Abstract
Activators of the Arp2/3 complex, termed nucleation-promoting factors (NPFs), are required for the proper spatial and temporal control of actin assembly in cells. Mammalian cells express several NPFs, each of which functions in a distinct cellular process, including WASP and N-WASP in phagocytosis and endocytosis, WAVE and JMY in cell migration, and WHAMM in ER-to-Golgi transport. Although another NPF called WASH was recently identified, the cellular localization and function of this protein were unclear. Here we demonstrated that human WASH alone potently activated the Arp2/3 complex in vitro and in cells, suggesting that the protein is not autoinhibited like N-WASP, but is likely regulated by interacting proteins. In cells, WASH was associated with Rab5-positive early endosomes and Rab11-positive recycling endosomes that were enriched for actin filaments. Silencing of WASH or Arp2/3 complex expression by RNAi, or disruption of actin function by drug treatments, caused enlargement and elongation of endosomes. Intriguingly, WASH silencing, as well as actin disruption, delayed EGF transport to LAMP1-positive late endosomes. These observations indicate that actin polymerization by WASH influences the shape and maturation of endosomes, and highlight a previously unrecognized role for WASH and the Arp2/3 complex in the degradative steps of endocytic trafficking.
Introduction
The actin cytoskeleton plays an important role in cellular behaviors such as migration and division (Barr and Gruneberg 2007; Pollard and Borisy 2003), as well as in intracellular processes including endocytosis and vesicle trafficking (Engqvist-Goldstein and Drubin 2003; Kaksonen et al. 2006; Robertson et al. 2009). Proper functioning of the actin cytoskeleton requires precise regulation of the polymerization and organization of actin filaments. One of the principal actin polymerizing and organizing factors in the cell is the Arp2/3 complex, a protein complex that nucleates new filaments from the sides of existing ones and cross-links filaments into Y-branched networks (Goley and Welch 2006). However, purified Arp2/3 complex does not display potent nucleating and Y-branching activity unless it is engaged by a class of proteins called nucleation-promoting factors (NPFs) (Welch and Mullins 2002). Mammalian cells express a diverse array of NPFs, each of which coordinates Arp2/3 complex activity during distinct cellular behaviors or processes.
The WASP and Scar homolog (WASH) protein was recently identified as an NPF (Linardopoulou et al. 2007) that belongs to a group called Class I. This group also includes the well-studied WASP, N-WASP, WAVE/Scar NPFs (Stradal et al. 2004; Takenawa and Suetsugu 2007), as well as the recently discovered WHAMM (Campellone et al. 2008), and JMY (Zuchero et al. 2009) proteins. All Class I NPFs share a common C-terminal WCA domain that includes a WASP-homology-2 (WH2 or W) element that binds to actin monomers, a connector (C) region that binds to both the Arp2/3 complex and actin monomers, and an acidic (A) region that binds to the Arp2/3 complex (Marchand et al. 2001). The WCA region of WASH, like that of other Class I NPFs, is sufficient to activate the Arp2/3 complex in vitro (Linardopoulou et al. 2007; Liu et al. 2009).
In contrast to their conserved C-terminal WCA domains, the N-terminal domains of Class I NPFs differ considerably. WASH contains two distinctive N-terminal domains, termed WASH homology domain 1 (WAHD1) and tubulin-binding region (TBR) (Gomez and Billadeau 2009), that are not present in other NPFs (Linardopoulou et al. 2007). The exact contribution of these domains to the function and regulation of WASH has not yet been defined. However, the various N-terminal sequences of other Class I NPFs are known to confer each protein with a distinct cellular function and mode of regulation. For example, under resting conditions WASP and N-WASP are autoinhibited by an intramolecular interaction between a central GTPase-binding domain and the WCA region (Kim et al. 2000; Miki et al. 1998; Prehoda et al. 2000), and are activated by Rho family GTPases like Cdc42 to promote endocytosis, phagocytosis, and filopodia formation (Stradal et al. 2004; Takenawa and Suetsugu 2007). In contrast, WAVEs are inhibited by association with a complex of interacting proteins (Derivery et al. 2009a; Eden et al. 2002; Ismail et al. 2009), and can be activated by the Rho family GTPase Rac (Eden et al. 2002; Ismail et al. 2009; Miki et al. 2000) to promote lamellipodia protrusion (Stradal et al. 2004; Takenawa and Suetsugu 2007). WHAMM has also been proposed to be regulated in a manner similar to the WAVE proteins (Campellone et al. 2008). The mechanism of WASH regulation, in contrast, is not well understood.
Nevertheless, recent studies have provided some insight into the function and regulation of WASH in cells. An initial study reported that GFP-tagged WASH localizes to lamellipodia and filopodia, suggesting that it may be involved in plasma membrane dynamics (Linardopoulou et al. 2007). Additionally, genetic and biochemical studies in Drosophila melanogaster indicate that WASH functions downstream of the Rho family GTPase Rho1 and interacts with the actin nucleators Spire and Cappuccino (Liu et al. 2009). These data suggest that WASH may regulate formation of both branched-actin networks with the Arp2/3 complex and unbranched assemblies with Spire and Cappuccino in cells (Liu et al. 2009). Moreover, WASH displays both F-actin and microtubule bundling activity in vitro (Liu et al. 2009), although the cellular functions of these activities are not yet defined. Finally, WASH was recently implicated in the internalization of the bacterial pathogen Salmonella typhimurium into host cells (Hanisch et al. 2009), raising the possibility that WASH functions in the endocytic pathway.
One of the central functions that have emerged for the Arp2/3 complex and NPFs is to facilitate the process of endocytosis (Engqvist-Goldstein and Drubin 2003; Kaksonen et al. 2006; Perrais and Merrifield 2005; Robertson et al. 2009). In mammalian cells, N-WASP and the Arp2/3 complex have been implicated in coordinating actin assembly to facilitate endocytic vesicle formation at the plasma membrane. For example, N-WASP and the Arp2/3 complex are found at sites of clathrin-mediated endocytosis (Merrifield et al. 2004; Rodal et al. 2005; Yarar et al. 2005), and cells lacking functional N-WASP exhibit reduced internalization kinetics of epidermal growth factor receptor (EGFR) (Benesch et al. 2005; Innocenti et al. 2005). In addition to a role in endocytic vesicle formation, evidence is accumulating that actin plays a role at later stages of the endocytic pathway (Soldati and Schliwa 2006). For instance, N-WASP is required for the actin-dependent propulsion of endosomes (Benesch et al. 2002; Rozelle et al. 2000; Taunton et al. 2000). Actin patches have also been observed on early endosomes, and are thought to be involved in the membrane remodeling events that accompany endosome biogenesis (Morel et al. 2009). However, the exact role of the Arp2/3 complex and NPFs in this process is not clear.
Here we investigated the function of WASH in mammalian cells, and discovered a role for WASH in the endocytic pathway. We found that WASH localized to early endosomes, and together with the Arp2/3 complex and actin, was critical for maintaining the shape of this compartment. We also showed that WASH and actin polymerization play an important functional role in trafficking of epidermal growth factor (EGF) to late endosomes. These data demonstrate a previously unappreciated role for WASH and the Arp2/3 complex during trafficking of cargo in the degradative endocytic pathway.
