Abstract
Low frequency transcranial ultrasound (<1MHz) is being investigated for a number of brain therapies, including stroke, tumor ablation, and localized opening of the blood brain barrier. However, lower frequencies have been associated with the production of undesired standing waves and cavitation in the brain. Presently, we examine an approach to suppress standing waves during continuous wave transcranial application. The investigation uses a small randomization in the frequency content of the signal for suppressing standing waves. The approach is studied in an ex-vivo human skull and in a plastic-walled chamber, representing idealized conditions. The approach is compared to single-frequency continuous wave operation as well as to a swept-frequency input. Acoustic field scans demonstrate that the swept-frequency method can suppress standing waves in the plastic chamber and skull by 3.4 and 1.6 times, respectively, compared to single-frequency continuous wave excitation. With random modulation, standing waves were reduced by 5.6 and 2 times, respectively, in the plastic chamber and skull. It is expected that the process may play a critical role in providing a safer application of the ultrasound field in the brain and may have application in other areas where standing waves may be created.
Index Terms: Random frequency modulation, standing wave suppression, transcranial ultrasound
I. Introduction
Transcranial ultrasound has demonstrated the potential to serve as a therapeutic tool in the treatment of a range of disorders [1]–[9]. At sub-megahertz frequencies, reduced aberration and absorption by the skull make such applications more practical [10]–[13]. Low frequency ultrasound, however, can be associated with certain risks for adverse bioeffects. Notably, the cavitation threshold reduces with frequency [14]. There is also an increased potential of inducing standing waves [15] as a result of longer wavelengths and reduced absorption in the brain tissue. The occurrence of such standing waves at and away from the therapeutic target locations has been suggested as a source of hemorrhaging during low frequency transcranial thrombolysis [16]–[18].
Motivated by these observations, we examine an approach for eliminating standing waves in the skull during continuous wave (CW), or near-CW application of ultrasound. We hypothesize that small randomization in the frequency content of the application signal is sufficient to significantly reduce standing waves. In this manner, the randomization will cause a break in the ultrasound symmetry between forward and reflected waves that does not allow standing waves to be established. It is further predicted that, given a sufficient spread in bandwidth, intensity fluctuations in the nearfield will be homogenized as a result of the frequency dependence on the spatial locations of nulls in the field.
Frequency sweeps have long been understood to reduce standing waves, and have been implemented in various ultrasound products, such as ultrasonic cleaners [19]. Mitri et al. [20] used a related approach to suppress standing waves in vibro-acoustography and Erpelding et al. [21] used a similar approach to inhibit standing waves in bubble-based radiation force measurements. Building on these studies, we compare the present randomized approach to the frequency sweep with similar frequency content.
II. MATERIALS AND METHODS
A. Signal generation
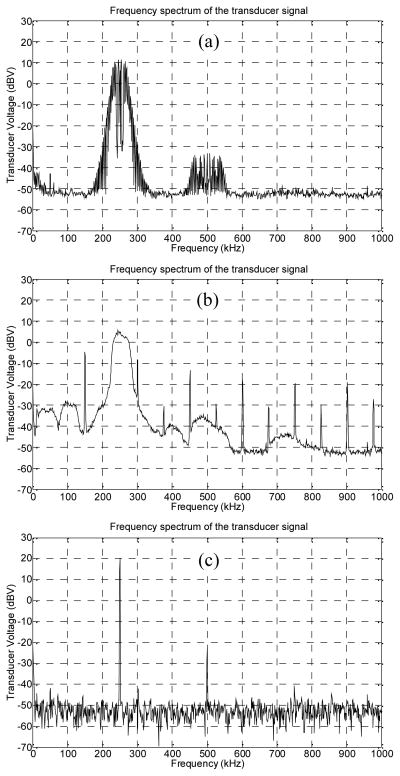
An air backed transducer (250 kHz, 50mm diameter, 10cm radius of curvature) with a −6 dB bandwidth of 50 kHz was used to generate all fields. Two different signal types were generated to compare the efficacy of the standing wave suppression. In the first signal, the frequency was swept linearly with time. This swept signal was created by feeding a triangular wave with a frequency of 3.9 kHz from a function generator (SRS, DS345) to another function generator (Agilent, 33250A) that modulated the 250 kHz carrier frequency with the triangular signal. The second signal was created by modulating a carrier signal with a random noise signal so that the instantaneous frequency deviated from the center frequency in a random manner. This random-modulated signal was generated by a custom-made circuit that consisted of a voltage-controlled-oscillator with a center frequency of 250 kHz and a bandwidth of 50 kHz (±10% of the center frequency) fed by a random noise modulation signal. Likewise, the center frequency and bandwidth of the swept signals were 250 kHz and 50 kHz. Both signals were amplified by an RF power amplifier (E&I, 240L) that excited the transducer. Control experiments with single-frequency CW excitation at 250 kHz were performed. The voltages across the transducer excited by the spread-spectrum and the single-frequency signals were set to 10Vrms and measured by an oscilloscope (Tektronix, TDS3014B). The power delivered to the transducer was less than 1W. The frequency spectra of the measured transducer voltages with different excitations are shown in Fig. 1, and the length of waveforms acquired for averaging for the single, swept and random frequency excitations were 2ms, 10ms and 100ms, respectively.
