Abstract
Objectives
The onset of preterm labor has been proposed to have survival value and to be adaptive in nature. This hypothesis would predict that induced preterm birth may be associated with higher rates of complications than spontaneous preterm birth. The purpose of this study was to determine if there is a difference in the frequency of neonatal respiratory distress syndrome (RDS), the most common neonatal complication, according to the etiology of preterm birth (e.g. preterm labor [PTL], preterm PROM, or pregnancies which ended because of maternal-fetal indications).
Study design
The relationship between the occurrence of RDS and the obstetrical circumstances leading to preterm birth was examined in 257 consecutive singleton preterm births (gestational age: 24-32 weeks). Cases with major congenital anomalies were excluded. The study population was divided into two groups according to the cause of preterm birth: 1) preterm birth due to PTL with intact membranes or preterm PROM (spontaneous preterm birth group); and 2) preterm birth due to maternal or fetal indications (indicated preterm birth group).
Results
1) RDS was diagnosed in 47% of cases; 2) RDS was more common in patients with indicated preterm birth than in those with spontaneous preterm birth group (58.1% vs. 38.4%, p=.002); 3) Patients with indicated preterm birth had a significantly higher mean gestational age at birth, but lower mean birth weight, lower rate of histological chorioamnionitis and higher rates of cesarean delivery, 5 min Apgar score of <7, and umbilical arterial blood pH of <7.15 than those with spontaneous preterm birth (p<0.05 for each); 4) Antenatal corticosteroids were used in 73.4% of cases with indicated preterm birth and in 76.9% of those with spontaneous preterm birth; 5) Multivariate analysis demonstrated that indicated preterm birth was associated with an increased risk of RDS after adjustment for confounding variables (OR=2.29, 95% CI 1.22-4.29).
Conclusions
1) The rate of RDS is greater following “indicated” rather than spontaneous preterm birth; 2) This observation supports the view that spontaneous preterm labor is adaptive in nature.
Keywords: Indicated preterm birth, neonatal respiratory distress syndrome (RDS), spontaneous preterm birth
Introduction
Preterm birth can result from spontaneous preterm labor with intact or rupture of membranes, or medically indicated preterm birth [2, 19, 21]. Among all singleton preterm births, spontaneous preterm births account for 65-70%, while 30-35% are due to indicated preterm delivery [1, 10].
The onset of spontaneous preterm birth has been proposed to have survival value and to be adaptive in nature. This hypothesis predicts that induced or indicated preterm birth may be associated with higher rates of complications than spontaneous preterm birth.
Neonatal respiratory distress syndrome (RDS) is the most common complication and the leading cause of neonatal mortality in preterm newborns. In neonates born at 28-30 weeks of gestation, the prevalence of RDS can be as high as 70% [12]. Neonatal RDS occurs in one-fifth of low birth-weight babies (<2,500gm) and two-thirds of very low birth-weight infants (<1,500gm) [20].
The purpose of this study was to determine if there is a difference in the frequency of neonatal RDS, as a function of the etiology of preterm birth (e.g. preterm labor [PTL], preterm PROM, or maternal-fetal indications).
Materials and Methods
Study design
A retrospective cohort study was conducted to examine the difference in the occurrence of RDS according to the etiology of preterm birth. The study population consisted of 257 consecutive patients who delivered preterm singleton live-born neonates (gestational age at birth: 24-32 weeks) in the participating institution from January 1998 to December 2006. Forty-two cases with chromosomal abnormalities or major congenital anomalies were excluded from this study. Neonates were divided into two groups according to the cause of preterm birth: 1) preterm birth due to PTL with intact membranes or preterm PROM (spontaneous preterm birth group); and 2) preterm birth due to maternal or fetal indications (indicated preterm birth group).
Clinical characteristics of the patients and diagnosis of RDS
We reviewed the medical records to determine the clinical and demographic characteristics of the mothers and their neonates. The demographic characteristics included age, parity and gestational age at delivery. Clinical characteristics consisted of antenatal corticosteroid administration, histopathological diagnosis of the placenta, and whether or not a cesarean delivery was performed. We also included gender, 1 minute Apgar score, 5 minute Apgar score, and umbilical arterial blood gas analysis. The Institutional Review Board of the Seoul National University approved the medical records review for this study.