Materials and Methods
Plasmids, Bacteria, and Cells
The genes encoding full-length WASH or WASH-WCA (nucleotides 912-1404) were amplified by PCR (for primers see Supplemental Table 1) from a human cDNA clone (accession BC048328, Open Biosystems). PCR products were digested with KpnI and NotI (New England Biolabs) and ligated into pKC-ET16b for bacterial expression or pKC-EGFP-C1 for mammalian expression (for a complete list of plasmids see Supplemental Table 2). Plasmids used to express N-WASP-WCA (pET-N-WASP-WCA), WAVE2-WCA (pET-WAVE2-WCA) and WHAMM-WCA (pET-WHAMM-WCA) were described previously (Campellone et al. 2008). Plasmids were maintained in E. coli XL-1 Blue (Stratagene), whereas E. coli BL21-Rosetta (EMD Biosciences) was used for recombinant protein expression. N-WASP+/+ and N-WASP-/- fibroblast-like cells (FLCs) were a kind gift from S. Snapper (Snapper et al. 2001). COS7 cells, NIH-3T3 cells, and FLCs were cultured in DMEM (Invitrogen) supplemented with 10% Fetal Bovine Serum (FBS; JR Scientific) at 37 °C in 5% CO2.
Protein Expression and Purification
E. coli transformed with pET16b-WASH were grown to OD600 = 0.6 and induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 12 h at 16 °C. Following expression, bacteria were resuspended in lysis buffer (8 M urea, 10 mM Tris pH 8.0, 250 mM KCl, 100 mM NaH2PO4, 10 μg/ml each of aprotonin, leupeptin, pepstatin, and 1 mM PMSF). Ni-NTA affinity purification was carried out for His-tagged protein purification under denaturing conditions (QIAGEN). Elutions were performed by lowering the lysis buffer pH to 4.5 with HCl. Protein refolding was performed by slowly dialyzing against 250 mM KCl, phosphate buffered saline (PBS) pH 7.4 with 10% glycerol. Gel filtration chromatography on a Superdex 75 (GE Healthcare) equilibrated with 250 mM KCl, PBS pH 7.4 with 10% glycerol was used to further purify the recombinant protein.
Full-length bovine N-WASP tagged at its N-terminus with 6xHis and FLAG epitopes was expressed in Sf9 insect cells using the Bac-to-Bac baculovirus expression system (Invitrogen). 72 h post-infection, cells were harvested by centrifugation at 500 × g for 10 min at 25 °C, resuspended in lysis buffer (50 mM Tris pH 8.0, 250 mM NaCl, 100 mM KCl, 20 mM imidazole, 1% NP-40 with protease inhibitors) and frozen in liquid N2. To prepare the lysate, cells were thawed and centrifuged at 200,000 × g, 4 °C, 20 min. The supernatant was incubated with Ni-NTA agarose (QIAGEN) to isolate His-N-WASP. His-N-WASP was eluted in 50 mM Tris pH 8.0, 250 mM NaCl, 100 mM KCl with 300 mM imidazole. His-tagged WCA domains were purified as described previously (Campellone et al. 2008).
Antibodies
Anti-WASH antibodies were generated by immunizing rabbits with full-length recombinant human WASH (Covance Inc.). Anti-N-WASP antibodies were generated by immunizing guinea pigs with full-length recombinant bovine N-WASP (Pocono Rabbit Farms). Polyclonal antibodies were affinity purified on WASH or N-WASP affinity columns as described previously (Welch et al. 1997). Antibodies recognizing the Arp2/3 subunits were also described previously (Welch et al. 1997). Antibodies raised against EEA1, Rab4, Rab5, and Rab11 (BD Biosciences), LAMP1 (Santa Cruz Biotechnology), GAPDH (Ambion), calnexin (Stressgen), and tubulin (Developmental Studies Hybridoma Bank at the University of Iowa) were purchased from the indicated vendors. Secondary antibodies conjugated to HRP (Jackson Immunoresearch) were used for immunoblotting. Alexa Fluor 488 and 568 conjugated secondary antibodies from Invitrogen were used for immunofluorescence.
Pyrene-Actin Polymerization Assays
Full-length recombinant WASH and WASH-WCA, N-WASP-WCA, WAVE2-WCA and WHAMM-WCA were dialyzed into 20 mM MOPS pH 7.0, 200 mM KCl, 2 mM MgCl2, 5 mM EGTA, 1 mM EDTA and 10% glycerol prior to assembly reactions. His-tagged Arp2/3 complex, rabbit skeletal muscle actin, and pyrene-actin were prepared as described previously (Goley et al. 2004). Actin assembly reactions were allowed to reach steady state, and the curves were normalized to differences in steady state fluorescence.
Cell/Tissue Extracts, Fractionation, and Immunoblotting
Cell extracts were prepared by lysing cells in 50 mM Tris pH 7.6, 150 mM NaCl, 1% Triton X-100, and protease inhibitors. Membrane and cytosol components were fractionated as previously described (Campellone et al. 2008). To determine tissue-specific expression of WASH, a mouse tissue blot (BioChain) was used. For immunoblotting, nitrocellulose membranes were blocked with 5% milk in PBS, probed with primary and secondary antibodies, and visualized by chemiluminescence (GE Healthcare).
Drug Treatments and Preparation for Fluorescence Microscopy
Cells grown on coverslips were treated with 10 μM latrunculin A or 10 μM cytochalasin D (EMD Bioscience), or DMSO (Sigma) for 10 min. Cells were fixed for 20 min in 2.5% paraformaldehyde (EMD Biosciences) at 37 °C. Immediately following fixation, cells were permeablized with 0.1% Triton X-100 for 5 min, incubated with primary antibodies diluted in PBS with 1% BSA (Sigma) and 5% normal goat serum (Invitrogen), washed in PBS, treated with Alexa Fluor-conjugated secondary antibodies, 1 μg/ml DAPI, and/or 4 U/ml Alexa Fluor 568-phalloidin (Invitrogen). Coverslips were mounted on glass slides using ProLong Gold anti-fade reagent (Invitrogen).
Image Acquisition and Analysis
Wide-field fluorescence images were taken on an Olympus IX71 inverted microscope with a 100× (1.35 NA) PlanApo objective lens equipped with a photometrics Coolsnap HQ camera. Images were captured using Metamorph software (Universal Imaging), converted to 8-bit tiff files and processed using ImageJ software (National Institutes of Health). Deconvolution images were taken using an Applied Precision DeltaVision 4 Spectris microscope with a 100× (1.4 NA) PlanApo objective equipped with a Photometrics CH350 CCD camera. Images were captured using SoftWoRx v3.3.6 software (Applied Precision), and were deconvolved with Huygens Professional v3.1.0p0 software (Scientific Volume Imaging). 3D reconstruction animations were made using Imaris 5.2 software (Bitplane). Areas of colocalization were quantified using ImageJ and the JACoP plugin (Bolte and Cordelieres 2006). 10 images from 3 independent experiments were quantified. The mean percentage of WASH structures that colocalized with each marker was determined with Prism software. Structured illumination microscopy images were captured on a DeltaVision OMX microscope (Applied Precision) with a 100× (1.4 NA) PlanApo objective equipped with a Sony ICX285 20 MHz CCD camera. SIM images were processed as described above for standard deconvolution images.
DNA and siRNA Transfections
To visualize WASH localization, COS7 cells were transfected with 250 ng of pEGFP-C1-WASH in a 6-well plate with Lipofectamine2000 (Invitrogen). For WASH silencing by RNAi, NIH-3T3 or FLCs were transfected twice, first at 0 h on day 1 when cells were 30-40% confluent and at 24 h on day 2. Transfections were carried out using a 20 nM final concentration of siRNA with RNAiMAX (Invitrogen). Either of two siRNAs (J-054931-09 and J-054931-12 from Dharmacon) were used independently with identical results for WASH depletions. Two siRNAs (I.D. numbers: 74834 and 77135 from Ambion) were used to target the ARPC3 and Arp3 subunits of the Arp2/3 complex. A 10 nM final concentration of each siRNA was used for each transfection. Transfections were performed as described above for WASH depletion. Endocytosis assays were performed and/or cells were fixed for microscopic analysis on day 3 (72 h after the first siRNA transfection).
