Abstract
Caveolin-1 (cav-1) is reportedly overexpressed in prostate cancer cells and is associated with disease progression. Specific oncogenic activities of cav-1 associated with Akt activation also occur in prostate cancer. A membrane-associated protein, cav-1, is nonetheless secreted by prostate cancer cells; results of recent studies showed that secreted cav-1 can stimulate cell survival and angiogenic activities, defining a role for cav-1 in the prostate cancer microenvironment. Serum cav-1 levels were also higher in prostate cancer patients than in control men without prostate cancer, and the preoperative serum cav-1 concentration had prognostic potential in men undergoing radical prostatectomy. Secreted cav-1 is therefore a potential biomarker and therapeutic target for prostate cancer.
Keywords: caveolin-1, progression, angiogenesis, biomarkers
Introduction
Caveolin-1 (cav-1) is a major structural component of caveolae, which are specialized plasma membrane invaginations involved in multiple cellular processes such as molecular transport, cell adhesion and signal transduction.1,2 Although under some conditions cav-1 may suppress tumorigenesis,3 cav-1 is associated with and contributes to malignant progression through various mechanisms.4–8 Specific proteins such as receptor tyrosine kinases, serine/threonine kinases, phospholipases, G protein-coupled receptors and Src family kinases are localized in lipid rafts and caveolar membranes, where they interact with cav-1 through the cav-1 scaffolding domain; the activities mediated by this domain result in the generation of platforms for compartmentalization of discrete signaling events.9 A high level of intracellular cav-1 expression is associated with metastatic progression of human prostate cancer10,11 and other malignancies, including lung,12 renal13 and esophageal squamous cell cancers.14
Virulent prostate cancer cell lines reportedly secrete biologically active cav-1 protein in vitro, and cav-1 promotes prostate cancer cell viability and clonal growth.15–17 The cancer-promoting effects of secreted cav-1 include antiapoptotic activities similar to those observed following enforced expression of cav-1 within the cells.15,18 In addition to showing cav-1-mediated autocrine activities, a recent study showed that recombinant cav-1 protein is taken up by prostate cancer cells and endothelial cells in vitro and that recombinant cav-1 increases angiogenic activities both in vitro and in vivo by activating Akt- and/or nitric oxide synthase-mediated signaling.6 Moreover, significantly higher serum cav-1 levels have been documented in men with prostate cancer than in men with benign prostatic hyperplasia19 and also in patients with elevated risk of cancer recurrence after radical prostatectomy.20
The concept of expression and secretion of cav-1 by prostate cancer cells in malignant progression is unique. The autocrine and paracrine activities of cav-1 mediated through the activation of Akt and/or nitric oxide synthase signaling may lead to pervasive engagement of the local tumor microenvironment, involving but not limited to the proangiogenic activities previously documented.
In this review, we discuss the unique functional properties of cav-1 in prostate cancer, especially on secreted cav-1 and its capacity to engage the prostate cancer microenvironment. We also discuss the potential for development of serum cav-1 as a biomarker for prostate cancer, with particular reference to the pre-operative cav-1 serum level as a prognostic tool. Finally, we discuss the possibility of biologically targeting cav-1 as the foundation for the development of novel effective therapies for prostate cancer.
Cav-1 upregulation in prostate cancer
We previously reported increased cav-1 immunostaining in prostate cancer relative to that in the normal adjacent prostatic epithelial cells, which express low-to-nondetectable levels of cav-1.10,11 An important finding was that increased cav-1 immunostaining had independent prognostic potential in men undergoing radical prostatectomy.11 These results were supported by those in two subsequent independent reports of studies that also evaluated prostate cancer tissue using immunohisto-chemical analysis and yielded similar conclusions.21,22 An important common observation from the cases examined in these studies is that cav-1 immunostaining in localized prostate cancer is focal and expressed only in a relatively small percentage of prostate cancer cells. These results also showed that cav-1 was positively correlated with Gleason grade, an important suggestion that even though it is focally expressed, cav-1 is a biomarker for clinically aggressive disease.
