Abstract
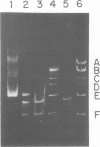
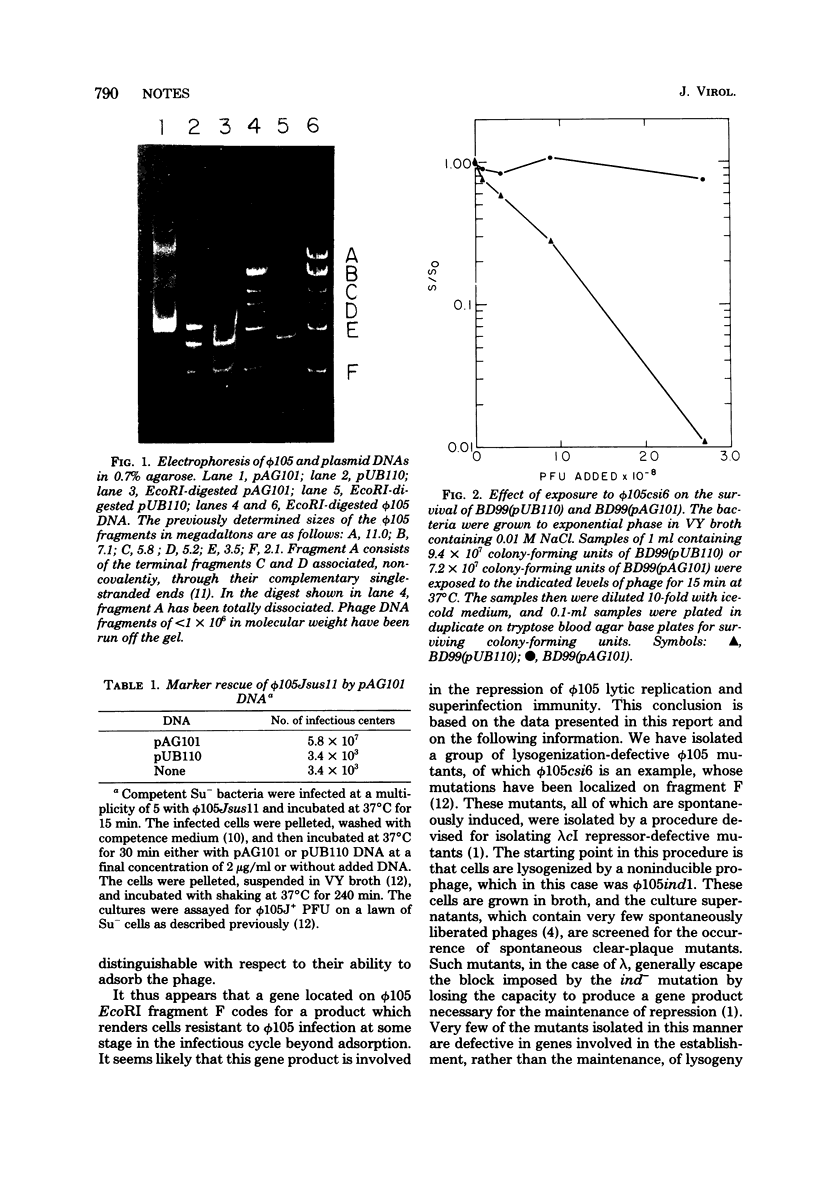
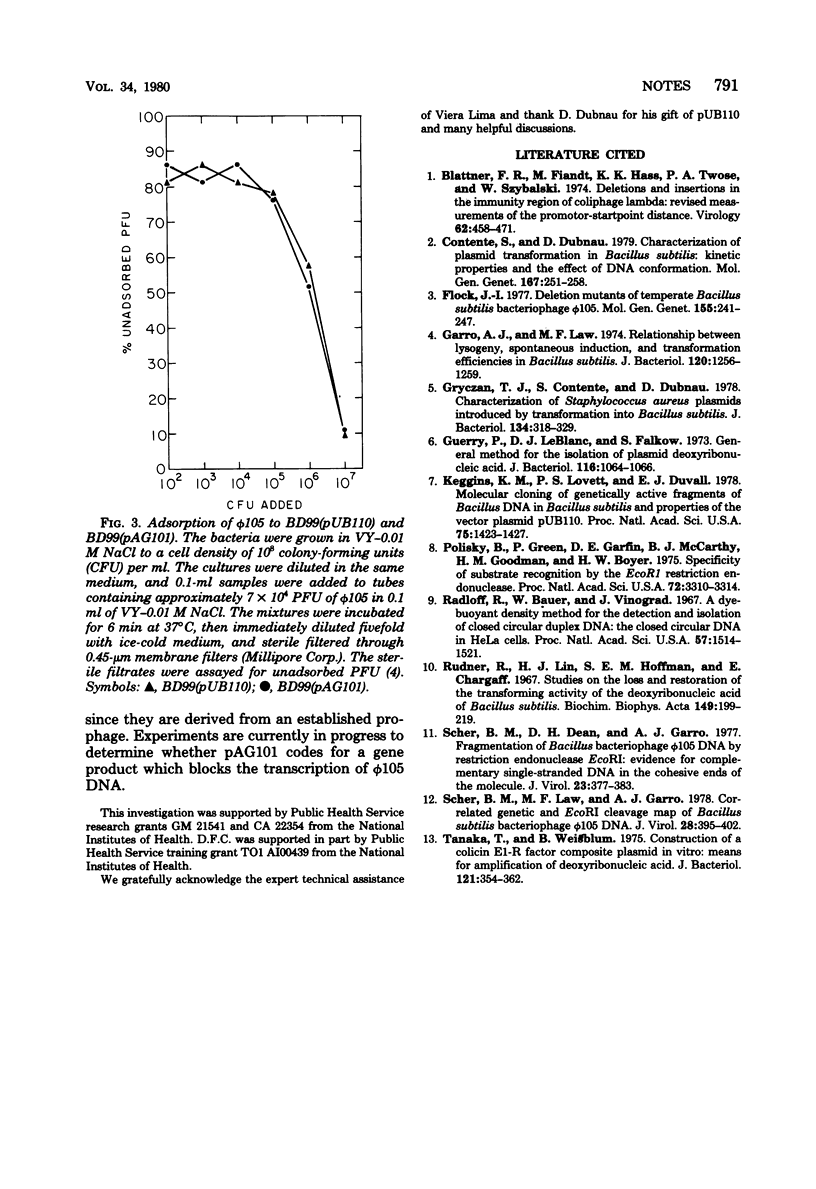
A 2.1-megadalton, EcoRI-generated fragment of Bacillus subtilis phage phi 105 DNA was cloned into plasmid pUB110. The hybrid plasmid produces a biologically active product which renders B. subtilis immune to infection by phi 105.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner F. R., Fiandt M., Hass K. K., Twose P. A., Szybalski W. Deletions and insertions in the immunity region of coliphage lambda: revised measurement of the promoter-startpoint distance. Virology. 1974 Dec;62(2):458–471. doi: 10.1016/0042-6822(74)90407-3. [DOI] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Characterization of plasmid transformation in Bacillus subtilis: kinetic properties and the effect of DNA conformation. Mol Gen Genet. 1979 Jan 2;167(3):251–258. doi: 10.1007/BF00267416. [DOI] [PubMed] [Google Scholar]
- Flock J. I. Deletion mutants of temperate Bacillus subtilis bacteriophage phi105. Mol Gen Genet. 1977 Oct 24;155(3):241–247. doi: 10.1007/BF00272803. [DOI] [PubMed] [Google Scholar]