Histological chorioamnionitis was defined as the presence of acute inflammatory changes in a membrane roll and the chorionic plate of the placenta. Funisitis was diagnosed in the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly using criteria previously published and used in our studies [27].
The primary outcome variable was the presence of RDS in the newborn which was defined as the presence of respiratory distress, increased oxygen requirement (FiO2>0.4), and diagnostic radiological and laboratory findings in the absence of evidence of any other causes of respiratory distress.
Statistical analysis
The Mann-Whitney U test or Student’s t test was used for the comparison of the continuous variables. For the dichotomized variables, χ2 test or Fisher’s exact test was performed. Multiple logistic regression analysis was used to examine the relationship between the etiology of preterm birth and the occurrence of RDS, controlled for the effect of potential confounding variables.
Results
Two hundred and fifty-seven mothers and their neonates met the entry criteria and 14 cases were excluded because the neonates died immediately after birth despite resuscitation. RDS was diagnosed in 47% (114/243) of newborns.
Table 1 describes the clinical and demographic characteristics of the study population according to the etiology of preterm birth. No significant differences were found in the maternal age, parity and the rate of the antenatal corticosteroid administration between the indicated preterm birth group and the spontaneous preterm birth group. Neonates in the indicated preterm birth group had a significantly higher mean gestational age at delivery, but lower mean birthweight than those in the spontaneous preterm birth group. Newborns who were delivered because of maternal and/or fetal indications had a significantly lower rate of histological chorioamnionitis and higher rates of cesarean delivery, 5 minute Apgar score of <7 and umbilical arterial blood pH of <7.15 than those who were delivered due to spontaneous labor or preterm PROM. There was no difference in neonatal mortality between the two groups. The prevalence of RDS in the indicated preterm birth group was significantly higher than in the spontaneous preterm birth group (58.1% vs. 38.4%, p=.002).
Table 1.
Characteristics of the study population according to the etiology of preterm birth
| Indicated preterm birth group ( n=110 ) |
Spontaneous preterm birth group ( n=147 ) |
P value | |
|---|---|---|---|
| Maternal age (mean±SD) | 32.0±4.5 | 31.3±4.4 | NS |
| Parity (nulliparity) (%) | 47.3 (52/110) | 47.6 (70/147) | NS |
| Gestational age at delivery (weeks) (mean±SD) |
29.3±1.8 | 28.5±2.3 | .005 |
| Antenatal steroid use* (%) | 73.4 (80/109) | 76.9 (113/147) | NS |
| Cesarean delivery (%) | 91.8 (101/110) | 51 (75/147) | <.001 |
| Sex (Male) (%) | 40.9 (45/110) | 64.6 (95/147) | <.001 |
| Birth weight (g) (mean±SD) | 1069.7±401.7 | 1286.9±408.0 | <.001 |
| 1-min Apgar score of < 7 (%) | 84.5 (93/110) | 76.9 (113/147) | NS |
| 5-min Apgar score of < 7 (%) | 64.5 (71/110) | 49.7 (73/147) | .017 |
| Umbilical arterial pH of < 7.15† (%) | 25.3 (25/99) | 10.1 (13/129) | .002 |
| Hypertensive disorders (%) | 74.5 (82/110) | 2.7 (4/147) | <.001 |
| Histological chorioamnionitis‡ (%) | 9.3 (9/97) | 59.9 (82/137) | <.001 |
| Funisitis§ (%) | 1.0 (1/98) | 33.8 (47/139) | <.001 |
| RDS¶ (%) | 58.1 (61/105) | 38.4 (53/138) | .002 |
| Neonatal mortality (%) | 16.4 (18/110) | 11.6 (17/147) | NS |
NS, not significant; RDS, respiratory distress syndrome.
One case was excluded from the analysis because of unavailable data for the use of antenatal corticosteroid.
Twenty-nine cases that did not have the results of the umbilical arterial blood gas analysis were excluded from the analysis.
