Abstract
The programmed death-1 (PD-1)/B7-H1 pathway acts as an important negative regulator of immune responses. We herein investigated the role of the PD-1/B7-H1 pathway in establishing an immunological spontaneous tolerance status in mouse liver allografting. B7-H1 is highly expressed on the donor-derived tissue cells and it is also associated with the apoptosis of infiltrating T cells in the allografts. Strikingly, a blockade of the PD-1/B7-H1 pathway via anti-B7-H1mAb or using B7-H1 knockout mice as a donor led to severe cell infiltration as well as hemorrhaging and necrosis, thus resulting in mortality within 12 days. Furthermore, the expression of the FasL, perforin, granzyme B, iNOS, and OPN mRNA in the liver allografts increased in the antibody-treated group in comparison to the controls. Taken together, these data revealed that the B7-H1 upregulation on the tissue cells of liver allografts thus plays an important role in the apoptosis of infiltrating cells, which might play a critical role of the induction of the spontaneous tolerance after hepatic transplantation in mice.
Keywords: apoptosis, B7-H1, orthotopic liver transplantation, regulatory cell, spontaneous tolerance
Introduction
The programmed death 1 (PD-1) receptor and its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) have been recently characterized (1, 2). PD-1 is induced on peripheral T cells, B cells and myeloid cells upon activation. B7-H1 is widely expressed on resting cells and up-regulated on activated B, T, myeloid, dendritic cells and many tissue cells of non-lymphoid organs, including the liver, whereas B7-DC is expressed exclusively on dendritic cells and monocytes. The parenchymal cells expression of B7-H1 may serve to regulate autoreactive T or B cell responses in peripheral tissues, and/or may serve to regulate the inflammatory responses at these sites. The PD-1/B7-H1 pathway plays an important role in regulating the alloimmune response in experimental models of skin and heart transplantation (3, 4), and graft vs. host disease (3). The suppressive activity of B7-H1 in alloimmune responses has also been demonstrated using the model of fully MHC-mismatched cardiac allografts in which blockade of PD-1/B7-H1 pathway resulted in the accelerated rejection in CD28 and B7-1/B7-2 double deficient recipients (4). More recently, Fife et al. reported that PD-1/B7-H1 signaling is required for protection from autoimmune diabetes in the NOD mouse model (5). These results prompted us to investigate the role and mechanisms of PD-1/B7-H1 pathway in mouse liver spontaneous transplantation tolerance.
Liver allografts in mice are accepted across MHC barriers without requirement for immunosuppressive therapy (6). Similar phenomena are found also in outbred pigs and rats (7, 8). A number of factors have been suggested to be involved in this unique behavior of liver allograft (9), including increased number of B-cell infiltration, production of soluble MHC class I antigens by the transplanted liver, role of passenger leukocytes, and development of regulatory cells. However, the mechanisms of these actions at the molecular level still remain unclear. In the current study, we demonstrated the preferential upregulation of B7-H1 expression on tissue cells of the accepted liver allograft, which contributes to the induction of apoptosis in the liver allograft infiltrating lymphocytes. Furthermore, mouse liver allografts from B7-H1 knockout mice and blocking of PD-1/B7-H1 interactions using anti-B7-H1 monoclonal antibody (mAb) resulted in acute liver allograft rejection. These were associated with increased CD8+ T cells infiltration and FasL, perforin, granzyme B, iNOS, and OPN mRNA expression in anti-B7-H1mAb-treated recipients. Therefore, the data suggest that the PD-1/B7-H1 pathway plays a key role in the spontaneous acceptance of liver allografts in mice.
Materials and Methods
Animals
Male C57BL/6 (B6; H-2b), C3H (H-2k), mice were purchased from the Jackson Laboratory (Bar Harbor, ME). B7-H1 knockout (B7-H1 KO) mice, established after backcrossing over 10 generations to B6, was kindly provided by Dr. Lieping Chen at Johns Hoskins University Medical School, Baltimore, MD. B10.BR (BR, H-2k) and B10.D2 (D2, H-2d) male mice were purchased from Shizuoka Laboratory Animal Center (Shizuoka, Japan). The animals were maintained under standard conditions and fed rodent food and water according to the laboratory animal care principles and the guide for the care and use of laboratory animals in our institutes.