Fig. 1.
Frequency spectrum of the measured voltage of the transducer excited by the signals with (a) swept frequency, (b) random-signal-modulation, and (c) single frequency without modulation.
B. Acoustic field scan experiments
The experiments were carried out inside a rubber-lined tank (tank dimensions = 111cm×45cm×40cm, rubber thickness = 6.35mm, and α = 8.6m−1 at 250kHz) filled with degassed deionized water. A 1mm diameter PVDF needle hydrophone (Precision Acoustics) was used to sense the acoustic pressure within the area of interest. This area was situated inside an ex-vivo human skull, in which the parietal bones of the skull were placed perpendicular to the transducer’s propagation axis.
The needle hydrophone was mounted on a three-axis positioning system controlled by a PC and was aligned 45° with respect to the ultrasound propagation direction. The transducer axis of symmetry was chosen as the Cartesian z-axis, with the origin at the center of the transducer. The sagittal plane and the transducer surface are parallel to the x-y plane, and the transverse plane is parallel to the x-z plane. The distance between the skull wall closest to the transducer and the transducer surface was 12mm. The ultrasound field in the x-z plane (y=0) in the cranium was scanned. The scan range was from (x, z) = (−30mm,30mm) to (30mm,70mm). With the single and swept-frequency excitations, the length of waveform data acquired at each hydrophone position were 2ms and 10ms, respectively, and the spatial resolution was 0.5mm for both excitations. The spatial resolution and the length of the waveform data acquired for the random-modulation were 1mm and 100ms, respectively.
Similar experiments were performed with two parallel acrylic plates replacing the ex-vivo human skull in order to evaluate the performance of the standing wave suppression methods in an idealized condition. The plates (150mm×90mm×5mm) were parallel to the transducer, and the plate separation was 129mm. The scan area ranged from (x, z) = (−30mm,20mm) to (30mm,70mm) with spatial resolution of 0.5mm.
III. RESULTS
A. Acoustic field Scans
The averaged relative acoustic pressure field plots in the x-z plane (y=0) inside an ex-vivo human cranium, with single frequency, swept frequency and random-signal modulation excitations, are shown in Figs. 2. The field patterns illustrate that the swept frequency method provided an improvement on the reduction of the standing wave effect compared to the single-frequency excitation. However, the standing wave effect with the swept excitation was still clearly visible. The distance between two adjacent peaks along the ultrasound propagation direction is 3mm which is half of the wavelength of the excitation center frequency. The plots also reveal that the random-modulation scheme substantially suppresses the standing wave effect in the ultrasound main beam (|x|<5mm). An inconspicuous standing wave effect was observed outside the main beam, but the intensity was significantly lower than that inside the main ultrasound beam. Both the average and the peak intensity at x=−15mm (outside the main beam) were more than 3 times less than that at x=0mm (inside the main ultrasound beam) from z=40m to 60mm.
Fig. 2.
