Abstract
Objectives
Determine the pathological importance of oxidative stress-induced injurious processes in chagasic heart dysfunction.
Background
Trypanosoma cruzi-induced inflammatory pathology and a feedback cycle of mitochondrial dysfunction and oxidative stress may contribute to Chagas disease.
Methods
Sprague Dawley rats were infected with T. cruzi, and treated with phenyl-α-tert-butylnitrone (PBN/antioxidant) and/or benzonidazole (BZ/anti-parasite). We monitored myocardial parasite burden, oxidative adducts, mitochondrial complex activities, respiration and ATP synthesis rates, and inflammatory and cardiac remodeling responses during disease development. Cardiac hemodynamics was determined for all rats.
Results
BZ (not PBN) decreased the parasite persistence and immune adverse events (proinflammatory cytokine expression, NADPH (β-Nicotinamide Adenine Dinucleotide Phosphate, reduced) oxidase and myeloperoxidase activities, and inflammatory infiltrate) in chronic hearts. PBN±BZ (not BZ alone) decreased the mtROS level, oxidative adducts (malonyldialdehyde, 4-hydroxynonenal, carbonyls), hypertrophic gene expression (ANP, BNP, αsk-Actin), and collagen deposition, and preserved the respiratory chain efficiency and energy status in chronic hearts. Subsequently, left ventricular dysfunction was prevented in PBN±BZ-treated chagasic rats.
Conclusions
BZ treatment after acute stage decreased the parasite persistence and inflammatory pathology. Yet, oxidative adducts, mitochondrial dysfunction and remodeling responses persisted and contributed to declining cardiac function in chagasic rats. Combinatorial treatment (PBN+BZ) was beneficial in arresting the T. cruzi-induced inflammatory and oxidative pathology and chronic heart failure in chagasic rats.
Keywords: Chagas disease, benzonidazole, mitochondrial oxidative stress, Trypanosoma cruzi, Antioxidant
INTRODUCTION
Chagas disease is an emerging parasitic disease in developed countries. In endemic countries, ~16–18 million people are infected with Trypanosoma cruzi, and at risk of disease development (1). In most patients, acute infection results in non-specific clinical symptoms. Several years later, 30–40% of seropositive individuals develop progressive chagasic cardiomyopathy with ventricular dilation, arrhythmia, and contractile dysfunction, leading to cardiac arrest and patient’s death (2).
Several lines of evidence establish that a low level of parasites remains in the host during the chronic disease phase (3). Direct detection of parasites in autopsy specimens and myocardial biopsies has been shown by immuno-histochemical and immuno-fluorescence techniques (4) and PCR (5). The reactivation of acute infection and parasitemia has been noted following immuno-suppression due to AIDS (6) or drug therapy (7). Blood transfusion and transplantation of infected organs obtained from asymptomatic individuals is a major cause of T. cruzi transmission in non-endemic countries (8). These studies indicated that parasite persistence result in consistent activation of inflammatory responses and development and/or propagation of pathological lesions in the heart.
Others and we have shown that chagasic myocardia sustain oxidative injuries. Phagocytes (macrophages and neutrophils), activated to control T. cruzi infection, are a major source of oxidative stress in acutely-infected host (9). Further, myocardial oxidative stress is enhanced due to increased mitochondrial production of reactive oxygen species (ROS) during Chagas disease (10). A compromised antioxidant status in T. cruzi-infected experimental animals (11) was associated with enhanced oxidative modification of cellular (11,12) and mitochondrial proteins and lipids (13). The plasma and intra-erythrocytic levels of glutathione and glutathione peroxidase are decreased (14), and glutathione disulfide and malonyldialdehyde (MDA) contents increased (15) in human chagasic patients. These studies indicated that oxidative injurious processes are of pathological significance in Chagas disease.
In this study, we aimed to evaluate the relative pathological importance of parasite persistence and oxidative injurious processes in the progression of Chagas disease and cardiac contractile dysfunction. Sprague Dawley rats were infected with T. cruzi and treated with PBN, a nitrone-based antioxidant that scavenges a wide variety of free radical species and inhibits free radical generation (16). Some of the infected rats were treated with BZ that is currently the treatment of choice for chagasic patients (17). We determined the efficacy of PBN intervention in reducing the generation or the effects of ROS, and of BZ in controlling parasite persistence, and subsequently, evaluated whether these treatments (individually or in combination) were beneficial in preventing progressive chagasic pathology and cardiac dysfunction in chronic rats.
METHODS
Animals and Parasites
Sprague Dawley rats (4–5-weeks old, Harlan Labs) were accustomed to animal facility for one-month and then infected with T. cruzi (SylvioX10/4, 50,000 trypomastigotes/rat, i.p.). Rats were given PBN (1.3mM, beginning day 0, throughout the course of infection) and/or BZ (0.7mM, beginning day 40 pi, for 3-weeks) in drinking water. Tissue parasite burden was determined by traditional and real-time PCR using T. cruzi 18SrDNA-specific oligonucleotides (18). The institutional Animal Care and Use Committee approved all animal experiments.
ROS and ATP
The myocardial and mitochondrial levels of ROS were determined using dihydroethidium (DHE) and amplex red/HRP fluorescent probes (19). Cryostat tissue-sections were stained with DHE to detect in-situ ROS. The ATP level in tissue homogenates and isolated mitochondria was determined by a luciferin/luciferase bioluminescence method (19). Mitochondria were energized with pyruvate/malate and succinate to monitor the rates of ROS and ATP production.
Lipid and protein oxidation
MDA level was determined by a TBARS assay (20). Protein carbonyls were derivatized with 2,4-dinitrophenylhydrazine (DNP), and estimated by ELISA (21) using a polyclonal anti-DNP antibody (Chemicon). Paraffin-embedded tissue-sections (5µm) were subjected to immune-staining with an anti-HNE antibody (Alpha-Diagnostics), and images analyzed by light microscopy.
Inflammation and tissue pathology
Formalin-fixed myocardial tissue-sections were stained with hematoxylin/eosin or Masson’s Trichrome, and scored for myocarditis, fibrosis, and parasitic-lesions (22). The enzymatic activities of xanthine oxidase (XOD), NADPH oxidase (NOX), and myeloperoxidase were monitored by spectrophotometry and/or in-gel catalytic staining. The myocardial mRNA levels for proinflammatory cytokines and hypertrophy markers was determined by real-time RT-PCR (18).
Heart function
Rats were monitored for heart function during the acute (25–40 dpi) and chronic (>150 dpi) stages of infection. After i.p. injection of heparin (1000-IU/kg) and administration of anesthesia, tracheotomy was performed to facilitate breathing, and a conductance Pressure-Volume (P-V) catheter (Scisense) was inserted into the left ventricle. Respiration (80-breaths/min, 3-L/min O2) was controlled through a tracheotomy cannula connected to a pressure-controlled respirator (RSP1002). The conductance and pressure signals were digitized and the volume calibration of the conductance system was performed. Hemodynamic measurements were made using EMKA cardiac P-V analysis software.