Twenty-three cases that did not have histopathologic examinations of the placenta were excluded from the analysis.
Twenty cases that had unavailable placental histological results for funisitis were excluded from the analysis.
Fourteen cases that died immediately at the delivery room regardless of resuscitation were excluded from the analysis.
Table 2 shows the characteristics of the neonates according to the presence or absence of RDS. Neonates who developed RDS were delivered at lower mean gestational ages, and had a lower mean birth weight than those without RDS. The rate of cesarean delivery in neonates with RDS was higher than in those without RDS. However, no significant difference was found in the rate of antenatal corticosteroid use between the two groups. Babies with RDS had significantly higher rates of 1 minute and 5 minute Apgar scores of <7 and umbilical arterial blood pH of <7.15 than those without RDS. The frequencies of histological chorioamnionitis and funisitis in neonates with RDS were significantly lower than in those without RDS.
Table 2.
Clinical characteristics of patients according to occurrence of neonatal RDS
| RDS (−) ( n=129 ) |
RDS (+) ( n=114 ) |
P value | |
|---|---|---|---|
| Maternal age (mean±SD) | 31.5±4.2 | 31.7±4.7 | NS |
| Parity (nulliparity) (%) | 49.5 (64/129) | 45.6 (52/114) | NS |
| Gestational age at delivery (weeks) (mean±SD) |
29.5±1.9 | 28.4±2.1 | <.001 |
| Antenatal steroid use (%) | 80.6 (104/129) | 71.9 (82/114) | NS |
| Cesarean delivery (%) | 61.2 (79/129) | 79.8 (91/114) | .002 |
| Sex (Male) (%) | 52.7 (68/129) | 53.5 (62/114) | NS |
| Birth weight (g) (mean±SD) | 1281.5±411.4 | 1146.9±403.7 | .007 |
| 1-min Apgar score of < 7 (%) | 68.2 (88/129) | 92.1 (105/114) | <.001 |
| 5-min Apgar score of < 7 (%) | 40.3 (52/129) | 68.4 (78/114) | <.001 |
| Umbilical arterial pH of < 7.15* (%) | 9.8 (11/112) | 23.6 (25/106) | .006 |
| Hypertensive disorders (%) | 27.9 (36/129) | 39.5 (45/114) | .056 |
| Histological chorioamnionitis† (%) | 46.7 (56/120) | 28.7 (31/108) | .005 |
| Funisitis‡ (%) | 25.4 (31/122) | 13.8 (15/109) | .027 |
Twenty-five cases that did not have the results of the umbilical arterial blood gas analysis were excluded from the analysis.
Fifteen cases that did not have histopathologic examinations of the placenta were excluded from the analysis.
Twelve cases that had unavailable placental histological results for funisitis were excluded from the analysis.
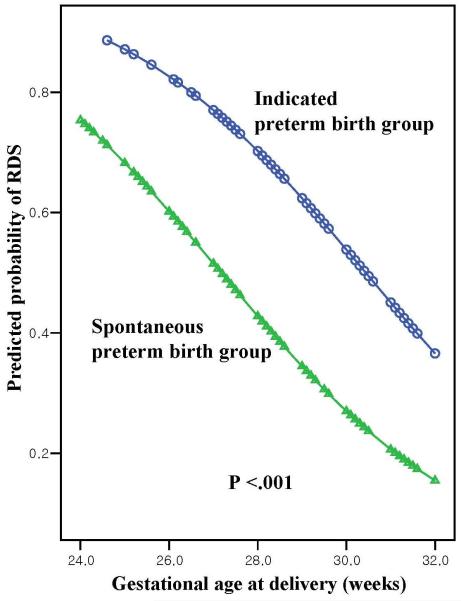
Using multiple logistic regression to adjust for the effect of gestational age at delivery, indicated preterm birth was associated with an increase in the odds ratio (OR) for the development of RDS (OR=3.16; 95% confidence interval (CI) 1.78-5.60; p <.001; Figure 1). The relationship remained significant (OR=2.29; 95% CI 1.22-4.29; p=.010; Table 3), even after adjusting for other variables that could have influenced the occurrence of RDS, such as cesarean delivery and antenatal corticosteroid use.