Orthotopic liver transplantation
BR, B6 or B7-H1 KO (B6 background) mice were used as donors and D2 or C3H mice were used as recipients. Transplantation surgery was performed in the mice under anesthesia with ether as describe previously (10). Anit-B7-H1 (clone MIH5), B7-DC (clone TY25) and PD1 (clone RMP1-14) neutralizing antibodies were intraperitoneally administered to the recipient D2 mice at day 0 (500 μg/mice) and than twice a week (250μg/mice) during 4 weeks.
Intra-abdominal vascularized heart transplantation
B6 mice were used as donors and C3H mice were used as recipients. Intra-abdominal vascularized heart transplantation was performed as described (11). Graft survival was assessed by daily transabdominal palpation.
Isolation of liver non-parenchymal cells (NPC)
The liver was perfused via the portal vein with 30 ml Ca++, Ma++ free Hanks balanced salt solution at a rate of 5 ml/min, followed by a perfusion of 1 ml collagenase type IV (1mg/ml, Sigma, St, Louis, MO). The liver was then removed, meshed and agitated in collagenase type IV (1 mg/ml) at 37°C for 30 minutes. The digested cell suspensions were filtered through a nylon mesh (pore size 40 μm). Liver nonparechymal cells (NPC) were then isolated by percoll density-gradient centrifugation, as previously described (12).
Flow cytometry analysis
To examine the expression of surface molecules, cells were stained with mAbs against CD45, CD4, CD8, CD80, CD86, MHC class-I (H-2b and H-2k), (BD. PharMingen, San Diego, CA), or B7-H1 antigen (eBioscience, San Diego, CA). The detection of apoptosis was performed using Annexin V-FITC Apoptosis Detection kit (BD. PharMingen). The cells were analyzed using a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA)
Histological studies
Liver tissues fixed in 10% phosphate-buffered formalin were embedded in paraffin, and their 1μm-thick sections were stained with hematoxylin and eosin for standard microscopy. The severity was graded from mild to severe according to the Banff scoring system for acute rejection (13), which assigns the cellular infiltration of portal triads, bile duct inflammation, and endothelial inflammation scores ranging from 1–3. Specimens measuring up to 1 cm3 were embedded in OCT compound (Tissue-Tek, Elkhart, IN) and stored at −80°C for immunohistochemical staining.
RNA isolation and quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from the liver or spleen of the recipient using RNeasy Mini Kit (QIAGEN Inc., Valencia, CA), according to the manufacturer’s protocol. Quantitative RT-PCR was performed using the TaqMan system on the Applied Biosystems PRISM 7700 (ABI Japan, Co., Ltd., Tokyo, Japan). The target-specific primers and probes were purchased from Applied Biosystems.
Statistical analysis
Student’s t-test was used to compare the paired and unpaired variables. A statistical evaluation for graft survival was performed using the Kaplan-Meier’s test. P values less than 0.05 were considered to be statistically significant.
