Abstract
Background
Retinal vessel caliber may be a novel risk marker of coronary heart disease (CHD) risk. However, the gender specific effect, magnitude of association and effect independent of traditional CHD disease risk factors remain unclear.
Purpose
To determine the association between retinal vessel caliber and risk of CHD.
Data sources
Relevant studies were identified through MEDLINE (1950 to June 2009) and EMBASE (1950 to June 2009) databases.
Study Selection
Studies were included if derived from a general population, retinal vessel caliber was measured from retinal photographs and incident CHD events were documented.
Data Extraction
Six population-based prospective cohort studies were identified and provided data for individual participant meta-analysis.
Data Synthesis
Proportional hazards models were constructed for retinal vessel caliber and incident CHD in women and men, while adjusting for traditional CHD risk factors. 2,219 (10.0%) incident CHD events were recorded from 22,159 individuals (mean age 62 years) free of CHD followed for 5–14 years. Retinal vessel caliber changes (wider retinal venules and narrower arterioles) were each associated with an increased risk of CHD in women but not men, with pooled multivariable-adjusted hazard ratios of 1.16 (95% confidence interval, CI, 1.06 to 1.26) per 20μm increase in venular caliber, and 1.17 (95% CI, 1.07 to 1.28) per 20μm decrease in arteriolar caliber in women, and 1.02 (95% CI, 0.94 to 1.10) per 20μm increase in venular caliber and 1.02 (95% CI, 0.95, 1.10) per 20μm decrease in arteriolar caliber in men. Higher hazard ratios were found amongst women without hypertension or diabetes.
Limitations
Error in the measurement of retinal vessel caliber and Framingham variables was not taken into account, and may over or underestimate the true association between retinal vessel caliber and CHD.
Conclusion
Retinal vessel caliber changes were independently associated with an increased risk of CHD events in women.
INTRODUCTION
Coronary heart disease (CHD) remains the leading cause of mortality in the United States despite advances in prevention, diagnosis and therapy. Further improvement in outcomes can be achieved through more accurate identification of persons at risk, enhanced understanding of pathogenesis, novel interventions and better implementation of existing preventive and therapeutic strategies.
Coronary microvascular dysfunction is increasingly recognized as an important contributor to CHD, particularly in women,(1) and there is considerable interest in noninvasive methods of assessing the coronary microcirculation.(2) The coronary vessels and retinal vessels undergo similar changes in hypertension (e.g. sclerosis)(3, 4) and assessment of retinal vessels may provide an indication of coronary microvascular damage.(5) With the advent of computer-assisted methods for measuring retinal vessel caliber from retinal photographs, retinal vascular imaging has been found to independently predict increased risk of CHD in prospective epidemiological studies,(6–10) raising the possibility of retinal vessel assessment as a novel risk marker. However, the results reported thus far have not been consistent. The Atherosclerosis Risk in Communities (ARIC) study, the first large epidemiological study to report associations of retinal vessel caliber with incident CHD, suggested that these associations were only present in middle-aged women.(6) Subsequent studies have produced conflicting results. The Cardiovascular Health Study (CHS) reported associations of narrower retinal arterioles and wider venules with incident CHD in both older women and men,(9) but other studies found associations mainly in younger women and men, with weak or no association in older people.(10)
Differences in study populations and inclusion criteria may account for the varying findings. For example, participants with diabetes or prevalent CHD were included in some,(10) but not other studies,(9, 11) while analytic methods and adjustment for traditional cardiovascular risk factors varied considerably between studies.
To provide robust evidence to address these discrepancies, we conducted a systematic review and an individual participant meta-analysis of population based cohort studies to determine the associations of retinal vessel caliber and CHD risk, while adjusting for traditional risk factors. We particularly examined if there were differences in associations between women and men.
METHODS
Data extraction
We reviewed the literature to identify all studies conducted in general populations that measured retinal vessel caliber and documented CHD events. A MEDLINE and EMBASE search was conducted of all studies published between 1950 and 4th June 2009. The MEDLINE search terms used were (exp Retinal Diseases/, (retina or retinal).tw., retinopathy.tw., Arteriolar narrowing.tw., Arterio-venous nicking.tw., Arteriovenous nicking.tw., venular dilatation.tw., venular dilation.tw., arterio-venular ratio.tw. ) and (Cardiovascular Diseases/,exp Heart Diseases/,exp Vascular Diseases/, cardiovascular.tw., coronary.tw., heart.tw., mortality.tw.) and (incidence/, exp Mortality/, exp epidemiologic studies/, prognos$.tw., Prognosis/, predict$.mp.,course.tw., (score or scoring or scored).tw., observ$.mp., risk:.mp., between group:.tw.) and (Photography/, Photomicrography/, photo$.tw., image$.tw.). We then searched the selected papers to identify studies that met the inclusion criteria: that they were carried out in general populations, had measured retinal vessel caliber from either photographic film or digital photographs using computer assisted methods and had recorded incident CHD events.
We contacted the principal or lead investigators of the chosen studies and obtained individual participant data from each of the studies to allow investigation of heterogeneity in published results, and, if appropriate, to calculate pooled estimates of the associations between retinal vessel caliber and CHD risk. Study investigators who agreed to participate in this collaborative project were then requested to provide original recorded data on the following variables – individual retinal vessel caliber measurements, fatal and non-fatal CHD events and time to these events, baseline measurements of variables included in the Framingham Risk Score (age, sex, systolic blood pressure, serum total cholesterol, high density lipoprotein, current smoking status, use of blood pressure lowering medications, presence of diabetes) plus body mass index, diastolic blood pressure, white blood cell count and previous CHD.