Figure 1.
Plot of predicted probability of RDS according to the gestational age at delivery and etiology of preterm birth. [After adjusting for gestational age at delivery, indicated preterm birth increased the odds of development of RDS (adjusted OR=3.16; 95% CI 1.78-5.60; p <.001)].
Table 3.
Relationship of various variables with the occurrence of RDS analyzed by overall logistic regression
| Odds ratio | 95% confidence interval | P value | |
|---|---|---|---|
| Gestational age at delivery | 0.70 | 0.60-0.81 | <.001 |
| Indicated preterm birth | 2.29 | 1.22-4.29 | .010 |
| Cesarean delivery | 2.26 | 1.13-4.53 | .022 |
| Antenatal corticosteroid use | 0.67 | 0.35-1.29 | .226 |
The frequency of RDS in the indicated preterm birth group was similar to that in the spontaneous preterm birth group among newborns delivered by cesarean section (58.2% vs. 47.2%, p=0.158) and also among newborns delivered vaginally (57.1% vs. 28.8%, p=.196). However, indicated preterm birth was significantly associated with the increase in the odds ratio for the development of RDS in both the cesarean and vaginal delivery groups, after the adjusting for gestational age at delivery and antenatal corticosteroid use (p<0.05 for each, Table 4).
Table 4.
Comparison of the occurrence of neonatal RDS according to the etiology of preterm birth and mode of delivery
| Indicated preterm births |
Spontaneous preterm births |
P- value | ||
|---|---|---|---|---|
| Unadjusted | Adjusted* | |||
| Among cases with cesarean delivery | ( n=101) | ( n=75 ) | ||
| Gestational age at delivery (weeks) (mean±SD) |
29.4±1.8 | 28.7±2.1 | .055 | |
| Antenatal steroid use† (%) | 74.0 (74/100) | 77.3 (58/75) | NS | |
| RDS‡ (%) | 58.2 (57/98) | 47.2 (34/72) | .158 | .048 |
| Among cases with vaginal delivery | ( n=9) | ( n=72 ) | ||
|---|---|---|---|---|
| Gestational age at delivery (weeks) (mean±SD) |
28.9±2.6 | 28.2±2.5 | .363 | |
| Antenatal steroid use (%) | 66.7 (6/9) | 76.4 (55/72) | NS | |
| RDS§ (%) | 57.1 (4/7) | 28.8 (19/66) | .196 | .033 |
NS, not significant; RDS, respiratory distress syndrome.
By logistic regression, adjusted for gestational age at delivery and antenatal corticosteroid use.
One case was excluded from the analysis because of unavailable data for the use of antenatal corticosteroid.
Six cases that died immediately at the delivery room regardless of resuscitation were excluded from the analysis.
Eight cases that died immediately at the delivery room regardless of resuscitation were excluded from the analysis.
Discussion
Principal findings of the study
The rate of RDS in the indicated preterm birth group was significantly higher than in the spontaneous preterm birth group among preterm singleton neonates whose gestational age at birth was less than 32 weeks. This remained significant after adjusting for the effect of confounding factors which could affect the development of RDS, such as gestational age at delivery, antenatal corticosteroid use, and cesarean delivery.
Why should RDS be less frequent in cases of spontaneous rather than indicated preterm birth
One possible explanation is that the rate of histological chorioamnionitis was higher in spontaneous preterm labor/delivery than in indicated preterm birth. Bry et al [3, 4] reported that intraamniotic interleukin-1 and endotoxin stimulate surfactant protein synthesis and lung maturation in fetal rabbits. Subsequently, many investigators [14-16, 18, 24] have demonstrated that chorioamnionitis accelerates fetal lung maturity. For example, Shimoya et al [24] reported that chorioamnionitis induces fetal lung maturation by increasing interleukin-6 and decreases the incidence of RDS. In this study, neonates born after spontaneous preterm birth had a significantly higher rate of histological chorioamnionitis than those in the indicated preterm birth group (59.9% vs. 9.3%; p<.001), and this relationship remained significant after adjusting for gestational age at delivery (adjusted OR=13.48; 95% CI 6.20-29.28).
