Abstract
Rationale:
Myocardial infarction (MI)-induced heart failure (HF) is characterized by central nervous system (CNS)-driven sympathoexcitation and deteriorating cardiac function. The paraventricular nucleus (PVN) of the hypothalamus is a key regulator of sympathetic nerve activity and is implicated in HF. Redox signaling in the PVN and other CNS sites is a primary mechanism of neuro-cardiovascular regulation, and excessive oxidant production by activation of NADPH oxidases (Nox) is implicated in some neuro-cardiovascular diseases.
Objective:
We tested the hypothesis that Nox-mediated redox signaling in the PVN contributes to MI-induced sympathoexcitation and cardiac dysfunction in mice.
Methods and Results:
Real-time PCR revealed that Nox4 was the most abundantly expressed Nox in PVN under basal conditions. Coronary arterial ligation (MI) caused a selective upregulation of this homologue compared to Nox1 and Nox2. Adenoviral gene transfer of Nox4 siRNA (AdsiNox4) to PVN (bilateral) attenuated MI-induced superoxide formation in this brain region (day 14) to the same level as that produced by PVN-targeted gene transfer of cytoplasmic superoxide dismutase (AdCu/ZnSOD). MI mice treated with AdsiNox4 or AdCu/ZnSOD in the PVN showed marked improvement in cardiac function as assessed by echocardiography and left ventricular hemodynamic analysis. This was accompanied by significantly diminished sympathetic outflow and apoptosis in the peri-infarct region of the heart.
Conclusions:
These results suggest that MI causes dysregulation of Nox4-mediated redox signaling in the PVN, which leads to sympathetic overactivation and a decline in cardiac function. Targeted inhibition of oxidant signaling in the PVN could provide a novel treatment for MI-induced HF.
Keywords: heart, reactive oxygen species, NADPH oxidase, brain, sympathetic nerves
Introduction
Despite recent decline in the incidence of many cardiovascular diseases in the United States, that of heart failure (HF) continues to rise.1 HF is characterized by progressively deteriorating cardiac function, in part due to persistent sympathetic overactivation that is initiated in response to impaired cardiac function.2 While increased sympathetic drive aids perfusion acutely following a cardiac insult such as myocardial infarction (MI), chronic sympathoexcitation contributes to fluid retention, as well as cardiac arrhythmias, hypertrophy, and apoptosis, all of which contribute to declining cardiac function over time.2
The paraventricular nucleus (PVN) of the hypothalamus is a critical site of autonomic and neuroendocrine regulation.3 The nucleus of the tractus solitarius (NTS) relays afferent information to the PVN, which, in turn, sends efferent projections to the rostral ventral lateral medulla (RVLM) and spinal cord to modulate sympathetic outflow.4 Neurons of the forebrain circumventricular subfornical organ (SFO) – a structure outside the blood-brain-barrier which interacts with peripheral circulating factors - also project to the PVN, which can further stimulate sympathoexcitation as well as the production and secretion of vasopressin.3, 4 Indeed, the PVN is strongly implicated in the neurohumoral dysregulation observed in HF5. Recently our studies in mice showed that PVN neurons are chronically activated after MI, and that this parallels the sustained elevations in sympathetic outflow during MI-induced HF.6
Substantial evidence implicates reactive oxygen species (ROS) as key signaling molecules in PVN and other central nervous system (CNS) nuclei in maintaining cardiovascular homeostasis7, 8 and in mediating cardiovascular diseases such as hypertension and HF.6, 9, 10 For example, increased redox signaling has been observed in SFO and RVLM in animal models of HF, and this parallels rising sympathetic outflow.6, 10, 11 Similarly, afferent reflexes involving neuronal signaling from the NTS to the PVN,12 a pathway that relies upon ROS formation in the PVN under normal conditions,13, 14 are elevated during HF.15, 16
Abundant evidence implicates the NADPH oxidase (Nox) family of enzymes as a key source of free radicals in the CNS during normal signaling processes and in the oxidative stress that contributes to some neuro-cardiovascular diseases.8, 16, 17 Tremendous diversity exists in the distribution and regulation of the Nox homologues,18 and indeed we have previously reported a distinct pattern of Nox homologue expression in murine fore-, mid-, and hindbrain regions involved in cardiovascular regulation.8 While several studies have shown that MI-induced cardiac dysfunction is significantly improved in transgenic mice in which various subunits of the NADPH oxidase complex have been globally deleted,19 these knockout models do not permit analysis of the impact of specific Nox-mediated redox signaling in specific brain regions such as the PVN.
Given the importance of the PVN in regulating sympathetic outflow and its involvement in HF, combined with the key role NADPH oxidases play in central neuro-cardiovascular regulation and disease, we tested the hypothesis that dysregulated Nox signaling in the PVN contributes to the enhanced sympathetic output and deteriorating cardiac function induced by MI. Using viral gene transfer to modulate Nox expression and ROS levels selectively in PVN, our results demonstrate that Nox4-generated O2•− in the PVN contributes to the persistent sympathoexcitation and cardiac dysfunction which accompanies the post-MI decline to HF.
Materials and Methods
An expanded Material and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Animals
Adult C57BL/6 mice (~18-20g, 8-10 weeks old) were used for all experiments. All procedures were approved by the Animal Care and Use Committee at Cornell University. Care of the mice met or exceeded the standards set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals, USDA regulations, and the AVMA Panel on Euthanasia.
Adenoviral vectors
Construction and characterization of recombinant E1-deleted adenoviral vectors encoding human copper-zinc superoxide dismutase (AdCu/ZnSOD),20 catalase (AdCat),21 bacterial β-galactosidase (AdLacZ),22 and siRNA targeted against eGFP (AdsiGFP) or Nox4 (AdsiNox4)23 have each been described previously. All vectors were obtained from the University of Iowa Gene Vector Core.
Targeted gene transfer to the PVN and induction of MI
Mice were anesthetized with sodium pentobarbital and placed in a stereotaxic apparatus. Viruses were delivered bilaterally to PVN (200 nL total) using titer-matched adenoviral stocks (1×109pfu/mL). Dual virus injections (AdCat experiments) utilized titer-matched stocks which were mixed prior to delivery (100nl each).
MI was induced by surgical ligation of the left anterior descending (LAD) coronary artery as described.6, 24 Briefly, mice were anesthetized with sodium pentobarbital and intubated. The heart was accessed from the third intercostal space and the LAD was ligated with 8-0 ethilon suture. Sham procedure was identical to MI except without LAD ligation.
Quantitative real-time PCR detection of Nox homologues
Mice were decapitated and brains flash frozen. PVN tissue was isolated by micropunch, total RNA harvested, and cDNA generated using random hexamers. 25ng templates were subjected in triplicate to real-time qPCR using ABI 7500FAST system, Power SYBR Green and Nox1-, Nox2-, or Nox4-specific primers.23, 25 β-actin was used for relative quantitation by ddCt method (expressed as 2(-ddCt)) or with 18S RNA for absolute quantitation (expressed as copy number relative to 18S copy number in 100ng RNA).25
In situ hybridization and Western blot analysis for Nox4 detection
Serial cryosections of O.C.T.-embedded brains containing PVN from sham and MI mice were labeled with antisense cRNA probes containing the 225 bp–901 bp region of mouse Nox4 mRNA (NM_015760). In situ hybridization was performed using digoxigenin-labeled probes as described previously.26
Nox4 protein was measured by Western blot performed on PVN micropunches lysates subjected to SDS-PAGE as described.23
Measurement of ROS in PVN
Dihyrdroethidium (DHE) staining was performed as described previously.6, 11 Fresh frozen sections containing PVN were mounted onto glass slides, incubated in 1μM DHE, rinsed, and imaged using a Zeiss LSM510 confocal microscope. DHE fluorescence intensity was analyzed using NIH ImageJ and normalized to sham animals.6, 9
Detection of Cu/ZnSOD in brain sections
SOD immunohistochemistry was performed as described.6 Mice were perfused transcardially, brains removed, and cryosections containing PVN were mounted directly onto glass slides. Samples were incubated with a sheep anti-human Cu/ZnSOD antibody followed by donkey-anti-sheep rhodamine-conjugated secondary antibody. Images were obtained using a Zeiss Axio Imager D1 fluorescence microscope.