Statistical analysis
We analyzed the data for women and men separately as our primary hypothesis was that retinal vessel caliber predicts incident CHD more strongly in women than men.(8) Also, separate Framingham Risk Scores are used for men and women which have different coefficients for the variables in the score.(12)
The standard deviation for the means of arteriolar and venular caliber was approximately 20 μm. We estimated the hazard ratio associated with per 20μm decrease in arteriolar caliber and per 20μm increase in venular caliber, each adjusted for the other retinal vessel caliber, and adjusted for the variables that comprise the Framingham Risk Score and other risk factors associated with CHD and retinal caliber.(13, 14) These were estimated separately for each study using a proportional hazards model. Data were then combined for all studies and a stratified proportional hazard model was used to test for interaction between the study stratification variable and retinal vessel caliber variables, as well as with gender and the CHD risk factors. This tests heterogeneity across studies in associations with retinal vessel caliber. Where no heterogeneity was present we obtained a pooled hazard ratio adjusted for the CHD risk factors. The stratified proportional hazards model allows the baseline hazard function to differ between the studies but assumes that the effect of the retinal vessel caliber and the other variables are fixed.
Non-fatal CHD events were defined as myocardial infarction, coronary artery bypass graft or coronary angioplasty. Fatal events coded using ICD-10 were classified as CHD deaths if the main or underlying cause of death was coded ICD-10 I21 to I25.
Within each study, the appropriate functional form of each of the continuous variables in the models was assessed using fractional polynomials and the proportional hazards assumption was tested using plots of the Schoenfeld residuals and by testing for the effect of adding time dependent covariates.(15)
In order to examine the robustness of these results, we repeated the main analysis by standardizing the retinal vessel caliber measurements by dividing them by the study specific standard deviations to allow for different mean and standard deviation of the retinal vessel caliber measurements in the different studies.(16) Also, we pooled the study specific hazard ratios using a random effects model.(17)
RESULTS
Characteristics of the studies identified
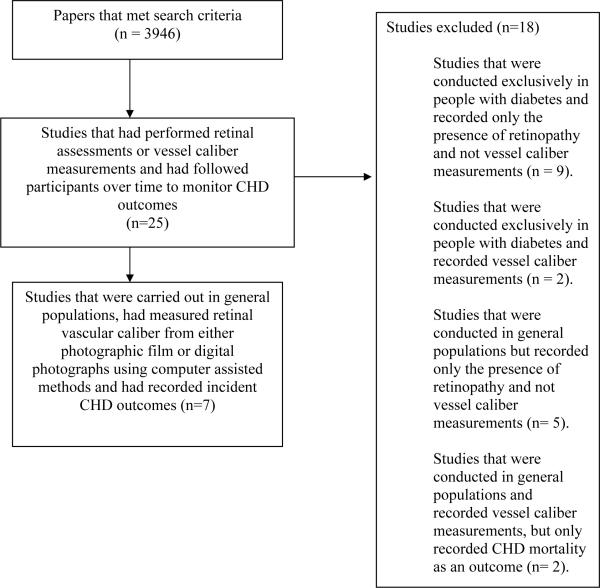
Our initial search strategy found 3946 papers. Twenty-five studies were then identified that had performed retinal assessments or vessel caliber measurements and had followed participants over time to monitor CHD events. Eighteen of these studies recorded only the presence of retinopathy and not retinal vessel caliber, or were conducted exclusively in people with diabetes or recorded only fatal events (Figure 1).(18–35) This left seven studies that met our inclusion criteria. One study, the Multi-Ethnic Study of Atherosclerosis (MESA), did not have sufficient outcome data available at the time of the analysis.(36) Investigators from all six of the other studies agreed to provide data for the individual participant meta-analysis. These six studies were the ARIC, the CHS, the Australian Diabetes, Obesity and Lifestyle (AusDiab) study, the Blue Mountains Eye Study (BMES), the Beaver Dam Eye Study (BDES), and the Rotterdam Study (RS). Table 1 shows the characteristics for 21,428 participants from each of the six studies included in the analysis. The measurement of retinal vessel caliber was similar in each study with some slight variations.(7, 37–41) Briefly, for participants of each study retinal photographs (film or digital) for a single, or both, eyes, centred on the optic disc and macula, were taken. The BDES and BMES both used the Zeis FF3 camera and 30° fields,(10) the ARIC and CHS used the Canon CR6-45NM with 45° fields,(37, 41) AusDiab used the Canon CR45UAF with 45° fields (38) and RS the Topcon TRC-SS2 with 20° fields.(11) Optic disc photographs were then viewed by trained graders masked to participant characteristics. Graders measured the diameters of all arterioles and venules coursing through a zone surrounding the optic disc, one half to one disc diameter away from the optic disc margin, using a computer-assisted software program specifically developed for this purpose.(37) The measurement module was custom programmed in Khoros (public domain image processing software from the University of New Mexico - Albuquerque) and utilized the green channel of the digital image to enhance contrast of the retinal vessels against the retinal pigment epithelium. The ARIC, BDES and BMES employed an earlier version of this software to measure the retinal vessel caliber, while the AusDiab, CHS and RS used a later version of the same software. Both versions are available on request from the authors or the Wisconsin Fundus Photograph Reading Center, University of Wisconsin-Madison. The individual mean retinal vessel calibers provided by each study were summarized for the present meta-analysis using the Parr-Hubbard formula.(37) Reproducibility statistics, based on repeat readings of the same retinal photograph, for these measurements were high with intra- and inter-grader reliability correlation coefficients of 0.73 and 0.72 for arteriolar caliber measurements, 0.86 and 0.76 for venular caliber measurements respectively. (37, 42)
Figure 1.
Flow chart of study selection
Table 1.