Transferrin Uptake and Recycling Assays
Uptake and recycling of biotinylated transferrin (B-SS-Tfn) was performed as described previously (Osborne et al. 2005) with the following modifications. FLCs or NIH-3T3 cells were serum-starved for 1 h in DMEM at 37 °C. Cells were then incubated with 1 μg/ml B-SS-Tfn in PBS + 0.2% BSA for 30 min at 4 °C, washed 3× with PBS + BSA, and chased with 100 μg/ml unlabeled transferrin in PBS/BSA at 31 °C for 0, 2, 4, 8, 16, 32, 48, or 64 min. The amount of internalized B-SS-Tfn at each time point was measured by ELISA and expressed as a percentage of the total surface-bound B-SS-Tfn prior to internalization.
EGF Transport Assays
Wild type FLCs were grown to 70-80% confluency and serum-starved for 1 h prior to internalization. Drug treatments were carried out using 1 μM latrunculin A, 1 μM cytochalasin D, or DMSO. Cells were allowed to internalize 20 ng/ml Alexa Fluor 488-EGF complex (Invitrogen) for 10 min at 37 °C, were washed 6× with PBS to remove surface bound EGF, and were chased for 30 min in serum-free DMEM with or without drug treatment. Cells were fixed, processed, and imaged as described above. The percentage of fluorescent EGF localized to LAMP1 structures was determined using ImageJ and JACoP. 10 images from 3 independent experiments were quantified for each treatment. The data were analyzed using Prism and experimental treatments were compared pairwise with control treatments using a one-way ANOVA. Differences with p-values < 0.05 were designated statistically significant.
Results
Human WASH is a potent Arp2/3 complex nucleation-promoting factor in vitro
WASH contains two distinct N-terminal domains, termed WAHD1 (WASH homology domain 1) and TBR (tubulin-binding region) (Gomez and Billadeau 2009), as well as a C-terminal WCA domain that is conserved among mammalian Class I NPFs (Figure 1A). Previous studies demonstrated that a truncated variant of human WASH consisting of the WCA domain (WASH-WCA) (Linardopoulou et al. 2007), as well as full-length D. melanogaster WASH or its WCA domain (Liu et al. 2009), activate the Arp2/3 complex to promote actin nucleation in vitro. However, the activity of full-length human WASH has not been evaluated. To assess the relative activity of full-length human WASH compared with the WASH-WCA domain, we purified His-tagged full-length recombinant WASH (His-WASH) (Figure 1B) and His-WASH-WCA from E. coli (Figure 1C), and tested their activity in pyrene-actin polymerization assays. In the presence of the Arp2/3 complex, increasing concentrations of both His-WASH and His-WASH-WCA resulted in a dose-dependent acceleration of actin assembly, although neither WASH nor the Arp2/3 complex alone were able to efficiently nucleate actin polymerization (Figure 1D). Full-length His-WASH was as potent at promoting Arp2/3 complex-mediated actin nucleation as WASH-WCA (Figure 1D). This observation suggests that recombinant WASH is not autoinhibited, a behavior that is similar to the NPFs WHAMM (Campellone et al. 2008) and WAVE (Machesky and Insall 1998), but different from N-WASP (Rohatgi et al. 1999).
Figure 1.
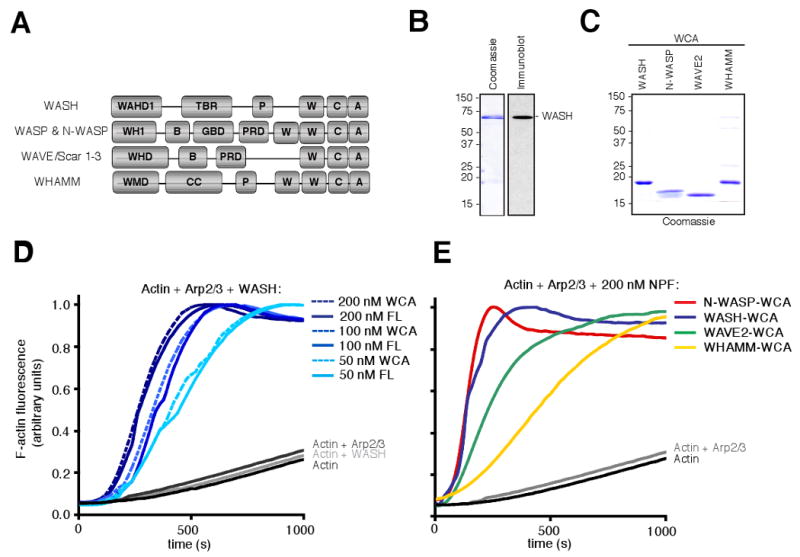
WASH is a potent Arp2/3 complex nucleation-promoting factor in vitro. (A) Schematic diagrams showing the domain organization of mammalian NPFs. (B) Purified recombinant full-length His-WASH was subjected to SDS-PAGE and stained with Coomassie blue or detected by immunoblotting with anti-WASH antibodies. (C) Purified His-WCA domains were separated by SDS-PAGE and stained with Coomassie blue. (D) Pyrene-actin assembly assays with 3 μM actin polymerized in the presence of 20 nM Arp2/3 complex and 50-200 nM full-length His-WASH (FL) or His-WASH-WCA (WCA). (E) Pyrene-actin assembly assays as in (D), but with 200 nM of the indicated NPF-WCA.
To compare the activity of WASH to that of other mammalian Class I NPFs, we purified recombinant human His-N-WASP-WCA, His-WAVE2-WCA and His-WHAMM-WCA from E. coli (Figure 1B) and again measured their activities in pyrene-actin assembly assays. WASH-WCA displayed comparable activity to N-WASP-WCA, and greater activity than either WAVE2-WCA or WHAMM-WCA (Figure 1E). Thus, WASH is a potent NPF like N-WASP and is constitutively active in vitro similar to WHAMM and WAVE2.
WASH is widely expressed in mammalian tissues and cell lines and fractionates with both cytosol and membranes
To investigate the expression pattern of WASH in mammalian tissues and cells, we generated antibodies against the full-length WASH protein and used these to detect WASH in extracts from mouse tissues and mammalian cultured cell lines by immunoblotting. These antibodies recognized purified recombinant WASH (Figure 1B), as well as one or a small number of WASH species in mammalian tissue or cell extracts (Figure 2). We found that WASH was expressed in most mouse tissues examined, with the highest expression levels observed in cardiac and renal tissue (Figure 2A). Additionally, WASH was expressed in all cultured cell lines that we tested, including mouse NIH-3T3, monkey COS7, and human HeLa cells (Figure 2B). We conclude that WASH is expressed widely in mammalian tissues and cells.
Figure 2.

WASH is widely expressed in mammalian tissues and cell lines and fractionates with both cytosol and membranes. (A) Cell extracts from mouse tissues or (B) from mammalian cultured cell lines were separated by SDS-PAGE and immunoblotted with anti-WASH antibodies. (C) NIH-3T3 cells were fractionated into membrane and cytosolic fractions by centrifugation and then separated by SDS-PAGE and immunoblotted with anti-WASH antibodies, antibodies against tubulin (cytosolic fraction marker), or antibodies recognizing calnexin (membrane fraction marker).