Hemodynamic and echocardiography measurements
Hemodynamic measurements were performed as described previously.11 Left ventricular (LV) pressures were recorded at 4KHz using a 1.4f Millar catheter and +dP/dt and −-dP/dt were calculated.11 A subset of mice was instrumented with jugular catheters for intravenous injection of isoproterenol over 30 seconds to a final dosage of 1ng/g. Peak ±dP/dt was measured over the subsequent 30 seconds and expressed relative to baseline just before injection.
Transthoracic echocardiography was performed under light anesthesia using a VEVO 770 high resolution imaging system and 707B transducer with 45MHz broadband frequency (VisualSonics).11 Retrospective B-mode cineloops obtained from parasternal long and short axes were used to calculate fractional shortening (FS) and ejection fraction (EF).11
Measurement of urinary norepinephrine
Urine was collected and norepinephrine (NE) measured by ELISA (IBL) according to manufacturer's instructions and as previously described.6
Power spectral and pharmacologic analysis of autonomic tone
A separate cohort of mice underwent implantation of radiotelemeters prior to sham/MI surgery to evaluate autonomic tone both by power spectral analysis and arterial pressure responses to ganglionic blockade. Briefly, mice were anesthetized and instrumented with telemetry devices as described.27 A subset of mice also underwent PVN-targeted viral gene transfer of AdsiNox4 at this time. Following 3-5 days recovery, telemeters were activated for continuous sampling of arterial pressure waveforms at 2KHz in conscious mice. After 3 days baseline recording, mice underwent MI or sham procedure. High-frequency sampling of pressure waveforms in conscious mice resumed the next day for an additional 14 days, at which time mice received acute hexamethonium injections (5mg/kg, i.p.) and arterial pressure responses were recorded. Power spectral analysis of arterial pressure variability before and after MI/sham surgery was performed as described.28, 29 Spectra were divided into low frequency (LF, 0.4-1.0 Hz) and high frequency (HF, 1.0-3 Hz).29 Data were calculated as a percentage in relation to baseline and presented as LF/HF relative to sham animals.
Quantification of apoptosis by DNA laddering
Mice were sacrificed by decapitation, hearts removed and immediately placed on ice, and peri- and non-infarct tissues were dissected. DNA was isolated, apoptotic fragments enriched, and the DNA laddering assay was performed (APO DNA1) as previously described.11
Statistical analyses
All data are expressed as mean ± SEM and were analyzed by ANOVA (after Bartlett's test of homogeneity of variance) followed by the Bonferroni post-test for multiple comparisons using Prism 5 (GraphPad Software, Inc.).
Results
Nox4 is the most abundant Nox homologue in the PVN and is selectively upregulated following MI
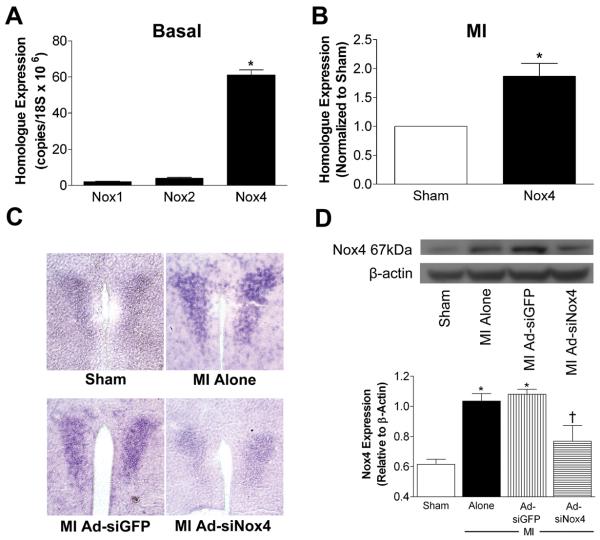
Excessive Nox1, Nox2, or Nox4 activation contributes to a number of cardiovascular diseases,18, 23 however the expression profiles of these homologues in the PVN have not been investigated. Using real-time RT-PCR in PVN micropunches, we show that Nox4 is the predominant isoform in PVN under basal conditions (Fig 1A). Nox1 and Nox2 are detectable, but Nox4 is expressed at levels 25-30-fold higher than these other homologues. This is consistent with our earlier findings that Nox4 is the most abundant Nox homologue in mid-brain,8 a region that encompasses PVN and other regions. We next measured levels of these homologues two weeks after MI, a time when there is significant sympathoexcitation and cardiac function is impaired. MI mice exhibited a two-fold increase in Nox4 expression in the PVN compared to sham animals (Fig 1B), while levels of Nox1 and Nox2 were unaffected (Nox1: 0.7 fold-sham; Nox2: 0.9 fold-sham; p>0.05, n=4-8 biological samples per group).
Figure 1. Nox4 is the most abundant Nox homologue in the PVN and is selectively upregulated following MI.
A) Summary of real-time RT-PCR analysis showing basal copy numbers of Nox1, Nox2, and Nox4 in PVN micropunches of naïve mice (n=6, each sample pooled from 3-4 mice); *p<0.05 vs. Nox1 or Nox2. B) Summary of Nox4 mRNA levels in PVN 14 days after MI expressed relative to sham mice (n=6 per group, each sample pooled from 3-4 mice); *p<0.05 vs. sham. C) Representative DIG staining (from 3 experiments) in PVN following in situ hybridization with antisense cRNA probes targeted against Nox4 from mice 14 days post-MI or sham surgery. MI mice had undergone PVN injections of AdsiGFP or AdsiNox4 three days before MI or sham. D) Representative Western blot (upper panel) and summary data (lower panel) of Nox4 protein levels from mice with or without PVN-targeted gene transfer of AdsiGFP or AdsiNox4 and 14 days after MI or sham surgery (n=4 per group, each sample pooled from 3 mice). *p<0.05 vs. Sham; †p<0.05 vs. MI alone and MI+AdsiGFP.
To provide more precise spatial resolution of Nox4 expression in PVN, and to verify the efficacy of AdsiNox4 in this brain region, we performed in situ hybridization using Nox4-specific cRNA probes. Sham animals showed punctate staining of Nox4 mRNA in PVN, and this was markedly increased at 14 days post-MI (Fig 1C). We have previously reported that AdsiNox4 effectively and selectively silences Nox4 transcription in cultured neurons and in SFO in vivo,23 however here we sought to confirm its fidelity in PVN using in situ hybridization. As shown in Fig 1C, bilateral PVN microinjection of AdsiNox4 attenuated the MI-induced increase in Nox4 signal, confirming the accurate targeting of our transgene and its ability to knock down Nox4 in this brain region. Importantly, injection of control siRNA (AdsiGFP) had no effect on MI-induced upregulation of Nox4. Finally, to verify effective Nox4 inhibition at the protein level, Western blot analysis on PVN homogenates was performed. Nox4 protein was significantly increased in PVN 14 days post-MI compared to sham controls (Fig 1D). AdsiNox4 caused a robust decrease in Nox4 levels in MI-treated mice, whereas AdsiGFP had no effect. Together these results demonstrate that Nox4 is the most abundant Nox homologue in the PVN, it becomes selectively upregulated after MI, and it can be silenced by injection of AdsiNox4.