Results
The accepted liver allografts are chimeric organs and expression of B7-H1 is enhanced on non- parenchymal cells (NPCs) of liver allografts
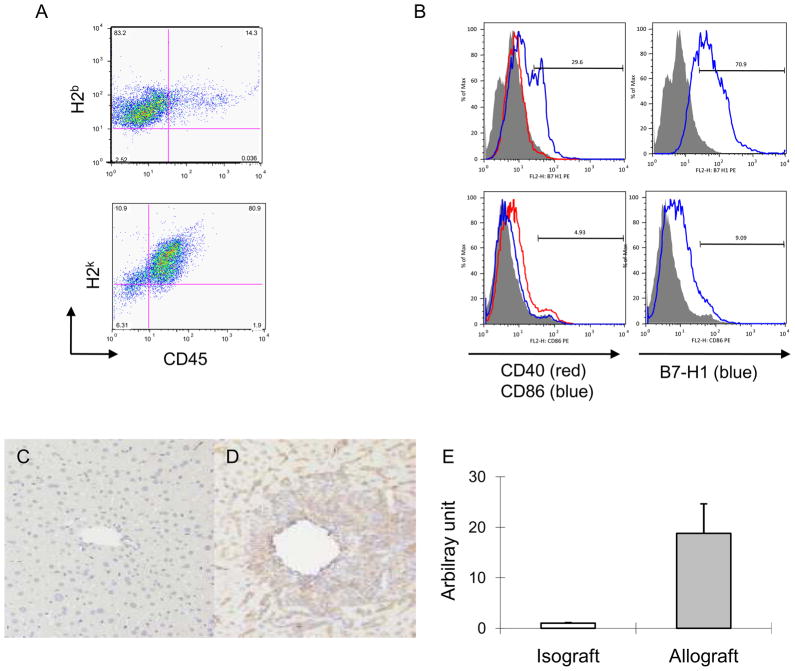
To determine the immune responses after allogeneic liver grafting, we analyzed the NPCs of B6 to C3H liver allografts at different time point of transplantation by flow cytometry. In consistent with previous report that massive T cells infiltrating from recipients in the liver grafts, in particular of CD8+ T cells, at 1–2 days after transplantation, then followed by gradual reduction of T cell infiltration (data not show). The NPCs from the accepted liver allografts (at day 83 post transplant) were isolated and double stained with mAbs anti-CD45 and H-2k or H2b. The results indicated that the accepted liver allografts are chimeric organs, in which CD45+ lymphocytes were recipient-derived (H-2k +), and CD45- tissue cells were donor original (H-2b +; Figure 1A). Next, the expressions of stimulatory and inhibitory surface molecules on liver graft NPC were defined. NPCs from B6 to C3H grafted liver were isolated and sorted into CD45+ and CD45− two populations with CD45 magnetic beads, and then stained with anti-CD45 and B7-H1 or other co-stimulatory molecules (CD40, CD86). In consistence with others’ reports that B7-H1 is expressed on most activated cells, in particular of tissue cells in a variety of organs, our results showed that B7-H1 was highly expressed on CD45− tissue cells isolated from liver allografts (Fig.1B upper), but not from normal liver (Fig 1. B lower), and relatively low levels of other co-stimulatory molecules (CD40 and CD86) were expressed in tissue cells derived from both the liver allograft and normal liver, indicating that the tissue cells from liver allografts preferentially expressed B7-H1, a inhibitory molecule of the B7 family. Furthermore, B7-H1 was barely identified in any syngeneic liver grafts by immunohistochemical staining (Fig. 1C), whereas allogeneic liver grafts showed the expression of B7-H1 in both the sinusoidal area and leukocyte infiltrative area (Fig. 1D). The expression of B7-H1 expression was further confirmed in the liver allografts on day 8 post grafting by real-time RT-PCR as shown in Figure 1E. The mRNA expression of B7-H1 in allografts was significantly higher than in syngeneic liver graft and was consistent with the FCM and immunohistochemical results.
Figure 1. Enhanced expression of B7-H1 on tissue cells of liver allografts.
(A) Liver NPCs were isolated from accepted liver allografts on day 83 after transplantation and double stained with mAbs anti-CD45 and H2b (donor) or H-2k (recipient). (B) The NPCs were sorted into CD45+ and CD45− two populations with CD45 magnetic beads, and CD45− cells were stained with mAbs anti-CD40, CD86 and B7-H1. A comparison of the expression of co-stimulatory molecules on the liver tissue cells (CD45−) from naïve liver (lower panel) with that from liver allograft (upper panel) indicated that enhanced expression of B7-H1 (blue line) but not CD40 (red line) and CD86 (blue line) on the liver allograft tissue cells. (C) Immunostaining of B7-H1 is negative or very faintly positive along the sinusoids in syngeneic graft, while, (D) Allogeneic grafts on day 8 after transplantation showed that B7-H1 is robustly positive. Magnification is 100×. The results are representative of three separate experiments. (E) Quantitative reverse transcriptase polymerase chain reaction (RT-PCR) for B7-H1 mRNA expression in the liver isografts and allografts at 8 days after transplantation. The relative quantity is presented as the ratio of the comparative cycle threshold (Ct) of the target genes against those of the housekeeping gene 18s.
