Abstract
Background
Inflammatory markers could be helpful in the management of patients with right iliac fossa pain, but the heterogeneity of designs and results precludes a definitive conclusion. A retrospective analysis of prospectively collected data was performed to assess the usefulness of laboratory data in the management of these patients.
Patients and methods
Patients with right iliac fossa pain referred to the surgeon were included. Blood samples were obtained for C-reactive protein, leukocyte, and granulocyte analysis. Clinical, surgical, and histopathologic data were collected. Analysis of inflammatory parameters was performed with logistic regression and areas under the receiver operating characteristic curve were compared.
Results
One hundred thirty-four patients were included. C-reactive protein increased with the severity of appendicitis and predicted accurately perforation (r2 = 0.613; P < 0.0005), showing the highest accuracy among inflammatory markers (areas under the ROC curve were 0.846, 0.753 and 0.685 for C-reactive protein, leukocyte and granulocytes, respectively; P < 0.001). Accuracy improved when C-reactive protein and leukocytes were combined (positive and negative predictive values were 93.2 percent and 92.3 percent, respectively).
Conclusions
C-reactive protein is a helpful marker in the management of patients with right iliac fossa pain. It increases with the evolution of the inflammatory process. Its predictive values improve in combination with the leukocyte count. A patient with normal C-reactive protein and leukocytes has a very low probability of appendicitis and should not undergo surgery.
Keywords: Abdominal Pain; blood; diagnosis; etiology; Acute Disease; Adolescent; Adult; Aged; Appendectomy; Appendicitis; complications; diagnosis; surgery; Biological Markers; blood; C-Reactive Protein; metabolism; Diagnosis, Differential; Diagnostic Errors; Disease Progression; Female; Follow-Up Studies; Humans; Laparotomy; Leukocyte Count; Male; Middle Aged; Pain Measurement; Prognosis; Prospective Studies; Rupture, Spontaneous; Sensitivity and Specificity; Severity of Illness Index; Time Factors
Keywords: C-reactive protein, white blood-cells count, acute appendicitis, right iliac fossa pain, abdominal pain, diagnosis, management
Introduction
Acute appendicitis is the most common abdominal surgical emergency. Misdiagnosis have a major impact in health care systems, as well as important legal consequences.1,2 Nowadays, the negative appendectomy rate is still about 15 percent and the perforation rate can be as high as 35 percent. Despite the wide use of imaging techniques, appendicitis remains a challenging diagnosis.3,4 The ability of computerized tomography to reduce the rate of negative appendectomies and perforations is still under discussion and remains to be proved in prospective trials.5–8 Some works have assessed the diagnostic accuracy of different inflammatory markers in appendicitis with heterogeneous designs and results (white blood cells, granulocytes, C-reactive protein, leukocyte elastase activity, D-lactate, phospholipase A2, interleukine-6…).9–13 Two meta-analyses have claimed that no conclusions can be reached from such a variety of populations, designs and results,14,15 even if leukocytes and C-reactive protein (CRP) emerge as the most accurate laboratory data in the diagnosis of acute appendicitis. We performed a retrospective analysis of prospectively collected data to assess and compare the accuracy of CRP, white blood cells and granulocytes in the management of adults with acute right iliac fossa pain.
Patients and methods
Aims and design of the study
The primary aim of the study was to assess inflammatory parameters’ usefulness in the management of adults with acute right iliac fossa pain (ARIFP). The secondary objective was to assess the correlation between levels of inflammatory markers and the severity of appendicitis when it was the final diagnosis. During one year and a half (October 2001 – April 2003) a single institution, observational, prospective study was performed.
Study population – inclusion and exclusion criteria
All consecutive patients referred to the surgeon on call for ARIFP in previously scheduled days (5 per month) were eligible. They were included if they fulfilled the inclusion criteria: age over 15 years, acute pain and suspected appendicitis after the clinical examination performed by the surgeon. Pregnant women, patients with previous appendectomy and those having a disease or a long-term treatment impairing the inflammatory response were excluded. All participants gave written informed consent for inclusion in the study.