Characteristics of study participants included in meta-analysisa
| Mean (standard deviation) |
Number (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Participants included in meta-analysis | Number of CHD events | Median follow-up, years | Arteriolar caliberb, μm | Venular caliberb, μm | Age years | Systolic blood pressure, mm/Hg | Serum Total Cholesterol, mmol/L | Body mass Index, kg/m2 | Diabetes | Taking blood pressure lowering medication | Current smoker | |
| Women | |||||||||||||
| ARICc | 5699 | 303 | 9.2 | 163 (17) | 192 (17) | 59 (6) | 123 (19) | 5.5 (1.0) | 29 (6) | 719 (13%) | 1737 (30%) | 954 (17%) | |
| AusDiabd | 813 | 23 | 5.0 | 179 (24) | 208 (23) | 57 (13) | 134 (20) | 5.8 (1.0) | 29 (6) | 216 (27%) | 210 (26%) | 73 (9%) | |
| BDESe | 1889 | 220 | 14.5 | 201 (21) | 227 (20) | 60 (11) | 130 (21) | 6.2 (1.2) | 28 (6) | 152 (8%) | 636 (34%) | 351 (19%) | |
| BMESf | 1135 | 33 | 4.9 | 195 (20) | 225 (20) | 64 (8.7) | 147 (21) | 6.2 (1.0) | 26 (5) | 57 (5%) | 362 (32%) | 115 (10%) | |
| CHSg | 844 | 129 | 8.3 | 166 (20) | 189 (18) | 78 (4) | 132 (20) | 5.5 (1.0) | 27 (5) | 99 (12%) | 456 (54%) | 48 (6%) | |
| Rotterdam | 2718 | 245 | 12.1 | 183 (18) | 219 (18) | 68 (8) | 139 (23) | 6.9 (1.2) | 27 (4) | 244 (9%) | 803 (30%) | 533 (20%) | |
| Men | |||||||||||||
| ARIC | 4153 | 565 | 9.1 | 161 (17) | 194 (17) | 60 (6) | 124 (17) | 5.2 (0.9) | 28 (4) | 607 (15%) | 1103 (27%) | 777 (19%) | |
| AusDiab | 600 | 29 | 5.0 | 172 (24) | 205 (22) | 58 (13) | 140 (19) | 5.6 (1.0) | 28 (5) | 210 (35%) | 152 (25%) | 88 (15%) | |
| BDES | 1373 | 225 | 14.4 | 202 (20) | 231 (20) | 59 (10) | 132 (18) | 5.9 (1.0) | 29 (5) | 114 (8%) | 386 (28%) | 310 (23%) | |
| BMES | 765 | 60 | 4.9 | 191 (21) | 225 (20) | 64 (9.0) | 144 (20) | 5.8 (1.0) | 26 (4) | 61 (8%) | 184 (24%) | 108 14%) | |
| CHS | 479 | 116 | 8.0 | 164 (19) | 190 (18) | 79 (4) | 129 (18) | 4.9 (0.9) | 27 (4) | 74 (16%) | 234 (49%) | 34 (7%) | |
| Rotterdam | 1691 | 271 | 11.8 | 181 (18) | 220 (18) | 67 (8) | 138 (22) | 6.3 (1.1) | 26 (3) | 134 (8%) | 350 (21%) | 503 (30%) | |
Excluding people with diabetes or previous CVD
Calculated using the Parr-Hubbard formula
Atherosclerosis Risk in Communities Study
Australian Diabetes, Obesity and Lifestyle Study
Beaver Dam Eye Study
Blue Mountains Eye Study
Cardiovascular Health Study
Fatal events in all studies were identified from searches of death registers, and were supplemented with contact with relatives and/or local medical providers in all studies.(43–47) For non-fatal CHD outcomes, the AusDiab, BDES and BMES identified non-fatal CHD events only among those who returned at subsequent visits.(47, 48) These participants were asked whether they had a CHD event. In BMES and AusDiab this was then verified from their medical records, but not in BDES. (47, 48) The remaining three studies – the ARIC, CHS and RS – identified non-fatal events using a process of continuous monitoring which included regular phone interviews and contacts with general practitioners and local hospitals.(43–45)
Participants in all studies completed baseline questionnaires on previous medical history and traditional CHD risk factors were measured. The ARIC, CHS and RS participants underwent a more extensive clinical examination than participants in the other studies.(43–45) All studies used standard methods to measure the traditional CHD risk factors of systolic blood pressure, smoking status, serum total cholesterol and high density lipoprotein. The only differences between studies were that BMES recorded systolic blood pressure taken from one measurement at the baseline visit, whereas all other studies used the average of at least two measurements taken at the baseline visit.(49) Also, the RS and BDES measured non-fasting cholesterol and HDL levels, while all other studies measured fasting values.(7, 10) In the CHS cholesterol, HDL and presence of diabetes were not recorded at the same visit as the retinal caliber measurements. Presence of diabetes was measured 2 years before the retinal caliber measurements and cholesterol and HDL were measured 5 years before this visit.
Assessment of heterogeneity of hazard ratios between studies
The proportional hazards assumption held for all variables in each study. No evidence of a non-linear relationship was found in any of the studies between any covariates and the log-hazard function. Table 2 shows the hazard ratios by quintile of retinal vessel caliber for women and men. Among women as the arteriolar caliber decreased the hazard ratio increased. Also, as the venular caliber increased the hazard ratio increased. No trend was evident amongst men.
Table 2.
Pooled hazard ratiosa and 95% confidence interval for CHD according to quintile of retinal vessel caliber
| Women | |||||
| Venular caliber, μm | <190 | 190 to 199 | 200 to 209 | 210 to 220 | ≥220 |
| Hazard ratio (95% CI) | 1 | 1.06 (0.85, 1.33) | 1.17 (0.92, 1.48) | 1.21 (0.94, 1.57) | 1.50 (1.16, 1.94) |
| Arteriolar caliber, μm | <160 | 160 to 169 | 170 to 179 | 180 to 189 | ≥190 |
| Hazard ratio (95% CI) | 1.62 (1.25, 2.10) | 1.31 (1.02, 1.68) | 1.21 (0.96, 1.53) | 1.17 (0.94, 1.45) | 1 |
| Men | |||||
| Venular caliber, μm | <190 | 190 to 199 | 200 to 209 | 210 to 220 | ≥220 |
| Hazard ratio (95% CI) | 1 | 0.89 (0.74, 1.08) | 0.98 (0.80, 1.18) | 0.98 (0.79, 1.22) | 0.96 (0.76, 1.21) |
| Arteriolar caliber, μm | <160 | 160 to 169 | 170 to 179 | 180 to 189 | ≥190 |
| Hazard ratio (95% CI) | 1.07 (0.85, 1.35) | 0.92 (0.73, 1.15) | 1.05 (0.86, 1.30) | 1.08 (0.88, 1.32) | 1 |
The hazard ratios are adjusted for age, systolic blood pressure, diastolic blood pressure, presence of diabetes, body mass index, serum cholesterol, serum HDL, current smoking status and current use of anti-hypertensive medication. The hazard ratios are also adjusted for the other retinal vessel caliber.
b. Excluding people with previous CHD.