The molecular basis for the initiation of cav-1 expression in prostate cancer and other malignancies is not clear. The cav-1 and cav-2 genes are co-localized to 7q31.1, a highly conserved region that encompasses a known fragile site that is deleted, associated with loss of heterozygosity, or amplified in a variety of human cancers, including prostate cancer.3,23–25 Although some investigators have used these data to support a case for both loss and gain of cav-1 expression, no convincing data specifically correlate genetic alterations at this site with changes in cav-1 expression for prostate cancer.26,27 The cav-1 gene promoter has multiple CpG sites, and alterations in gene methylation have been shown in prostate cancer.28 However, patterns of cav-1 gene methylation have not, thus far, provided a convincing argument for the upregulation of cav-1 in prostate cancer. It is interesting that a recent article suggests that the loss of function for a tumor suppressor miRNA (miR-205) may lead to upregulation of cav-1 in prostate cancer.29
As many genetic alterations that occur in primary prostate cancer have also been documented in premalignant disease, such as high-grade prostatic intraepithelial neoplasia, it would be interesting to analyze cav-1 in those premalignant lesions. Although it is focally expressed in primary prostate cancer, it is important to note that cav-1 is expressed in most metastatic cells.15 This focal expression in primary prostate cancer and significantly increased cav-1 expression in associated metastases fit well with the notion that cav-1 is more aligned with the criteria of a progression-related protein than with those of a protein that significantly affects localized tumor growth.30 The idea of association of cav-1 with clinically significant prostate cancer is novel, and the prospect that cav-1 expression may segregate clinically significant prostate cancer from clinically insignificant prostate cancer is exciting.31
Although the association between cav-1 overexpression in prostate cancer and aggressive, clinically significant disease has been found consistently in multiple studies, the relationship between cav-1 overexpression and androgen sensitivity is less clear. Early studies showed that cav-1 overexpression was inversely associated with androgen sensitivity and positively associated with tumor growth in mouse models of prostate cancer.32 The cav-1 gene is transcriptionally upregulated in androgen-sensitive prostate cancer cells, although the level of induction was modest.5 In general, cav-1 has been associated with the stimulatory effects of steroid receptors, including the androgen receptor, suggesting a point of convergence for further mechanistic studies.33,34 Overall, the available information on cav-1 expression fits the hypothesis that prostate cancer progression, even in the presence of normal levels of circulating testosterone, is coincidental with the development of androgen insensitivity. Certainly, the development of castrate-resistant prostate cancer involves selection for unique malignant properties that allow prostate cancer cells to metastasize in the presence of castrate levels of androgens. However, the emergence of castrate-resistant prostate cancer does not preclude co-selection of metastatic and androgen-insensitive prostate cancer in men who have not undergone hormone therapy.
Mechanisms of cav-1-mediated oncogenic activities in prostate cancer
Overexpression of cav-1 was reported in various malignancies, including cancer of the colon,35 kidney,13 bladder,36 lung,12 pancreas37 and ovary,38 and in some types of breast cancer.39 The level of cav-1 expression may depend on the tumor type and stage; for example, high cav-1 levels were reported in late or advanced squamous cell carcinoma40 and in metastatic prostate cancer.15,32 These results have led many investigators to attempt to identify cav-1-related oncogenic pathways for various malignancies. Although cav-1 activities impinge on various oncogenic pathways and can inhibit or activate these pathways, depending on the cell type and context,3 the results of multiple studies now indicate that Akt activation has an important role in cav-1-mediated oncogenic functions in prostate cancer. The first demonstration of a direct association between cav-1 expression and Akt indicated that the overexpression of cav-1 increased binding to and inhibited the serine/ threonine phosphatases, PP1 and PP2A, in human prostate cancer cells. These interactions, which were likely mediated through cav-1 binding to a cav-1 scaffolding domain-binding site on PP1 and PP2A and inhibition of their activities, led to significantly increased levels of phospho-Akt and sustained activation of downstream oncogenic Akt targets.18 Findings from a recent independent study supported this mechanism and further showed that the putative oncogene inhibitor of differentiation-1 induced Akt activation by promoting the binding activity of cav-1 and PP-2A.41 It is important to consider that the activation of Akt has been previously associated with prostate cancer and is clearly one of the most important oncogenic activities that underlie prostate cancer progression.42 It is worthwhile to consider the idea that activated Akt contributes to the expression and secretion of multiple growth factors (GFs) that have important roles in the growth, survival and progression of prostate cancer cells through autocrine and paracrine activities42 (Figure 1). The molecular mechanisms that may connect cav-1 upregulation to GF expression and secretion through the activation of Akt are worthy of future investigation.