B7-H1 mediated T cells apoptosis in liver allograft affects graft outcome
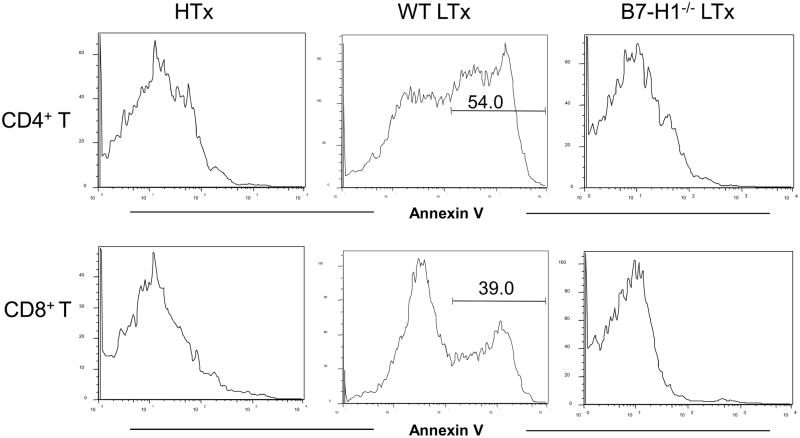
B7-H1 can induce apoptosis of PD-1 expressing cells (4). The fate of CD4/CD8 T cells in accepted liver allograft and rejected hearts allograft was determined by Annexin V staining to determine the role of B7-H1 expressed on donor tissue cells in anti-donor immune responses. As shown in Figure 2, there was a high incidence of apoptotic CD4+ (54%) and CD8+ (39%) T cells in the spontaneously accepted liver grafts, but not in the rejected cardiac allografts. B7-H1−/− allogeneic livers were acutely rejected (MSD 5+/−1.0 day) in association with massive infiltration in the liver (data not shown). The level of CD8+ and CD4+ T cell apoptosis in B7-H1−/− liver allografts are lower in comparison to the wild type liver allograft.
Figure 2. T cells apoptosis in the liver allografts is B7-H1-dependent.
Liver and heart GICs from recipients of allogeneic liver and heart graft on day 6 after transplantation were isolated and staining with CD4, CD8 and Annexin V. Apoptosis of CD4+ and CD8+ T cell from liver allograft was greater than heart allograft and T cells from a liver allograft without B7-H1 expression showed no apoptosis. The GICs from 3 mice per group were pooled and the results are representative of two separate experiments.
Spontaneous acceptance of liver transplants is dependent on B7-H1 expression
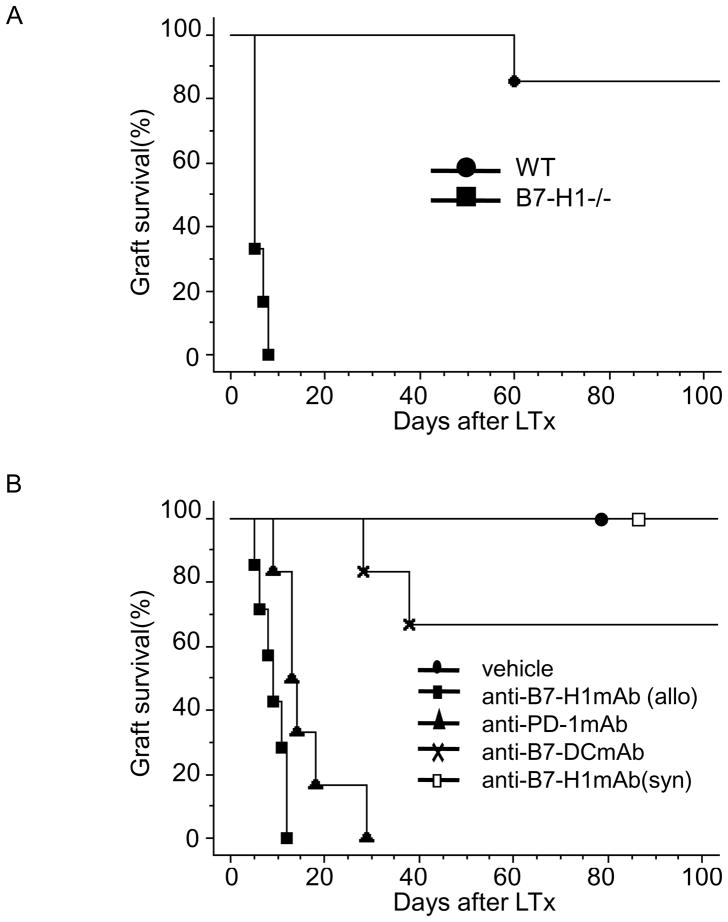
The liver obtained from B7-H1−/− (B6) mice was transplanted into allogeneic (C3H) mice without any immunosuppressants and the survival of the recipients was monitored to examine the role of B7-H1 expression in spontaneous tolerance of the mouse liver allograft. As demonstrated in Figure 3A, more than 85% of WT B6 livers transplanted into WT C3H mice (n =7) survived at 100 days post transplant (MST >100 days). In contrast, B7-H1−/− B6 liver allografts transplanted into WT C3H mice (n = 6) resulted in rejection within 7 days (MST = 5 ± 1.3 days; p<0.01).
Figure 3. The B7-H1 expression is required in mouse liver allograft spontaneous tolerance.
(A) WT or B7-H1−/− B6 liver was transplanted to WT C3H mice, and graft rejection was monitored daily. Each combination of donor and recipient was described as follow: WT to WT (●; n = 7; MST > 100 days), (B7-H1 −/−to WT; ■; n = 6; MST = 5 ± 1.3 days; p < 0.01). (B) B7-H1, PD-1 and B7-DC blockade abrogates the mouse spontaneous liver transplantation tolerance. The D2 recipients received BR liver donors with anti-B7-H1mAb (■; n =7; MST = 9 ± 2.8 days; p < 0.01), anti-PD-1 mAb (▲; n = 6; MST = 14 ± 7.0 days; p < 0.01), anti-B7-DC mAb (×; n = 6; MST = 78 ± 34.7 days; p < 0.01), vehicle treatment (●; n = 7; MST > 100 days) and D2 recipients received D2 liver donor with anti-B7-H1 mAb (□; n = 7; MST > 100 days).