Data analysis
Data were expressed as mean ± SD (n=9 animals/group, triplicate observations/experiment), analyzed by SPSS software (14.0 version, SPSS Inc.). Normally distributed data were analyzed by student’s t-test (comparison between two groups) and one-way ANOVA with Tukey’s post-hoc test (comparison between multiple groups). Mann Whitney and Kruskal-Wallis tests were employed when data were not normally distributed. Significance is shown by *normal-versus-infected/untreated) and #infected/untreated-versus-infected/treated (*,#p<0.05, **,##p<0.01, ***,###p<0.001).
RESULTS
Parasite burden
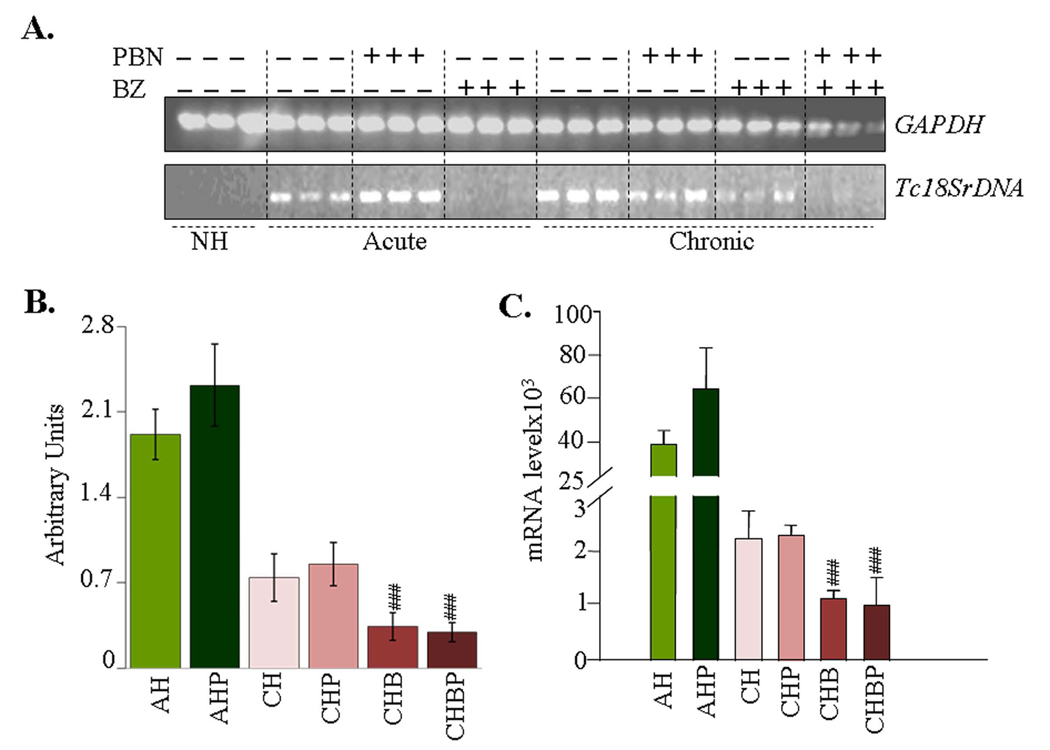
Semiquantitative PCR showed a significant level of Tc18SrDNA signal in the myocardium of acutely- and chronically-infected rats (Fig-1A&B). The extent of Tc18SrDNA amplification was similar in the heart tissue of infected/PBN-treated and infected/untreated rats. In comparison, Tc18SrDNA-specific signal was barely detectable in the myocardium of BZ-treated/chronic rats. Specificity of the PCR reaction was confirmed by no amplification of Tc18SrDNA with normal rat DNA. A real-time PCR was employed to obtain a quantitative measure of parasite burden in infected rats. Our data showed a 15-fold higher parasite burden in acute hearts compared to chronic hearts (Fig-1C). BZ treatment (±PBN) resulted in 55% decline in Tc18SrDNA signal in infected rats (Fig-1C, #p<0.01ANOVA-Tukey’s). These results showed that BZ was effective in controlling the parasite persistence, and PBN alone had no effect on parasites’ replication and/or survival in the heart.
Figure-1. Tissue burden of T. cruzi in infected rats (±PBN/BZ).
Myocardial total DNA was used as a template, and T. cruzi 18SrDNA amplified by PCR (A&B) and real-time PCR (C). Densitometric quantification of Tc18SrDNA PCR-amplicons (28-cycles), normalized to rat GAPDH, is shown in panel-B. Abbreviations: NH-normal heart, AH-acutely-infected/untreated (25-dpi), AHP-acutely-infected/PBN-treated, CH-chronically-infected/untreated (>150 dpi), CHP-chronically-infected/PBN-treated, CHB-chronically-infected/benzonidazole-treated, CHBP-chronically-infected/PBN+BZ-treated. Data in all figures are presented as mean ± SD (n=9, *, #p<0.05, **, ##p<0.01, ***, ###p<0.001).