Should preeclampsia decrease the rate of RDS after an indicated preterm birth?
Traditionally, it has been believed that chronic intrauterine stress such as preeclampsia, accelerates fetal lung maturity [5, 23]. However, recent studies [6, 22, 26] indicate that this may not be the case. Moreover, Chang et al [7] observed that the risk of RDS in neonates at <32 weeks of gestation is increased in mothers with preeclampsia. In this study, there was a trend towards a higher rate of RDS in newborns whose mothers had a hypertensive disorder compared to those whose mothers were normotensive during pregnancy (55.6% vs. 42.6%; p=.056), although it was not statistically significant. The percentage of those with a hypertensive disorder in the indicated preterm birth group was higher than in the spontaneous preterm birth group (74.5% vs. 2.7%). This point could explain the higher rate of RDS in the indicated preterm birth group.
Results of other studies
Our findings are in keeping with those of a large population-based study [17], even though this study did not adjust for confounding factors, except for gestational age at birth, which could have influenced the occurrence of RDS. The authors [17] reported that “indicated preterm birth” was associated with both neonatal respiratory and gastrointestinal diseases, which may decrease survival.
In preterm twin gestations, several investigators [11, 25] have observed that second twins have higher rates of RDS, chronic lung disease and neonatal mortality than first twins. In preterm PROM, the first twin is more likely to be exposed to the effect of microbial products and cytokines than the second twin, and this may be an explanation for these findings.
In this study, spontaneous preterm labor with intact membranes, preterm PROM, and preterm birth due to maternal/fetal indications accounted for 32.7%, 24.5%, and 42.8% of total preterm births before 32 weeks of gestation. The frequency of indicated preterm birth was relatively high in our study compared with previous reports [1, 10]. However, this probably reflects the fact that the study population was restricted to a gestational age at birth of 24-32 weeks.
Roberts and Dalziel [20] have demonstrated that antenatal corticosteroid administration to mothers with impending preterm delivery decreases the occurrence of RDS. However, this effect did not reach statistical significance in our study (adjusted OR 0.67; 95% CI 0.35-1.29; p=.226). One explanation for this finding is a selection bias because of the small size of population studied or a suboptimal effect because of an incomplete course of corticosteroids [8, 9, 13].
In conclusion, the rate of RDS is greater in “indicated” rather than in spontaneous preterm birth. This observation supports the view that spontaneous preterm labor is adaptive in nature.
Acknowledgement
This research was supported by the Korea Science and Engineering Foundation (KOSEF) grand funded by the Korea government (No. R01-2006-000-10607-0) and also, in part, by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.l
References
- 1.Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstetrics and gynecology. 2005;105:1084–91. doi: 10.1097/01.AOG.0000158124.96300.c7. [DOI] [PubMed] [Google Scholar]
- 2.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 3.Bry K, Lappalainen U. Intra-amniotic endotoxin accelerates lung maturation in fetal rabbits. Acta Paediatr. 2001;90:74–80. doi: 10.1080/080352501750064914. [DOI] [PubMed] [Google Scholar]
- 4.Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest. 1997;99:2992–9. doi: 10.1172/JCI119494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bustos R, Kulovich MV, Gluck L, Gabbe SG, Evertson L, Vargas C, Lowenberg E. Significance of phosphatidylglycerol in amniotic fluid in complicated pregnancies. Am J Obstet Gynecol. 1979;133:899–903. doi: 10.1016/0002-9378(79)90309-0. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho MA, Faundes A, Santos LC. Pregnancy-induced hypertension and hyaline membrane disease. Int J Gynaecol Obstet. 1997;58:197–202. doi: 10.1016/s0020-7292(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 7.Chang EY, Menard MK, Vermillion ST, Hulsey T, Ebeling M. The association between hyaline membrane disease and preeclampsia. Am J Obstet Gynecol. 2004;191:1414–7. doi: 10.1016/j.ajog.2004.06.097. [DOI] [PubMed] [Google Scholar]