Blockade of the PD-1/B7-H1 interaction with anti-B7-H1 or anti-PD-1mAb broke mouse liver transplant spontaneous tolerance
The effect of anti-B7-H1mAb on liver allograft survival was investigated to further evaluate the effect of B7-H1 and to exploit the possible therapeutic applications in treatment of liver diseases. Blocking monoclonal antibodies were administered to recipients that received a transplant with an allogeneic liver. In these cases, normal BR liver allografts were accepted by D2 recipients without immunosuppressive therapy (vehicle treatment, MST >100 days; n = 7), whereas grafts were rejected acutely within 12 days in the anti-B7-H1mAb treated recipients (MST = 9 ± 2.6 days; n=7; Fig. 3B). The effect of anti-B7-H1mAb on syngeneic liver grafts was examined to determine the specificity of anti-B7-H1mAb treatment. Anti-B7-H1mAb treatment did not affect syngeneic liver graft survival (MST >100 days; n=7). Furthermore, anti-PD-1 or B7-DCmAb was administrated to mice after liver allografting. As shown in Figure 3B, allografts were rejected within 29 days in the anti-PD-1mAb treated recipients (MST = 16 ± 7.0 days; n = 6), while, anti-B7-DCmAb treatment showed a slighter effect on inhibition of the induction of spontaneous tolerance in mouse liver allograft (MST = 77 ± 34.7 days; n=6). Therefore, these data indicated that blockade of B7-H1 signaling pathway by administration of anti-B7-H1 or anti-PD-1 mAb leads to enhancement of anti-donor immune responses. Giving the factor that the PD-1/B7-H1 signaling pathway was necessary for the induction of allogeneic mouse liver transplant tolerance, immune responses in the inflamed liver could be modulated by controlling PD-1/B7-H1 signaling pathway
Histological analysis of rejecting liver allografts induced by anti-B7-H1 mAb
As shown in Table 1, using a modification of the Banff grading system for liver allograft rejection, higher scores for inflammation were observed in the grafts harvested from the anti-B7-H1mAb treatment recipients in comparison to the control recipients. At days 8 after transplant, BR liver allografts in the D2 mice treated with anti-B7-H1mAb manifested a greater infiltration of portal triads, inflammation of the veins, and invasion of the bile duct epithelium by lymphocytes. There was no hemorrhage or parenchymal necrosis in the control recipients, but there was extensive necrosis with some congestion and hemorrhage in livers being rejected by the anti-B7-H1mAb treated recipients (data not shown).