MI causes a robust increase in Nox4-mediated superoxide radical formation in PVN
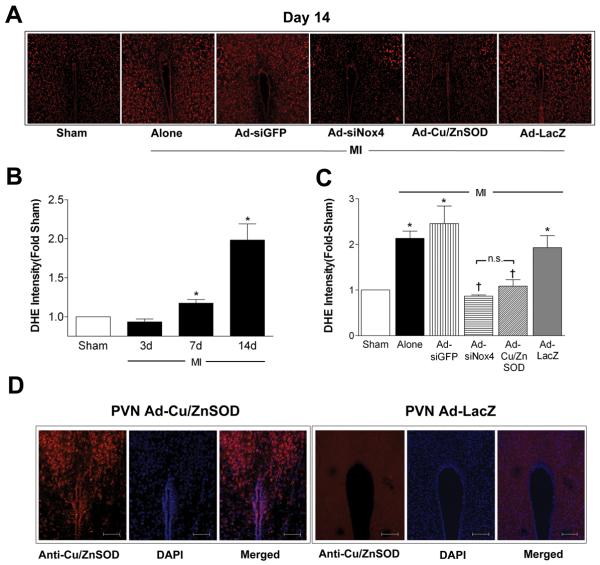
To begin to examine the functional consequences of MI-induced Nox4 upregulation in PVN, we analyzed the intracellular ROS profile in PVN using DHE fluorescence confocal microscopy at 3, 7, and 14 days after MI or sham surgery. As shown in representative photomicrographs in Figure 2A (day 14) and summary data in Figure 2B, ROS were not altered at day 3 post-MI but were significantly increased by 7 days and became even more pronounced by 2 weeks post-MI. Bilateral PVN-targeted AdsiNox4 attenuated the MI-induced increase in DHE fluorescence at 14 days to the same level as that produced by viral gene transfer of the O2•− scavenger Cu/ZnSOD (AdCu/ZnSOD) to this brain region (Fig 2A and 2C). Importantly, these effects were not due to a non-specific effect of the adenoviruses or indiscriminate activation of RNA silencing machinery since MI mice injected with the control vectors AdsiGFP or AdLacZ exhibited DHE intensities that were nearly identical to untreated MI mice (Fig 2A and 2C). We have shown previously that AdCu/ZnSOD not only causes robust and localized Cu/ZnSOD expression in CNS at the 2 week time-point, but it also results in a robust increase in SOD activity.6, 9 To confirm robust PVN-localized transgene expression here, Cu/ZnSOD immunohistochemistry was performed in AdLacZ- and Ad-CuZn/SOD-treated mice. Figure 2D shows high level Cu/ZnSOD expression in PVN of AdCu/ZnSOD- but not AdLacZ-treated mice. Collectively, these results strongly suggest that MI-mediated upregulation of Nox4 in PVN results in a marked increase in O2•− in this brain region.
Figure 2. MI causes a robust increase in Nox4-mediated ROS formation in PVN.
A) Representative images of DHE fluorescence in PVN of mice that had undergone sham or MI surgery 2 wks earlier. PVN-targeted gene transfer occurred 3 days before MI. B) Summary of DHE intensity in PVN of mice 3, 7 and 14 days post-MI (n=5 per group). Data are expressed relative to sham controls (n=5); *p<0.05 vs sham. C) Summary of DHE intensity in PVN 14 days following MI surgery (n=10) or MI combined with PVN-targeted injections of AdsiGFP (n=6), AdsiNox4 (n=10), AdCu/ZnSOD (n=10) or AdLacZ (n=9). Data are expressed relative to shams (n=10); *p<0.05 vs sham; †p<0.05 vs. MI alone, MI+AdsiGP, or MI+AdLacZ; n.s., not significant. D) Representative photomicrographs (from 4 experiments) of Cu/ZnSOD immunoreactivity (red) in the PVN 14 days following bilateral injection of AdLacZ or AdCu/ZnSOD. Sections were co-stained with DAPI (blue). Scale bar = 100μm.
AdsiRNA-mediated silencing of Nox4 in PVN improves MI-induced cardiac dysfunction
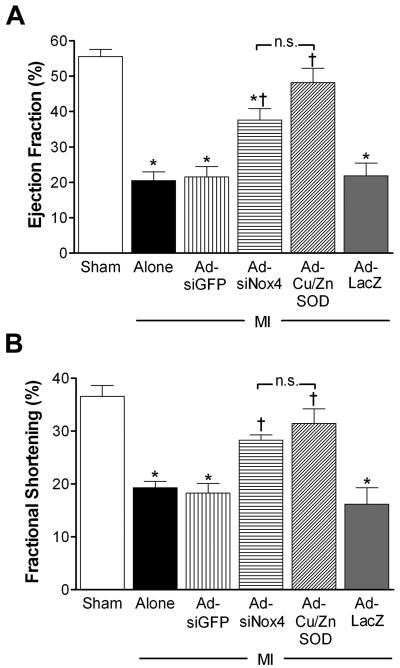
To investigate the cardiovascular consequences of MI-induced Nox4 upregulation in PVN, we measured the effects of PVN-targeted AdsiNox4 on echocardiographic and LV hemodynamic endpoints in mice 2 weeks after MI or sham surgery. High-resolution ultrasonographic analysis demonstrated normal cardiac function in sham animals as evidenced by unaltered ejection fraction (EF) and fractional shortening (FS) compared to naïve mice (EF: naïve, 59.1±2.7% vs. sham, 54.7±3.4%; FS: naïve, 37.5±2.0% vs. sham, 35.5±1.2%; p>0.05, n=11 and 15, respectively). In contrast, a marked diminution in EF (Fig 3A) and FS (Fig 3B) was observed in mice that underwent MI surgery. These findings are consistent with what we11 and others19 have reported previously in this model of MI. Importantly, AdsiNox4 microinjected into PVN led to significant improvement in both EF and FS in MI-treated animals, while the control AdsiGFP did not alter either parameter (Fig 3A and 3B).
Figure 3. AdsiRNA-mediated silencing of Nox4 in PVN improves MI-induced cardiac dysfunction as measured by echocardiography.
Summary of ejection fraction (EF, panel A) and fractional shortening (FS, panel B) calculated using high-resolution ultrasound 14 days after sham surgery (n=13), MI surgery (n=11), or MI combined with PVN-targeted AdsiGFP (n=8), AdsiNox4 (n=8), AdCuZnSOD (n=8) or AdLacZ (n=9); *p<0.05 vs. Sham; †p<0.05 vs. MI Alone, MI+AdsiGFP, or MI+AdLacZ; n.s., not significant.
Since AdsiNox4 inhibits ROS formation in PVN (see above), these data suggest that the beneficial effect of AdsiNox4 on cardiac function is due to this antioxidant action. However, to provide further evidence for this, we compared these AdsiNox4 ultrasound data to those obtained in a separate cohort of MI mice injected with AdCu/ZnSOD in the PVN. As shown in Figure 3, the positive effects of AdCu/ZnSOD on EF and FS were similar to those produced by AdsiNox4, suggesting that Nox4-mediated O2•− production in the PVN is involved in the post-MI decline in heart function. Furthermore, since AdLacZ, the control for AdCu/ZnSOD, had no effect on EF and FS in MI mice, we could exclude non-specific side-effects of adenoviral gene transfer. It is also important to note that infarct sizes, as estimated by ultrasound at 48 hrs post-surgery, were similar between all MI groups (MI alone: 27.8±2.1%; MI+AdsiGFP: 31.1±2.1%; MI+ AdsiNox4: 30.1±1.8%; MI+AdCu/ZnSOD: 26.9±1.5%; MI+AdLacZ: 28.9±2.1%; p>0.05, n=7-9 per group), confirming that the differences in cardiac function observed with AdsiNox4 and AdCu/ZnSOD were not due to different degrees of infarction at the start of the experiment.
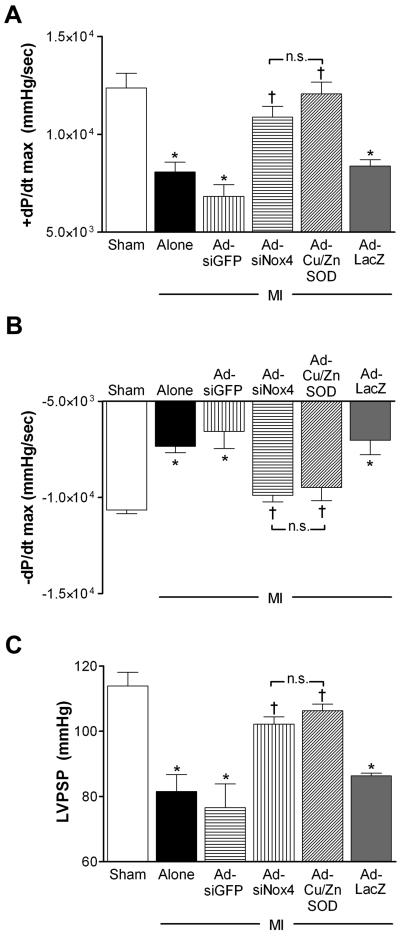
To further evaluate the effects of PVN-targeted Nox4 inhibition on cardiac function using another method, separate cohorts of mice were instrumented with cardiac catheters to measure basal LV hemodynamic parameters 14 days post-MI or sham surgery. Similar to the MI-induced cardiac dysfunction observed with high-resolution ultrasound, coronary artery ligation caused significant LV impairment as indicated by diminished LV peak contractility (+dP/dt max, Fig 4A), relaxation (−dP/dt max, Fig 4B) and systolic pressure (LVPSP, Fig 4C) compared to shams. Transduction of the PVN with either AdsiNox4 or AdCu/ZnSOD restored these parameters toward normal (sham) levels (Fig 4). Collectively, these data provide evidence that chronic cardiac dysfunction caused by MI can be significantly improved by inhibiting Nox4-generated O2•− in the PVN.