ROS and oxidative damage
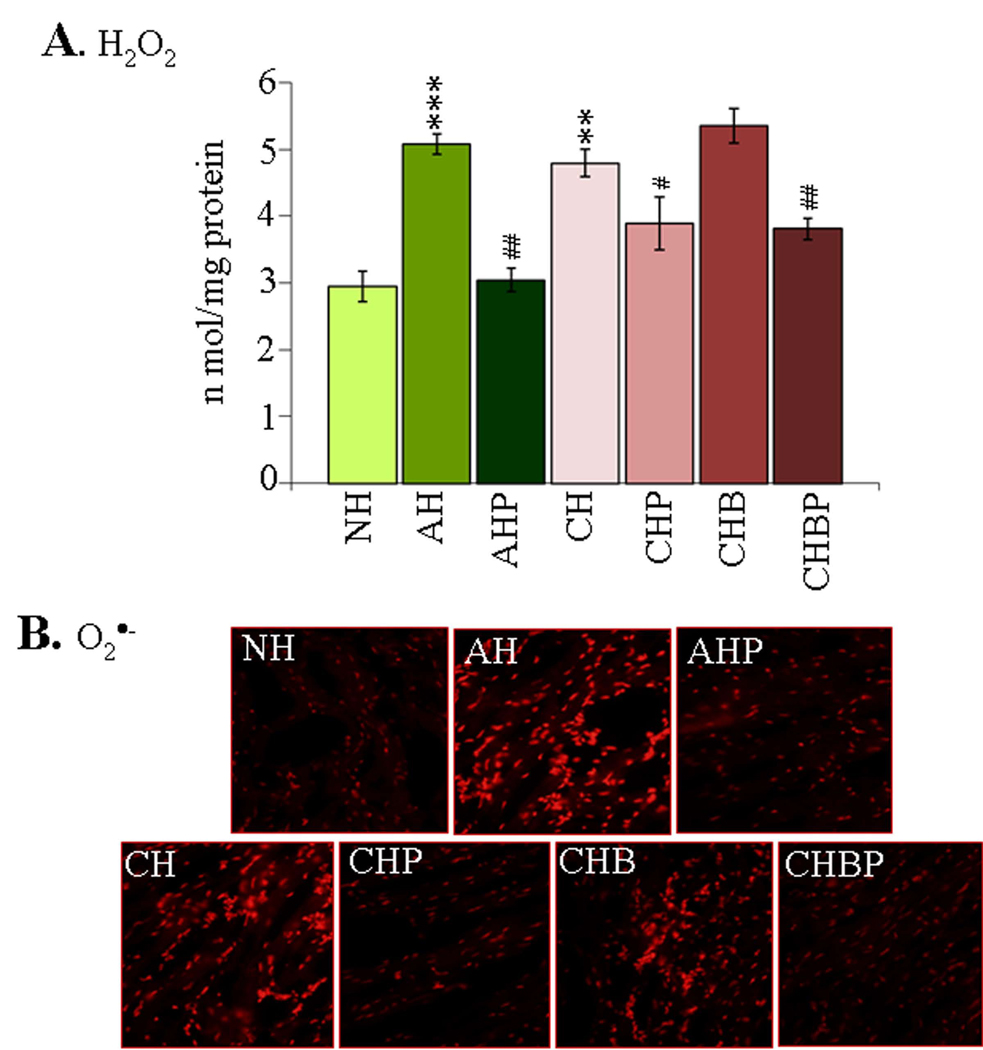
Fluorometric evaluation of ROS showed 72% and 63% increase in H2O2 levels in the myocardial homogenates of acutely- and chronically-infected rats (Fig-2A, *p<0.01t-test) that was not controlled in infected/BZ-treated rats. When treated with PBN (±BZ), the enhanced level of H2O2 was controlled by 96% in acute myocardium, and by 49–53% in chronic hearts (Fig-2A, all #p<0.01t-test/ANOVA-Tukey’s). In situ studies verified the above results. We noted a substantial DHE oxidation to ethidium red in the myocardial sections of acutely- and chronically-infected rats that were not treated or treated with BZ only (Fig-2B). Infected rats treated with PBN (±BZ) exhibited a significant decline in ethidium fluorescence during the acute and chronic stages of infection and disease development (Fig-2B).
Figure-2. ROS determination in chagasic rats (±PBN/BZ).
(A) The myocardial H2O2 levels were determined by amplex red/HRP method. (B) Frozen myocardial-sections were loaded with DHE (oxidizes to fluorescent ethidium), and micrographs obtained at 20× magnification (abbreviations/Figure-1).
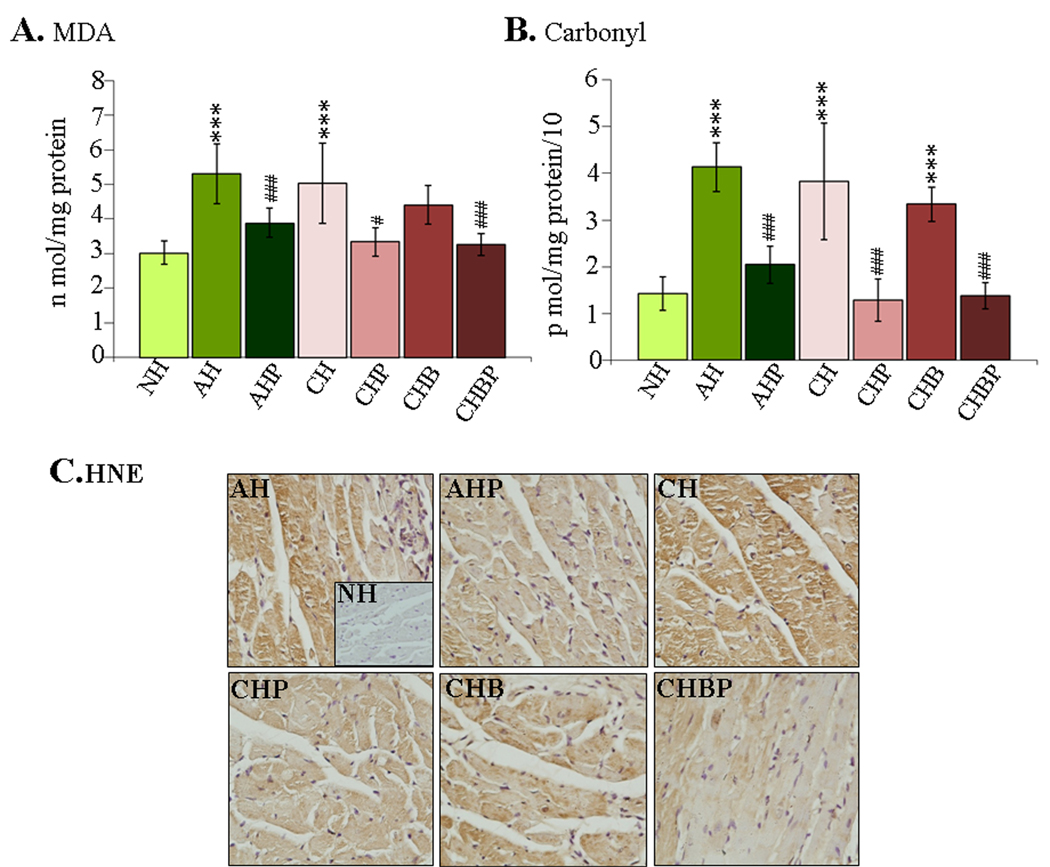
In agreement with ROS levels, the myocardial contents of MDA- and carbonyl-adducts were increased by 75% and 189%, respectively, in the acutely-infected rats, and by 66% and 168%, respectively, in chronic rats (Fig-3A&B, *p<0.001t-test). The PBN(±BZ) treatment resulted in a decline in myocardial MDA and carbonyl contents by 62% and 77%, respectively, in the acute stage, and by 84–88% and 100%, respectively, in the chronic stage ( all #p<0.001t-test/ANOVA-Tukey’s). Rats treated with BZ only exhibited a slight control of MDA (31% decrease) and no decline in carbonyl-adducts as compared to infected/untreated rats. Immuno-staining showed extensive HNE-adducts (intensity score 2–3) in acutely- and chronically-infected rats and in chronic/BZ-treated rats (Fig-3C). HNE adducts were significantly decreased (intensity score 0–1) in the myocardium of PBN-treated (±BZ) rats at both acute and chronic stages of infection and disease development (Fig-3C). These data (Figs-2&3) showed that PBN (but not BZ) treatment decreased the ROS level and oxidative damage in the myocardium of infected rats.