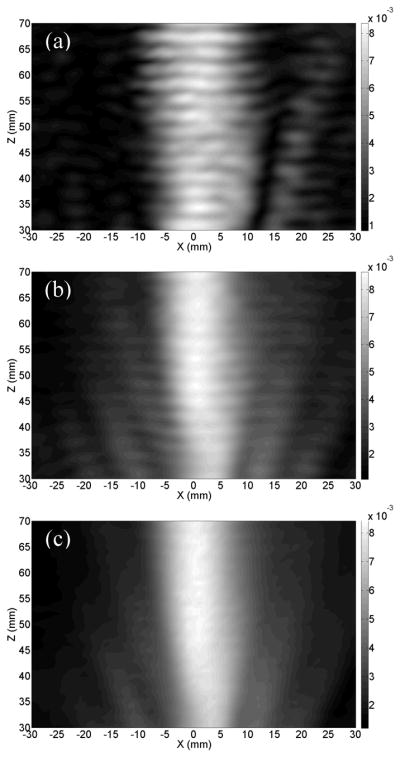
Measured hydrophone rms voltage in the x-z plane in an ex-vivo human cranium when the transducer was excited by the signal with (a) single frequency, (b) swept-frequency, and (c) random-signal modulation in linear scale.
B. Effectiveness of Standing Wave Suppression
The effectiveness of the standing wave suppression method is quantified by a comparison of the amplitude peak, pmax, to minimum, pmin, over a region with the average amplitude of pavg,
| (1) |
which provides a quantification of the deviation from the mean pressure. The ratio R in the region between 40 mm and 60 mm from the transducer face was investigated for the estimation of the effectiveness of the standing wave suppression, and was evaluated from the experiments with the ex-vivo human skull and the plastic plates. For reference, the standing wave ratio (SWR) between the wave node and antinode is also presented. The SWR quantifies the extent to which the wave is a pure or partial standing wave [22]. These results are summarized in Table I. From the skull experiments, the standing wave effect was reduced by 1.6 and 2 times respectively in ratio R with the swept frequency and the random frequency excitations. A similar trend was recorded in the plastic plates, the standing wave effect is reduced by 3.4 times with the swept-frequency method, and 5.6 times with the random frequency method in the plastic chamber situation.
Table I.
Standing wave effect with different excitations quantified in terms of the ratio R and the SWR
| Excitation | Skull | Plastic Plates | ||
|---|---|---|---|---|
| Ratio R | SWR | Ratio R | SWR | |
| Single Frequency | 0.276 | 2.37 dB | 0.354 | 1.42 dB |
| Swept-Frequency | 0.174 | 1.57 dB | 0.102 | 0.89 dB |
| Random Modulation | 0.135 | 1.20 dB | 0.063 | 0.54 dB |
IV. DISCUSSION AND CONCLUSION
The study was designed to demonstrate a potential method for reducing standing waves that may be induced with low-frequency transcranial ultrasound. The approach was examined at 250 kHz, due to the frequency’s direct implications from recent studies in transcranial tumor ablation, sonothrombolysis and targeted opening of the blood brain barrier. The method, however, is expected to readily generalize to any application where it would be advantageous to eliminate standing waves.
Experiments indicated the effect of inducing random-frequency modulations on ultrasound standing waves. Results indicate the approach’s ability to inhibit standing waves both within a human skull, as well as within a plastic cavity of parallel walls. The approach was found to be superior to the established swept-frequency method. The method may have application in stroke treatment, transcranial ablative therapies and in studies using ultrasound to open the blood brain barrier. Application is further expected to translate to an assortment of situations where suppression of standing waves is desired.
Acknowledgments
This work was supported by NIH grants U41 RR019703 and R01 EB003268.
Contributor Information
Sai Chun Tang, Email: sct@bwh.harvard.edu.
Gregory T. Clement, Email: gclement@hms.harvard.edu.