Figure 4. Silencing of Nox4 in the PVN improves basal LV hemodynamics post-MI.
Summary of peak LV contractility (+dP/dt max, panel A), relaxation (−dP/dt max, panel B), and systolic pressure (LVPSP, panel C) 14 days after mice underwent surgery for sham (n=6), MI (n=8) or MI combined with PVN microinjection of AdsiGFP (n=6), AdsiNox4 (n=8), AdCu/ZnSOD (n=6) or AdLacZ (n=7); *p<0.05 vs. Sham; †P<0.05 vs. MI Alone, MI+AdsiGFP or MI+AdLacZ; n.s., not significant.
Heart rate was not different between groups during the hemodynamic studies (Sham: 416±29; MI alone: 400±44; MI+AdsiGFP: 441±27; MI+AdsiNox4: 411±32; MI+AdCu/ZnSOD: 444±30; MI+AdLacZ: 441±35 bpm; p>0.05, n=6-9 per group) or during the echocardiography experiments (Sham: 439±18; MI alone: 460±14; MI+AdsiGFP: 485±19; MI+AdsiNox4: 464±15; MI+AdCu/ZnSOD: 442±21; MI+AdLacZ: 455±21 bpm; p>0.05, n=7-14 per group). This suggests that improvement in cardiac function after transduction of the PVN with either AdsiNox4 or AdCu/ZnSOD is not explained by a differential cardio-depressant effect of anesthesia in these experiments.
PVN-targeted Nox4 silencing attenuates MI-induced sympathoexcitation and restores LV responsiveness to β-receptor challenge
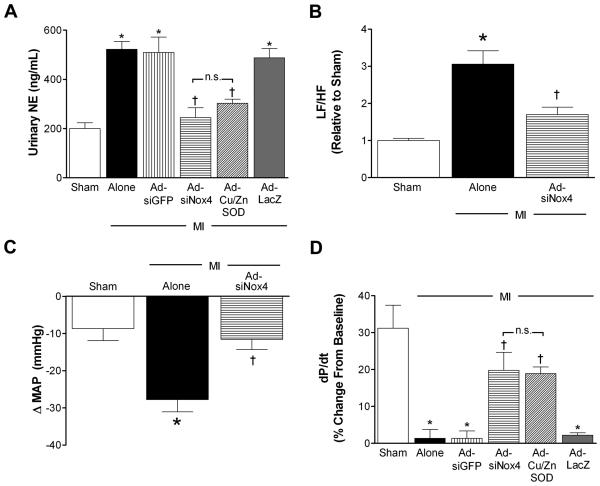
Given the evidence that PVN plays a key role in the sympathoexcitatory response in various models of HF,5, 30 we hypothesized that a mechanism by which AdsiNox4 improves cardiac performance in post-infarct mice is by modulating sympathetic outflow. First, urinary NE levels were measured 14 days after MI or sham surgery as an indirect index of sympathetic tone. Similar to observations reported by us 6, 11 and others,31 NE concentrations were not different between naïve and sham mice (naïve 168.5±15.8 vs. sham 162.8±24ng/mL, p>0.05, n=5 per group), but were significantly elevated following MI (Fig 5A). These MI-induced increases in NE were nearly normalized either by silencing Nox4 (AdsiNox4) or by scavenging O2•− (AdCu/ZnSOD) in the PVN (Fig 5A). Neither of the control vectors (AdsiGFP or AdLacZ) had any significant effects on MI-induced increases in urinary NE.
Figure 5. Nox4 knockdown in the PVN attenuates MI-induced sympathoexcitation and restores LV responsiveness to βAR challenge.
A) Summary of urinary norepinephrine (NE) concentrations 14 days after sham (n=11), MI (n=9), or MI combined with PVN-targeted AdsiGFP (n=12), AdsiNox4 (n=12), AdCu/ZnSOD (n=9), or AdLacZ (n=11). B) Power spectral analysis of arterial pressure variability 14 days after sham (n=4), MI (n=4) or MI combined with PVN-targeted AdsiGFP (n=4). Data were calculated as a percentage in relation to baseline and presented as LF/HF relative to sham animals. C) Summary of peak decreases in MAP to hexamethonium 14 days after sham (n=4), MI (n=4), or MI+AdsiNox4 (n=4). D) Summary of peak LV contractility following acute isoproterenol stimulation (expressed as percent of baseline) 14 days after sham (n=6), MI (n=5), or MI combined with PVN-targeted AdsiGFP (n=7), AdsiNox4 (n=6), AdCu/ZnSOD (n=7), or AdLacZ (n=6). *p<0.05 vs. Sham; †p<0.05 vs. MI alone, MI+Ad-siGFP, or MI+Ad-LacZ; n.s., not significant.
To further investigate the contributions of Nox4 in the PVN on sympathetic outflow following MI, we next performed power spectral analysis of arterial pressure variability as an additional index of sympathetic tone. Increased low frequency (LF)/high frequency (HF) oscillations of arterial pressure reflect increased sympathetic activity.29 By two weeks post-surgery, MI caused a three-fold increase in the LF/HF ratio compared to sham animals (Fig 5B), providing additional evidence that MI induces marked sympathoexcitation. Bilateral injection of AdsiNox4 into the PVN significantly attenuated the MI-induced increase in LF/HF, suggesting that Nox4-mediated signaling in the PVN contributes to increased sympathetic activity following MI.
A third strategy was used to evaluate the role of Nox4 signaling in the PVN on sympathetic drive. Increased depressor responses to ganglionic blockade with hexamethonium are taken to indicate enhanced contribution of the sympathetic nervous system to basal blood pressure.6 As seen in Fig 5C, mice that had undergone MI 14 days earlier exhibited augmented decreases in mean arterial pressure (MAP) to hexamethonium compared to sham animals. PVN-targeted silencing of Nox4 normalized this response, suggesting that Nox4 contributes to enhanced sympathetic control of basal blood pressure in MI-treated mice. It is important to note that baseline MAPs were not different among the groups early after sham/MI surgery (Day 3, Sham: 89±9; MI: 88±15; MI+AdsiNox4: 89±10 mmHg; p>0.05, n=4 per group) nor at 14 days post-surgery just prior to injection of hexamethonium (Sham: 88±6; MI: 88±3; MI+AdsiNox4: 84±11 mmHg; p>0.05, n=4 per group).
Persistent sympathetic stimulation has been shown to decrease myocardial catecholamine sensitivity via down-regulation of cardiac β-adrenergic receptors (βAR).32 This translates to diminished LV contractility upon acute βAR challenge. To provide a final marker of MI-induced sympathoexcitation and LV function in these studies, we examined LV contractile responses to the non-selective βAR agonist isoproterenol in the various groups. As shown in Figure 5D, isoproterenol caused a 30% increase in peak contractility (+dP/dt) in sham animals, and this response was significantly blunted in mice that had undergone MI. This is consistent with previous reports in mice.33 AdsiNox4 injected into the PVN restored the LV contractile response to βAR stimulation in MI mice, and did so to the same extent as scavenging O2•− with AdCu/ZnSOD in this brain region. It is important to note that heart rates immediately before and after isoproterenol injection were not statistically different among any of the treatment groups (data not shown), eliminating the possibility of this variable altering cardiac preload and therefore LV contractility. These results provide additional evidence that Nox4-mediated O2•− formation in the PVN contributes to sustained sympathoexcitation following MI, which leads to a loss of inotropic reserve.