Given that other NPFs activate Arp2/3-mediated actin assembly adjacent to cellular membranes, we next assessed whether WASH associated with cellular membranes by biochemical fractionation. NIH-3T3 cell lysates were fractionated into membrane and cytosolic components. The fidelity of the fractionation was confirmed by immunoblotting for the endoplasmic reticulum marker calnexin, which was found exclusively in the membrane fraction, and tubulin, which was only detected in the cytosolic fraction (Figure 2C). Interestingly, WASH was slightly enriched in the membrane fraction, although a significant portion was also observed in the cytosolic fraction (Figure 2C). The distribution of WASH was similar to that previously observed for WAVE2 (Suetsugu et al. 2006), but different from that of N-WASP and WHAMM which are more heavily enriched on membranes (Campellone et al. 2008). Thus, although WASH associates with cellular membranes, a significant pool of WASH is either free in the cytoplasm or associates with small vesicles that do not pellet in our assay.
WASH associates with perinuclear vesicles enriched with F-actin
Previously published work suggested that WASH-GFP colocalizes with actin in cellular filopodia and lamellipodia (Linardopoulou et al. 2007). To assess the subcellular localization of endogenous WASH, we used polyclonal antibodies raised against full-length human WASH for immunofluorescence microscopy. Surprisingly, we did not observe WASH staining at the cell periphery in lamellipodia or filopodia in COS7 cells. Instead, WASH was almost exclusively associated with perinuclear punctae that overlapped with a region enriched in F-actin (Figure 3A). To further verify this localization pattern, we generated an N-terminally EGFP-tagged variant of WASH (GFP-WASH) and observed its localization in COS7 cells. Importantly, GFP-WASH accumulated in the perinuclear region in a pattern similar to endogenous WASH (Figure 3B). Together with the fractionation data (Figure 1), the immunofluorescence staining and GFP-WASH localization data indicate that WASH primarily localizes to a perinuclear membrane-bound compartment.
Figure 3.
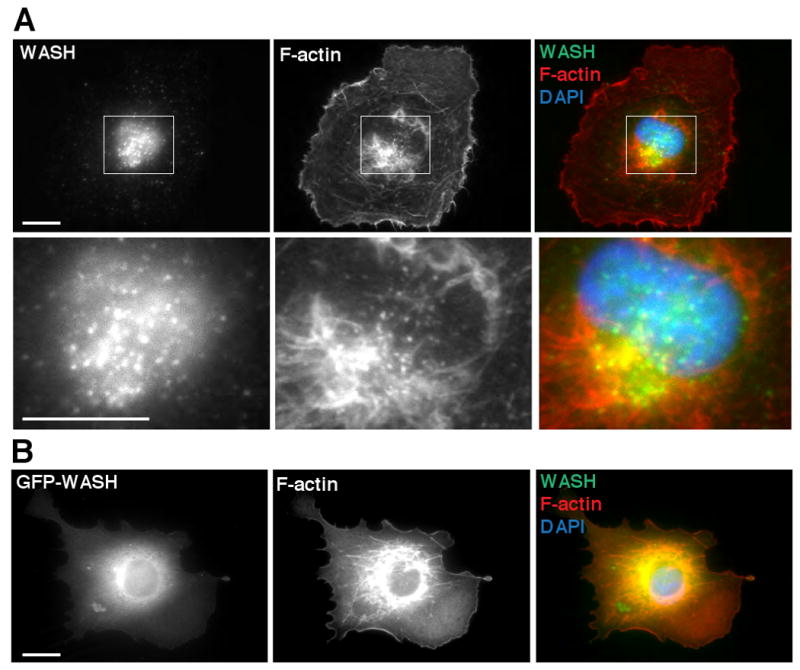
WASH associates with perinuclear vesicles enriched with F-actin. (A) WASH visualized by immunofluorescence (green) colocalizes with perinuclear F-actin visualized by Alexa Fluor 568-phalloidin (red) in COS7 cells. (B) GFP-WASH (green), F-actin stained with Alexa Fluor 568-phalloidin (red), and DNA stained with DAPI (blue) in COS7 cells. Scale bars, 10 μm.
Notably, GFP-WASH expression resulted in a striking accumulation of F-actin in the perinuclear region of transfected cells (Figure 3B). A similar accumulation of F-actin was observed after overexpressing WHAMM and WAVE2, but not N-WASP (Campellone et al. 2008). This result is consistent with the notion that WASH is not autoinhibited in cells, similar to the behavior of the recombinant WASH protein in vitro (Figure 1).
WASH is asymmetrically distributed on early endosomes
To determine which organelle WASH associates with, we sought to colocalize WASH with markers of perinuclear membrane-bound compartments by immunofluorescence staining together with deconvolution microscopy. We found that WASH associates with several proteins of compartments in the endocytic pathway, including the early endosome markers early endosome antigen 1 (EEA1) and Rab5, the recycling endosome marker Rab11, and the fast recycling vesicle marker Rab4 (Stenmark 2009) (Figure 4). We quantified the percentage of WASH-containing punctae that colocalized with each of these proteins. We found that WASH most extensively colocalized with EEA1- and Rab5-positive early endosomes, and less frequently colocalized with Rab11 recycling endosomes and Rab4-positive vesicles (Figure 4C).
Figure 4.

WASH is asymmetrically distributed on endosomes. (A) Maximum intensity projections from 3D deconvolved images of COS7 cells in which WASH (green) and various endosome markers (red) were visualized by immunofluorescence, and DNA was stained with DAPI (blue). Scale bars, 10 μm. (B) 3D reconstruction of WASH (green) and EEA1 (red) visualized by immunofluorescence. Inset show the asymmetric distribution of WASH on EEA1-positive vesicles. Movie of the 3D reconstruction in (B) can be viewed in Figure4.mov. Gridlines are 1 μm apart. (C) The percentage of WASH structures that colocalized with each endosomal marker expressed as the mean ± SD. 10 images from 3 independent experiments were counted.
The EEA1/Rab5-positive early endosomes with which WASH colocalizes represent a crossroads of the endocytic pathway that sort and move materials to recycling endosomes, late endosomes, and the Golgi apparatus (Maxfield and McGraw 2004; Perret et al. 2005). Recent studies have shown that annexin A2 organizes into cholesterol-rich platforms on early endosomes (Mayran et al. 2003), suggesting that other proteins may be organized into subdomains on these vesicles. To determine if WASH is uniformly distributed on endosomes or is concentrated in subdomains, we closely examined the relative distributions of WASH and EEA1 on early endosomes using deconvolution microscopy and 3D reconstruction (Figure 4B; Figure4.mov). Interestingly, WASH staining did not completely overlap with EEA1, but instead appeared asymmetrically distributed relative to EEA1 staining, suggesting that WASH is targeted to a subdomain of the early endosome.
The actin cytoskeleton controls the morphology of WASH- containing compartments and EEA1-positive endosomes
The observations that WASH acts as an actin nucleation-promoting factor and localizes to endosomes suggested that actin assembly might influence the physical characteristics of endosomes. To investigate the role of actin polymerization in determining the shape of WASH-containing compartments, we treated COS7 cells with cytochalasin D, a drug that binds to actin filament barbed ends and blocks polymerization, or latrunculin A, a toxin that sequesters actin monomers and leads to rapid filament disassembly. Treated cells or untreated controls were stained for WASH or EEA1 by immunofluorescence and for actin by fluorescent phalloidin, and then imaged by super-resolution structured illumination microscopy (Figure 5). In control cells, we often observed F-actin staining colocalizing with WASH vesicles. Treatment with cytochalasin D resulted in partial actin disassembly and the formation of enlarged and elongated WASH structures compared to untreated controls. Moreover, treatment with latrunculin A resulted in more complete actin disassembly and very enlarged WASH-positive compartments consisting of long interconnected tubules. Additionally, treatment with both drugs resulted in enlargement and lengthening of some EEA1-positive early endosomes, although the effect was not as pronounced as it was for WASH-positive compartments. These observations indicate that actin polymerization plays an important role in maintaining the size and shape of WASH- and EEA-positive compartments.