References
- 1.Hynynen K, Clement G. Clinical applications of focused ultrasound - The brain. Int J Hyperthermia. 2007;23(2):193–202. doi: 10.1080/02656730701200094. [DOI] [PubMed] [Google Scholar]
- 2.Clement GT, White PJ, King RL, McDannold N, Hynynen K. A magnetic resonance imaging-compatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound in Med. 2005;24(8):1117–1125. doi: 10.7863/jum.2005.24.8.1117. [DOI] [PubMed] [Google Scholar]
- 3.Hynynen K, McDannold N, Clement G, Jolesz FA, Zadicario E, Killiany R, Moore T, Rosen D. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain - A primate study. Eur J Radiology. 2006;59(2):149–156. doi: 10.1016/j.ejrad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7:166–181. doi: 10.1109/iret-me.1960.5008041. [DOI] [PubMed] [Google Scholar]
- 5.Fry WJ. Ultrasound in neurology. Neurology. 1956;6(10):693–704. doi: 10.1212/wnl.6.10.693. [DOI] [PubMed] [Google Scholar]
- 6.Daffertshofer M, Fatar M. Therapeutic ultrasound in ischemic stroke treatment: experimental evidence. Eur J Ultrasound. 2002;16(1–2):121–130. doi: 10.1016/s0929-8266(02)00049-6. [DOI] [PubMed] [Google Scholar]
- 7.Mikulik R, Alexandrov AV. Acute stroke: therapeutic transcranial Doppler sonography. Front Neurol Neurosci. 2006;21:150–161. doi: 10.1159/000092397. [DOI] [PubMed] [Google Scholar]
- 8.Kawata H, Naya N, Takemoto Y, Uemura S, Nakajima T, Horii M, Takeda Y, Fujimoto S, Yamashita A, Asada Y, Saito Y. Ultrasound accelerates thrombolysis of acutely induced platelet-rich thrombi similar to those in acute myocardial infarction. Circ J. 2007;71(10):1643–1648. doi: 10.1253/circj.71.1643. [DOI] [PubMed] [Google Scholar]
- 9.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 10.Ammi AY, Mast TD, Huang IH, Abruzzo TA, Coussios CC, Shaw GJ, Holland CK. Characterization of ultrasound propagation through ex vivo human temporal bone. Ultrasound Med Biol. 2008;34(10):1578–1589. doi: 10.1016/j.ultrasmedbio.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fry FJ, Goss SA, Patrick JT. Transkull focal lesions in cat brain produced by ultrasound. J Neurosurg. 1981;54(5):659–663. doi: 10.3171/jns.1981.54.5.0659. [DOI] [PubMed] [Google Scholar]
- 12.McDannold N, Zadicario E, Pilatou MC, Jolesz FA. Preclinical testing of a second-generation MRI-guided focused ultrasound system for transcranial brain tumor ablation. Proc 16th Scientific Meeting, International Society for Magnetic Resonance in Medicine; 2008. (unpublished observations) [Google Scholar]
- 13.Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA, Sheikov N. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg. 2006;105(3):445–454. doi: 10.3171/jns.2006.105.3.445. [DOI] [PubMed] [Google Scholar]
- 14.Azuma T, Kawabata K, Umemura S, Ogihara M, Kubota1 J, Sasaki A, Furuhata H. Bubble Generation by Standing Wave in Water Surrounded by Cranium with Transcranial Ultrasonic Beam; Jpn J Appl Phys; 2005. pp. 4625–4630. [Google Scholar]
- 15.Azuma T, Kawabata K, Umemura S, Ogihara M, Kubota J, Sasaki A, Furuhata H. Schlieren observation of therapeutic field in water surrounded by cranium radiated from 500 kHz ultrasonic sector transducer. Proc IEEE Ultrason Symp. 2004;2:1001–1004. [Google Scholar]
- 16.Daffertshofer M, Gass A, Ringleb P, Sitzer M, Sliwka U, Els T, Sedlaczek O, Koroshetz WJ, Hennerici MG. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke. 2005;36(7):1441–1446. doi: 10.1161/01.STR.0000170707.86793.1a. [DOI] [PubMed] [Google Scholar]
- 17.Culp WC, McCowan TC. Ultrasound Augmented Thrombolysis. Current Med Imaging Reviews. 2005;1(1):5–12. [Google Scholar]
- 18.Aubry JF, Fink M. Transcranial Ultrasound-Mediated Thrombolysis: Safety Issue. Proc 7th Int Symp on Therapeutic Ultrasound; 2007. (unpublished observations) [Google Scholar]
- 19.Louis B, Long GB. Sonic washer. 2985003. US Patent. 1961
- 20.Mitri FG, Greenleaf JF, Fatemi M. Chirp imaging vibro-acoustography for removing the ultrasound standing wave artifact. IEEE Trans Med Imaging. 2005;24(10):1249–1255. doi: 10.1109/TMI.2005.854518. [DOI] [PubMed] [Google Scholar]
- 21.Erpelding TN, Hollman KW, O’Donnell M. Bubble-based acoustic radiation force using chirp insonation to reduce standing wave effects. Ultrasound Med Biol. 2007;33(2):263–269. doi: 10.1016/j.ultrasmedbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackstock DT. Fundamentals of Physical Acoustics. Vol. 141. Wiley–IEEE; 2000. [Google Scholar]