Nox4 silencing in the PVN ameliorates peri-infarct apoptosis
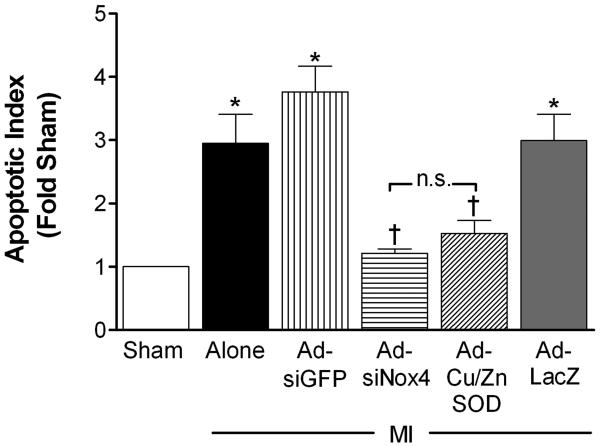
Since high sympathetic drive to the heart has been linked to cardiomyocyte apoptosis,2, 32 we next sought to determine if MI-induced upregulation of Nox4 in PVN (and concomitant sympathoexcitation) is involved in myocardial apoptosis. Two weeks following MI, there was a ~3-fold increase in apoptotic DNA fragment density in LV tissue compared to sham controls (Fig 6). This was mostly restricted to the peri-infarct border of the LV, as non-infarcted myocardium from all treatment groups exhibited very little DNA laddering (data not shown). PVN-targeted AdsiNox4 or AdCu/ZnSOD caused significant attenuation of the apoptotic response, while the control vectors had little effect. This suggests that Nox4-mediated O2•− signaling in the PVN is involved in the increased myocardial apoptosis observed after MI.
Figure 6. Silencing of Nox4 in the PVN ameliorates peri-infarct apoptosis.
Summary of densitometry analysis of 200-, 400-, and 600-basepair DNA fragments (normalized to sham, n=6) isolated from peri-infarct tissue 14 days after MI (n=6) or MI combined with PVN-targeted AdsiGFP (n=6), AdsiNox4 (n=7), AdCu/ZnSOD (n=9), or AdLacZ (n=8). *p<0.05 vs. Sham; †p<0.05 vs. MI alone, MI+Ad-siGFP, or MI+Ad-LacZ; n.s., not significant.
Hydrogen peroxide scavenging in PVN has no impact on ROS formation or cardiac performance post-infarct
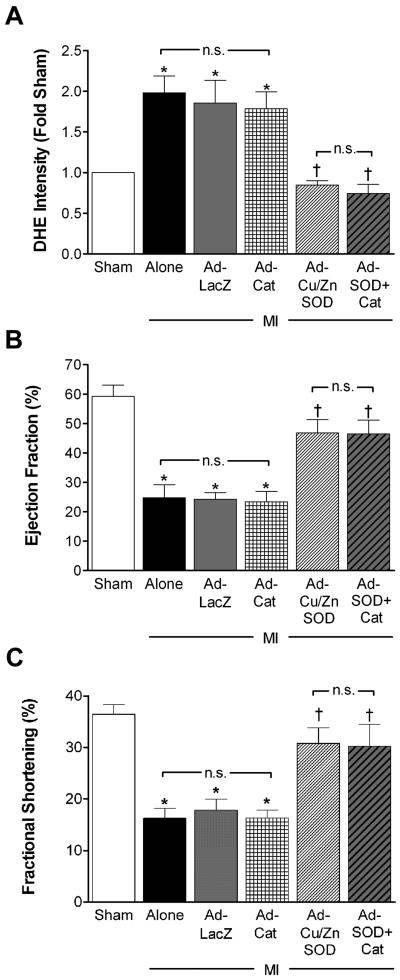
Our evidence thus far suggests that inhibition of O2•− formation in PVN by AdsiNox4 or AdCu/ZnSOD attenuates cardiac dysfunction, sympathoexcitation and cardiac apoptosis following MI. However, since H2O2 is a byproduct of Cu/ZnSOD dismutation of O2•− and Nox4 has been linked to H2O2 production,34 we considered the possibility that H2O2 in the PVN may be involved in the effects we observed. To test this, we performed bilateral PVN injections of adenoviral vectors encoding the H2O2 scavenger catalase (AdCat) alone or in combination with AdCu/ZnSOD prior to the MI procedure. As shown in Figure 6A, the MI-induced increase in DHE fluorescence intensity in PVN at 14 days was significantly attenuated by AdCu/ZnSOD but not by AdCat. Furthermore, the co-injection of AdCat with AdCu/ZnSOD had no further effect on ROS formation than AdCu/ZnSOD alone. Similarly, scavenging O2•− with AdCu/ZnSOD was the most effective intervention for improving post-MI EF (Fig 6B) and FS (Fig 6C), as AdCat had little impact on these parameters when administered either alone or in combination with AdCu/ZnSOD. These results suggest that the functional benefit observed in these studies is due to diminished O2•− signaling and not enhanced H2O2 levels in the PVN.
Discussion
It is well established that CNS-driven autonomic dysfunction participates in the post-MI decline to HF,2, 35 however the precise sites and molecules involved still have yet to be fully elucidated. Here we report that MI causes an upregulation of Nox4 expression in the PVN, which leads to increased O2•− formation in this brain region. Inhibiting this response selectively in the PVN, either by silencing Nox4 or by increasing Cu/ZnSOD levels in this region, improves post-infarct cardiac performance by ameliorating chronic sympathoexcitation and concomitant loss of LV βAR responsiveness. This was associated with reduced apoptosis in the peri-infarct zone. Importantly, PVN-targeted delivery of the H2O2 scavenger catalase, either alone or in combination with CuZn/SOD, had no effect on ROS formation in the PVN or MI-induced cardiac dysfunction, suggesting that Nox4-generated O2•− in this brain region, and not H2O2, is the likely culprit in this model of HF.
Increasing evidence supports a role for NADPH oxidases as key signaling intermediates in a variety of CNS sites and centrally-mediated cardiovascular responses. For example, we have demonstrated that dominant-negative inhibition of a Rac1-containing NADPH oxidase in the SFO abolishes the pressor, bradycardiac, and dipsogenic responses to central angiotensin II (Ang-II) administration,17 and that Nox2 and Nox4 each play distinct roles in mediating these responses.23 Recently, NADPH oxidase activation in the PVN has been shown to be required for activation of the cardiac sympathetic afferent reflex,12, 14 although the specific Nox homologues involved were not identified. Moreover, Wang and colleagues have shown that Nox2-derived ROS in the NTS are critical in mediating the effects of Ang-II on calcium signaling and neuronal activation.36
NADPH oxidase-derived ROS signaling is also strongly implicated in HF, with Nox2 emerging as a critical homologue thus far. Global knockout of Nox2 or related subunits improves cardiac function and autonomic dysfunction when HF in induced in these mice.19 A correlation between upregulation of Nox2 in RVLM and rising sympathetic outflow in a rabbit model of HF has been reported,10 and this is thought to contribute to enhanced carotid body chemoreceptor sensitivity observed in these animals.37 In other studies by this group, chronic central delivery of Ang-II has been shown to increase expression of Nox2 and related subunits in the RVLM, which is associated with an increase in O2•− production and enhanced renal sympathetic activity.38 Since Ang-II levels are increased in the CNS during HF,39, 40 the implication is that Nox2 activation in RVLM is important in the sympathoexcitation that accompanies this disease.