Table 3 provides hazard ratios for CHD event outcomes for each study adjusted for the CHD risk factors of age, systolic blood pressure, diastolic blood pressure, serum cholesterol, serum HDL, presence of diabetes, smoking status, current use of anti-hypertensive medication and body mass index. Among women, wider venules and narrower arterioles were both associated with an increased risk of CHD events in the ARIC, CHS and RS. The hazard ratios for retinal vessel caliber measures were significant for men only in the CHS.
Table 3.
Adjusteda hazard ratios and 95% confidence interval for CHD, according to retinal vessel caliber variables
| Study | Participantsb included in CHD events meta-analysis | CHD events | Arteriolar caliberc | Venular caliberd | |
|---|---|---|---|---|---|
| Women | |||||
| ARICe | 5699 | 303 | 1.34 (1.14, 1.59) | 1.22 (1.04, 1.43) | |
| AusDiabf | 813 | 23 | 0.70 (0.46, 1.09) | 0.79 (0.51, 1.22) | |
| BDESg | 1889 | 220 | 1.04 (0.87, 1.23) | 1.09 (0.92, 1.29) | |
| BMESh | 1135 | 33 | 1.10 (0.69, 1.74) | 1.19 (0.80, 1.77) | |
| CHSi | 844 | 129 | 1.31 (1.04, 1.65) | 1.32 (1.03, 1.70) | |
| Rotterdam | 2718 | 245 | 1.14 (0.95, 1.36) | 1.11 (0.93, 1.31) | |
| Pooled | 13098 | 953 | 1.17 (1.07, 1.28) | 1.16 (1.06, 1.26) | |
| P-value for test of heterogeneity of study specific hazard ratios | 0.38 | 0.92 | |||
| Men | |||||
| ARICe | 4153 | 565 | 1.09 (0.96, 1.23) | 1.01 (0.89, 1.14) | |
| AusDiabf | 600 | 29 | 0.90 (0.61, 1.31) | 0.71 (0.47, 1.08) | |
| BDESg | 1373 | 225 | 1.05 (0.89, 1.25) | 1.12 (0.94, 1.33) | |
| BMESh | 765 | 60 | 0.94 (0.70, 1.27) | 0.78 (0.58, 1.06) | |
| CHSi | 479 | 116 | 1.17 (0.91, 1.51) | 1.24 (0.94, 1.62) | |
| Rotterdam | 1691 | 271 | 0.87 (0.74, 1.02) | 0.99 (0.84, 1.16) | |
| Pooled | 9061 | 1266 | 1.02 (0.94, 1.10) | 1.02 (0.95, 1.10) | |
| P-value for test of heterogeneity of study specific hazard ratios | 0.31 | 0.17 |
The hazard ratios are adjusted for age, systolic blood pressure, diastolic blood pressure, presence of diabetes, body mass index, serum cholesterol, serum HDL, current smoking status and current use of anti-hypertensive medication. The hazard ratios are also adjusted for the other retinal vessel caliber.
Excluding people previous CHD.
Per 20μm decrease in arteriolar caliber.
Per 20μm increase in venular caliber.
Atherosclerosis Risk in Communities Study
Australian Diabetes, Obesity and Lifestyle Study
Beaver Dam Eye Study
Blue Mountains Eye Study
Cardiovascular Health Study
There was no evidence that the associations of retinal vessel caliber with CHD were heterogeneous between studies, among either men or women. Also, there was no evidence that the effect of any of the Framingham variables varied between studies for men or women, except for age, serum cholesterol and serum HDL cholesterol among women. When interactions between study site and these variables were included in the model the estimated hazard ratio for the retinal vessel calibers did not change.
Pooled hazard ratios for CHD
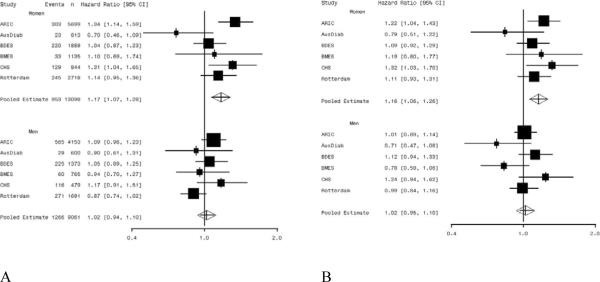
Among women there was evidence that retinal vessel caliber changes (both wider venules, pooled hazard ratio 1.16, 95% confidence interval [CI] 1.06, 1.26, and narrower arterioles, pooled hazard ratio 1.17, 95% CI 1.07, 1.28) were associated with an increased risk of CHD (Table 3). There was no evidence that retinal vessel caliber was associated with CHD events in men (venules hazard ratio 1.02, 95% CI 0.94, 1.10; arterioles hazard ratio 1.02, 95% CI 0.95, 1.10). Study specific and pooled hazard ratios are summarized in Figures 2A and 2B. There was evidence that the hazard ratios for venular caliber and arteriolar caliber differed between women and men (p=0.03 and 0.02, respectively).
Figure 2.
Forest plots of the adjusted* hazard ratios for CHD events per 20μm decrease in retinal arteriolar caliber (A) and per 20μm increase in retinal venular caliber (B).
* adjusted for age, systolic blood pressure, use of anti-hypertensives, total cholesterol, HDL-cholesterol, current smoking status, diastolic blood pressure, presence of diabetes, body mass index and the other retinal caliber. ARIC refers to Atherosclerosis Risk in Communities study; AusDiab to the Australian Diabetes, Obesity and Lifestyle Study; BDES to the Beaver Dam Eye Study; BMES to the Blue Mountains Eye Study; CHS to the Cardiovascular Health Study.
