet L, Del Giudice L, Dall’Acqua F, Basso G. Central role of p53 and mitochondrial damage in PUVA-induced apoptosis in human keratinocytes. Toxicol. Appl. Pharmacol. 2008;227:84–96. doi: 10.1016/j.taap.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Molecular Operating Environment (MOE 2008.10) Montreal, Quebec, Canada: Chemical Computing Group, Inc.; http://www.chemcomp.com. [Google Scholar]
- 32.Korb O, Stützle T, Exner TE. PLANTS: Application of Ant Colony Optimization to Structure-Based Drug Design. In: Dorigo M, Gambardella LM, Birattari M, Martinoli A, Poli R, Stützle T, editors. Ant Colony Optimization and Swarm Intelligence, 5th International Workshop, ANTS 2006; Sep 4–7, 2006; Brussels, Belgium. Berlin: Springer; 2006. pp. 247–258. LNCS 4150. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.