To the best of our knowledge, this is the first report that Nox4 activation in the CNS is also critical in the post-MI deterioration in cardiac function. Our studies focused on the PVN, which like the RVLM, is strongly implicated in the autonomic dysfunction associated with HF.5, 6 We showed that although Nox1 and Nox2 were detectable in the PVN, they were expressed at low levels and were not altered following MI. Nox4, on the other hand, was the most abundant homologue in the PVN under basal levels, and its expression was increased two-fold after ligation of the LAD coronary artery. This is consistent with evidence that Nox4 may be regulated at the transcriptional level18, 41 since it does not require assembly of cytosolic subunits to confer enzymatic activity.41
There are conflicting reports regarding the oxidant species - O2•− or H2O2- produced by Nox4 in different cells and tissues. For example, Nox4 produces mainly H2O2 in rat aortic smooth muscle cells,34 whereas Nox4-dependent O2•− generation has been reported in intracellular compartments of human embryonic kidney cells.41 Recent evidence demonstrates that siRNA-mediated knockdown of Nox4 inhibits both O2•− and H2O2 production in kidney glomerular mesangial cells,42 suggesting that the active enzyme is capable of producing multiple oxidant species. In our studies, injection of either AdsiNox4 or the O2•− scavenger AdCu/ZnSOD normalized DHE fluorescence in the PVN of MI-treated mice, suggesting that intracellular O2•− is the oxidant species produced by Nox4. Moreover, our findings that H2O2 scavenging in the PVN with AdCat had no effect on DHE staining or cardiac function, either alone or in combination with AdCu/ZnSOD, further supports a role for O2•− as the Nox4-generated ROS in this model. However, depending on the level of H2O2 and its subcellular localization, catalase may not be the most appropriate scavenger and therefore we cannot entirely rule out a role for H2O2. Studies are ongoing in our laboratory using adenoviruses encoding other H2O2 scavengers, including glutathione peroxidase-1 (GPx-1).
Progressive deterioration of cardiac function following MI is the result of multiple events, including ventricular hypertrophy, cardiac arrhythmias, and increased cardiac apoptosis.32, 43 Our findings of a significant increase in peri-infarct apoptosis accompanying the LV dysfunction after MI are consistent with reports that LV apoptosis correlates with declining cardiac function in humans with HF.19, 44 Furthermore, our data showing that PVN-targeted Nox4 silencing or O2•− scavenging reduced LV apoptosis and was associated with marked improvement in cardiac function are in line with studies showing a correlation between reduced cardiomyocyte apoptosis after MI and improved LV function in other animal models of HF.45 Since excessive NE stimulation induces cell death in cardiomyocytes through activation of protein kinase A and excessive calcium influx via voltage-dependent calcium channels,43, 46 and apoptosis can be attenuated by blockade of cardiac β-adrenergic receptors,47 we speculate that the sympathoinhibitory effects of Nox4 silencing in the PVN of post-infarct mice may help explain the reduction in peri-infarct apoptosis in this study. However, further studies will be required to fully understand how Nox4 silencing in the PVN impacts key elements of the cardiac apoptotic cascade.
In addition to increased sympathetic outflow, we also observed decreased LV responsiveness to βAR stimulation with isoproterenol in post-infarct mice. These results are consistent with human studies in which cardiac βAR are downregulated in response to elevated catecholamine concentrations.43, 48 This is thought to be protective, since chronic catecholaminergic stimulation increases cardiac hypertrophy and cardiomyocyte apoptosis which lead to further cardiac dysfunction.43 The success of clinical β-blocker therapy in HF patients is largely due to competition for available β-receptors in the surviving myocardium, which not only reduces the detrimental effects of chronic catecholamine stimulation but also inhibits further downregulation of these receptors as levels of NE remain elevated.32 In our model, Nox4 silencing or O2•− scavenging in the PVN resulted in improved contractile reserve, which we speculate was due to attenuation of CNS-driven sympathetic outflow to the heart and preservation of the chronotropic and inotropic effects of NE. Although clinical trials (MOXCON) using pharmacological inhibition of central sympathetic outflow have not had favorable outcomes in HF patients, it should be noted that the drug used (moxonadine) caused global inhibition of CNS sympathetic activity, which may have precluded extra-cardiac autonomic functions that are necessary to preserve post-MI homeostasis. It is possible that targeted disruption of selective central regulatory circuits such as the PVN, the neurons of which project to T1-T3 and T9-T11 segments of the spinal cord, which in turn give rise to sympathetic efferents projecting to the heart and kidney, respectively,5 may provide a more specific approach to attenuating post-MI autonomic dysfunction.
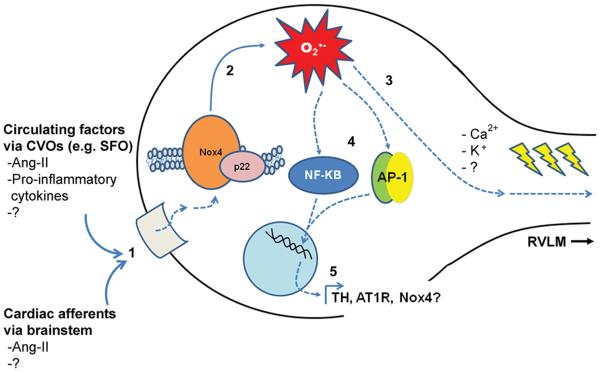
Questions that remain unanswered by our studies are what brain region(s) and molecular signaling event(s) are upstream of Nox4 activation in the PVN following MI, and what are the downstream effectors that lead to altered sympathetic outflow (see Figure 7). Myocardial ischemia, cardiac cell death and declining cardiac output collectively evoke the release of numerous vaso- and neuro-active substances from multiple cells and tissues, including Ang-II, vasopressin, catecholamines, and pro-inflammatory cytokines, all of which help to sustain tissue perfusion and cardiac output.16, 39, 49 These peptides act locally in peripheral tissues, but some of them can further influence cardiovascular regulation by interfacing with regions of the brain that lack a blood-brain-barrier (circumventricular organs, CVOs), including the SFO and organum vasculosum of the lamina terminalis.39, 49 These centers have robust projections to the PVN and, therefore, serve as circuits through which circulating signals can evoke CNS-driven responses.35, 49, 50 Indeed, we have previously shown that MI-induced redox-signaling events in the SFO result in chronic activation of PVN neurons.6 In addition to actions at CVOs, peripheral circulating and neural signals can evoke Ang-II and cytokine production in CNS sites inside the blood-brain-barrier,51 including the PVN,39 which also leads to modulation of autonomic outflow in HF.38, 49 Given that it is now well established that the actions of Ang-II in the CNS – both inside and outside the CVOs – require redox signaling,7, 16 and that Ang-II is known to activate Nox4,34 we speculate that Ang-II may be a critical upstream stimulus of MI-induced Nox4 activity in the PVN. Whether this would occur through circulating Ang-II interfacing with the SFO,6, 11, 49 causing subsequent activation of angiotensinergic projections from SFO to PVN,49, 52 or stimulation of local Ang-II production within the PVN itself will require further investigation. In addition, since intrinsic reflexes such as the cardiac sympathetic afferent reflex involve neural signaling from the NTS to the PVN through Ang-II-mediated ROS formation in PVN neurons,13 and given that the cardiac sympathetic afferent reflex is enhanced during HF15 and involves NADPH oxidase activation,14 it is also possible that augmented Ang-II signaling via this pathway contributes to the increased Nox4 activity we report.
Figure 7. Hydrogen peroxide scavenging in PVN has no impact on ROS formation or cardiac performance post-infarct.
A) Summary of DHE fluorescence in PVN 14 days following MI (n=10) or MI combined with AdLacZ (n=5), Ad-Cat (AdCat, n=5), AdCu/ZnSOD (n=6), or AdCu/ZnSOD+AdCat (n=5). Data are normalized to sham animals (n=5). Summary of ejection fraction (panel B) and fractional shortening (panel C) calculated using high-resolution ultrasound 14 days after sham (n=12), MI (n=11), or MI in combination with PVN-targeted injection of AdLacZ (n=11), AdCat (n=6), AdCu/ZnSOD, n=6), or AdCu/ZnSOD+Ad-Cat (n=5). *p<0.05 vs sham; †p<0.05 vs. MI alone, MI+AdLacZ, or MI+AdCat; n.s., not significant.