Data collection
Demographic information was collected for enrolled patients (age, sex), as were medical histories and clinical data (body temperature, time passed between the onset of pain and the admission to the emergency department and presence of peritoneal signs at clinical examination by the surgeon). Blood samples were obtained on admission to the emergency room for all patients: C-reactive protein was measured by immuno-turbidimetry (Cobas Integra 700, Roche Diagnostic, Switzerland); routine blood counts including white blood cells count and percentage of granulocytes were performed with an automatic analyser (ABX Pentra 120, ABX Diagnostic, France). A pregnancy test was performed in all fertile women. Clinical and laboratory data were recorded, as well as surgical and pathological results in operated patients. In our laboratory CRP was considered normal under 6 mg/L; white blood cells count (WBC) was normal between 4.500 and 9.600 per mm3 and granulocytes were normal if they were less than 75 percent of WBC.
Management of patients
WBC and granulocytes were available in the emergency setting, whereas CRP was not. The surgeon decided for further imaging (abdominal ultrasonography or CT-scan), examination by a gynaecologist, observation with serial clinical exams or direct surgery at his own criteria.
Definitions and diagnosis
Patients were considered included in the “surgical disease group” if the pathologist confirmed the diagnosis of appendicitis or if other surgical disease was proved at laparotomy. Staging of appendicitis was macroscopically performed always by the same surgeon (P.O.D.) during appendectomy and classified as phlegmonous (inflammation), gangrenous (necrotic areas) or perforated. Patients were included in the “no surgical disease group” if: 1) negative appendectomy was confirmed by the pathologist, 2) a disease not requiring surgical treatment was found at laparotomy or imaging, 3) in-hospital observation without surgical or antibiotic treatment for 24 hours was followed by clinical improvement and discharge (these patients were contacted by phone 72 hours after discharge to assess the absence of pain).
Statistical analysis
Laboratory levels are given as mean values followed by the 95 percent confidence interval. Decimals were always conserved for calculation, but only the first decimal is shown for CRP and granulocytes; they were neglected for WBC. Comparison of mean values between both groups was performed with a Student t test (if normal distribution according to Kolmogorov-Smirnov and Shapiro-wilk tests) or with non-parametric tests (Wilcoxon or Mann-Whitney U test) as appropriate. Comparison of mean values of CRP, leukocytes and granulocytes between different degrees of appendicitis were performed with ANOVA or Kruskal-Wallis (as appropriate). Univariate and multivariate step-by-step forward logistic regression were performed to assess the diagnostic accuracy of the different predictive models for surgical disease (with Nagelkerke r2 coefficient), as well as area under the ROC curve (AUC) and its significance were calculated. Multinomial regression was performed to assess the ability of every laboratory data to predict the staging of disease in case of appendicitis. A two-tailed P of < 0.05 was considered significant for all tests. Data collection and statistical calculations were performed using SPSS (version 10.0) software.
Results
Characteristics of patients and diagnosis
Among patients referred to the Emergency Department for ARIFP during the period analysis, the surgeon on call evaluated 149 patients with suspected appendicitis prompting clinical observation, further imaging or direct surgery; 135 of them fulfilled the inclusion criteria. One operated patient was later excluded because of the unavailability of pathological examination and the absence of final diagnosis. Thus the final population study was composed of 134 patients: 60 men and 74 women, mean age: 33 years (CI 95 percent: 31.7 to 34.3 years; range: 15 to 75 years). Median evolution time after onset of symptoms was 22 hours.
Final diagnosis was acute appendicitis in 88 patients (65.7 percent), other surgical diseases in 11 patients (8.2 percent) and no surgical disease in 35 patients (26.1 percent) (distribution of diagnosis in Table 1). All patients with surgical diseases were operated on by McBurney or midline laparotomy, as well as 18 of 35 patients without surgical disease (negative appendectomy rate of 15.4 percent).
Table 1.
Distribution of diagnosis in the 134 patients included in the study.
|
Surgical diseases other than appendicitis (n = 11) |
| Bowel perforation: 2 |
| Diverticulitis: 3 |
| Bowel ischemia: 2 |
| Crohn’s disease with abcess: 1 |
| Perforated caecum carcinoma: 1 |
| Right adnexal endometriosis: 1 |
| Anisakiasis: 1 |
|
Appendicitis and severity (n = 88) |
| Phlegmonous appendicitis: 52 |
| Gangrenous appendicitis: 21 |
| Perforated appendicitis: 15 (17 percent) |
|
Operated patients without surgical disease (n = 18) |
| No findings: 15 |
| Non-specific ileitis: 2 |
| Mesenteric adenitis: 1 |
|
Non-operated patients (n = 17) |
| Non-specific abdominal pain: 12 |
| Non-specific ileitis: 1 |
| Anisakiasis: 1 |
| Mesenteric adenitis: 2 |
| Crohn’s disease: 1 |
Patients with surgical diseases other than acute appendicitis (n = 11) did not have routine appendectomy. Their CRP values were significantly higher than those for patients with appendicitis (CRP levels of 127.8 vs. 60.2 mg/L, P = 0.013). Mean WBC and granulocytes were also higher in those patients but P values were not significant.