Figure-3. Myocardial oxidative damage in T. cruzi-infected rats (±PBN/BZ).
(A) TBARS determination of myocardial malonlydialdehydes. (B) DNP-derivatized carbonyl-proteins were measured by an ELISA. (C) Immuno-histochemical staining of heart tissue-sections with anti-HNE antibody (magnification-40×).
Mitochondrial dysfunction
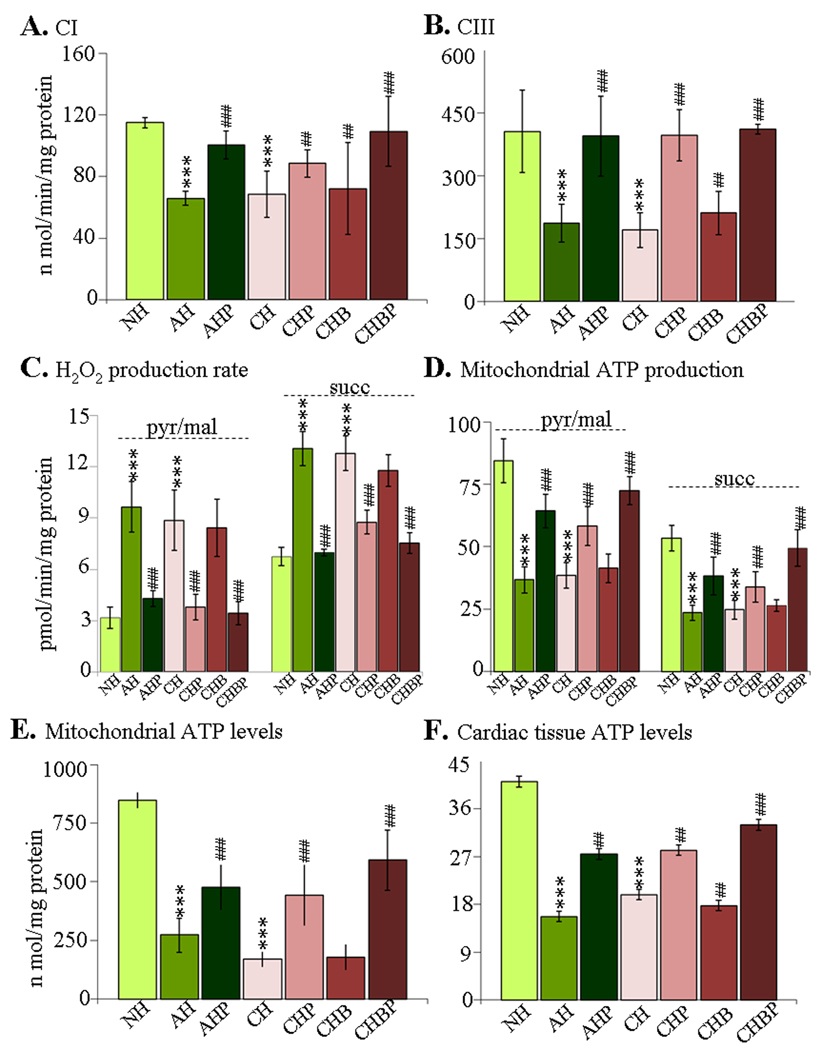
Cardiac mitochondria of infected rats exhibited 41–43% and 54–58% decline in the CI (NADH-quinone-oxidoreductase) and CIII (cytochrome-c-oxidoreductase) respiratory complex activities, respectively, during the course of infection and disease development (Fig-4A&B, *p<0.01t-test). The respiratory chain inefficiency was associated with enhanced mitochondrial ROS production. The pyr/mal- and succinate-stimulated mtROS production was distinctly increased in infected myocardium (acute: 2-fold and 95%, respectively; chronic: 1.8-fold and 91%, respectively, Fig-4C, *p<0.001t-test). Treatment with PBN (±BZ) resulted in 70% and 43–88% improvement in CI activity in acutely- and chronically-infected rats, respectively (Fig-4A) and normalization of CIII activity in infected myocardium (Fig-4B). Subsequently, pyr/mal- and succinate-dependent ROS formation was significantly controlled in PBN-treated (±BZ) rats during acute (82% and 96% decline, respectively) and chronic (57% and 32% decline, respectively) stages (Fig-4C, all #p<0.001t-test/ANOVA-Tukey’s). Treatment with BZ alone failed to preserve the respiratory complex activities or avert the increased rate of ROS production in cardiac mitochondria of infected rats.
Figure-4. Mitochondrial functional analysis in chagasic rats (±PBN/BZ).
(A&B) Spectrophotometric measurement of complex-I (A) and complex-III (B) activities in isolated cardiac mitochondria. (C&D) The rate of H2O2 (C) and ATP (D) production was monitored in pyr/mal- and succinate-energized isolated cardiac mitochondria. (E&F) The mitochondrial (E) and myocardial (F) ATP levels were determined by a luciferin/luciferase-bioluminescence method (abbreviations/Figure-1).
The pyr/mal- and succinate-dependent mitochondrial rate of ATP synthesis was decreased by 54–57% in infected myocardium (Fig-4D), and associated with 68–80% and 52–62% decline in mitochondrial and myocardial ATP levels, respectively (Fig-4E&F). PBN treatment (±BZ) improved the pyr/mal- and succinate-dependent mtATP production by 49–61% in infected hearts (Fig-4E, all #p<0.01t-test/ANOVA-Tukey’s). Subsequently, mitochondrial and cardiac ATP contents were improved in PBN-treated (±BZ) rats by 35–46% (Fig-4E&F, all #p<0.01t-test/ANOVA-Tukey’s). Treatment with BZ alone resulted in no significant improvement in mtATP synthesis and myocardial ATP levels in infected rats (Fig-4D–F). These data (Fig-4) demonstrated that PBN (but not BZ) preserved the mitochondrial respiratory chain activity and ATP production, and prevented enhanced ROS formation in the myocardium of acutely- and chronically-infected rats.
BZ kills T. cruzi via oxidative stress (23), and, thereby may have standby effects on host mitochondria. To determine if such is the case, we compared myocardial mitochondrial respiration in normal and normal/BZ-treated rats. We observed no significant differences in the rate of substrate-stimulated oxygen consumption in left-ventricle slices (in vivo) of normal and normal/BZ-treated rats. Likewise, state-3 respiration and state-3/state-4 ratio of isolated cardiac mitochondria from normal/BZ-treated were not compromised (Supplement file-2/Fig-1, Supplement file-2/Table-1). Further, BZ-treated/normal rats exhibited no significant increase in MDA and carbonyl adducts (data not shown). Thus, BZ alone did not contribute to mitochondrial dysfunction and oxidative stress in the host myocardium.