- Garro A. J., Law M. F. Relationship between lysogeny, spontaneous induction, and transformation efficiencies in Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1256–1259. doi: 10.1128/jb.120.3.1256-1259.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keggins K. M., Lovett P. S., Duvall E. J. Molecular cloning of genetically active fragments of Bacillus DNA in Bacillus subtilis and properties of the vector plasmid pUB110. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1423–1427. doi: 10.1073/pnas.75.3.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner R., Lin H. J., Hoffmann E. M., Chargaff E. Studies on the loss and the restoration of the transforming activity of the deoxyribonucleic acid of Bacillus subtilis. Biochim Biophys Acta. 1967 Nov 21;149(1):199–219. doi: 10.1016/0005-2787(67)90702-2. [DOI] [PubMed] [Google Scholar]
- Scher B. M., Dean D. H., Garro A. J. Fragmentation of Bacillus bacteriophage phi105 DNA by complementary single-stranded DNA in the cohesive ends of the molecule. J Virol. 1977 Aug;23(2):377–383. doi: 10.1128/jvi.23.2.377-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher B. M., Law M. F., Garro A. J. Correlated genetic and EcoRI cleavage map of Bacillus subtilis bacteriophage phi105 DNA. J Virol. 1978 Oct;28(1):395–402. doi: 10.1128/jvi.28.1.395-402.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]