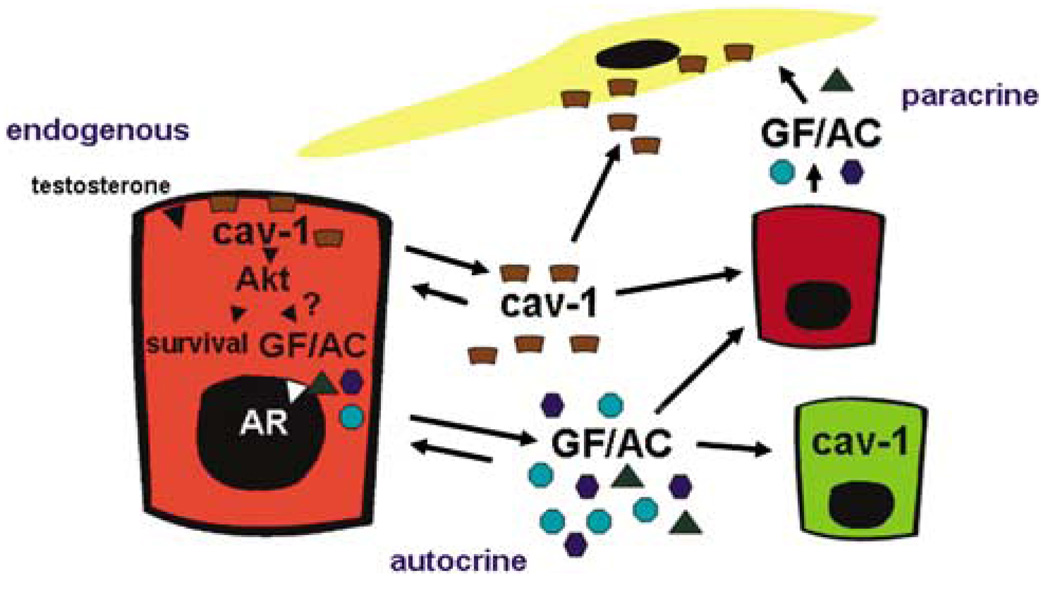
Figure 1.
Caveolin-1 (cav-1) upregulation in prostate cancer promotes cell survival through Akt-mediated activities. Prostate cancer-derived, secreted cav-1, which is taken up by prostate cancer cells and tumor-associated endothelial cells, stimulates angiogenesis. The unique autocrine and paracrine activities associated with cav-1 in prostate cancer present a novel paradigm for the development of biomarkers and therapeutic approaches for prostate cancer. GFs, growth factors; ACs, angiogenic cytokines; AR, androgen receptor.
Recent studies further showed that alterations in Akt activities regulate the expression of fatty acid synthase, a putative metabolic oncogene, and its co-localization with cav-1 in lipid rafts in prostate cancer cells.43 The same article reported that Src, an oncogenic tyrosine kinase, has an important role in this process. It is important to note that cav-1 was initially identified as a v-Src substrate, P-Y14cav-1.44 Overall, these recent articles suggest that an interactive and interdependent network of oncogenic proteins, including cav-1, Akt, fatty acid synthase and Src, has an important role in prostate cancer. Although initiation and maintenance of this oncogenic protein network appear to be a critical mechanism through which cav-1 promotes malignant activities in prostate cancer, there are certainly others. Results of a recent study showed that cellular levels of P-Y14cav-1 are critically associated with Rho/ROCK-and Src-dependent regulation of tumor cell motility and invasion.45
Prostate cancer cells secrete cav-1
Cav-1, which is secreted by mouse and human prostate cancer cell lines, promotes cancer cell survival in vitro.15 These results were validated in independent studies and were extended to include perineural cells in the prostate cancer microenvironment.17,46 At the time these results were reported, a previous study had shown that cav-1 was secreted by normal pancreatic acinar cells in vitro,47 but to our knowledge, there were no previous reports of cav-1 secretion by malignant cells. These results raised the question of the mechanism responsible for cav-1 secretion from cancer cells and whether this mechanism was specific to prostate cancer cells or the prostate cancer microenvironment. An intriguing article reported that cav-1 was found in ‘prostasomes,’ which are vesicular organelles enriched with raft components, of PC-3 cells, suggesting that cav-1 is secreted by prostate cancer cells through a unique mechanism.48 The results of a more recent study supported the concept that cav-1 is secreted by prostate cancer cells through a unique exosome–prostasome-mediated pathway.49 Additional studies are warranted to further characterize the specificity and mechanism(s) involved in cav-1 secretion by prostate cancer cells and potentially by specific stromal cells within the prostate cancer microenvironment.
