Abstract
The fluorophilicity of a series of hydrocarbon and fluorocarbon-functionalized nicotinic acid esters (nicotinates) is measured from their partitioning behavior (log KP) in the biphasic solvent system of perfluoro(methylcyclohexane) (PFMC) and toluene. The chain length of the hydrocarbon or fluorocarbon alkyl group of the ester ranges from one to twelve carbon atoms. Knowledge of the fluorophilicity of these solutes is relevant to the design of these prodrugs for fluorocarbon-based drug delivery. The experimental log Kp values range from −1.72 to −3.40 for the hydrocarbon nicotinates and −1.64 to 0.13 for the fluorinated nicotinates, where only the prodrug with the longest fluorinated chain (2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-pentadecafluorooctyl nicotinic acid ester) partitions preferentially into the fluorinated phase (log Kp = 0.13). Predictions of the partition coefficients using solubility parameters calculated from group contribution techniques or molecular dynamics simulation are in reasonable agreement for the perhydrocarbon nicotinates and short chained perfluorinated nicotinates (≈ 0.3%-39% deviation). Significant deviations from experimental partition coefficients (greater than 100%) are observed for the longest chain perfluoroalkyl nicotinates.
Keywords: Fluorophilicity, fluorinated solutes, nicotinates, phase behavior, prodrug
1.0 Introduction
Fluorocarbons and perfluorinated moieties have demonstrated potential for a variety of novel industrial [1, 2] and pharmaceutical applications, including liquid ventilation therapy, diagnostic ultrasound imaging and as blood contrast agents [3-8]. The potential to extend fluorinated solvent applications to drug delivery is limited by the poor solubility of typical hydrocarbon-based polar and non-polar pharmaceuticals in these solvents [9, 10]. The use of a prodrug, in which cleavable functional groups facilitate solubility of the drug in the fluorocarbon solvent, is a viable approach for the delivery of pharmaceuticals [6, 11]. The prodrug or functionalized drug molecule is clinically inactive and conversion to the parent drug compound, typically through enzymatic cleavage, is necessary to induce the desired pharmacological effects [6]. The fluorocarbon solvent system must provide sufficient solubility of the prodrug, while promoting its subsequent extraction from solution and delivery to the tissues. In addition, the prodrug/fluorocarbon solvent systems are selected to minimize the biological toxicity and maximize the prodrug efficacy. Previously, our group studied the viability of nicotinic acid ester (nicotinate) prodrugs functionalized with hydrocarbon and fluorinated side groups to facilitate delivery in a fluorocarbon medium (perfluorooctyl bromide; PFOB) for administration through the pulmonary route [11]. In passive diffusion to the target lung cells, the nicotinate prodrugs are expected to encounter immiscible fluorocarbon, organic and aqueous solvent layers, making biphasic partition studies particularly relevant to the study of prodrug uptake through a cellular matrix. Knowledge of partition coefficients provides a thermodynamic interpretation of drug delivery and cytotoxicity. Limited studies address the partitioning behavior of homologous series of fluorinated solutes and provide direct comparisons to their hydrocarbon analogues. The ability to predict partitioning behavior has the potential to improve drug design for delivery by fluorinated solvents.