Inflammatory pathology
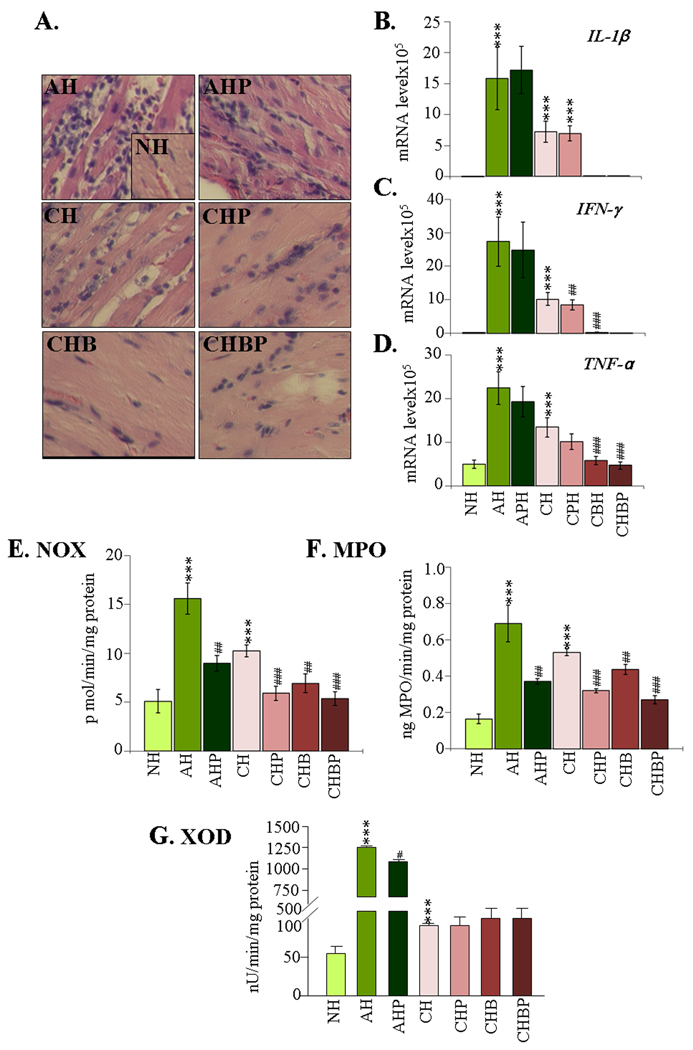
Histological studies showed parasitic foci and intense inflammatory infiltrate constituted of mononuclear (macrophages, T cells) and polymorphonuclear (neutrophils) cells in acute myocardium (histological score 2–4, Fig-5A). The inflammatory sequel was moderate and diffused in chronic hearts (histological score 1–2). The mRNA levels for proinflammatory cytokines IL-1β, IFN-γ, and TNF-α were significantly increased in acutely- and chronically-infected myocardium (Fig-5B–D, all *p<0.001t-test). PBN treatment of infected rats resulted in a moderate decline in myocardial inflammatory infiltrate (histological score 2–3) and marginal-to-no change in cytokine mRNA levels. In comparison, infected rats treated with BZ (±PBN) exhibited a considerable decline in myocardial inflammatory infiltrate (histological score 0–1). Accordingly, the myocardial mRNA levels for IL-1β, IFN-γ and TNF-α-were significantly decreased in chronic rats given BZ alone (100%, 99%, and 89.5% decline, respectively, #p<0.01ANOVA-Tukey’s) or in combination with PBN (normalized to normal control level, #p<0.01ANOVA-Tukey’s) (Fig-5).
Figure-5. Cardiac inflammatory response in T. cruzi-infected rats (±PBN/BZ).
(A) H&E staining of heart-sections (blue-nuclear and pink-muscle/cytoplasm/keratin). (B–D) Myocardial levels of IL-1β (B), IFN-γ (C) and TNF-α (D) mRNAs, determined by real-time RT-PCR. Specific activities of (E) NADPH oxidase (NOX), (F) myleoperoxidase (MPO) and (G) xanthine oxidase (XOD) were measured by spectrophotometry (abbreviations/Figure-1).
NOX, MPO, and XOD are indicators of macrophage, neutrophil, and endothelial cell activation, respectively. Spectrophotometric studies showed a substantial increase in NOX, MPO, and XOD activities in the infected myocardium (acute: 7.6-fold, 3.2-fold, and 43.1-fold increase, respectively; chronic: 110%, 2.2-fold, and 108% increase, respectively, Fig-5E–G, *p<0.01t-test). PBN treatment (±BZ) led to a significant control of NOX and MPO activities in the acute (60–70% and 63% respectively, all #p<0.01t-test) and chronic (55–95% and 58–71%, respectively, all #p<0.01ANOVA-Tukey’s) hearts. Likewise, BZ treatment was effective and resulted in 16–95% decline in NOX and 25–71% decline in MPO activities respectively, in chronic myocardium (#p<0.01ANOVA-Tukey’s). PBN and BZ treatments (individually or in combination) did not alter the XOD activity in infected rats (Fig-5G). In-gel catalytic staining assays validated the spectrophotometric results, and showed that PBN and BZ (individually or in combination) controlled the enhanced activities of NOX and MPO in infected myocardium (Supplement file-2/Fig-2). These data demonstrated that the parasite-dependent infiltration of inflammatory infiltrate and cytokine expression in the heart were significantly controlled by BZ. PBN was primarily effective in limiting the inflammatory oxidative stress in chagasic hearts as evidenced by a decline in NOX and MPO activities.