Figure 5.
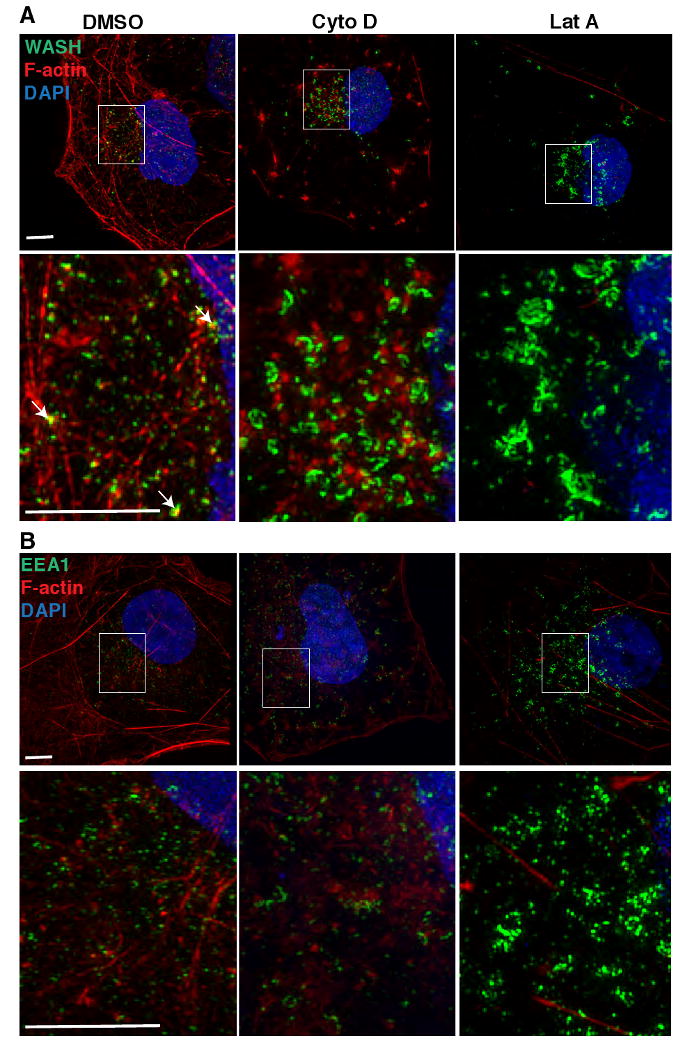
Disruption of the actin cytoskeleton results in an enlarged and elongated WASH-containing and EEA1-positive endosomes. (A) WASH or (B) EEA1 visualized by immunofluorescence (green), F-actin stained with Alexa Fluor 568-phalloidin (red) and DNA stained with DAPI in COS7 cells treated with DMSO alone, or DMSO with 10 μM cytochalasin D (Cyto D), or 10 μM latrunculin A (Lat A). Imaging was by 3D structured illumination microscopy. Each panel is a representative maximum intensity projection of the 3D images. Actin colocalized with WASH structures (see arrows) in control (DMSO) treatment. Scale bars, 5 μm.
WASH and the Arp2/3 complex regulate endosome shape
Because actin assembly appears to be crucial for determining the shape of WASH-positive compartments and early endosomes, the actin nucleating activity of WASH and the Arp2/3 complex might also be important for regulating endosome shape. To assess the role of the Arp2/3 complex, we transfected mouse fibroblast-like cells (FLCs) with siRNAs targeting the Arp3 and ARPC3 subunits of the complex, and confirmed depletion of the complex by immunoblotting for the Arp3 subunit (Figure 6A). The effect on the morphology of WASH-associated compartments was then examined by immunofluorescence. Interestingly, WASH-containing structures were enlarged and extended when compared with similar compartments in FLCs treated with control siRNAs (Figure 6B). We conclude that the Arp2/3 complex, like actin, is critical for determining shape of WASH compartments on endosomes.
Figure 6.
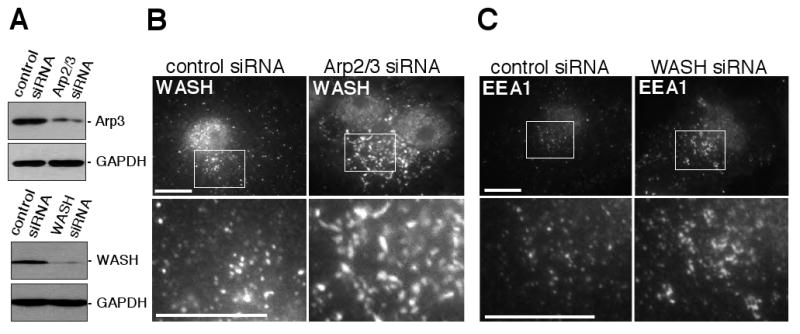
WASH and the Arp2/3 complex control endosome shape. (A) Extracts from fibroblast-like cells (FLCs) treated with control non-specific siRNAs, siRNAs targeting the Arp3 and ARPC3 subunits of the Arp2/3 complex, or WASH siRNAs were separated by SDS-PAGE and immunoblotted with anti-Arp3, anti-WASH, or anti-GAPDH (loading control) antibodies. (B) RNAi silencing of Arp2/3 complex expression in FLCs lead to enlarged WASH-positive compartments visualized by immunofluorescence. (C) RNAi silencing of WASH in FLCs lead to enlarged EEA1-positive endosomes visualized by immunofluorescence. Scale bars, 10 μm.
Next, we sought to ascertain if WASH also plays a role in endosome shape determination. Therefore, we transfected FLCs with siRNA targeting WASH, which resulted in substantial depletion of WASH protein levels (Figure 6A), and visualized early endosomes by immunofluorescence staining for EEA1 (Figure 6C). In contrast to cells transfected with control siRNAs, which had small, round EEA1-positive vesicles, WASH-depleted cells contained many larger and elongated EEA1-positive structures. Collectively, these data demonstrate that actin assembly by WASH and the Arp2/3 complex is important for maintenance of endosome geometry.
WASH depletion does not affect the kinetics of transferrin uptake or recycling
To determine if the ability of WASH to influence endosome shape is correlated with a role in modulating endocytic trafficking, we first examined the effect of WASH depletion on the kinetics of internalization and recycling of the endocytic cargo protein transferrin. Mouse NIH-3T3 cells were transfected with control or WASH siRNAs, and depletion of WASH was confirmed by immunoblotting (Figure 7A). To measure a single round of transferrin internalization and recycling, biotinylated transferrin (B-SS-Tfn) was prebound to cell surface receptors at 4 °C, then chased at 31 °C for various times, and the percentage of B-SS-Tfn internalized was determined by ELISA for each time point (Figure 7B). Unexpectedly, WASH depletion had no effect on the kinetics of transferrin internalization or trafficking. Thus, the defect in endosome morphology observed in WASH-depleted cells does not perturb transferrin trafficking (Figure 7B), consistent with previous data showing that severe disruption of endosome geometry is not always predictive of transferrin trafficking defects (Ceresa et al. 2001).
Figure 7.

WASH depletion does not affect the kinetics of transferrin uptake or recycling in the presence or absence of N-WASP. (A) Extracts from NIH-3T3s or fibroblast-like cells (FLCs) transfected with control or WASH siRNAs were separated by SDS-PAGE and immunoblotted with anti-WASH or anti-GAPDH antibodies. (B) Single-round kinetics of uptake and recycling of prebound biotinylated transferrin (B-SS-Tfn) in NIH-3T3 cells, N-WASP+/+ FLCs, and N-WASP-/- FLCs. Graph shows percent of total B-SS-Tfn that is internalized (mean of 3 independent experiments ± SD) versus time. (C) WASH (green) and N-WASP (red) stained by immunofluorescence and DNA (blue) stained by DAPI were visualized in COS7 cells by deconvolution microscopy. Scale bar, 5 μm.