This work examines the partitioning behavior in perfluoro(methylcyclohexane); PFMC)) – toluene of a series of hydrocarbon and fluorocarbon-functionalized nicotinic acid esters (Scheme 1). The PFMC-toluene partition coefficients are widely adopted benchmark of fluorophilicity in partitioning studies [12, 13]. The nicotinates are classified according to the alkyl chain of the ester as perhydrocarbon (C1F0 – C12F0); perfluorinated, each linked by one methylene chain to the carboxyl group (C2F3 – C8F15); and partially fluorinated, with either a terminal hydrogen atom (C3F4 and C5F8) or two methylene group (C8F13) linkages in the fluorinated chain of the ester. Nicotinic acid, the parent drug compound of the nicotinic acid esters (nicotinates), has clinical benefit in the treatment of cancer. It is a precursor of cofactors, nicotinamide adenine dinucleotide (NAD) and NADP, that could alleviate injury to lungs caused by poisons and natural toxic compounds [6, 14]. The PFMC-toluene partition coefficient (log KP) is an established measure of fluorophilicity [12, 13, 15-17] and is calculated from the concentration ratio of prodrug in fluorocarbon phase (PFMC) to hydrocarbon phase (toluene). Similar to the octanol/water partition coefficient for the measurement of lipophilicity and the interpretation of drug pharmacokinetics [18, 19], fluorocarbon/hydrocarbon partition coefficients have the potential to be a predictive tool to describe the ability to deliver drugs from a fluorinated solvent to target cells. This study compares the experimental partition values of prodrugs in PFMC-toluene with liquid-liquid partitioning predicted from regular solution theory (RST). Two methods of solubility parameter estimations are compared: Fedors Group Contribution [20, 21] and molecular dynamic simulations using (Materials Studio (Accelrys Inc. (California), Version 4.0). Predictive methods provide a screening tool to assess the effect of functional group chain length and structure on partitioning behavior, allowing the design of prodrug candidates for fluorocarbon drug delivery.
Scheme 1.
2.0 Experimental
2.1 Materials
Perfluoro(methylcyclohexane) (PFMC, (CF3C6C11) ≥ 95%) was purchased from Sigma Aldrich and toluene, ((CH3C6H5) of ≈100% purity) was purchased from Mallinckrodt Baker Inc. (Paris, Kentucky). Synthesis of the nicotinate acid ester prodrugs (nicotinates) was described previously [6, 22] and involves the addition of anhydrous dicyclohexylcarbodiimide (DCC) and dimethylaminopyridine (DMAP) to a mixture of nicotinic acid and the corresponding alcohol in anhydrous dichloromethane. The nicotinic acid esters or nicotinate prodrugs were synthesized with greater than 98% purity as determined by GC/MS analysis.
2.2 Apparatus and Procedure
The partition coefficients for the nicotinate prodrugs in perfluoro(methylcyclohexane)-toluene system were determined from the depletion of the prodrug in the fluorocarbon phase into a known volume of toluene (as measured by FID gas chromatography (Varian CP-3800 FID)). The nicotinic acid esters were initially dissolved in 3 ml or 5.5 ml volumes of fluorinated solvent (PFMC), resulting in a known prodrug concentration in the range of 1 mM to 4 mM. Volume ratios of 1:1 and 5:1 in PFMC-toluene systems were used to achieve measurable equilibrium concentration differences in the fluorocarbon phase after contacting with the hydrocarbon phase. The stir flask method [19] was employed, where only the denser fluorocarbon phase was gently stirred to facilitate equilibration, with a goal of avoiding emulsion formation. All experiments were performed at 25 °C. More than one hour was allowed for equilibration before the fluorocarbon phase samples were drawn with a Hamilton syringe into vials lined with PTFE septums.
Duplicate initial and equilibrium samples (0.5 ml) of the fluorocarbon phase were injected onto a 15 m capillary column of 95% dimethylpolysiloxane stationary phase (Varian fused silica column/CP-SIL8 CB) with internal diameter of 0.25 mm. A high purity helium flow of 2 ml/min and FID temperature of 300°C were employed in the analysis. All the samples were spiked with 5 μl of custom internal standard (naphthalene-d8) in methylene chloride. The equilibrium nicotinate concentration in the fluorocarbon phase (i.e. perfluoromethylcyclohexane) was determined by GC from a calibration curve; the equilibrium concentration of the nicotinate in the corresponding organic phase concentration was determined by material balance using the known amount of nicotinate and the volumes of the immiscible phases. The partition coefficient, log Kp was then calculated as given in Eq. 1 [12, 13, 15-17].