Prostate cancer-derived, secreted cav-1 alters the tumor microenvironment through proangiogenic activities
Prostate cancer is unique in its capacity to influence and become dependent on stromal cells that reside in the tumor microenvironment. GFs derived from prostate cancer cells, including vascular endothelial growth factor, transforming GF-β1 and multiple fibroblast GFs, are known to significantly affect, through autocrine and paracrine activities, the capacity of prostate cancer cells to growand metastasize.50–53 Various mechanisms are reportedly involved in the deregulation of these GFs in cancer cells, including transcriptional regulation54 and alteration of mRNA stability. 55–58
We recently found, unexpectedly, that prostate cancer-derived secreted cav-1 is also capable of significantly altering the tumor microenvironment by stimulating angiogenesis. Specifically, cav-1 is taken up by cav-1-negative tumor cells and/or endothelial cells, leading to stimulation of specific angiogenic activities through the PI3K–Akt–eNOS signaling module.6 This study followed a previous study which had shown greater angiogenesis in cav-1-positive prostate cancer than in cav-1-negative prostate cancer and that also showed co-localization of cav-1 with vascular endothelial growth factor receptor-2 in tumor-associated endothelial cells.59 The combined action of prostate cancer-derived, secreted cell-derived GFs and/or angiogenic cytokines and cav-1 could have a profound effect on prostate cancer microenvironment (Figure 1). The combined effect could potentially result in structural modification of the pre-existing signaling pathways through the interaction of cav-1 with specific signaling molecules. As many of the molecules involved in angiogenic signaling pathways possess cav-1 scaffolding domain-binding sites, for example, vascular endothelial growth factor receptor-2 and Src,9 these interactions are likely mediated in part through the cav-1 scaffolding domain–scaffolding domain-binding site interface. It is interesting to note that an abnormal augmentation of these signaling pathways could further potentiate the downstream autocrine and/or paracrine activities of GFs and/or angiogenic cytokines secreted by prostate cancer cells, representing unique co-stimulatory activities with profound potential to alter the tumor microenvironment toward a progressively increasing malignant state.
Secreted cav-1 as a biomarker and therapeutic target
The expression and secretion of cav-1 by prostate cancer cells presents an opportunity for the development of cav-1-based biomarkers for prostate cancer. We previously developed an immunoassay for measuring serum cav-1 levels and showed that the median serum cav-1 level in men with clinically localized prostate cancer was significantly higher than that in healthy control men (that is, in those with normal findings on digital rectal examination and serum prostate-specific antigen levels of ⩽1.5 ng ml−1 over a period of 2 years) and in men with clinical benign prostatic hyperplasia.19 Further, in a larger population study in men with a serum prostate-specific antigen of > 10 ng ml−1, high pretreatment levels of cav-1 in the serum were associated with a shorter time to biochemical recurrence (defined as a serum prostate-specific antigen level of ⩾ 0.2 ng ml−1 on two consecutive measurements).20 High pretreatment serum cav-1 levels were established using a cutoff determined by using the minimum P-value method.