Table 4 shows the associations after adjusting for different baseline covariates. Among women there was a moderate decrease in the hazard ratio for venular caliber but not arteriolar caliber when the traditional CHD risk factors of cholesterol, HDL, smoking status and diabetes are included. When systolic blood pressure was then included, the hazard ratio for arteriolar caliber declined more than that for venular caliber. Among men a similar effect was observed although the hazard ratios were smaller.
Table 4.
Pooled hazard ratiosa,b and 95% confidence interval for CHD, according to retinal vessel caliber variables
| Arteriolar caliberc | Venular caliberd | ||
|---|---|---|---|
| Women Adjusted for | |||
| Age | 1.18 (1.09, 1.29) | 1.26 (1.16, 1.37) | |
| Age, cholesterol, HDL, smoking status, diabetes | 1.22 (1.10, 1.31) | 1.17 (1.07, 1.27) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI | 1.20 (1.10, 1.30) | 1.16 (1.06, 1.26) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI, SBP | 1.15 (1.05, 1.25) | 1.14 (1.05, 1.24) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI, SBP, DBP, BP meds | 1.17 (1.07, 1.28) | 1.16 (1.06, 1.26) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI, SBP, DBP, BP meds, WBCe | 1.20 (1.09, 1.31) | 1.17 (1.08, 1.28) | |
| Men Adjusted for | |||
| Age | 1.06 (0.98, 1.14) | 1.12 (1.04, 1.20) | |
| Age, cholesterol, HDL, smoking status, diabetes | 1.06 (0.98, 1.14) | 1.03 (0.96, 1.11) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI | 1.05 (0.98, 1.13) | 1.03 (0.95, 1.11) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI, SBP | 1.00 (0.94, 1.09) | 1.02 (0.94, 1.10) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI, SBP, DBP, BP meds | 1.02 (0.94, 1.10) | 1.02 (0.95, 1.10) | |
| Age, cholesterol, HDL, smoking status, diabetes, BMI, SBP, DBP, BP meds, WBCe | 1.02 (0.95, 1.11) | 1.04 (0.96, 1.12) |
Stratified by study.
Excluding people previous CHD.
Per 20μm decrease in arteriolar caliber.
Per 20μm increase in venular caliber.
The AusDiab data were not included in these estimates as white blood cell count was not measured in the AusDiab study.
Table 5 shows, separately for men and women, the pooled hazard ratios for subgroups stratified by age, presence of diabetes and presence of hypertension status. The highest hazard ratios were observed amongst women without hypertension or diabetes.
Table 5.
Pooled hazard ratiosa and 95% confidence interval for CHD, according to retinal vessel caliber variables, for participants categorised by age, presence of hypertension and diabetes
| Number at riskb | CHD Events | Arteriolar caliberc | Venular caliberd | ||
|---|---|---|---|---|---|
| Women | |||||
| Hypertension | 6445 | 654 | 1.10 (1.00, 1.23) | 1.10 (0.99, 1.22) | |
| No hypertension | 6653 | 299 | 1.31 (1.12, 1.54) | 1.28 (1.10, 1.50) | |
| Age < 60 | 5396 | 191 | 1.16 (0.95, 1.43) | 1.26 (1.04, 1.53) | |
| 60–69 | 4783 | 335 | 1.22 (1.05, 1.42) | 1.18 (1.02, 1.37) | |
| ≥70 | 2919 | 427 | 1.10 (0.97, 1.25) | 1.09 (0.96, 1.23) | |
| Diabetes | 1487 | 207 | 1.00 (0.83, 1.20) | 1.04 (0.87, 1.25) | |
| No Diabetes | 11611 | 746 | 1.22 (1.11, 1.35) | 1.18 (1.08, 1.30) | |
| Hypertension or diabetes | 6884 | 694 | 1.09 (0.99, 1.21) | 1.09 (0.99, 1.21) | |
| No hypertension or diabetes | 6214 | 259 | 1.34 (1.13, 1.59) | 1.33 (1.12, 1.57) | |
| Men | |||||
| Hypertension | 4164 | 724 | 1.06 (0.96, 1.17) | 1.03 (0.93, 1.13) | |
| No hypertension | 4897 | 542 | 0.94 (0.84, 1.06) | 0.98 (0.87, 1.11) | |
| Age < 60 | 3796 | 372 | 1.13 (.098, 1.32) | 1.11 (0.96, 1.28) | |
| 60–69 | 3559 | 557 | 0.99 (0.88, 1.11) | 0.98 (0.88, 1.10) | |
| ≥70 | 1706 | 337 | 0.94 (0.82, 1.08) | 0.98 (0.85, 1.14) | |
| Diabetes | 1200 | 257 | 1.05 (0.89, 1.24) | 0.91 (0.77, 1.08) | |
| No Diabetes | 7861 | 1009 | 1.01 (0.93, 1.10) | 1.05 (0.96, 1.14) | |
| Hypertension or diabetes | 4647 | 825 | 1.04 (0.95, 1.15) | 1.01 (0.92, 1.10) | |
| No hypertension or diabetes | 4414 | 441 | 0.95 (0.83, 1.08) | 1.04 (0.90, 1.19) |
The hazard ratios are adjusted for age, systolic blood pressure, diastolic blood pressure, presence of diabetes, body mass index, serum cholesterol, serum HDL, current smoking status and current use of anti-hypertensive medication. The hazard ratios are also adjusted for the other retinal vessel caliber.
Additional analyses
Findings were similar when the data were analyzed per standard deviation change in retinal vessel caliber and also when the study specific hazard ratios were combined using a random effects model. Excluding the CHS, which did not record some risk factors at the same visit as the retinal vessel caliber, also did not affect the overall results.
DISCUSSION
In this individual participant level meta-analysis of 22,159 participants from six population-based studies, we show that variations in retinal vessel caliber (both wider retinal venules and narrower retinal arterioles) were associated with an increased risk of incident CHD in women, but not in men. There was no apparent heterogeneity across study results. The risk associated with changes in retinal vessel caliber was higher among women without diabetes or hypertension.