The downstream effectors that are activated by Nox4/ROS in the PVN and lead to long-term changes in sympathetic activity are also up for speculation (see Figure 7). It is now well established that ROS can modulate neuronal signaling through alterations in Ca2+ 36, 53 or K+ channels,54 so it is possible that the MI-mediated Nox4/ROS induction leads to such changes in RVLM-projecting PVN neurons. In addition, since there is compelling evidence that activation of transcription factors (TFs) such as activator protein-1 (AP-1) and nuclear factor κ-B (NFκB) in CNS nuclei contribute to cardiovascular disease,55, 56 and these transcription factors are redox-sensitive,57 we are currently using in vivo bioluminescence58 to test the hypothesis that Nox4-mediated activation of one or both of these TFs in PVN is causally linked to MI-induced sympathoexcitation and cardiac decline. It is interesting to note that Zucker and colleagues have recently demonstrated increased AP-1 binding in RVLM of rabbits with HF,51 and activation of brain NFκB in PVN has been observed in response to Ang-II infusion.56
Finally, while our studies implicate Nox4-mediated redox signaling in the PVN in the autonomic dysfunction that occurs after MI, we did not investigate the effect of ROS scavenging in this site on the synthesis and secretion of vasopressin. The PVN is comprised of two distinct sets of neurons which serve diverse roles in cardiovascular regulation: parvocellular neurons that regulate autonomic outflow via efferent neural projections to the RVLM and spinal cord and magnocellular neurons that influence cardiovascular and volume homeostasis by production and secretion of vasopressin.59 Elevated plasma levels of vasopressin have been reported after MI,39 however to date a link between redox signaling in the PVN and increased vasopressin production or release has not been investigated. Since it is likely that our method of gene transfer results in transduction of both PVN neuronal subtypes,60 treatment with AdCu/ZnSOD or AdsiNox4 could alter the redox state of magnocellular neurons, which could, in turn, influence vasopressin levels. Further investigation into the effects of PVN-targeted Nox4/ROS inhibition on plasma vasopressin levels after MI will be necessary to address this possibility.
In conclusion, we report that Nox4-mediated oxidative stress in the PVN plays a causal role in the cardiac dysfunction that occurs following MI. Our results demonstrate that targeted O2•− scavenging in PVN, either enzymatically with AdCu/ZnSOD or by selective knockdown of Nox4 with AdsiNox4, attenuates CNS-driven sympathetic outflow, which is associated with restoration of βAR responsiveness in the surviving myocardium and reduced cardiac apoptosis. We speculate that these beneficial effects translate into the improved post-infarct cardiac performance observed in AdsiNox4 or AdCu/ZnSOD-treated mice. These findings suggest that targeted inhibition of redox signaling in the PVN could provide a novel treatment for the post-MI decline in cardiac function.
Novelty and Significance.
What is known?
Over-activation of the sympathetic nervous system and declining cardiac function are hallmarks of myocardial infarction (MI)-induced heart failure.
The paraventricular nucleus (PVN) of the hypothalamus in the brain is involved in sympathoexcitation during heart failure.
Oxidative stress in the PVN and other brain sites may be involved in the pathogenesis of certain cardiovascular diseases.
What new information does this article contribute?
There are direct causal links between oxidative stress in the PVN, sympathetic overactivity and declining cardiac function after myocardial infarction -(MI).
Nox4-containing NADPH oxidase is the primary source of free radicals in the PVN after MI.
Selective silencing of Nox4 in the PVN improves MI-induced cardiac dysfunction by diminishing sympathoexcitation and apoptosis in the heart.
Morbidity and mortality associated with acute MI and heart are linked to unchecked neurohumoral excitation that eventually fuels a downward spiral of cardiovascular deterioration. Several discrete regions of the CNS have emerged as primary culprits in driving this neural dysfunction, although the underlying molecular mechanisms remain poorly understood. Here, we report, for the first time, that the Nox4 homologue of NADPH oxidase in the hypothalamus is a key molecule underlying MI-induced neural excitation and cardiac decline. Although NADPH oxidase has been implicated in MI-induced autonomic and cardiac dysfunction, the studies have relied upon non-specific inhibitors and/or global null mutations in mice. Our experiments are unique in that they combine brain site-selective gene transfer of Nox4-specific siRNA with sophisticated in vivo cardiovascular assessment in mice. This strategy allowed us to link the MI-induced upregulation of Nox4 in the hypothalamus with declining cardiac function and other cardiovascular parameters in vivo. Further investigation into anti-oxidant therapies targeted to the hypothalamus may provide a novel strategy for the treatment of MI-induced heart failure.
Supplementary Material
Figure 8. Schematic depicting possible Nox4-mediated signaling mechanisms in PVN following MI.
1) PVN neurons are activated either by way of upstream circumventricular organs (e.g. SFO) and the circulating factors that interface with them post-MI, or through stimulation of cardiac afferent pathways which utilize Ang-II and perhaps other signaling factors. 2) Stimulation of PVN neurons leads to increased expression and activation of Nox4, causing elevated O2•− in this nucleus. 3) This could lead to changes in firing of RVLM-projecting PVN neurons through modulation of Ca2+, K+ or other currents, and/or 4) activation of redox-sensitive transcription factors such as AP-1 or NFκB. 5) Increased AP-1 or NFκB-mediated transcription of many possible targets in PVN, including tyrosine hydroxylase (TH), the Ang-II receptor AT1R or even Nox4 itself could lead to neuronal changes that sustain increased sympathetic output through projections to the RVLM. PVN (paraventricular nucleus); RVLM (rostral ventral lateral medulla); SFO (subfornical organ). Solid blue lines indicate established findings, whereas dotted lines depict pathways still under investigation.
Acknowledgements
We wish to thank Dr. Valdir Braga for assistance with the brain microinjections, Dr. Mark Rishniw for help with the ultrasound analysis, Dr. David Lin for advice in carrying out the in situ hybridization experiments, and Dr. Allyn Mark for helpful discussions.
Sources of Funding
The work described herein was funded by grants to RLD from the NIH (HL63887 and HL84624) and the American Heart Association (0540114N). DWI was supported by an American Heart Association Founders Affiliate Predoctoral Research Grant (0815714D).
Non-standard Abbreviations and Acronyms
- Ad
Adenovirus
- Ang
Angiotensin
- AP-1
Activator protein-1
- Cat
Catalase
- CNS
Central nervous system
- CVO
Circumventricular organ
- DHE
Dihydroethidium
- EF
Ejection fraction
- NE
Norepinephrine
- FS
Fractional shortening
- GPx-1
Glutathione peroxidase 1
- H2O2
Hydrogen peroxide
- HF
Heart failure
- LAD
Left anterior descending artery
- LV
Left ventricle
- LVPSP
Left ventricular peak systolic pressure
- MAP
Mean arterial pressure
- MI
Myocardial infarction
- NADPH Nicotinamide
adenine dinucleotide phosphate
- NFκB
Nuclear factor kappa-B
- Nox
NADPH oxidase
- NTS
Nucleus tractus solitarius
- O.C.T.