Mean age and sex were not different between patients with and without surgical disease. Median evolution time after onset of symptoms was slightly different between both groups (20 hours for patients with surgical diseases vs. 24 hours for patients without surgical diseases; P = 0.03) as well as sex distribution (43 women and 56 men in the group with surgical diseases vs. 25 women and 10 men in the group without surgical diseases; P = 0.003).
Inflammatory markers and surgical disease
Mean levels of CRP, WBC and granulocytes were all significantly higher in the group of patients with surgical diseases (Table 2), both with parametric and non-parametric tests. Predictive models in univariate analysis are shown in Table 3. All inflammatory markers were significant, CRP having the highest diagnostic accuracy (AUC) and the best correlation in regression analysis. The diagnostic accuracy improved when CRP and WBC were combined, rising up to a diagnostic accuracy of 86.8 percent (P < 0.0005), whereas it did not improve with other combinations of laboratory data.
Table 2.
Mean values of inflammatory markers in patients with and without surgical disease.
| Surgical disease | No surgical disease | P value | |
|---|---|---|---|
| CRP (mg/L) | 67.7 | 12.2 | < 0.0005 |
| WBC (per mm3) | 14846 | 10791 | < 0.0005 |
| Granulocytes (percent) | 79.9 | 71.9 | 0.001 |
CRP: C-reactive protein; WBC: white blood-cells
Table 3.
Predictive value of laboratory parameters (univariant analysis).
| r2(*) | P for r2 | AUC (P for AUC) | |
|---|---|---|---|
| CRP | 0.29 | P < 0.00005 | 0.846 (P < 0.0005) |
| WBC | 0.20 | P < 0.00005 | 0.753 (P < 0.0005) |
| Granulocytes | 0.15 | P = 0.0002 | 0.685 (P < 0.001) |
| CRP + WBC | 0.48 | P < 0.00005 | 0.868 (P < 0.0005) |
Nagelkerke coefficient.
CRP: C-reactive protein; WBC: white blood-cells; AUC: area under the ROC curve.
After multivariate analysis, CRP was the only inflammatory marker retained as significant (P < 0.0005), whereas WBC and granulocytes were not (P = 0.30 and P = 0.27, respectively).
Does CRP correlate with the severity of appendicitis?
When only patients with appendicitis were considered, CRP, WBC and granulocytes were found to be different following the severity of inflammation (see Table 4). CRP levels progressively increased with the severity of appendicitis, whereas WBC and granulocytes did not and even decreased in perforated cases as compared with gangrenous appendicitis (see Figure 1). Multinomial regression showed that CRP was the only single significant predictor of perforation among the laboratory data (P < 0.005).
Table 4.
Data following the severity of appendicitis.
| Sex (M/F) | Evolution time | Mean CRP (CI 95 percent) | Mean WBC count (CI 95 percent) | Mean percent granulocytes (CI 95 percent) | |
|---|---|---|---|---|---|
| No appendicitis | 10/25 | 42.1 | 12.2 (3.9–20.5) | 10791 (9380–12202) | 71.9 (67.6–76.2) |
| Phlegmonous | 32/20 | 20.1 | 27 (18.9–35.1) | 14486 (13313–15660) | 78.7 (75.9–81.5) |
| Gangrenous | 10/11 | 24.7 | 60.6 (41.1–80) | 15972 (13714–18231) | 83.2 (80.1–86.3) |
| Perforated | 8/7 | 50.1 | 174.8 (107.8–241.8) | 15765 (12986–18545) | 80.5 (76.8–84.2) |
| P value | > 0.05* | < 0.0005+ | < 0.0005+ | < 0.0005+ | < 0.0005+ |
P value of Chi-square test.
P value of ANOVA.
Figure 1.