Our findings have several clinical implications. First, we confirm the gender difference in the associations of retinal vessel caliber with CHD. This finding provides strong support for our hypothesis that microvascular dysfunction is a greater contributor to CHD pathogenesis in women than men,(50–52) and could explain gender differences in CHD presentation (women with nonobstructive coronary angiograms have more chest pain) and outcome with revascularization (this is worse in women).(53–56) As compared to men, women have smaller coronary arteries with more diffuse atherosclerosis, and more impaired arteriolar vasodilator responses.(52). Arteriolar narrowing in response to ageing, elevated blood pressure and endothelial dysfunction may further compromise myocardial perfusion leading to increased CHD risk in women.(6, 51, 56) The pathophysiological implications that wider retinal venules are associated with an increased CHD risk only in women is less clear but is consistent with reported associations of this retinal vessel change with inflammatory markers, endothelial dysfunction and increased aortic and large arterial wall stiffness.(5, 33, 57, 58) Our findings suggest that an assessment of the pathophysiology of venular dilation could provide new insights into microvascular CHD pathology.
Second, our findings provide suggestive evidence for the need to evaluate the microvasculature particularly in women with nonobstructive coronary angiograms.(51, 56) Retinal arteriolar narrowing can be reversed with antihypertensive therapy,(59) and has potential as a visible secondary endpoint of end-organ damage in trials of antihypertensive agents. Nonetheless, whether this translates into a meaningful CHD risk reduction is unknown at present and is being investigated in the retinal component of the Action in Diabetes and Vascular Disease (ADVANCE) trial.(33)
Differences between the first and last quintile of retinal arteriolar and venular caliber independently convey 50–62% higher risk of incident CHD in women. However whether such differences in retinal vessel caliber can be reliably estimated using fundoscopy in clinical examinations is unclear. A meta-analysis found no studies that assessed the reliability of direct fundoscopy in detecting microvascular changes and that only hemorrhages and exudates could be reliably assessed from retinal photographs.(60) The measurement of retinal vessels using the computer-assisted software program utilized by the studies in our paper show similar levels of reliability.(37, 42, 61–63) Whether the quantitative evaluation of retinal vessel caliber from retinal photographs adds value to CHD risk prediction in women remains to be determined and cannot yet be recommended for clinical practice.
The moderate decrease in the pooled hazard ratio for venular caliber but not arteriolar caliber when the traditional CHD risk factors of cholesterol, HDL, smoking status and diabetes were included in the models is consistent with data that venular calibre is influenced by dyslipidemia, inflammation and hyperglycemia. (2, 5, 7) The greater decline in hazard ratio for arteriolar caliber than for venular caliber when systolic blood pressure was included in the models is also consistent with reports that only narrower arteriolar calibre is associated with elevated blood pressure and may play a role in maintaining peripheral resistance and blood pressure. (2, 5, 7)
The strengths of this meta-analysis include access to the individual participant data records from all population studies to date which met our entry criteria, resulting in a large sample of 21,159 individuals and 2,219 CHD events, and standardized methods of retinal vessel caliber analysis and covariate adjustment. As the measurement of the retinal vessel caliber using retinal photography is a relatively new technique we have been able to collaborate with all of the researchers worldwide who have reported using this technology in cohort studies that have recorded CHD events. We have also included data from studies that have yet to publish results on the relationship between retinal vessel caliber and incident CHD. We thus feel that publication bias is highly unlikely to be present in our review and meta-analysis.
A number of limitations deserve mention. Two of the studies measured non-fasting rather than fasting cholesterol and HDL-cholesterol levels. However, the effect of normal food intake on lipid levels is small(64), and hence unlikely to affect the estimates of the association between retinal caliber and CHD risk. Error in the measurement of retinal vessel caliber and Framingham variables was not taken into account, and may have led to an over or underestimate of the true association between retinal vessel caliber and CHD.(65) These errors may differ between the studies due to the different photographic procedures and software used. We summarized retinal vessel caliber using the Parr-Hubbard formula because all six studies provided these data;(37) a revised formula is available although it is not believed to affect the estimated relationship between retinal vessel caliber and CHD outcomes.(66) In three of the studies non-fatal events were only recorded among those who returned for a subsequent visit. The hazard ratios for these three studies were lower than for the three studies that used a process of continual monitoring. This may mean we have underestimated the true hazard ratio. We were not able to include data from the MESA study, however the number of events in this study is smaller than in the smallest included study, the AusDiab study and so inclusion would have little impact on the results.(67)
In summary, our meta-analysis utilizing individual data records on 22,159 middle to older aged individuals confirmed that retinal vascular caliber changes (both wider retinal venules and narrower retinal arterioles) were independently associated with increased risk of CHD events in women, but not men. These findings further emphasize the role, contribution and importance of the microvasculture in the pathogenesis of CHD in women.
Acknowledgements
Ruth Mitchell, Trials Search Co-ordinator, Cochrane Renal Group, Centre for Kidney Research, Children's Hospital at Westmead, Australia provided advice on the construction of the Medline and Embase search strategies.
Funding/Support: The Screening and Test Evaluation Program is supported by the Australian National Health and Medical Research Council (Program Grants No.s 402764 & 358395).
The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Financial disclosures: None reported.