| (Eq. 1) |
2.3 Prediction of Prodrug Partitioning in Liquid-Liquid Systems
The following expression describes the activity coefficient of the prodrug solute in solution using regular solution theory (RST) [13, 23]:
| (Eq. 2) |
where γi is the activity coefficient of the solute i in solvent j (PFMC-rich phase (a) or toluene-rich phase (b)), δ is the Hildebrand solubility parameter, Vi is the molar volume of the solute, and T is the PFMC-toluene biphasic system temperature, maintained at 298 K. Equating the activity of the solute in each phase and applying the RST expression for the activity coefficient of the solute results in the following expression:
| (Eq. 3) |
The solubility parameter (δ) or cohesive energy density (c, where δ = c1/2) defines the cohesive forces between solute and solvent molecules in solution (e.g., fluorous solute molecules in either PFMC or toluene) [21]. In the partitioning of solutes between two immiscible liquid phases, preference will be given to the phase with comparable cohesive forces to the solute. For systems of regular solutions, maximum solubility is achieved when the solute and solvent solubility parameters are similar.
Regular solution theory strictly applies to molecules that interact via only dispersion forces [21, 23]. However, the application of RST has been extended to numerous systems, such as partially fluorinated organic compounds [13]. In the case of the solvents in this investigation (PFMC (b) and toluene (a)), the molecules are nonpolar with no hydrogen bonding capacity, fulfilling some of the criteria of regular solution theory.
3.0 Calculational
3.1 Group Contribution Methods for the Estimation of the Nicotinate Solubility Parameters
Group contribution methods have been successfully applied to the partitioning of a broad range of fluorous compounds including those which bear structural similarities with the nicotinic acid esters [12, 13]. The Hildebrand solubility parameter and molar volume of the solutes were estimated by Fedors group incremental method [21]. The Hildebrand solubility parameters were calculated by summing the contributions of distinct molecular units to the molar vaporization energies and molar volumes [21]. The actual sub units applied in the calculations are listed in Table 1 of the supplementary information.
3.2 Molecular Dynamics Simulations for the Estimation of the Nicotinate Solubility Parameters
The solubility parameters of the nicotinic acid esters were predicted using molecular dynamics simulations (Materials Studio (Accelrys Inc. (California), Version 4.0). Representative 3D models of the solutes were constructed in the Amorphous Cell module using the software’s standard protocol. Cubic unit cells (21 – 30 Å) of the pure nicotinates were built in the amorphous state, under periodic boundary conditions [24]. The densities of the nicotinates were calculated by dividing the molecular weight by the molar volume, with the latter estimated from the Group Contribution method discussed in the previous section. Before performing the molecular dynamics simulations to calculate the Hildebrand solubility parameters or cohesive energy density, δ, the cells were subjected to energy minimizations or geometry optimization steps to reduce the structural conformation from the initial high energy state to a state more representative of experimental conditions. The Smart Minimizer, which combines the three iterative procedures, Steepest descent, Conjugate gradient and Newton methods, with a medium convergence level (0.1 kcal/mol/ Å) was applied. The Steepest descent method is most applicable to systems high above thermodynamic equilibrium, that is structures far from their optimum potential energy surface and the conjugate gradient procedure for those close to equilibrium [25, 26]. The Smart Minimizer selects the appropriate energy minimization method depending on the initial configuration of the structure drawn in the amorphous cell.
Molecular dynamics simulations were performed on the Discover program using the COMPASS force field. COMPASS (condensed-phase optimized molecular potentials for atomistic simulation studies) is an ab initio force field [27, 28]. COMPASS is a class II force field, which has been demonstrated to make accurate predictions of thermodynamic properties such as cohesive energy density [29]. The force fields for the potential energy calculations are automatically assigned by the COMPASS program to the atoms prior to the energy calculations. In COMPASS, the total energy of the system is described as the sum of bonding/valence interactions and non-bonding terms (VdW and coulombic).
| (Eq. 4) |
The valence or bonding terms accounted for bond bending, bond angle bending, dihedral angle torsion, inversion out of plane interactions and a Urey-Bradley (UB) term used to account for interactions between atom pairs involved in 1-3 configurations. In COMPASS, the van der Waals interactions or dispersive forces are modeled by a Lenard-Jones potential using an atom based cutoff (8-10 Å), which reflects the short-range nature of these interactions. The Ewald summation method was used to evaluate the long-range electrostatic interactions [24, 26]. The van der Waals off-diagonal parameters were calculated using geometric mean rule in the Ewald method.