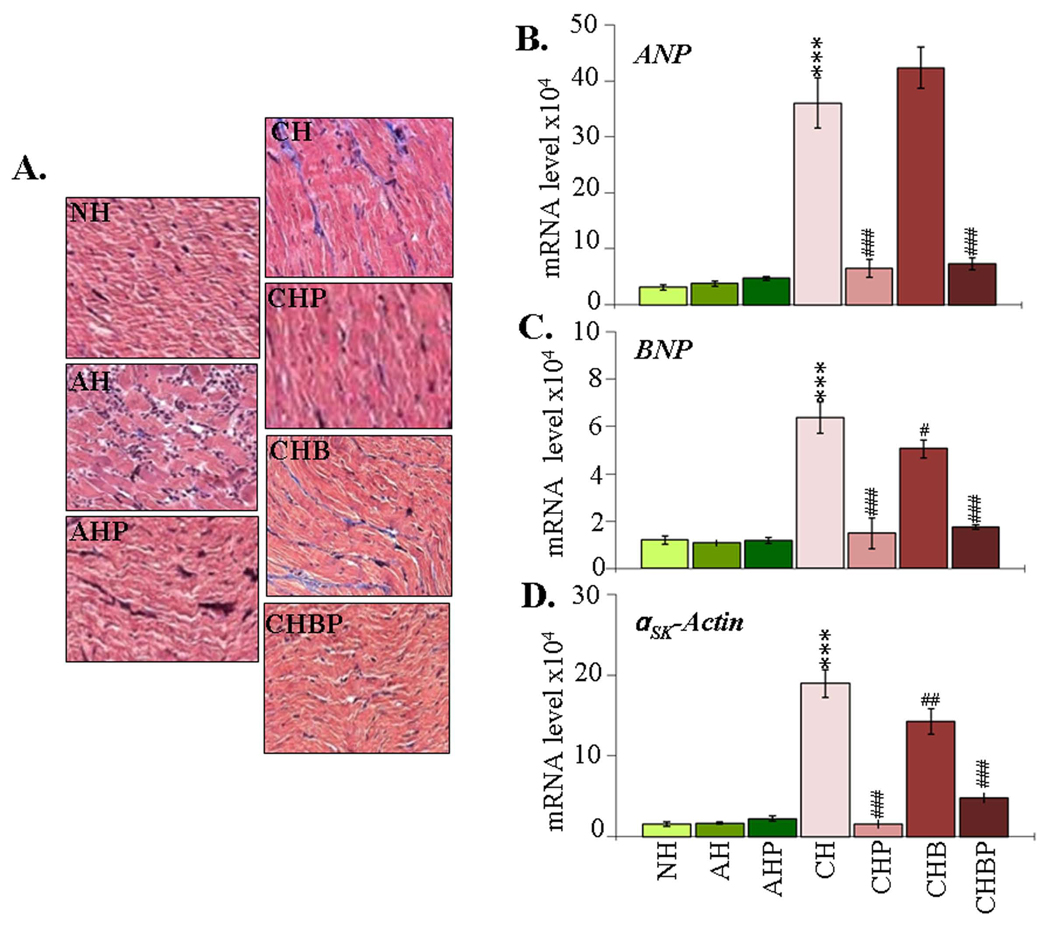
Cardiac remodeling
We performed Masson's Trichrome staining of heart sections to detect collagen deposition. In acute rats, scattered foci of myocyte necrosis associated with inflammatory cells were visible; however, no significant evidence of fibrosis was detectable (Fig-6A). Histological observation of extensive collagen deposition in chronic rats (histological score 2–4) was associated with 10.4-fold, 4.4-fold and 11.2-fold increase in ANP, BNP, and αsk-Actin mRNAs, respectively (Fig-6B–D, all *p<0.001t-test). We observed a moderate decline (histological score 2–3) in collagen deposition (Fig-6A), and a distinct decline in the enhanced ANP, BNP and αsk-Actin mRNA levels in infected rats treated with PBN (82%, 77% and 92%, respectively) or PBN/BZ (79.7%, 72.5% and 74.8%, respectively) (Fig-6B–D, all #p<0.00t-test/ANOVA-Tukey’s). Treatment with BZ alone resulted in a marginal decline in collagen deposition (histological score 3–4) and BNP and αsk-Actin mRNAs in chronic myocardium. These results suggested that adjunct therapy with PBN and BZ was effective in limiting cardiac remodeling responses in chagasic rats.
Figure-6. Myocardial remodeling in T. cruzi-infected rats (±PBN/BZ).
(A) Masson’s Trichrome staining of heart-sections (blue-collagen, red-muscle/cytoplasm). (B–D) Real-time RT-PCR measurement of myocardial mRNA levels for atrial-natriuretic-peptide (ANP) (B), brain-natriuretic-peptide (BNP) (C), and α-skeletal-actin (αsk-Actin) (D) (abbreviations/Figure-1).
Hemodynamic measurements
Pressure-volume loops were analyzed at steady state and following inferior vena cava occlusion (Supplement file-2/Table-2). Baseline measurements showed no significant differences in hemodynamics of normal and acute rats (data not shown). The pressure shifts of the P-V loops from baseline (mmHg: 82.3-normal, 48.1-chronic) indicated a decrease in cardiac contractility in chronic stage. The left ventricular +dP/dtmax (peak rate of pressure rise), −dP/dtmax (peak rate of pressure decline), stroke volume (SV, blood volume pumped with each beat), cardiac output (CO, blood volume pumped/min), ejection fraction (EF, fraction of blood ejected/contraction), and heart rate (HR) were decreased by 28%, 43%, 13%, 46%, 34% and 38%, respectively, in chronic rats (all *p<0.01Mann-Whitney). Notable elevation in end-systolic-volume (ESV) and end-dystolic-volume (EDV) occurred in chronic hearts with 174% and 44% increase, respectively, compared to controls. No significant gain in cardiac function was observed in chronic/BZ-treated rats. In comparison, a gain in cardiac function by PBN treatment was evident by a significant elevation of +dp/dtmax, −dp/dtmax, SV, CO, EF and HR in chronic, PBN-treated (48%, 59%, 26%, 70% 93% and 87% respectively, all #p<0.01Kruskal-Wallis) and PBN/BZ-treated (52%, 53%, 100%, 96%, 91% and 90%, respectively, all #p<0.001Kruskal-Wallis) rats. Subsequently, ESV and EDV elevation noted in chronic chagasic hearts was normalized to control level by PBN (±BZ) treatment (all #p<0.01Kruskal-Wallis).
P-V loops with inferior vena cava occlusion showed that the end systolic pressure-volume relationship (ESPVR, indicator of systolic performance) was decreased by 48% and end diastolic pressure-volume relationship (EDPVR, indicator of diastolic stiffness) was increased by 68% in chronic rats and no benefit was afforded by BZ-treatment. PBN treatment (±BZ) was beneficial as evidenced by <10% decline in ESPVR and <15% increase in EDPVR when compared to normal controls. Thus, hemodynamic results showed that PBN markedly prevented the chagasic cardiac dysfunction.
DISCUSSION
In this study, we demonstrate that diminishing the oxidative stress-induced alterations in myocardial structure, gene expression, and mitochondrial function are beneficial in preventing the chronic evolution of cardiac dysfunction in Chagas disease. We also provide molecular and biochemical evidence for inefficacy of benzonidazole during chronic Chagas disease.