These initial clinical and basic laboratory study results, together with those of pathology-based tissue analysis, show the potential of serum cav-1 as a prognostic biomarker for the identification of men with clinically aggressive prostate cancer. Specifically, the pretreatment serum cav-1 concentration may be used to identify men with clinically significant prostate cancer who are likely to experience a rapid recurrence of the cancer following radical prostatectomy. Although further studies are necessary to validate these results, it is conceivable that serum cav-1 analysis would contribute to the identification of the subset of the men undergoing localized therapy for presumed localized disease who would benefit from neoadjuvant or adjuvant therapy, for example, local radiotherapy, localized biologic therapy, androgen-deprivation therapy and/or targeted systemic therapy.60–63
We have considered, in addition to the potential use of serum cav-1 analysis as a prognostic biomarker for clinically aggressive prostate cancer, the possibility that secreted cav-1 is a therapeutic target for prostate cancer. Our recent studies revealed that systemic treatment of mice with cav-1 antisera significantly reduced the development and growth of primary site tumors and metastases in both orthotopic and experimental metastasis mouse models of prostate cancer. 15,64 These studies further showed that metastatic prostate cancer cells may survive and grow partly through the uptake of secreted cav-1. As targeted systemic antibody therapy has been used successfully to treat specific malignancies,65,66 the development of cav-1-targeted antibody therapy should be further pursued as a potential therapy for prostate cancer.
Summary
The initial observations that prostate cancer cells overexpress cav-1 and that cav-1 is associated with clinically significant prostate cancer have led to extensive basic laboratory and clinical studies of the role of cav-1 in prostate cancer and other malignancies. Although the molecular and cellular biology of cav-1 is complex, these studies thus far have shown that the overexpression and secretion of cav-1 leads to the amplification of the tumor-promoting effects of cav-1 through the activation of endogenous oncogenic pathways and engagement of tumor microenvironment. The association between cav-1 and clinically significant prostate cancer is unique, and the prospect that cav-1 expression may segregate clinically significant prostate cancer from clinically insignificant prostate cancer is exciting. By virtue of the capacity of prostate cancer cells to secrete cav-1, specific cav-1-based biomarker and therapeutic strategies have been proposed and tested. The initial results are promising and indicate that further studies may lead to clinically useful prognostic and therapeutic tools for prostate cancer.
Acknowledgements
This work was supported in part by NIH R01 CA68814 and Department of Defense Grant DAMD PC051247.
Footnotes
Conflict of interest The data included in this paper are relevant to intellectual property that has been licensed by Baylor College of Medicine to Progression Therapeutics Inc., a private biotechnology start-up. The association to the start-up is indirect and does not in any way constitute a conflict of interest.
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
References
- 1.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol. 1998;275:L843–L851. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg PW, Schmid SL. Caveolin, cholesterol and Ras signalling. Nat Cell Biol. 1999;1:E35–E37. doi: 10.1038/10028. [DOI] [PubMed] [Google Scholar]
- 3.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 4.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Yang G, Ebara S, Satoh T, Nasu Y, Timme TL, et al. Caveolin-1 mediates testosterone-stimulated survival/clonal growth and promotes metastatic activities in prostate cancer cells. Cancer Res. 2001;61:4386–4392. [PubMed] [Google Scholar]
- 6.Tahir SA, Yang G, Goltsov AA, Watanabe M, Tabata K, Addai J, et al. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008;68:731–739. doi: 10.1158/0008-5472.CAN-07-2668. [DOI] [PubMed] [Google Scholar]