Table 1.
Banff score of mouse liver grafts□
| Control | anti-B7-H1mAb | p-value* | |
|---|---|---|---|
| Portal triad inflammation | 2.0±0.0 | 2.8±0.4 | < 0.05 |
| Venous inflammation | 1.4±0.5 | 2.6±0.5 | < 0.05 |
| Bile duct inflammation | 0.8±0.4 | 2.0±0.7 | < 0.05 |
| Necrosis | 0.0±0.0 | 1.4±0.5 | < 0.005 |
| Total | 4.2±0.8 | 7.4±1.7 | < 0.005 |
The severity was graded from mild to severe according to the Banff scoring system for acute rejection, which assigns the cellular infiltration of portal triads, bile duct inflammation, and endothelial inflammation scores ranging from 1–3 thus indicating; 1, minimal changes; 2, moderate changes; or 3, severe changes. Additional findings not included in the Banff scoring system were also examined. The extent of necrosis was scored as the percentage of parenchymal involvement (0, no necrosis present; 1, <25% of the total parenchyma involved; 2, 25–50% of total parenchyma involved; 3, 50–75% of the total parenchyma involved and 4, >75% of total parenchyma involved).
Significant difference was calculated at day 8 using Mann–Whitney test between the control and anti-B7-H1mAb administration groups.
Cytotoxic T lymphocyte (CTL) infiltration and its related mRNAs expression in liver allograft
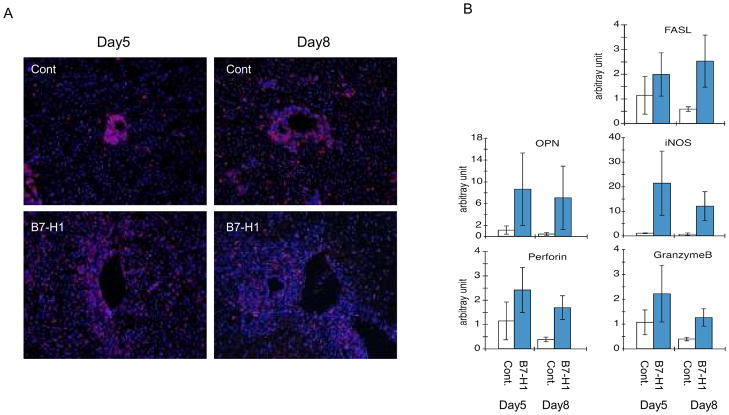
Consistent with pathological findings, the CD8+ cytotoxic T lymphocyte (CTL) infiltration was dramatically increased and the cells that migrated to the sinusoidal area in grafts harvested from the anti-B7-H1 mAb treatment recipients, while the CTL infiltration limited around the portal area in the control grafts (Fig. 4A). Furthermore, the expression of a number of genes associated with CTLs (granzyme B, FasL and perforin) and other inflammatory cytokines (Osteopontin; OPN and inducible nitric oxide synthase; iNOS) in liver allografts at days 5 and 8 post grafting were determined by real-time RT-PCR analysis, and were compared with the anti-B7-H1 mAb treated-group (Fig. 4B). The expression of all genes was massively elevated in the liver allografts and all mRNA levels were significantly higher in the anit-B7-H1 mAb treated group.
Figure 4. Cytotoxic T lymphocyte (CTL) infiltration and the expression of related mRNAs in a liver allograft.