We have used a rodent model of infection and disease development that exhibited the classical hallmarks of chronic Chagas disease. Infected rats showed a high level of acute parasite burden (Fig-1) resulting in extensive infiltration of inflammatory infiltrate and expression of proinflammatory cytokines in the heart (Fig-5). During the chronic stage, diffused inflammatory response persisted in the heart. Cardiac remodeling in chronic rats was evidenced by an increased expression of hypertrophy markers (ANP, BNP, and αsk-Actin) and collagen deposition (Fig-6). Subsequently, ventricular performance was compromised, as evidenced by 34–46% decline in cardiac output and ejection fraction and alterations in other parameters proposed as a fair index of contractility (Supplement file-2/Table-2) (24). The baseline hemodynamics of chronic rats, obtained with the P-V loops, were in accordance with the results from human Chagasic patients (25).
A systematic review of the literature indicates that pathogenesis of Chagas disease is dependent on a low-grade, systemic infection with documented immune-adverse reactions (discussed in (3)). Only acutely-infected patients, irrespective of their ages, are shown to respond to treatment with anti-parasite drug (BZ) and be cured, defined by the control of acute parasitemia and myocarditis (17). No convincing evidence exists demonstrating the efficacy of BZ in indeterminate-to-chronic patients. Further, it is not known whether drug therapies are ineffective in chronic patients, because either BZ fails to kill tissue parasites or other host events persist independent of parasites, and result in evolution of chronic heart failure. To investigate this, we examined in our experimental model the effect of BZ treatment on parasites and host events. Our data showed that oral delivery of BZ for three weeks during an indeterminate phase precluded the tissue parasite persistence and associated inflammation in chronic hearts (Figs-1&5). Activated neutrophils and macrophages express NOX, iNOS, and MPO and participate in parasite control through a release of cytotoxic ROS, NO, and HOCl, respectively (26). Our observation of a decline in NOX and MPO activities (Fig-5 and supplement file-2/Fig-2) in BZ-treated chronic rats provided further evidence that anti-parasite therapy was effective in averting the parasite-mediated inflammatory pathology. Nonetheless, BZ-treated/chronic rats exhibited cardiac remodeling (Fig-6) and deterioration of ventricular contractility (Supplement file-2/Table-2) comparable to that exhibited by untreated/chronic rats. These data provide molecular evidence that a lack of control of chronic Chagas disease by BZ is not due to its inefficacy in controlling parasite persistence and parasite-dependent inflammatory responses. Importantly, the observations in BZ-treated rats of consistent oxidative stress-induced myocardial adducts (Fig-3) and mitochondrial inefficiency (Fig-4) strongly indicate a role of oxidative stress and mitochondrial/cellular injuries in LV dysfunction during progressive Chagas disease. The mitochondrial production of ROS was significantly enhanced in BZ-treated and untreated chronic rats (Fig-4), thus, providing evidence for the mitochondrial origin of pathologic oxidative stress in chagasic hearts.
PBN treatment of infected rats provided a direct evidence for the detrimental effects of oxidative stress and mitochondrial dysfunction in Chagas disease. Oral delivery of PBN (±BZ) resulted in preservation of LV function (Supplement file-2/Table-2). It is important to note that beneficial effects of PBN in preserving cardiac function were observed despite no decline in parasite persistence (Fig-1) and moderate-to-no change in myocardial inflammatory infiltrate and proinflammatory cytokines in chronic hearts (Fig-5). Instead, PBN-treated/chronic rats exhibited a substantial increase in mitochondrial function as evidenced by improved complex activities and ATP synthesis, and decreased ROS production (Fig-4). The decline in mtROS levels was associated with a significant decline in the myocardial accumulation of MDA-, HNE-, and carbonyls-adducts in chronic, PBN-treated/infected rats (Fig-3). The maximal benefits were obtained when rats were treated with PBN and BZ together; thus, suggesting that combinatorial therapies, including anti-parasite drugs and antioxidants, will be effective in preserving the LV function in chagasic hearts.
Cardiac hypertrophy is a key phenotype of the failing heart and a major independent risk factor predictive of cardiovascular mortality and morbidity. In both experimental models and human chagasic patients, clinical evolution of heart failure is preceded by anatomo-pathological changes that include ventricular thickness and interstitial fibrosis, followed by ventricular dilation, and reduced contractility (27). The re-expression of fetal genes (ANP, BNP, αsk-Actin and β- MHC) is a hallmark of hypertrophic remodeling, and a considerable body of evidence shows the redox regulation of remodeling responses in cardiac diseases of various etiologies (28). Besides ROS, experimental studies have shown that the inflammatory cytokines, TNF-α, IL-1β, and MCP-1, also promote myocardial hypertrophy, and contribute to the development and progression of heart failure (29). Our observation of a decline in the expression of hypertrophic markers and collagen deposition in response to PBN treatment (±BZ) (Fig-6) suggests that ROS signals hypertrophic remodeling in chagasic myocardium. The role of ROS of mitochondrial, but not of inflammatory, origin in signaling hypertrophy in chagasic hearts is provided by the observation that NOX and MPO, the classical mediators of inflammatory ROS were equally depressed in BZ- and PBN-treated rats (Fig-5), and yet hypertrophic phenotype was depressed in PBN-treated rats only (Fig-6). Further studies would identify whether inflammatory cytokines synergistically enhance the ROS-mediated signaling cascades involved in activation of hypertrophic responses in chagasic hearts.
In summary, we have shown that complex inflammatory and oxidative processes in the heart contribute to the evolution of chronic hypertrophy and LV dysfunction in Chagas disease. Specifically, PBN treatment enhanced the antioxidant/oxidant balance, preserved the mitochondrial respiratory chain activity and ATP synthesis, and averted the ROS-induced hypertrophy and LV dysfunction in chronic rats. BZ treatment was effective in controlling parasite persistence and associated inflammation, but the feedback cycle of mitochondrial dysfunction and oxidative stress persisted, and, therefore, LV function was not significantly improved in BZ-treated/chronic rats. Thus, we propose that a combination of antioxidants capable of modulating or delaying the onset of oxidative insult and mitochondrial deficiencies and anti-parasite drugs capable of abolishing parasites and parasite-associated inflammation in the myocardium would prove beneficial in preventing cardiac pathology and loss of LV function in Chagas disease.
Supplementary Material
Acknowledgments
This work was supported by a grant (AI054578) from the National Institutes of Health/National Institute of Allergy and Infectious Disease to NJG
Commonly used abbreviations
- BZ
benzonidazole
- DHE
dihydroethidium
- HNE
4-hydroxynonenal
- HRP
horseradish-peroxidase
- IL-1β
interleukin-1beta
- IFN-γ
interferon-gamma
- MDA
malonyldialdehyde
- MPO
myeloperoxidase
- NOX
NADPH oxidase
- PBN
phenyl-α-tert-butylnitrone
- pyr/mal
pyruvate/malate
- ROS
reactive oxygen species
- T. cruzi
Trypanosoma cruzi
- TNF-α
tumor necrosis factor-alpha
- XOD
xanthine oxidase
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.World Health Organization. Report of Scientific Working Group on Chagas Disease. UNDP/World Bank/WHO; 2006:4.