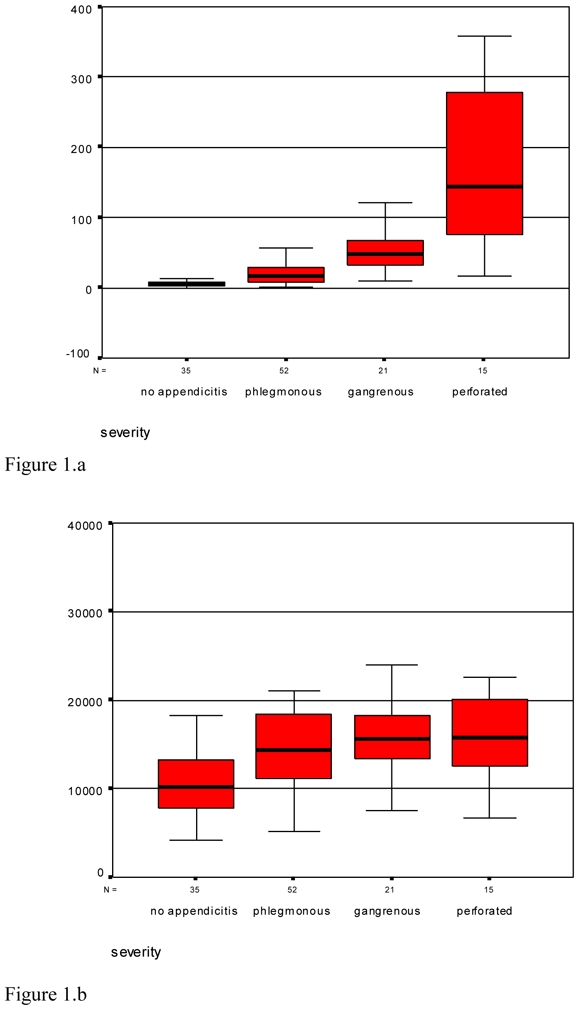

Distribution of C-reactive protein (CRP) (1.a), white blood-cell count (1.b) and percentage of granulocytes (1.c) following the severity of appendicitis.
Influence of evolution time
Patients were stratified following the evolution time in a group with more than 12 hours of evolution since the onset of pain and another one with less than 12 hours. CRP and WBC had better diagnostic accuracy in patients with more than 12 hours of evolution (areas under the ROC curve were 0.896 vs. 0.779 for CRP, P < 0.0005; they were 0.795 vs. 0.609 for WBC, P < 0.0005). The combination of CRP and WBC also showed higher accuracy in patients with more than 12 hours of evolution (0.913 vs. 0.845; P < 0.0005).
Predictive value of inflammatory parameters
The predictive values of laboratory data are shown in Table 5. The population studied had an overall prevalence of appendicitis of 65.7 percent. The positive predictive value when CRP and WBC were elevated was 93.2 percent, while the negative predictive value if both markers were normal was 92.3 percent.
Table 5.
Predictive values of laboratory data.
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| CRP | 90.9 % | 74.3 % | 79.8 % | 74.3 % |
| WBC | 86.4 % | 42.9 % | 73.6 % | 62.5 % |
| Granulocytes | 82.8 % | 45.7 % | 73.3 % | 48.5 % |
| CRP + WBC | 85.8 %(*) | 37.1 %(+) | 93.2 %(*) | 92.3 %(+) |
PPV: positive predictive value. NPV: negative predictive value.
Both elevated.
Both normal.
Discussion
Acute appendicitis remains nowadays a challenging diagnosis. Up to one third of patients have atypical clinical features.16 The wide use of ultrasonography and CT-scan has not effectively decreased the rate of perforated appendicitis or the number of negative appendectomies in large population studies,3,4 despite the hopeful results of some series in tertiary care academic hospitals.16,17
Andersson showed in a recent meta-analysis that diagnostic accuracy was higher for laboratory data than for clinical signs and symptoms, even when recorded by an experienced surgeon15. Some authors have assessed the diagnostic value of inflammatory markers with quite different designs and results.9–11,16–28 Most studies assessing its accuracy were retrospective and included only operated patients (introducing therefore an important selection bias);14 thus their results should not be transferred to patients complaining of abdominal pain. Many of them also included children, which are known to have a different kinetics for CRP and other inflammatory markers.16,17,30 Variety of designs explains the lack of evidence in the two meta-analysis published to date about this topic.14,15
This single institution study was prospective and included consecutive adults referred by the emergency physician to the general surgeon on call for ARIFP. These selection criteria explain a high prevalence of surgical diseases in our study (73.8 percent) and reflect accurately the daily practise of a general surgeon (usually examining the patient after the family or emergency physician). Moreover, performing the study within this selected population allows the transfer of results to this setting.37 Negative appendectomy and perforation rates in the current study were consistent with the literature.1,4
Diagnostic value
Inflammatory markers should not been considered as specific for any medical or surgical disease. However, when applied in patients with ARIFP after clinical examination by a senior surgeon (a selected population), they could be a helpful diagnostic tool because of the rising probability of appendicitis in this setting.