(A) CD8+ CTLs infiltration was dramatically increased in grafts harvested from the anti-B7-H1mAb treatment recipients (B7-H1) in comparison to the control no-treatment recipients (Cont) on days 5 and 8 after transplantation. Original magnification is 100x. (B) Changes in mRNA expression vs. one sample of the control group were calculated. The relative quantity is presented as the ratio of the comparative cycle threshold (Ct) of the target genes against those of the housekeeping gene 18s. Data are representative of three independent experiments and indicate the mean ratio of triplicate results in each experiment.
Discussion
The mechanisms underlying mouse liver allograft spontaneous tolerance remain largely unclear. Consistent with previous studies (14, 15), although massive infiltration of CD8+ T cells in both liver allografts as well as cardiac allografts, the number of infiltrated CD8+ T cells in the liver allografts declined sequentially (data not shown), thus indicating that as many other allogeneic organs the liver transplantation induces anti-alloimmune responses, which are attenuated gradually through unclear mechanisms. The current data indicated that the decreased CD8+ T cells underwent apoptosis dependent on B7-H1 expression in allogeneic liver graft (Fig. 2). Many studies in mouse islet, corneal, skin and cardiac transplant models have demonstrated that the PD-1/B7-H1 signaling pathway is required for the induction and maintenance of established graft tolerance (3, 4). The present study mainly focused on the inductive phase of transplant tolerance. The necessity of the PD-1/B7-H1 costimulation pathway was examined in liver transplantation tolerance induction by blocking the B7-H1 signal using B7-H1 KO mouse and anti-B7-H1mAb in a spontaneous mouse liver tolerance model. Our results, for the first time, clearly demonstrate the critical role of B7-H1 upregulated in the liver grafts tissue cells in spontaneous mouse liver allograft tolerance and the lack of this signaling results in liver allograft acute rejection. The liver allografts were rejected within 7 days when a liver graft deficient in B7-H1 was transplanted into an allogeneic recipient (Fig. 3A). Similarly, the abrogation of PD-1/B7-H1 signal by the administration of anti-B7-H1 or anti-PD-1mAbs inhibited the spontaneous tolerance induction (Fig. 3B). Meanwhile, the administration of anti-B7-H1mAb did not affect the survival of syngeneic liver grafts, thus indicating that the destructive effect of anti-B7-H1mAb on liver allograft is not due to the direct non-specific toxicity, and it may be mediated by regulating alloimmune responses. B7-H1 mediated downregulation of T cell immune response in vivo remain to be fully elucidated. It has been proposed that B7-H1 negative signaling pathway downregulates T cell immune responses in vivo by directly ligation with PD-1 upregulated on the surface of activated T cells, thus resulting in the induction of the apoptosis of effector CTL (16). We demonstrated that the CD8+ CTL infiltration dramatically increased in the portal triads, central vein and bile duct and extensive necrosis with some congestion and hemorrhage in livers being rejected by the anti-B7-H1mAb treated recipients, accompanied with its higher related genes mRNA expression (Fig. 4). These findings may imply that B7-H1 signaling thus plays a role in T cell activation and survival, which both determine the outcome of liver allografts. The current data consistent with the previous reports (17, 18) show that B7-H1 was expressed in the sinusoidal endothelial region and mononuclear on allogeneic liver graft, but not on syngeneic liver grafts (Fig. 1C, E), thus suggesting inflammatory factors enhance B7-H1 expressions. The signal between PD-1 on effector T cells and B7-H1 on liver nonparenchymal cells such as Kupffer cells (KC) and liver sinusoidal endothelial cells (LSEC) have been reported to be able to inhibit effector T cell accumulation without suppressing their activation (17). Our recent study also demonstrated that hepatic stellate cells (HSC) expressed higher levels of B7-H1 upon IFNγ-stimulation, which inhibited alloimmune responses both in vitro and in vivo by the induction of activated T cell apoptosis via B7-H1 pathway (19). The cotransplantation of activated HSC with allogeneic islets effectively protected islet allografts from rejection by elimination of alloreactive CD8+ T cells, while quiescent and B7-H1 KO HSC lost the protective effect (20). The PD1/B7-H1 signal may allow effector T cells to accomplish their primary functions, but blocks their accumulation in the tissue to avoid excessive or destructive immune reactions. Therefore, B7-H1 acts as a safeguard to restrict the ability to induce strong immune responses against infectious agents. The temporary blockade of the PD-1/B7-H1 pathway may open new therapeutic approach for chronic viral infections.