- 7.Williams TM, Hassan GS, Li J, Cohen AW, Medina FA, Philippe GF, et al. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in TRAMP mice. J Biol Chem. 2005;10:1074. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 8.Woodman SE, Ashton AW, Schubert W, Lee H, Williams TM, Medina FA, et al. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am J Pathol. 2003;162:2059–2068. doi: 10.1016/S0002-9440(10)64337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shatz M, Liscovitch M. Caveolin-1: a tumor-promoting role in human cancer. Int J Radiat Biol. 2008;84:177–189. doi: 10.1080/09553000701745293. [DOI] [PubMed] [Google Scholar]
- 10.Yang G, Truong LD, Timme TL, Ren C, Wheeler TM, Park SH, et al. Elevated expression of caveolin is associated with prostate and breast cancer. Clin Cancer Res. 1998;4:1873–1880. [PubMed] [Google Scholar]
- 11.Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59:5719–5723. [PubMed] [Google Scholar]
- 12.Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol. 2002;161:1647–1656. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo HJ, Oh DK, Kim YS, Lee KB, Kim SJ. Increased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int. 2004;93:291–296. doi: 10.1111/j.1464-410x.2004.04604.x. [DOI] [PubMed] [Google Scholar]
- 14.Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, et al. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94:929–933. [PubMed] [Google Scholar]
- 15.Tahir SA, Yang G, Ebara S, Timme TL, Satoh T, Li L, et al. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–3885. [PubMed] [Google Scholar]
- 16.Wu D, Foreman TL, Gregory CW, McJilton MA, Wescot GG, Ford OH, et al. Protein kinase cepsilon has the potential to advance the recurrence of human prostate cancer. Cancer Res. 2002;62:2423–2429. [PubMed] [Google Scholar]
- 17.Bartz R, Zhou J, Hsieh JT, Ying Y, Li W, Liu P. Caveolin-1 secreting LNCaP cells induce tumor growth of caveolin-1 negative LNCaP cells in vivo. Int J Cancer. 2008;122:520–525. doi: 10.1002/ijc.23142. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tahir SA, Ren C, Timme TL, Gdor Y, Hoogeveen R, Morrisett JD, et al. Development of an immunoassay for serum caveolin-1: a novel biomarker for prostate cancer. Clin Cancer Res. 2003;9:3653–3659. [PubMed] [Google Scholar]
- 20.Tahir SA, Frolov A, Hayes TG, Mims MP, Miles BJ, Lerner SP, et al. Preoperative serum caveolin-1 as a prognostic marker for recurrence in a radical prostatectomy cohort. Clin Cancer Res. 2006;12:4872–4875. doi: 10.1158/1078-0432.CCR-06-0417. [DOI] [PubMed] [Google Scholar]
- 21.Goto T, Nguyen BP, Nakano M, Ehara H, Yamamoto N, Deguchi T. Utility of Bcl-2, P53, Ki-67, and caveolin-1 immunostaining in the prediction of biochemical failure after radical prostatectomy in a Japanese population. Urology. 2008;72:167–171. doi: 10.1016/j.urology.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67:614–622. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]
- 23.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 24.Engelman JA, Zhang XL, Lisanti MP. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998;436:403–410. doi: 10.1016/s0014-5793(98)01134-x. [DOI] [PubMed] [Google Scholar]
- 25.Nupponen NN, Kakkola L, Koivisto P, Visakorpi T. Genetic alterations in hormone-refractory recurrent prostate carcinomas. Am J Pathol. 1998;153:141–148. doi: 10.1016/S0002-9440(10)65554-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bachmann N, Haeusler J, Luedeke M, Kuefer R, Perner S, Assun G, et al. Expression changes of CAV1 and EZH2, located on 7q31 approximately q36, are rarely related to genomic alterations in primary prostate carcinoma. Cancer Genet Cytogenet. 2008;182:103–110. doi: 10.1016/j.cancergencyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Hurlstone AF, Reid G, Reeves JR, Fraser J, Strathdee G, Rahilly M, et al. Analysis of the CAVEOLIN-1 gene at human chromosome 7q31.1 in primary tumours and tumour-derived cell lines. Oncogene. 1999;18:1881–1890. doi: 10.1038/sj.onc.1202491. [DOI] [PubMed] [Google Scholar]