CRP was the most accurate single laboratory data for the diagnosis of appendicitis in the current study (accuracy was 84.6 percent, P < 0.0005), which is consistent with the literature.14,15
Wu et al. have recently assessed different cut-off values for inflammatory parameters, as well as the usefulness of their changes over time during a in-hospital observation period.9,29 There was a lower incidence of appendicitis in their study as compared with the present one. They concluded that the change of total neutrophil count was useful in the first day while CRP was most accurate in the second and third days after onset of symptoms. In our work, neutrophil count did not improve the predictive model, even after stratification following evolution time; this difference can be explained by the fact that median evolution time was 22 hours in the current study and few patients could have this parameter assessed during the first day after onset of symptoms.
CRP predictive values for appendicitis have been very heterogeneous in medical literature (sensitivity ranging from 40 percent to 99 percent and specificity from 27 percent to 90 percent).14 Sensitivity in the current study was consistent with values obtained recently by other authors but specificity was higher.10,27 This can be explained by our higher prevalence of appendicitis caused by the selection criteria; specificity is well-known to increase with the prevalence of disease.
CRP diagnostic accuracy increased if the WBC count was added, rising to a positive predictive value of 93.2 percent when both markers were elevated and a negative predictive value of 92.3 percent when both were normal. This conclusion is consistent with data from other authors who obtained negative predictive values as high as 100 percent with the adjunction of WBC to CRP.15, 24, 28 Birchley reported recently a small prospective study with better positive than negative predictive values; this was probably the result of the use of inflammatory markers as categorical variables in that study.36
A diagnostic test for appendicitis should have a high sensitivity to avoid a late diagnosis, but also a high specificity to avoid unnecessary further studies or negative appendectomies. The combination of CRP and WBC fulfils this requirements when used in this setting; it could be a safe criteria to select patients for further imaging techniques.37
Diagnostic laparoscopy was not routinely used in an emergency setting in our department when the study was conducted. Even nowadays its place in the management of abdominal pain is not well defined.38 It could probably be useful in patients with atypical or discordant clinical, laboratory and imaging data, when clinical observation is not appropriate.
The under-representation of gynaecological diseases in our population can be explained by the fact that some fertile women were seen by a gynaecologist at the request of the emergency physician and then by the surgeon after the elimination of gynaecological diseases.
Influence of evolution time in laboratory data
Some authors have claimed that CRP is less accurate than WBC in the first hours after the onset of pain but its sensitivity rises to 100 percent after 12 hours; appendicitis could be excluded if CRP is normal after 12 hours.21,25,35 In the current study, accuracy was 77.9 percent in patients with less than 12 hours after onset of pain and increased to 89.6 percent after 12 hours. This is slightly higher than obtained in other series but it can also be explained by our lower cut-off value (6 mg/L) as compared with 10 mg/L in most works.24,28 During the first 12 hours after onset of pain it is specially indicated to consider CRP together with WBC; if one of them is normal and the other is elevated, patients could benefit from clinical observation or further imaging.
Wu et al. obtained areas under the curve as high as 0.97 and 0.91 for CRP during days 2 and 3 after onset of symptoms.29 These results are consistent with the current study (0.896 for patients with more than 12 hours after onset of pain), even if cut-off values were higher in their study (9.5 and 17 mg/L as compared with 6 mg/L in the current study).