In conclusion, the current study indicated that the PD-1/B7-H1 signaling pathway is required for the induction of peripheral tolerance in a mouse liver transplant model. In the liver allograft infiltrating CD8+ T cells underwent apoptosis, which was dependent on the B7-H1 expression on the liver graft tissue cells. Blockade of the PD-1/B7-H1 signal by administration of anti-B7-H1mAb or liver donor deficient in B7H1 resulted in the acute rejection of the allograft, associated with an increase of infiltrating CTLs and inflammation related cytokines mRNA expression. This study confirms, for the first time, the role of PD-1/B7-H1 signaling in the induction of spontaneous liver tolerance, thereby suggesting that selectively sparing the PD-1/B7-H1 signal may be beneficial in the development of a tolerance strategy in organ transplantation. Furthermore, the implications of these findings may pertain not only to the mouse spontaneous acceptance of liver transplants, but also to the immunopathogenesis of chronic viral hepatitis.
Acknowledgments
The authors gratefully acknowledge Dr. H. Kimura for his critical comments and useful suggestion.
Abbreviations
- HSC
hepatic stellate cell
- KC
Kupffer cells
- LSEC
liver sinusoidal endothelial cells
- PD-1
programmed death-1
- qRT-PCR
quantitative real time reverse-transcription polymerase chain reaction
- NPC
non parenchymal cells
- Treg
Regulatory T
References
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 3.Kitazawa Y, Fujino M, Wang Q, Kimura H, Azuma M, Kubo M, et al. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83(6):774–782. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- 4.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174(11):6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 5.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203(12):2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugioka A, Morita M, Fujita J, Hasumi A, Shiroishi T. Graft acceptance and tolerance induction in mouse liver transplantation using wild mice. Transplant Proc. 2001;33(1–2):137–139. doi: 10.1016/s0041-1345(00)01942-4. [DOI] [PubMed] [Google Scholar]
- 7.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223(5205):472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 8.Kamada N. The immunology of experimental liver transplantation in the rat. Immunology. 1985;55(3):369–389. [PMC free article] [PubMed] [Google Scholar]
- 9.Fujino M, Li XK. Immune tolerance induction in experimental liver transplantation (in Japanese) Surgery Frontier. 2006;13(2):36–40. [Google Scholar]
- 10.Morita M, Fujino M, Li XK, Kimura H, Nakayama T, Taniguchi M, et al. Spontaneous tolerance involving natural killer T cells after hepatic grafting in mice. Transpl Immunol. 2007;18(2):142–145. doi: 10.1016/j.trim.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Lu L, Woo J, Rao AS, Li Y, Watkins SC, Qian S, et al. Propagation of dendritic cell progenitors from normal mouse liver using granulocyte/macrophage colony-stimulating factor and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179(6):1823–1834. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25(3):658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 14.Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158(10):4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 15.Sharland A, Shastry S, Wang C, Rokahr K, Sun J, Sheil AG, et al. Kinetics of intragraft cytokine expression, cellular infiltration, and cell death in rejection of renal allografts compared with acceptance of liver allografts in a rat model: early activation and apoptosis is associated with liver graft acceptance. Transplantation. 1998;65(10):1370–1377. doi: 10.1097/00007890-199805270-00015. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198(1):39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oikawa T, Takahashi H, Ishikawa T, Hokari A, Otsuki N, Azuma M, et al. Intrahepatic expression of the co-stimulatory molecules programmed death-1, and its ligands in autoimmune liver disease. Pathol Int. 2007;57(8):485–492. doi: 10.1111/j.1440-1827.2007.02129.x. [DOI] [PubMed] [Google Scholar]
- 19.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40(6):1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44(5):1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]