- 2.Rassi A, Jr, Rassi A, Little WC. Chagas heart disease. Clin Cardiol. 2000;23:883–889. doi: 10.1002/clc.4960231205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zacks MA, Wen JJ, Vyatkina G, Bhatia V, Garg NJ. An overview of chagasic cardiomyopathy: pathogenic importance of oxidative stress. An Acad Bras Cienc. 2005;77:695–715. doi: 10.1590/s0001-37652005000400009. [DOI] [PubMed] [Google Scholar]
- 4.Mortara RA, daSilva S, Patricio FR, et al. Imaging Trypanosoma cruzi within tissues from chagasic patients using confocal microscopy. Parasitol Res. 1999;85:800–808. doi: 10.1007/s004360050636. [DOI] [PubMed] [Google Scholar]
- 5.Salomone OA, Juri D, Omelianiuk MO, et al. Prevalence of circulating Trypanosoma cruzi detected by PCR in patients with Chagasic cardiomyopathy. Am J Cardiol. 2000;85:1274–1276. doi: 10.1016/s0002-9149(00)00747-5. [DOI] [PubMed] [Google Scholar]
- 6.Sartori AM, Lopes MH, Caramelli B, et al. Simultaneous occurrence of acute myocarditis and reactivated Chagas disease in an AIDS patient. Clin Infect Dis. 1995;21:1297–1299. doi: 10.1093/clinids/21.5.1297. [DOI] [PubMed] [Google Scholar]
- 7.Jardim E, Takayanagui OM. Chagasic meningoencephalitis with detection of Trypanosoma cruzi in cerebrospinal fluid of an immunodepressed patient. J Trop Med Hyg. 1994;97:367–370. [PubMed] [Google Scholar]
- 8.Leiby DA, Rentas FJ, Nelson KE, et al. Evidence of Trypanosoma cruzi infection (Chagas disease) among patients undergoing cardiac surgery. Circulation. 2000;102:2978–2982. doi: 10.1161/01.cir.102.24.2978. [DOI] [PubMed] [Google Scholar]
- 9.Locksley RM, Klebanoff SJ. Oxygen-dependent microbicidal systems of phagocytes and host defense against intracellular protozoa. J Cell Biochem. 1983;22:173–185. doi: 10.1002/jcb.240220306. [DOI] [PubMed] [Google Scholar]
- 10.Wen J-J, Garg NJ. Mitochondrial generation of ROS is enhanced at Qo site of complex-III in myocardium of Trypanosoma cruzi-infected mice: beneficial effects of an antioxidant. J Bioenerg Biomembr. 2008;40:587–598. doi: 10.1007/s10863-008-9184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen J-J, Vyatkina G, Garg NJ. Oxidative damage during chagasic cardiomyopathy: Role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med. 2004;37:1821–1833. doi: 10.1016/j.freeradbiomed.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Wen JJ, Dhiman M, Whorton EB, Garg NJ. Tissue-specific oxidative imbalance and mitochondrial dysfunction during Trypanosoma cruzi infection in mice. Microbes Infect. 2008;10:1201–1209. doi: 10.1016/j.micinf.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen J-J, Garg NJ. Oxidative modifications of mitochondrial respiratory complexes in response to stress of Trypanosoma cruzi infection. Free Radic Biol Med. 2004;37:2072–2081. doi: 10.1016/j.freeradbiomed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 14.deOliveira TB, Pedrosa RC, Filho DW. Oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol. 2007;116:357–363. doi: 10.1016/j.ijcard.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Wen J-J, Yachelini PC, Sembaj A, Manzur RE, Garg NJ. Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Rad Biol Med. 2006;41:270–276. doi: 10.1016/j.freeradbiomed.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Floyd RA, Hensley K, Forster MJ, Kelleher-Anderson JA, Wood PL. Nitrones as neuroprotectants and anti-aging drugs. Ann NY Acad Sci. 2002;959:321–329. doi: 10.1111/j.1749-6632.2002.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 17.Caldas IS, Talvani A, Caldas S, et al. Benznidazole therapy during acute Chagas disease reduces parasite load but does not prevent chronic cardiac lesions. Parasitol Res. 2008;103:413–421. doi: 10.1007/s00436-008-0992-6. [DOI] [PubMed] [Google Scholar]
- 18.Garg NJ, Bhatia V, Gerstner A, deFord J, Papaconstantinou J. Gene expression analysis in mitochondria from chagasic mice: Alterations in specific metabolic pathways. Biochemical J. 2004;381:743–752. doi: 10.1042/BJ20040356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen J-J, Bhatia V, Popov VL, Garg NJ. Phenyl-alpha-tert-butylnitrone reverses mitochondrial decay in acute Chagas disease. Am J Pathol. 2006;169:1953–1964. doi: 10.2353/ajpath.2006.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Buss IH, Winterbourn CC. Protein carbonyl measurement by ELISA. Methods Mol Biol. 2002;186:123–128. doi: 10.1385/1-59259-173-6:123. [DOI] [PubMed] [Google Scholar]
- 22.Garg NJ, Popov VL, Papaconstantinou J. Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts. Biochim Biophys Acta. 2003;1638:106–120. doi: 10.1016/s0925-4439(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira FB, Ruiz JC, Robello C, Romanha AJ, Murta SM. Molecular characterization of cytosolic and mitochondrial tryparedoxin peroxidase in Trypanosoma cruzi populations susceptible and resistant to benznidazole. Parasitol Res. 2009;104:835–844. doi: 10.1007/s00436-008-1264-1. [DOI] [PubMed] [Google Scholar]
- 24.Zabalgoitia M, Ventura J, Lozano JL, et al. Myocardial contrast echocardiography in assessing microcirculation in baboons with Chagas disease. Microcirculation. 2004;11:271–278. doi: 10.1080/10739680490425976. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi AT, Sugimachi M, Sunagawa K, Bergsland J, Koide S, Batista RJ. Improved LV contraction and energetics in a Chagasic patient undergoing partial left ventriculectomy. J Card Surg. 2001;16:30–33. doi: 10.1111/j.1540-8191.2001.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 26.Forlenza M, Scharsack JP, Kachamakova NM, Taverne-Thiele AJ, Rombout JH, Wiegertjes GF. Differential contribution of neutrophilic granulocytes and macrophages to nitrosative stress in a host-parasite animal model. Mol Immunol. 2008;45:3178–3189. doi: 10.1016/j.molimm.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Marin-Neto JA, Cunha-Neto E, Maciel BC, Simoes MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 28.Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in redox regulation of cell signal transduction pathways. Front Biosci. 2009;14:4809–4814. doi: 10.2741/3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gullestad L, Aukrust P. Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am J Cardiol. 2005;95:17C–23C. doi: 10.1016/j.amjcard.2005.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.