Previous studies have shown that the NPF N-WASP also associates with endosomes and is important for vesicle rocketing (Chang et al. 2003; Taunton et al. 2000), raising the possibility that the failure to observe a defect in transferrin trafficking in WASH-depleted cells may be due to functional redundancy between N-WASP and WASH. To test this hypothesis, we measured the kinetics of transferrin uptake and recycling, as described above, in cells lacking both N-WASP and WASH. N-WASP+/+ and N-WASP-/- FLCs (Snapper et al. 2001) were treated with control siRNAs or siRNAs targeting WASH, and depletion of WASH was confirmed by immunoblotting (Figure 7A). Surprisingly, both N-WASP-/- cells treated with control siRNAs and N-WASP-/- cells depleted for WASH displayed transferrin uptake and recycling kinetics that were indistinguishable from control cells (Figure 7B). These results indicate that neither WASH nor N-WASP plays a discernable role in transferrin trafficking in this cell type.
To further investigate whether WASH and N-WASP might perform a redundant function in cells, we examined whether the two proteins colocalized in cells by immunofluorescence staining. To this end, we generated an anti-N-WASP antibody that recognized a single band in N-WASP+/+ FLCs, but not N-WASP-/- FLCs (Supplementary Figure 1). Notably, we did not observe any colocalization between the two proteins, in contrast to the colocalization between WASH and endosomal marker proteins (Figure 7C). These data indicate that N-WASP and WASH reside in different compartments and suggest that they perform distinct cellular functions.
WASH depletion or actin cytoskeleton disruption delays EGF transport to late endosomes
Because WASH silencing did not affect transferrin internalization or recycling, we next investigated whether WASH and/or actin played a role in trafficking of EGF in the degradative pathway. Previous studies have shown that EGFR and EGF could be internalized either by a clathrin-dependent pathway, which resulted in recycling back to the cell surface (Sigismund et al. 2008), or a clathrin-independent pathway, which leads to degradation in lysosomes (Orth et al. 2006; Sigismund et al. 2008; Sigismund et al. 2005). In our experiments, FLCs were treated with high concentrations of EGF to enhance the degradative pathway, as described previously (Sigismund et al. 2008). To measure the transport of EGF to LAMP1-positive late endosomes, FLCs were pulsed with fluorescent EGF for 10 min to allow internalization, chased with unlabeled EGF for 30 min to allow transport to late endosomes, and then fixed and stained for the late endosome marker LAMP1, and imaged by deconvolution microscopy (Figure 8A). The percentage of fluorescent EGF that was transported to late endosomes was then quantified (Figure 8C).
Figure 8.

WASH depletion or disruption of the actin cytoskeleton delays EGF transport to LAMP1-positive late endosomes. (A) Fibroblast-like cells (FLCs) were transfected with nonspecific siRNA (control) or WASH siRNA, or treated with latrunculin A (Lat A) or cytochalasin D (Cyto D). After Alexa Fluor 488 EGF complex (green) was internalized for 10 min at 37 °C and chased for 30 min at 37 °C, cells were fixed, and LAMP1 (red) was stained by immunofluorescence and DNA (blue) was stained with DAPI. Scale bar, 5 μm. (B) Extracts from FLCs transfected with control or WASH siRNAs were separated by SDS-PAGE and immunoblotted with anti-WASH or anti-GAPDH antibodies. (C) The amount of fluorescent EGF colocalized with LAMP1-positive vesicles as a percentage of the total fluorescent EGF is shown for each treatment. 10 cells were counted for each condition, and data are mean ± SD of three independent experiments. Asterisk (*) indicates a p-value < 0.05.
In this assay, disrupting actin polymerization by treating cells with cytochalasin D, or depolymerizing actin with latrunculin A, dramatically reduced the transport of fluorescent EGF to LAMP1-positive vesicles compared to control cells (Figure 8A, C). Importantly, silencing WASH expression by RNAi (Figure 8B) also caused a significant reduction in the percentage of EGF reaching LAMP1-positive structures (Figure 8C). Intriguingly, WASH depletion and actin disruption also resulted in a redistribution of LAMP1-positive structures from the perinuclear region to more peripheral regions in the cell (Figure 8A). However, there was no statistically significant difference in the total number of LAMP1 compartments after either treatment (data not shown). Together these results suggest that WASH and F-actin play an important role in both transport to late endosomes and positioning these compartments in the cell.
Discussion
Nucleation-promoting factors regulate the Arp2/3 complex to coordinate actin assembly during diverse cellular behaviors. Here we show that the NPF WASH and the Arp2/3 complex initiate actin assembly on endosomes. Moreover, WASH, the Arp2/3 complex, and actin dynamics are important for determining endosome morphology and trafficking to late endosomes.
Our results demonstrate that human WASH is a potent NPF in vitro. The nucleation-promoting activity of WASH-WCA is comparable to that of N-WASP-WCA, the most potent of the mammalian NPFs, and greater than that of WAVE2-WCA or WHAMM-WCA. The relative potency of the human protein is consistent with the activity of D. melanogaster WASH-WCA, which has also been reported to be as active as fly WASP-WCA and more potent than WAVE/Scar-WCA (Liu et al. 2009). Thus, WASH activity appears to be well-conserved among metazoans.
Our results also suggest a potential mechanism for WASH regulation. We observe that the NPF activities of full-length WASH and WASH-WCA are comparable in vitro. Similarly, it has been reported that full-length D. melanogaster WASH is slightly more active than WASH-WCA (Liu et al. 2009). These results imply that WASH may not be regulated by an autoinhibitory mechanism like that of N-WASP. Furthermore, we find that overexpression of GFP-WASH in cells results in ectopic actin assembly. This activity of WASH in cells is shared with several other NPFs including the WAVEs (Machesky and Insall 1998) and WHAMM, but not N-WASP (Campellone et al. 2008). It is now well-established that the activity of native WAVE2 is suppressed in cells by its association with a complex of interacting proteins (Derivery et al. 2009a; Eden et al. 2002; Ismail et al. 2009). Therefore, WASH NPF activity is likely to be similarly regulated in trans by interacting proteins to tightly control actin polymerization. In support of this idea, the results of two recent studies suggest that WASH exists in a stable complex with other proteins in cells (Derivery et al. 2009b; Gomez and Billadeau 2009).
In contrast to a previous report that a C-terminally GFP-tagged WASH derivative localizes to filopodia and lamellipodia (Linardopoulou et al. 2007), we find that endogenous WASH and an N-terminally GFP-tagged variant localize to a subpopulation of endosomes. This discrepancy could be due to the location of the GFP tag (N- or C-terminal) or differences in expression levels of these constructs. Nevertheless, the fact that we observe similar localization patterns for endogenous WASH and the N-terminally GFP-tagged protein supports our observations. Using deconvolution microscopy we show that endogenous WASH colocalizes with markers of several endosomal compartments, including the early endosome markers EEA1 and Rab5, the recycling endosome marker Rab11, and to a lesser extent, the fast recycling pathway marker Rab4. Our observations are in broad agreement with two recent studies that also concluded that WASH colocalizes with markers of early endosomal compartments (Derivery et al. 2009b; Gomez and Billadeau 2009). Nevertheless, some differences in the localization patterns have been reported, most notably the observation of either strong (Derivery et al. 2009b) or weak (our study) association with Rab4-positive fast recycling endosomes. Such differences might be attributed to the distinct cell types, experimental conditions, and antibodies used in each study. In our study, WASH is particularly enriched on early endosomes, suggesting that it functions during the early stages of endocytic trafficking.