References
- 1.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356(8):830–40. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 2.Liew G, Wang JJ, Mitchell P, Wong TY. Retinal Vascular Imaging: A New Tool in Microvascular Disease Research. Circ Cardiovas Imaging. 2008;1(2):156–161. doi: 10.1161/CIRCIMAGING.108.784876. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka M, Fujiwara H, Onodera T, Wu DJ, Matsuda M, Hamashima Y, et al. Quantitative analysis of narrowings of intramyocardial small arteries in normal hearts, hypertensive hearts, and hearts with hypertrophic cardiomyopathy. Circulation. 1987;75(6):1130–9. doi: 10.1161/01.cir.75.6.1130. [DOI] [PubMed] [Google Scholar]
- 4.Tso MOM, Abrams GW, Jampol LM. Hypertensive retinopathy, choroidopathy, and optic neuropathy: A clinical and pathophysiological approach to classification. In: Singerman LJ, Jampol LM, editors. Retinal and choroidal manifestations of systemic disease. Williams & Wilkins; Baltimore: 1991. [Google Scholar]
- 5.Liew G, Sharrett AR, Wang JJ, Klein R, Klein BE, Mitchell P, et al. Relative importance of systemic determinants of retinal arteriolar and venular caliber: the atherosclerosis risk in communities study. Arch Ophthalmol. 2008;126(10):1404–10. doi: 10.1001/archopht.126.10.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287(9):1153–9. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 7.Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–34. doi: 10.1167/iovs.03-1390. [DOI] [PubMed] [Google Scholar]
- 8.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Sharrett AR, et al. Risk prediction of coronary heart disease based on retinal vascular caliber (from the Atherosclerosis Risk In Communities [ARIC] Study) Am J Cardiol. 2008;102(1):58–63. doi: 10.1016/j.amjcard.2008.02.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch Intern Med. 2006;166(21):2388–94. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 10.Wang JJ, Liew G, Klein R, Rochtchina E, Knudtson MD, Klein BE, et al. Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J. 2007;28(16):1984–92. doi: 10.1093/eurheartj/ehm221. [DOI] [PubMed] [Google Scholar]
- 11.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66(9):1339–43. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. see comment. [DOI] [PubMed] [Google Scholar]
- 13.Liew G, Sharrett AR, Kronmal R, Klein R, Wong TY, Mitchell P, et al. Measurement of retinal vascular caliber: issues and alternatives to using the arteriole to venule ratio. Invest Ophthalmol Vis Sci. 2007;48(1):52–7. doi: 10.1167/iovs.06-0672. [DOI] [PubMed] [Google Scholar]
- 14.Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54(1):74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Sauerbrei W, Meier-Hirmer C, Benner A, Royston P. Multivariable regression model building by using fractional polynomials: Description of SAS, STATA and R programs. Computational Statistics & Data Analysis. 2006;50(12):3464–3485. [Google Scholar]
- 16.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294(14):1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Altman DG. Systematic reviews in health care : meta-analysis in context. 2nd ed BMJ; London: 2001. [Google Scholar]
- 18.Van Hecke MV, Dekker JM, Nijpels G, Moll AC, Van Leiden HA, Heine RJ, et al. Retinopathy is associated with cardiovascular and all-cause mortality in both diabetic and nondiabetic subjects: the hoorn study. Diabetes Care. 2003;26(10):2958. doi: 10.2337/diacare.26.10.2958. [DOI] [PubMed] [Google Scholar]
- 19.Klein BE, Klein R, McBride PE, Cruickshanks KJ, Palta M, Knudtson MD, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med. 2004;164(17):1917–24. doi: 10.1001/archinte.164.17.1917. [DOI] [PubMed] [Google Scholar]
- 20.van Hecke MV, Dekker JM, Stehouwer CD, Polak BC, Fuller JH, Sjolie AK, et al. Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB prospective complications study. Diabetes Care. 2005;28(6):1383–9. doi: 10.2337/diacare.28.6.1383. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Archives of Ophthalmology. 2004;122(11):1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Looker HC, Krakoff J, Knowler WC, Bennett PH, Klein R, Hanson RL. Longitudinal studies of incidence and progression of diabetic retinopathy assessed by retinal photography in pima indians. Diabetes Care. 2003;26(2):320–6. doi: 10.2337/diacare.26.2.320. [DOI] [PubMed] [Google Scholar]
- 24.Voutilainen-Kaunisto RM, Terasvirta ME, Uusitupa MI, Niskanen LK. Occurrence and predictors of retinopathy and visual acuity in Type 2 diabetic patients and control subjects. 10-year follow-up from the diagnosis. Journal of Diabetes & its Complications. 2001;15(1):24–33. doi: 10.1016/s1056-8727(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 25.McCarty DJ, Fu CL, Harper CA, Taylor HR, McCarty CA. Five-year incidence of diabetic retinopathy in the Melbourne Visual Impairment Project. Clinical & Experimental Ophthalmology. 2003;31(5):397–402. doi: 10.1046/j.1442-9071.2003.00685.x. [DOI] [PubMed] [Google Scholar]
- 26.Leske MC, Wu SY, Hyman L, Li X, Hennis A, Connell AM, et al. Diabetic retinopathy in a black population: the Barbados Eye Study. Ophthalmology. 1999;106(10):1893–9. doi: 10.1016/s0161-6420(99)90398-6. [DOI] [PubMed] [Google Scholar]
- 27.Ling R, Ramsewak V, Taylor D, Jacob J. Longitudinal study of a cohort of people with diabetes screened by the Exeter Diabetic Retinopathy Screening Programme. Eye. 2002;16(2):140–5. doi: 10.1038/sj.eye.6700081. [DOI] [PubMed] [Google Scholar]
- 28.Agardh CD, Agardh E, Torffvit O. The prognostic value of albuminuria for the development of cardiovascular disease and retinopathy: A 5-year follow-up of 451 patients with type 2 diabetes mellitus. Diabetes Research and Clinical Practice. 1996;32(1-2):35–44. doi: 10.1016/0168-8227(96)01218-1. [DOI] [PubMed] [Google Scholar]
- 29.Yanko L, Goldbourt U, Michaelson IC, Shapiro A, Yaari S. Prevalence and 15-year incidence of retinopathy and associated characteristics in middle-aged and elderly diabetic men. British Journal of Ophthalmology. 1983;67(11):759–65. doi: 10.1136/bjo.67.11.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy MS, Peng B, Roy A. Risk factors for coronary disease and stroke in previously hospitalized African-Americans with Type 1 diabetes: a 6-year follow-up. Diabetic Medicine. 2007;24(12):1361–8. doi: 10.1111/j.1464-5491.2007.02293.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Wang YX, Xie XW, Jonas JB. Retinopathy and mortality. The Beijing Eye Study. Graefes Archive for Clinical & Experimental Ophthalmology. 2008;246(6):923–5. doi: 10.1007/s00417-008-0773-z. [DOI] [PubMed] [Google Scholar]