- 8.Costa S, Zecca E, De Luca D, De Carolis MP, Romagnoli C. Efficacy of a single dose of antenatal corticosteroids on morbidity and mortality of preterm infants. Eur J Obstet Gynecol Reprod Biol. 2007;131:154–7. doi: 10.1016/j.ejogrb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Elimian A, Figueroa R, Spitzer AR, Ogburn PL, Wiencek V, Quirk JG. Antenatal corticosteroids: are incomplete courses beneficial? Obstet Gynecol. 2003;102:352–5. doi: 10.1016/s0029-7844(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacking D, Watkins A, Fraser S, Wolfe R, Nolan T. Respiratory distress syndrome and birth order in premature twins. Archives of disease in childhood. 2001;84:F117–21. doi: 10.1136/fn.84.2.F117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjalmarson O. Epidemiology and classification of acute, neonatal respiratory disorders. A prospective study. Acta Paediatr Scand. 1981;70:773–83. doi: 10.1111/j.1651-2227.1981.tb06228.x. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol. 2005;25(Suppl 2):S31–5. doi: 10.1038/sj.jp.7211317. [DOI] [PubMed] [Google Scholar]
- 14.Jobe AH, Newnham JP, Willet KE, Moss TJ, Gore Ervin M, Padbury JF, Sly P, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162:1656–61. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 15.Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L527–36. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- 16.Kramer BW, Kramer S, Ikegami M, Jobe AH. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2002;283:L452–9. doi: 10.1152/ajplung.00407.2001. [DOI] [PubMed] [Google Scholar]
- 17.Morken NH, Kallen K, Jacobsson B. Outcomes of preterm children according to type of delivery onset: a nationwide population-based study. Paediatr Perinat Epidemiol. 2007;21:458–64. doi: 10.1111/j.1365-3016.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- 18.Moss TJ, Nitsos I, Kramer BW, Ikegami M, Newnham JP, Jobe AH. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. Am J Obstet Gynecol. 2002;187:1059–65. doi: 10.1067/mob.2002.126296. [DOI] [PubMed] [Google Scholar]
- 19.Moutquin JM. Classification and heterogeneity of preterm birth. BJOG. 2003;110(Suppl 20):30–3. doi: 10.1016/s1470-0328(03)00021-1. [DOI] [PubMed] [Google Scholar]
- 20.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD004454.pub2. CD004454. [DOI] [PubMed] [Google Scholar]
- 21.Savitz DA, Blackmore CA, Thorp JM. Epidemiologic characteristics of preterm delivery: etiologic heterogeneity. Am J Obstet Gynecol. 1991;164:467–71. doi: 10.1016/s0002-9378(11)80001-3. [DOI] [PubMed] [Google Scholar]
- 22.Schiff E, Friedman SA, Mercer BM, Sibai BM. Fetal lung maturity is not accelerated in preeclamptic pregnancies. Am J Obstet Gynecol. 1993;169:1096–101. doi: 10.1016/0002-9378(93)90262-h. [DOI] [PubMed] [Google Scholar]
- 23.Shah DM, Shenai JP, Vaughn WK. Neonatal outcome of premature infants of mothers with preeclampsia. J Perinatol. 1995;15:264–7. [PubMed] [Google Scholar]
- 24.Shimoya K, Taniguchi T, Matsuzaki N, Moriyama A, Murata Y, Kitajima H, Fujimura M, Nakayama M. Chorioamnionitis decreased incidence of respiratory distress syndrome by elevating fetal interleukin-6 serum concentration. Hum Reprod. 2000;15:2234–40. doi: 10.1093/humrep/15.10.2234. [DOI] [PubMed] [Google Scholar]
- 25.Shinwell ES, Blickstein I, Lusky A, Reichman B. Effect of birth order on neonatal morbidity and mortality among very low birthweight twins: a population based study. Archives of disease in childhood. 2004;89:F145–8. doi: 10.1136/adc.2002.021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winn HN, Klosterman A, Amon E, Shumway JB, Artal R. Does preeclampsia influence fetal lung maturity? J Perinat Med. 2000;28:210–3. doi: 10.1515/JPM.2000.028. [DOI] [PubMed] [Google Scholar]
- 27.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–70. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]