- 28.Cui J, Rohr LR, Swanson G, Speights VO, Maxwell T, Brothman AR. Hypermethylation of the caveolin-1 gene promoter in prostate cancer. Prostate. 2001;46:249–256. doi: 10.1002/1097-0045(20010215)46:3<249::aid-pros1030>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Gandellini P, Folini M, Longoni N, Pennati M, Binda M, Colecchia M, et al. miR-205 exerts tumor-suppressive functions in human prostate through down-regulation of protein kinase Cepsilon. Cancer Res. 2009;69:2287–2295. doi: 10.1158/0008-5472.CAN-08-2894. [DOI] [PubMed] [Google Scholar]
- 30.Thompson TC, Park SH, Timme TL, Ren C, Eastham JA, Donehower LA, et al. Loss of p53 function leads to metastasis in ras+myc-initiated mouse prostate cancer. Oncogene. 1995;10:869–879. [PubMed] [Google Scholar]
- 31.Dall’era MA, Cooperberg MR, Chang JM, Davies BJ, Albertsen PC, Klotz LH, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 32.Nasu Y, Timme TL, Yang G, Bangma CH, Li L, Ren C, et al. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells [see comments] Nat Med. 1998;4:1062–1064. doi: 10.1038/2048. [DOI] [PubMed] [Google Scholar]
- 33.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–13451. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 34.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patlolla JM, Swamy MV, Raju J, Rao CV. Overexpression of caveolin-1 in experimental colon adenocarcinomas and human colon cancer cell lines. Oncol Rep. 2004;11:957–963. [PubMed] [Google Scholar]
- 36.Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK. Caveolin-1 expression is associated with high-grade bladder cancer. Urology. 2001;58:811–814. doi: 10.1016/s0090-4295(01)01337-1. [DOI] [PubMed] [Google Scholar]
- 37.Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140–1144. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davidson B, Goldberg I, Givant-Horwitz V, Nesland JM, Berner A, Bryne M, et al. Caveolin-1 expression in ovarian carcinoma is MDR1 independent. Am J Clin Pathol. 2002;117:225–234. doi: 10.1309/u40r-1bn4-6kj3-bdg3. [DOI] [PubMed] [Google Scholar]
- 39.Van den Eynden GG, Van Laere SJ, Van der Auwera I, Merajver SD, Van Marck EA, Van Dam P, et al. Overexpression of caveolin-1 and -2 in cell lines and in human samples of inflammatory breast cancer. Breast Cancer Res Treat. 2006;95:219–228. doi: 10.1007/s10549-005-9002-1. [DOI] [PubMed] [Google Scholar]
- 40.Yoo SH, Park YS, Kim HR, Sung SW, Kim JH, Shim YS, et al. Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung Cancer. 2003;42:195–202. doi: 10.1016/s0169-5002(03)00287-3. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Ling MT, Wang Q, Lau CK, Leung SC, Lee TK, et al. Identification of a novel inhibitor of differentiation-1 (ID-1) binding partner, caveolin-1, and its role in epithelial-mesenchymal transition and resistance to apoptosis in prostate cancer cells. J Biol Chem. 2007;282:33284–33294. doi: 10.1074/jbc.M705089200. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Ittmann MM, Ayala G, Tsai MJ, Amato RJ, Wheeler TM, et al. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005;8:108–118. doi: 10.1038/sj.pcan.4500776. [DOI] [PubMed] [Google Scholar]
- 43.Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, et al. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. 2008;7:2257–2267. doi: 10.4161/cc.7.14.6475. [DOI] [PubMed] [Google Scholar]
- 44.Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989;108:2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joshi B, Strugnell SS, Goetz JG, Kojic LD, Cox ME, Griffith OL, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–8220. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 46.Ayala GE, Dai H, Tahir SA, Li R, Timme T, Ittmann M, et al. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–5164. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- 47.Liu P, Li WP, Machleidt T, Anderson RG. Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nat Cell Biol. 1999;1:369–375. doi: 10.1038/14067. [DOI] [PubMed] [Google Scholar]
- 48.Llorente A, de Marco MC, Alonso MA. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci. 2004;117:5343–5351. doi: 10.1242/jcs.01420. [DOI] [PubMed] [Google Scholar]