- 32.Qiu C, Cotch MF, Sigurdsson S, Garcia M, Klein R, Jonasson F, et al. Retinal and cerebral microvascular signs and diabetes: the age, gene/environment susceptibility-Reykjavik study. Diabetes. 2008;57(6):1645–1650. doi: 10.2337/db07-1455. [DOI] [PubMed] [Google Scholar]
- 33.Stolk RP, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, Juming L, et al. Rationale and design of the AdRem study: evaluating the effects of blood pressure lowering and intensive glucose control on vascular retinal disorders in patients with type 2 diabetes mellitus. Contemp Clin Trials. 2007;28(1):6–17. doi: 10.1016/j.cct.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Tillin T, Evans RM, Witt NW, Sharp PS, McKeigue PM, Chaturvedi N, et al. Ethnic differences in retinal microvascular structure. Diabetologia. 2008;51(9):1719–22. doi: 10.1007/s00125-008-1096-7. [DOI] [PubMed] [Google Scholar]
- 35.Miller RG, Prince CT, Klein R, Orchard TJ. Retinal vessel diameter and the incidence of coronary artery disease in type 1 diabetes. American Journal of Ophthalmology. 2009;147(4):653–60. doi: 10.1016/j.ajo.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47(6):2341–50. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–80. doi: 10.1016/s0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 38.Tikellis G, Wang JJ, Tapp R, Simpson R, Mitchell P, Zimmet PZ, et al. The relationship of retinal vascular calibre to diabetes and retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetologia. 2007;50(11):2263–71. doi: 10.1007/s00125-007-0822-x. [DOI] [PubMed] [Google Scholar]
- 39.Wong TY, Knudtson MD, Klein R, Klein BE, Meuer SM, Hubbard LD. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–90. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 40.Leung H, Wang JJ, Rochtchina E, Tan AG, Wong TY, Klein R, et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44(7):2900–4. doi: 10.1167/iovs.02-1114. [DOI] [PubMed] [Google Scholar]
- 41.Wong TY, Klein R, Sharrett AR, Manolio TA, Hubbard LD, Marino EK, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: The Cardiovascular Health Study. Ophthalmology. 2003;110(4):658–66. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 42.Couper DJ, Klein R, Hubbard LD, Wong TY, Sorlie PD, Cooper LS, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. American Journal of Ophthalmology. 2002;133(1):78–88. doi: 10.1016/s0002-9394(01)01315-0. [DOI] [PubMed] [Google Scholar]
- 43.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 44.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 45.Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA. Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol. 1991;7(4):403–22. doi: 10.1007/BF00145007. [DOI] [PubMed] [Google Scholar]
- 46.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116(2):151–7. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 47.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care. 2002;25(10):1790–4. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65(7):1005–9. doi: 10.1212/01.wnl.0000179177.15900.ca. [DOI] [PubMed] [Google Scholar]
- 49.Wang JJ, Liew G, Wong TY, Smith W, Klein R, Leeder SR, et al. Retinal vascular calibre and the risk of coronary heart disease-related death. Heart. 2006;92(11):1583–7. doi: 10.1136/hrt.2006.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342(12):829–35. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 51.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. Jama. 2005;293(4):477–84. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 52.Pepine CJ, Kerensky RA, Lambert CR, Smith KM, von Mering GO, Sopko G, et al. Some thoughts on the vasculopathy of women with ischemic heart disease. J Am Coll Cardiol. 2006;47(3 Suppl):S30–5. doi: 10.1016/j.jacc.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 53.Cordero A, Alegria E. Sex differences and cardiovascular risk. Heart. 2006;92(2):145–6. doi: 10.1136/hrt.2005.069187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation. 2007;115(7):823–6. doi: 10.1161/CIRCULATIONAHA.106.685859. [DOI] [PubMed] [Google Scholar]
- 55.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47(6):1019–26. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- 56.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 57.Cheung N, Islam FM, Jacobs DR, Jr., Sharrett AR, Klein R, Polak JF, et al. Arterial compliance and retinal vascular caliber in cerebrovascular disease. Ann Neurol. 2007;62(6):618–24. doi: 10.1002/ana.21236. [DOI] [PubMed] [Google Scholar]
- 58.Cheung N, Sharrett AR, Klein R, Criqui MH, Islam FM, Macura KJ, et al. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension. 2007;50(4):617–22. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 59.Hughes AD, Stanton AV, Jabbar AS, Chapman N, Martinez-Perez ME, Mc GTSA. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. J Hypertens. 2008;26(8):1703–7. doi: 10.1097/HJH.0b013e328304b072. [DOI] [PubMed] [Google Scholar]
- 60.van den Born BJ, Hulsman CA, Hoekstra JB, Schlingemann RO, van Montfrans GA. Value of routine funduscopy in patients with hypertension: systematic review. Bmj. 2005;331(7508):73. doi: 10.1136/bmj.331.7508.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikram MK, Witteman JC, Vingerling JR, Breteler MM, Hofman A, de Jong PT. Retinal vessel diameters and risk of hypertension: the Rotterdam Study. Hypertension. 2006;47(2):189–94. doi: 10.1161/01.HYP.0000199104.61945.33. [DOI] [PubMed] [Google Scholar]
- 62.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–40. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 63.Sherry LM, Wang JJ, Rochtchina E, Wong T, Klein R, Hubbard L, et al. Reliability of computer-assisted retinal vessel measurementin a population. Clin Experiment Ophthalmol. 2002;30(3):179–82. doi: 10.1046/j.1442-9071.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 64.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118(20):2047–56. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 65.Bennett DA. Review of analytical methods for prospective cohort studies using time to event data: single studies and implications for meta-analysis. Stat Methods Med Res. 2003;12(4):297–319. doi: 10.1191/0962280203sm319ra. [DOI] [PubMed] [Google Scholar]
- 66.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–9. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 67.Folsom AR, Kronmal RA, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168(12):1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]