Severity of appendicitis
The dynamic usefulness of inflammatory parameters has been pointed out by some authors which performed repeated laboratory examinations in patients with equivocal signs.22,29 It has been suggested that CRP could accurately predict perforated appendicitis in children and adults.24 Our results confirm a close correlation between CRP and the severity of appendicitis, which is consistent with the results reported by Grönroos et al.24 Distinguishing between perforated and non-perforated appendicitis becomes more important as an increasing number of authors claim that these are different diseases: perforated appendicitis requires emergent surgery whereas non-perforated appendicitis can be accurately delayed some hours;39 some authors even suggest that medical treatment could be suitable in uncomplicated appendicitis, but this is not still a standard of care40,41. WBC and granulocytes were not accurate in this setting, as they reached their highest level in gangrenous appendicitis and decreased in perforated appendicitis. Detecting complicated forms with CRP (values > 100 mg/L) could save time avoiding further imaging and prompting emergent surgery. On the other hand, patients with CRP levels between 10 and 40 mg/L and elevated WBC have probably phlegmonous appendicitis; further imaging can be safely performed if the clinical picture is atypical. Delaying surgery some hours would not harm these patients39.
We conclude that C-reactive protein is the most useful single laboratory parameter in the management of adults sent to the surgeon for suspected appendicitis, particularly after 12 hours of evolution. It adds helpful information in the decision analysis identifying patients that require surgery and those that can benefit from clinical observation or further imaging. Its accuracy and predictive values improve when combined with the leukocyte count, whereas granulocytes did not add any information. A patient with clinically suspected appendicitis and both elevated C-reactive protein and leukocyte count does not require further imaging and should undergo surgery. A patient with both normal C-reactive protein and leukocytes has a very low probability of appendicitis; emergent surgery is not indicated and clinical observation or further imaging are the best management choice in this setting. When laboratory data are discordant, further imaging is warranted (even more if the clinical picture is not typical). C-reactive protein levels are strongly correlated to the severity of the inflammation and detect accurately perforated appendicitis. A prospective study assessing the impact of CRP and leukocytes in the negative appendectomy rate is warranted.
Acknowledgments
The authors thank José M. Rodriguez-Barbero M.D. for the review of the histopathological diagnosis, and to T. Pascual-Duran M.D. for his help with the laboratory techniques. They also thank the Nursing Team of the Emergency Department in the University Hospital of Getafe.
Footnotes
No conflict of interest declared.
Presented in part at the 16th World Congress of the International Association of Surgeons & Gastroenterologists (I.A.S.G.), held in Madrid (Spain), 2006, May 25th–27th.
References
- 1.Flum DR, Koepsell T. The clinical and economic correlates of misdiagnosed appendicitis. Nationwide analysis. Arch Surg. 2002;137:799–804. doi: 10.1001/archsurg.137.7.799. [DOI] [PubMed] [Google Scholar]
- 2.Gill B, Jenkins J. Cost-effective evaluation and management of acute abdomen. Surg Clin North Am. 1996;76:71–82. doi: 10.1016/s0039-6109(05)70423-0. [DOI] [PubMed] [Google Scholar]
- 3.Flum DR, Morris A, Koepsell T, Dellinger EP. Has misdiagnosis of appendicitis decreased over time? A population-based analysis. JAMA. 2001;286:1748–53. doi: 10.1001/jama.286.14.1748. [DOI] [PubMed] [Google Scholar]