Optimal cutting temperature®
- O2•−
Superoxide
- PVN
Paraventricular nucleus
- ROS
Reactive oxygen species
- RVLM
Rostral ventral lateral medulla
- SFO
Subfornical organ
- SOD
Superoxide dismutase
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2009 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Watson AM, Hood SG, May CN. Mechanisms of sympathetic activation in heart failure. Clin Exp Pharmacol Physiol. 2006;33:1269–1274. doi: 10.1111/j.1440-1681.2006.04523.x. [DOI] [PubMed] [Google Scholar]
- 3.Kenney MJ, Weiss ML, Haywood JR. The paraventricular nucleus: An important component of the central neurocircuitry regulating sympathetic nerve outflow. Acta Physiol Scand. 2003;177:7–15. doi: 10.1046/j.1365-201X.2003.01042.x. [DOI] [PubMed] [Google Scholar]
- 4.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 5.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- 6.Lindley TE, Doobay MF, Sharma RV, Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res. 2004;94:402–409. doi: 10.1161/01.RES.0000112964.40701.93. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: Distribution, regulation and function. Antioxid Redox Signal. 2006 doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: Roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 11.Lindley TE, Infanger DW, Rishniw M, Zhou Y, Doobay MF, Sharma RV, Davisson RL. Scavenging superoxide selectively in mouse forebrain is associated with improved cardiac function and survival following myocardial infarction. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1–8. doi: 10.1152/ajpregu.00078.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong MK, Duan YC, Chen AD, Xu B, Gao XY, De W, Zhu GQ. Paraventricular nucleus is involved in the central pathway of cardiac sympathetic afferent reflex in rats. Exp Physiol. 2008;93:746–753. doi: 10.1113/expphysiol.2007.041632. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Zhang Y, Wang HJ, Gao XY, Wang W, Zhu GQ. Reactive oxygen species in paraventricular nucleus modulates cardiac sympathetic afferent reflex in rats. Brain Res. 2005;1058:82–90. doi: 10.1016/j.brainres.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Yu Y, Zhang F, Zhong MK, Shi Z, Gao XY, Wang W, Zhu GQ. NAD(P)H oxidase in paraventricular nucleus contributes to the effect of angiotensin II on cardiac sympathetic afferent reflex. Brain Res. 2006;1082:132–141. doi: 10.1016/j.brainres.2006.01.113. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Schultz HD, Patel KP, Zucker IH, Wang W. Augmented input from cardiac sympathetic afferents inhibits baroreflex in rats with heart failure. Hypertension. 2005;45:1173–1181. doi: 10.1161/01.HYP.0000168056.66981.c2. [DOI] [PubMed] [Google Scholar]
- 16.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–539. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- 18.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 19.Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 20.Zwacka RM, Dudus L, Epperly MW, Greenberger JS, Engelhardt JF. Redox gene therapy protects human IB-3 lung epithelial cells against ionizing radiation-induced apoptosis. Hum Gene Ther. 1998;9:1381–1386. doi: 10.1089/hum.1998.9.9-1381. [DOI] [PubMed] [Google Scholar]
- 21.Lam EW, Zwacka R, Engelhardt JF, Davidson BL, Domann FE, Jr, Yan T, Oberley LW. Adenovirus-mediated manganese superoxide dismutase gene transfer to hamster cheek pouch carcinoma cells. Cancer Res. 1997;57:5550–5556. [PubMed] [Google Scholar]
- 22.Davidson BL, Allen ED, Kozarsky KF, Wilson JM, Roessler BJ. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- 23.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, Sharma RV, Davisson RL. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH oxidase homologues in the vasopressor and dipsogenic effects of brain angiotensin II. Hypertension. 2009;54:1106–1114. doi: 10.1161/HYPERTENSIONAHA.109.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salto-Tellez M, Yung Lim S, El-Oakley RM, Tang TP, ALmsherqi ZA, Lim SK. Myocardial infarction in the C57BL/6J mouse: A quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 2004;13:91–97. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 25.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez S, Sickles HM, Deleonardis C, Alcaraz A, Gridley T, Lin DM. Notch2 is required for maintaining sustentacular cell function in the adult mouse main olfactory epithelium. Dev Biol. 2008;314:40–58. doi: 10.1016/j.ydbio.2007.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: A physiological genomics tool. Physiol Genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- 28.Bissonnette JM, Knopp SJ, Maylie J, Thong T. Autonomic cardiovascular control in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Auton Neurosci. 2007;136:82–89. doi: 10.1016/j.autneu.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baudrie V, Laude D, Elghozi JL. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R904–12. doi: 10.1152/ajpregu.00488.2006. [DOI] [PubMed] [Google Scholar]
- 30.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-alpha blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol. 2007;293:H599–609. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 31.Ito K, Kimura Y, Hirooka Y, Sagara Y, Sunagawa K. Activation of rho-kinase in the brainstem enhances sympathetic drive in mice with heart failure. Auton Neurosci. 2008;142:77–81. doi: 10.1016/j.autneu.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Eschenhagen T. Beta-adrenergic signaling in heart failure-adapt or die. Nat Med. 2008;14:485–487. doi: 10.1038/nm0508-485. [DOI] [PubMed] [Google Scholar]
- 33.Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. Control of myocardial contractile function by the level of beta-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem. 1998;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 34.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leenen FH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res. 2007;101:221–223. doi: 10.1161/CIRCRESAHA.107.158261. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–489. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- 37.Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res. 2007;75:546–554. doi: 10.1016/j.cardiores.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao L, Wang W, Li Y, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 39.Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: New perspectives. Am J Physiol Regul Integr Comp Physiol. 2003;284:R259–R276. doi: 10.1152/ajpregu.00317.2002. [DOI] [PubMed] [Google Scholar]
- 40.Zhu GQ, Gao L, Li Y, Patel KP, Zucker IH, Wang W. AT1 receptor mRNA antisense normalizes enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1828–35. doi: 10.1152/ajpheart.01245.2003. [DOI] [PubMed] [Google Scholar]
- 41.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev. 2008 doi: 10.1007/s10741-008-9132-8. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci. 2007;334:197–205. doi: 10.1097/MAJ.0b013e318157388f. [DOI] [PubMed] [Google Scholar]
- 45.von Harsdorf R. “Fas-ten” your seat belt: Anti-apoptotic treatment in heart failure takes off. Circ Res. 2004;95:554–556. doi: 10.1161/01.RES.0000143717.70275.8f. [DOI] [PubMed] [Google Scholar]
- 46.Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat cardiomyocytes through a reactive oxygen species-TNF alpha-caspase signaling pathway. Cardiovasc Res. 2004;62:558–567. doi: 10.1016/j.cardiores.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 47.Sabbah HN, Sharov VG, Gupta RC, Todor A, Singh V, Goldstein S. Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis in dogs with heart failure. J Am Coll Cardiol. 2000;36:1698–1705. doi: 10.1016/s0735-1097(00)00913-x. [DOI] [PubMed] [Google Scholar]
- 48.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 49.Huang BS, Leenen FH. The brain renin-angiotensin-aldosterone system: A major mechanism for sympathetic hyperactivity and left ventricular remodeling and dysfunction after myocardial infarction. Curr Heart Fail Rep. 2009;6:81–88. doi: 10.1007/s11897-009-0013-9. [DOI] [PubMed] [Google Scholar]
- 50.Weiss ML, Kenney MJ, Musch TI, Patel KP. Modifications to central neural circuitry during heart failure. [review] [92 refs] Acta Physiol Scand. 2003;177:57–67. doi: 10.1046/j.1365-201X.2003.01047.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu D, Gao L, Roy SK, Cornish KG, Zucker IH. Neuronal angiotensin II type 1 receptor upregulation in heart failure: Activation of activator protein 1 and jun N-terminal kinase. Circ Res. 2006;99:1004–1011. doi: 10.1161/01.RES.0000247066.19878.93. [DOI] [PubMed] [Google Scholar]
- 52.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: Functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- 53.Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]
- 54.Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96:659–666. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- 55.Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD, Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–910. doi: 10.1161/HYPERTENSIONAHA.107.095216. [DOI] [PubMed] [Google Scholar]
- 56.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zwacka RM, Zhou W, Zhang Y, Darby CJ, Dudus L, Halldorson J, Oberley L, Engelhardt JF. Redox gene therapy for ischemia/reperfusion injury of the liver reduces AP1 and NF-kappaB activation. Nat Med. 1998;4:698–704. doi: 10.1038/nm0698-698. [DOI] [PubMed] [Google Scholar]
- 58.Peterson JR, Infanger DW, Braga VA, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Longitudinal noninvasive monitoring of transcription factor activation in cardiovascular regulatory nuclei using bioluminescence imaging. Physiol Genomics. 2008;33:292–299. doi: 10.1152/physiolgenomics.00296.2007. [DOI] [PubMed] [Google Scholar]
- 59.Kozniewska E, Romaniuk K. Vasopressin in vascular regulation and water homeostasis in the brain. J Physiol Pharmacol. 2008;59(Suppl 8):109–116. [PubMed] [Google Scholar]
- 60.Sinnayah P, Lindley TE, Staber PD, Davidson BL, Cassell MD, Davisson RL. Targeted viral delivery of cre recombinase induces conditional gene deletion in cardiovascular circuits of the mouse brain. Physiol Genomics. 2004;18:25–32. doi: 10.1152/physiolgenomics.00048.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.