Early endosomes are the sorting station for most internalized cargo and are thought to be organized into multiple structural and functional domains (Gruenberg 2001) to facilitate the efficient trafficking of cargo destined for degradation, recycling back to the plasma membrane, or delivery to the trans-Golgi network (Maxfield and McGraw 2004; Perret et al. 2005). Interestingly, we find that WASH is not uniformly distributed on early endosomes. Instead, it is asymmetrically distributed relative to EEA1, suggesting that it localizes to specific subdomains of endosomes. Two recent studies also documented the asymmetric localization of WASH relative to endosomal membranes (Derivery et al. 2009b; Gomez and Billadeau 2009). How these specialized WASH-associated domains are organized remains an open question. One possibility is suggested by the observations that the actin-binding protein annexin A2 interacts specifically with cholesterol-rich membrane platforms on early endosomes (Harder et al. 1997; Mayran et al. 2003), and that these domains are associated with F-actin (Morel et al. 2009). Thus, WASH may also be associated with cholesterol-rich subdomains, since WASH colocalizes with F-actin on endosomes. It is also possible that actin itself plays a role in organizing these subdomains. We propose that WASH localization and actin polymerization on early endosomes coordinates localized membrane remodeling.
Consistent with a role for WASH in membrane remodeling, we show that WASH, the Arp2/3 complex, and actin are critical for controlling early endosome morphology in cells. Depletion of WASH or the Arp2/3 complex by RNAi, or disruption of actin assembly by drug treatments, results in the appearance of enlarged WASH- or EEA1-positive endosomes that exhibit an exaggerated tubular morphology. This result is similar to what was recently reported for WASH-depleted cells (Derivery et al. 2009b; Gomez and Billadeau 2009), and to the recent observation that actin disassembly by drug treatment causes elongated tubules to emanate from early endosomes as visualized by electron microscopy (Morel et al. 2009). Our results complement these studies and further support a direct role for WASH and the Arp2/3 complex in modulating endosome shape and tubulation.
Despite the disruption of early endosome shape in WASH-depleted cells, we do not observe any defect in the uptake or recycling of transferrin, even if WASH is depleted in cells genetically lacking N-WASP. Two recent studies also examined the role of WASH in transferrin trafficking (Derivery et al. 2009b; Gomez and Billadeau 2009). Consistent with our results, one group observed that surface transferrin levels were unaffected in WASH-depleted cells (Gomez and Billadeau 2009). In contrast, the second group observed that transferrin recycling was impaired in cells silenced for WASH expression (Derivery et al. 2009b). It is noteworthy that all three studies examined transferrin trafficking using different assays. Moreover, our study examined transferrin recycling kinetics in NIH-3T3 cells, whereas HeLa cells were used in the study that observed an effect of WASH depletion (Derivery et al. 2009b). Therefore, it is likely that differences in experimental procedures and/or cell type contributed to any apparent discrepancies.
Although we do not detect a role for WASH in transferrin uptake and recycling, we find that WASH is important for endocytic trafficking of EGF. WASH depletion by RNAi or actin disassembly by drug treatment impairs EGF trafficking to late endosomes. In agreement with our observations, it has recently been shown that disrupting the actin cytoskeleton inhibits EGFR degradation (Morel et al. 2009). These observed defects in EGF trafficking upon WASH depletion or actin disruption, together with the appearance of enlarged and tubulated endosomes (this study; Morel et al. 2009), raise the possibility that that tubular extensions observed on early endosomes represent exaggerated intermediates in the endosomal maturation process. A second possibility arises from the recent observation that WASH regulates retromer-mediated retrograde transport of CI-MPR (Gomez and Billadeau 2009). In light of this finding, it is also possible that the tubular extensions we observe represent arrested retromer traffic or recycling cargo, which delays the transition from early-to-late endosomes in our assay. Future work will be required to distinguish between possibilities.
Our results suggest a model in which WASH, the Arp2/3 complex, and actin dynamics are involved in membrane remodeling events that are required for the transition from early-to-late endosomes. Consistent with this notion, annexin A2, Spire1, and the Arp2/3 complex were recently proposed to contribute to actin assembly and remodeling or severing of early endosomes (Morel et al. 2009). Our work expands on this idea by implicating WASH in the activation of the Arp2/3 complex on endosomes. WASH may also work cooperatively with other actin nucleators such as Spire and formins, as has been suggested to occur during D. melanogaster oogenesis (Liu et al. 2009). We propose that WASH localization to subdomains of early endosomes may reflect an association with emerging membrane tubules on this compartment. Because interfering with WASH, the Arp2/3 complex, or actin function results in elongated endosome tubules, we speculate that actin assembly by WASH and the Arp2/3 complex is involved in severing these nascent tubules. While the precise mechanism of membrane severing by WASH, the Arp2/3 complex, and actin is not clear, this process may be analogous to how N-WASP and branched actin networks function during endocytic vesicle scission at the plasma membrane (Kaksonen et al. 2006; Perrais and Merrifield 2005).
Although our study sheds light on the cellular function of WASH and actin in endocytic trafficking, numerous questions remain. One question centers on the regulation of WASH activity in cells. Most other mammalian NPFs are regulated by small GTPases (Stradal and Scita 2006), and it has been proposed that WASH is regulated by the Rho family GTPase Rho1 in D. melanogaster (Liu et al. 2009). Mammalian WASH may also be regulated by Rho, or alternatively by one of the many Rab-family GTPases that coordinate endocytic trafficking events. A second question centers on the mechanism by which actin assembly contributes to endosome morphogenesis and trafficking. The contribution of actin filaments may be to act as a scaffold for the recruitment of other factors, or to generate force by polymer assembly or via the activity of motor proteins. Furthermore, microtubules may also play a role in this process, and WASH may contribute to coordinating the activity of multiple cytoskeletal systems, as D. melanogaster WASH displays both actin filament and microtubule bundling activities in vitro (Liu et al. 2009) and mammalian WASH has been shown to interact with tubulin (Gomez and Billadeau 2009). Future experiments will reveal how WASH acts in concert with other actin assembly factors and different cytoskeletal elements to coordinate membrane remodeling in the endocytic pathway.
Conclusions
A general consensus is emerging that WASH localizes to endosomes and is involved in endocytic trafficking. However, WASH has now been implicated in three separate endocytic pathways: transferrin recycling (Derivery et al. 2009b), retromer-mediated retrograde CI-MPR trafficking (Gomez and Billadeau 2009), and EGF trafficking (this study). As early endosomes serve as a crossroads for each of these processes, it is possible that WASH activity directly or indirectly influences all of these trafficking pathways. It is also possible that WASH acts in distinct pathways for different endocytic cargoes or in diverse cell types. We are clearly only beginning to understand the role of actin in endocytic trafficking in mammalian cells, and the next few years are likely to bring exciting developments as we learn more details about the role of NPFs and the Arp2/3 complex in controlling actin and its interacting proteins to shape and reorganize intracellular membranes during membrane trafficking processes.
Supplementary Material
Acknowledgments
We thank members of the Welch Lab for helpful discussion; Ken Campellone, Taro Ohkawa, Alisa Serio, and Cat Haglund for comments on the manuscript; members of Heald, Weis, and Drubin Labs for technical assistance; Ken Campellone, Karsten Weis, and Leanna Ferrand for experimental assistance. S.N.D. was supported by an NSF Graduate Research Fellowship. M.D.W. is supported by NIH/NIGMS grant R01-GM059609.