- 4.Flum DR, McClure TD, Morris A, Koepsell T. Misdiagnosis of appendicitis and the use of diagnostic imaging. J Am Coll Surg. 2005;201:933–9. doi: 10.1016/j.jamcollsurg.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 5.Rao PM, Rhea JT, Rattner DW, Venus LG, Novelline RA. Introduction of appendiceal CT: impact on negative appendectomy and appendiceal perforation rates. Ann Surg. 1999;229:344–9. doi: 10.1097/00000658-199903000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balthazar EJ, Rofsky NM, Zucker R. Appendicitis: the impact of computed tomography imaging on negative appendectomy and perforation rates. Am J Gastroenterol. 1998;93:768–71. doi: 10.1111/j.1572-0241.1998.222_a.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee SL, Walsh AJ, Ho HS. Computed tomography and ultrasonography do not improve and may delay the diagnosis and treatment of acute appendicitis. Arch Surg. 2001;136:556–562. doi: 10.1001/archsurg.136.5.556. [DOI] [PubMed] [Google Scholar]
- 8.Walker S, Haun W, Clark J, McMillin K, Zeren F, Gilliland T. The value of limited computed tomography with rectal contraste in acute appendicitis. Am J Surg. 2000;180:450–4. doi: 10.1016/s0002-9610(00)00540-7. [DOI] [PubMed] [Google Scholar]
- 9.Wu HP, Lin CY, Chang CF, Chang YJ, Huang CY. Predictive value of C-reactive protein at different cutoff levels in acute appendicitis. Am J Emerg Med. 2005;23:449–53. doi: 10.1016/j.ajem.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Yang HR, Wang YC, Chung PK, Chen WK, Jeng LB, Chen RJ. Role of leukocyte count, neutrophil percentage, and C-reactive protein in the diagnosis of acute appendicitis in the elderly. Am Surg. 2005;71:344–7. [PubMed] [Google Scholar]
- 11.Eriksson S, Olander B, Pira U, Granstrom L. White blood cell count, leucocyte elastase activity, and serum concentrations of interleukin-6 and C-reactive protein after open appendicectomy. Eur J Surg. 1997;163:123–7. [PubMed] [Google Scholar]
- 12.Caglayan F, Cakmak M, Caglayan O, Cavusoglu T. Plasma D-lactate levels in diagnosis of appendicitis. J Invest Surg. 2003;16:233–7. [PubMed] [Google Scholar]
- 13.Grönroos JM, Forsstrom JJ, Irjala K, Nevalainen TJ. Phospholipase A2, C-reactive protein, and white blood cell count in the diagnosis of acute appendicitis. Clin Chem. 1994;40:1757–60. [PubMed] [Google Scholar]
- 14.Hallan S, Asberg A. The accuracy of C-reactive protein in diagnosing acute appendicitis - a meta-analysis. Scand J Clin Lab Invest. 1997;57:373–80. doi: 10.3109/00365519709084584. [DOI] [PubMed] [Google Scholar]
- 15.Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28–37. doi: 10.1002/bjs.4464. [DOI] [PubMed] [Google Scholar]
- 16.Marchand A, Van Lente F, Galen RS. The assessment of laboratory tests in the diagnosis of acute appendicitis. Am J Clin Pathol. 1983;80:369–74. doi: 10.1093/ajcp/80.3.369. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson S, Granstrom L, Bark S. Laboratory tests in patients with suspected acute appendicitis. Acta Chir Scand. 1989;155:117–20. [PubMed] [Google Scholar]
- 18.Dueholm S, Bagi P, Bud M. Laboratory aid in the diagnosis of acute appendicitis. A blinded, prospective trial concerning diagnostic value of leukocyte count, neutrophil differential count, and C-reactive protein. Dis Colon Rectum. 1989;32:855–9. doi: 10.1007/BF02554555. [DOI] [PubMed] [Google Scholar]
- 19.Oosterhuis WP, Zwinderman AH, Teeuwen M, et al. C reactive protein in the diagnosis of acute appendicitis. Eur J Surg. 1993;159:115–9. [PubMed] [Google Scholar]
- 20.Söndenaa K, Buan B, Söreide JA, et al. Rapid C-reactive protein (CRP) measurements in the diagnosis of acute appendicitis. Scand J Clin Lab Invest. 1992;52:585–9. doi: 10.1080/00365519209115500. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson S, Granstrom L, Carlstrom A. The diagnostic value of repetitive preoperative analyses of C-reactive protein and total leucocyte count in patients with suspected acute appendicitis. Scand J Gastroenterol. 1994;29:1145–9. doi: 10.3109/00365529409094902. [DOI] [PubMed] [Google Scholar]
- 22.Andersson RE, Hugander A, Ravn H, et al. Repeated clinical and laboratory examinations in patients with an equivocal diagnosis of appendicitis. World J Surg. 2000;24(4):479–85. doi: 10.1007/s002689910076. [DOI] [PubMed] [Google Scholar]
- 23.Körner H, Söreide JA, Söndenaa K. Diagnostic accuracy of inflammatory markers in patients operated on for suspected acute appendicitis: a receiver operating characteristic curve analysis. Eur J Surg. 1999;165:679–85. doi: 10.1080/11024159950189744. [DOI] [PubMed] [Google Scholar]