- 49.Lu Q, Zhang J, Allison R, Gay H, Yang WX, Bhowmick NA, et al. Identification of extracellular delta-catenin accumulation for prostate cancer detection. Prostate. 2009;69:411–418. doi: 10.1002/pros.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung LW, Huang WC, Sung SY, Wu D, Odero-Marah V, Nomura T, et al. Stromal-epithelial interaction in prostate cancer progression. Clin Genitourin Cancer. 2006;5:162–170. doi: 10.3816/CGC.2006.n.034. [DOI] [PubMed] [Google Scholar]
- 51.Kwabi-Addo B, Ozen M, Ittmann M. The role of fibroblast growth factors and their receptors in prostate cancer. Endocr Relat Cancer. 2004;11:709–724. doi: 10.1677/erc.1.00535. [DOI] [PubMed] [Google Scholar]
- 52.Morrissey C, Vessella RL. The role of tumor microenvironment in prostate cancer bone metastasis. J Cell Biochem. 2007;101:873–886. doi: 10.1002/jcb.21214. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds AR, Kyprianou N. Growth factor signalling in prostatic growth: significance in tumour development and therapeutic targeting. Br J Pharmacol. 2006;147 Suppl 2:S144–S152. doi: 10.1038/sj.bjp.0706635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Josko J, Mazurek M. Transcription factors having impact on vascular endothelial growth factor (VEGF) gene expression in angiogenesis. Med Sci Monit. 2004;10:RA89–RA98. [PubMed] [Google Scholar]
- 55.Cash J, Korchnak A, Gorman J, Tandon Y, Fraizer G. VEGF transcription and mRNA stability are altered by WT1 not DDS(R384W) expression in LNCaP cells. Oncol Rep. 2007;17:1413–1419. [PubMed] [Google Scholar]
- 56.Kanies CL, Smith JJ, Kis C, Schmidt C, Levy S, Khabar KS, et al. Oncogenic Ras and transforming growth factor-beta synergistically regulate AU-rich element-containing mRNAs during epithelial to mesenchymal transition. Mol Cancer Res. 2008;6:1124–1136. doi: 10.1158/1541-7786.MCR-07-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song QH, Klepeis VE, Nugent MA, Trinkaus-Randall V. TGF-beta1 regulates TGF-beta1 and FGF-2 mRNA expression during fibroblast wound healing. Mol Pathol. 2002;55:164–176. doi: 10.1136/mp.55.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Touriol C, Morillon A, Gensac MC, Prats H, Prats AC. Expression of human fibroblast growth factor 2 mRNA is post-transcriptionally controlled by a unique destabilizing element present in the 3′-untranslated region between alternative polyadenylation sites. J Biol Chem. 1999;274:21402–21408. doi: 10.1074/jbc.274.30.21402. [DOI] [PubMed] [Google Scholar]
- 59.Yang G, Addai J, Wheeler TM, Frolov A, Miles BJ, Kadmon D, et al. Correlative evidence that prostate cancer cell-derived caveolin-1 mediates angiogenesis. Hum Pathol. 2007;38:1688–1695. doi: 10.1016/j.humpath.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 60.Ayala G, Satoh T, Li R, Shalev M, Gdor Y, Aguilar-Cordova E, et al. Biological response determinants in HSV-tk + ganciclovir gene therapy for prostate cancer. Mol Ther. 2006;13:716–728. doi: 10.1016/j.ymthe.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 61.Efstathiou E, Troncoso P, Wen S, Do KA, Pettaway CA, Pisters LL, et al. Initial modulation of the tumor microenvironment accounts for thalidomide activity in prostate cancer. Clin Cancer Res. 2007;13:1224–1231. doi: 10.1158/1078-0432.CCR-06-1938. [DOI] [PubMed] [Google Scholar]
- 62.Pisters LL, Pettaway CA, Troncoso P, McDonnell TJ, Stephens LC, Wood CG, et al. Evidence that transfer of functional p53 protein results in increased apoptosis in prostate cancer. Clin Cancer Res. 2004;10:2587–2593. doi: 10.1158/1078-0432.ccr-03-0388. [DOI] [PubMed] [Google Scholar]
- 63.Sonpavde G, Chi KN, Powles T, Sweeney CJ, Hahn N, Hutson TE, et al. Neoadjuvant therapy followed by prostatectomy for clinically localized prostate cancer. Cancer. 2007;110:2628–2639. doi: 10.1002/cncr.23085. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe M, Yang G, Cao G, Tahir SA, Naruishi K, Tabata K, et al. Functional analysis of secreted caveolin-1 in mouse models of prostate cancer progression. Mol Can Res. 2009 doi: 10.1158/1541-7786.MCR-09-0071. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 66.Weiner GJ. Monoclonal antibody mechanisms of action in cancer. Immunol Res. 2007;39:271–278. doi: 10.1007/s12026-007-0073-4. [DOI] [PubMed] [Google Scholar]