References
- Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131(5):847–60. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem. 2002;277(40):37771–6. doi: 10.1074/jbc.M204145200. [DOI] [PubMed] [Google Scholar]
- Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118(Pt 14):3103–15. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–32. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Campellone KG, Webb NJ, Znameroski EA, Welch MD. WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell. 2008;134(1):148–61. doi: 10.1016/j.cell.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa BP, Lotscher M, Schmid SL. Receptor and membrane recycling can occur with unaltered efficiency despite dramatic Rab5(q79l)-induced changes in endosome geometry. J Biol Chem. 2001;276(13):9649–54. doi: 10.1074/jbc.M010387200. [DOI] [PubMed] [Google Scholar]
- Chang FS, Stefan CJ, Blumer KJ. A WASp homolog powers actin polymerization-dependent motility of endosomes in vivo. Curr Biol. 2003;13(6):455–63. doi: 10.1016/s0960-9822(03)00131-3. [DOI] [PubMed] [Google Scholar]
- Derivery E, Lombard B, Loew D, Gautreau A. The Wave complex is intrinsically inactive. Cell Motil Cytoskeleton. 2009a doi: 10.1002/cm.20342. [DOI] [PubMed] [Google Scholar]
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev Cell. 2009b;17(5):712–23. doi: 10.1016/j.devcel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418(6899):790–3. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein AE, Drubin DG. Actin assembly and endocytosis: from yeast to mammals. Annu Rev Cell Dev Biol. 2003;19:287–332. doi: 10.1146/annurev.cellbio.19.111401.093127. [DOI] [PubMed] [Google Scholar]
- Goley ED, Rodenbusch SE, Martin AC, Welch MD. Critical conformational changes in the Arp2/3 complex are induced by nucleotide and nucleation promoting factor. Mol Cell. 2004;16(2):269–79. doi: 10.1016/j.molcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7(10):713–26. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Gomez TS, Billadeau DD. A FAM21-containing WASH complex regulates retromer-dependent sorting. Dev Cell. 2009;17(5):699–711. doi: 10.1016/j.devcel.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2(10):721–30. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Hanisch J, Ehinger J, Ladwein M, Rohde M, Derivery E, Bosse T, Steffen A, Bumann D, Misselwitz B, Hardt WD, et al. Molecular dissection of Salmonella-induced membrane ruffling versus invasion. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01380.x. [DOI] [PubMed] [Google Scholar]
- Harder T, Kellner R, Parton RG, Gruenberg J. Specific release of membrane-bound annexin II and cortical cytoskeletal elements by sequestration of membrane cholesterol. Mol Biol Cell. 1997;8(3):533–45. doi: 10.1091/mbc.8.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti M, Gerboth S, Rottner K, Lai FP, Hertzog M, Stradal TE, Frittoli E, Didry D, Polo S, Disanza A, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol. 2005;7(10):969–76. doi: 10.1038/ncb1304. [DOI] [PubMed] [Google Scholar]
- Ismail AM, Padrick SB, Chen B, Umetani J, Rosen MK. The WAVE regulatory complex is inhibited. Nat Struct Mol Biol. 2009;16(5):561–3. doi: 10.1038/nsmb.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7(6):404–14. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature. 2000;404(6774):151–8. doi: 10.1038/35004513. [DOI] [PubMed] [Google Scholar]
- Linardopoulou EV, Parghi SS, Friedman C, Osborn GE, Parkhurst SM, Trask BJ. Human Subtelomeric WASH Genes Encode a New Subclass of the WASP Family. PLoS Genet. 2007;3(12):e237. doi: 10.1371/journal.pgen.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Abreu-Blanco MT, Barry KC, Linardopoulou EV, Osborn GE, Parkhurst SM. Wash functions downstream of Rho and links linear and branched actin nucleation factors. Development. 2009;136(16):2849–60. doi: 10.1242/dev.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8(25):1347–56. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Marchand JB, Kaiser DA, Pollard TD, Higgs HN. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat Cell Biol. 2001;3(1):76–82. doi: 10.1038/35050590. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5(2):121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mayran N, Parton RG, Gruenberg J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. Embo J. 2003;22(13):3242–53. doi: 10.1093/emboj/cdg321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Qualmann B, Kessels MM, Almers W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur J Cell Biol. 2004;83(1):13–8. doi: 10.1078/0171-9335-00356. [DOI] [PubMed] [Google Scholar]
- Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391(6662):93–6. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- Miki H, Yamaguchi H, Suetsugu S, Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408(6813):732–5. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- Morel E, Parton RG, Gruenberg J. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev Cell. 2009;16(3):445–57. doi: 10.1016/j.devcel.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 2006;66(7):3603–10. doi: 10.1158/0008-5472.CAN-05-2916. [DOI] [PubMed] [Google Scholar]
- Osborne A, Flett A, Smythe E. Endocytosis assays in intact and permeabilized cells. Curr Protoc Cell Biol. 2005;Chapter 11:Unit 11–18. doi: 10.1002/0471143030.cb1118s27. [DOI] [PubMed] [Google Scholar]
- Perrais D, Merrifield CJ. Dynamics of endocytic vesicle creation. Dev Cell. 2005;9(5):581–92. doi: 10.1016/j.devcel.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Perret E, Lakkaraju A, Deborde S, Schreiner R, Rodriguez-Boulan E. Evolving endosomes: how many varieties and why? Curr Opin Cell Biol. 2005;17(4):423–34. doi: 10.1016/j.ceb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290(5492):801–6. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- Robertson AS, Smythe E, Ayscough KR. Functions of actin in endocytosis. Cell Mol Life Sci. 2009;66(13):2049–65. doi: 10.1007/s00018-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal AA, Kozubowski L, Goode BL, Drubin DG, Hartwig JH. Actin and septin ultrastructures at the budding yeast cell cortex. Mol Biol Cell. 2005;16(1):372–84. doi: 10.1091/mbc.E04-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97(2):221–31. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10(6):311–20. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15(2):209–19. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102(8):2760–5. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper SB, Takeshima F, Anton I, Liu CH, Thomas SM, Nguyen D, Dudley D, Fraser H, Purich D, Lopez-Ilasaca M, et al. N-WASP deficiency reveals distinct pathways for cell surface projections and microbial actin-based motility. Nat Cell Biol. 2001;3(10):897–904. doi: 10.1038/ncb1001-897. [DOI] [PubMed] [Google Scholar]
- Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7(12):897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Stradal TE, Rottner K, Disanza A, Confalonieri S, Innocenti M, Scita G. Regulation of actin dynamics by WASP and WAVE family proteins. Trends Cell Biol. 2004;14(6):303–11. doi: 10.1016/j.tcb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Stradal TE, Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol. 2006;18(1):4–10. doi: 10.1016/j.ceb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Kurisu S, Oikawa T, Yamazaki D, Oda A, Takenawa T. Optimization of WAVE2 complex-induced actin polymerization by membrane-bound IRSp53, PIP(3), and Rac. J Cell Biol. 2006;173(4):571–85. doi: 10.1083/jcb.200509067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8(1):37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J Cell Biol. 2000;148(3):519–30. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol. 1997;138(2):375–84. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MD, Mullins RD. Cellular control of actin nucleation. Annu Rev Cell Dev Biol. 2002;18:247–88. doi: 10.1146/annurev.cellbio.18.040202.112133. [DOI] [PubMed] [Google Scholar]
- Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16(2):964–75. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Coutts AS, Quinlan ME, Thangue NB, Mullins RD. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat Cell Biol. 2009;11(4):451–9. doi: 10.1038/ncb1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