- 24.Grönroos JM, Grönroos P. Leucocyte count and C-reactive protein in the diagnosis of acute appendicitis. Br J Surg. 1999;86:501–4. doi: 10.1046/j.1365-2168.1999.01063.x. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson S, Granstrom L, Olander B, Wretlind B. Sensitivity of interleukin-6 and C-reactive protein concentrations in the diagnosis of acute appendicitis. Eur J Surg. 1995;161:41–5. [PubMed] [Google Scholar]
- 26.Paajanen H, Mansikka A, Laato M, Ristamaki R, Pulkki K, Kostiainen S. Novel serum inflammatory markers in acute appendicitis. Scand J Clin Lab Invest. 2002;62:579–84. doi: 10.1080/003655102764654312. [DOI] [PubMed] [Google Scholar]
- 27.Tepel J, Sommerfeld A, Klomp HJ, Kapischke M, Eggert A, Kremer B. Prospective evaluation of diagnostic modalities in suspected acute appendicitis. Langenbecks Arch Surg. 2004;389:219–24. doi: 10.1007/s00423-003-0439-6. [DOI] [PubMed] [Google Scholar]
- 28.Kessler N, Cyteval C, Gallix B, et al. Appendicitis: evaluation of sensitivity, specificity, and predictive values of US, Doppler US, and laboratory findings. Radiology. 2004;230:472–8. doi: 10.1148/radiol.2302021520. [DOI] [PubMed] [Google Scholar]
- 29.Wu HP, Huang CY, Chang YJ, Chou CC, Lin CY. Use of changes over time in serum inflammatory parameters in patients with equivocal appendicitis. Surgery. 2006;139:789–96. doi: 10.1016/j.surg.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Paajanen H, Mansikka A, Laato M, Kettunen J, Kostiainen S. Are serum inflammatory markers age dependent in acute appendicitis? J Am Coll Surg. 1997;184:303–8. [PubMed] [Google Scholar]
- 31.Gurleyik E, Gurleyik G, Unalmiser S. Accuracy of serum C-reactive protein measurements in diagnosis of acute appendicitis compared with surgeon’s clinical impression. Dis Colon Rectum. 1995;38:1270–4. doi: 10.1007/BF02049151. [DOI] [PubMed] [Google Scholar]
- 32.Lane MJ, Mindelzun RE. Appendicitis and its mimickers. Semin Ultrasound CT MR. 1999;20:77–85. doi: 10.1016/s0887-2171(99)90039-2. [DOI] [PubMed] [Google Scholar]
- 33.Rao PM, Rhea JT, Rattner DW, Venus LG, Novelline RA. Introduction of appendiceal CT: impact on negative appendectomy and appendiceal perforation rates. Ann Surg. 1999;229:344–9. doi: 10.1097/00000658-199903000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balthazar EJ, Rofsky NM, Zucker R. Appendicitis: the impact of computed tomography imaging on negative appendectomy and perforation rates. Am J Gastroenterol. 1998;93:768–71. doi: 10.1111/j.1572-0241.1998.222_a.x. [DOI] [PubMed] [Google Scholar]
- 35.Albu E, Miller BM, Choi Y, Lakhanpal S, Murthy RN, Gerst PH. Diagnostic value of C-reactive protein in acute appendicitis. Dis Colon Rectum. 1994;37:49–51. doi: 10.1007/BF02047214. [DOI] [PubMed] [Google Scholar]
- 36.Birchley D. Patients with clinical acute appendicitis should have pre-operative full blood count and C-reactive protein assays. Ann R Coll Surg Engl. 2006;88:27–32. doi: 10.1308/003588406X83041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwig L, Bossuyt P, Glasziou P, et al. Designing studies to ensure that estimates of test accuracy are transferable. BMJ. 2002;324:669–71. doi: 10.1136/bmj.324.7338.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morino M, Pellegrino L, Castagna E, Farinella E, Mao P. Acute nonspecific abdominal pain: A randomized, controlled trial comparing early laparoscopy versus clinical observation. Ann Surg. 2006;244:881–6. doi: 10.1097/01.sla.0000246886.80424.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abou-Nukta F, Bakhos C, Arroyo K, et al. Effects of delaying appendectomy for acute appendicitis for 12 to 24 hours. Arch Surg. 2006;141:504–6. doi: 10.1001/archsurg.141.5.504. [DOI] [PubMed] [Google Scholar]
- 40.Livingston EH, Woodward WA, Sarosi GA, Haley RW. Disconnect between incidence of nonperforated and perforated appendicitis. Implications for pathophysiology and management. Ann Surg. 2007;245:886–92. doi: 10.1097/01.sla.0000256391.05233.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersson RE. The natural history and traditional management of appendicitis revisited: spontaneous resolution and predominance of prehospital perforations imply that a correct diagnosis is more important than an early diagnosis. World J Surg. 2007;31:86–92. doi: 10.1007/s00268-006-0056-y. [DOI] [PubMed] [Google Scholar]