Equations of motion for this NVT (number of molecules, volume and temperature) ensemble were calculated using the Velocity Verlet numerical algorithm to determine the interaction energy of the pure nicotinate molecules in the amorphous cell. The simulations were allowed sufficient number of integration steps with a 1.0 femtosecond timestep to reach equilibrium or the minimum potential energy surface. Direct velocity scaling was used to control the temperature and bring the system to equilibrium at 298 K. Full trajectory files from the molecular dynamics simulations, with information on energy, pressure, velocities and coordinates, were stored in dynamic frames and used by the Dynamics program to compute the cohesive energy density. The cohesive energy densities and solubility parameters were calculated from an ensemble average of the trajectory frames and standard deviation values deduced from the frames. Standard deviation values reported for the molecular dynamics values in Table 1 correspond to the solubility parameters estimated in the final frames (50 – 100) of the simulation.
Table 1.
Hildebrand and Molecular Dynamics solubility parameters of nicotinate acid ester prodrugs
| Compound | Molar Vaporization Energy1 (by Fedors Group Contribution) (kJ/mol) |
Molar Volume1 (by Fedors Group Contribution) (cm3/mol) |
δ (by Group Contribution) (J/cm3)1/2 |
δ (by Simulation) (J/cm3)1/2 |
|---|---|---|---|---|
| Perhydrocarbon nicotinates (Scheme 1A) |
||||
| C1F0 | 62.02 | 114.4 | 23.28 | 22.47 ± 0.33 |
| C2F0 | 66.92 | 130.5 | 22.65 | 21.82 ± 0.10 |
| C4F0 | 76.84 | 162.7 | 21.73 | 20.70 ± 0.10 |
| C6F0 | 86.72 | 194.9 | 21.09 | 20.28 ± 0.06 |
| C8F0 | 96.60 | 227.1 | 20.62 | 19.93 ± 0.05 |
| C10F0 | 106.48 | 259.3 | 20.26 | 18.88 ± 0.10 |
| C12F0 | 116.36 | 291.5 | 19.98 | 19.40 ± 0.08 |
| Perfluoroalkyl nicotinates (Scheme 1B) |
||||
| C2F3 | 66.52 | 154.5 | 20.75 | 19.97 ± 0.08 |
| C3F5 | 70.79 | 177.5 | 19.97 | 19.51 ± 0.10 |
| C4F7 | 75.06 | 200.5 | 19.35 | 18.82 ± 0.08 |
| C8F15 | 92.14 | 292.5 | 17.75 | 17.26 ± 0.09 |
| Perfluoroalkyl nicotinates (Scheme 1C) |
||||
| C3F4 | 77.78 | 162 | 21.91 | 22.43 ± 0.13 |
| C5F8 | 86.33 | 208 | 20.37 | 19.82 ± 0.18 |
| C8F13 | 92.81 | 285.6 | 18.03 | 17.85 ± 0.09 |
Values taken from Fedors group contribution method in ref [21]
Studies have demonstrated the ability of class II force fields such as COMPASS to predict cohesive energy densities that compare favorably with experimental values [29]. The COMPASS program assigns the appropriate force field parameters based on the structure and type of atoms present. Of particular concern was the parameterization of the force fields for fluorinated groups. Our procedure was validated by using this simulation procedure to estimate the solubility parameter of our fluorinated solvent, perfluoromethylcyclohexane, which has a reported solubility parameter value of δ = 12.5 (J/cm3)1/2 [21]. The solubility parameter estimate from this dynamics simulation approach is 12.51 ± 0.13.
4.0 Results and discussion
4.1 Partitioning behavior of nicotinate prodrugs
The perhydrocarbon nicotinates demonstrated two orders of magnitude increase in fluorophilicity with increasing chain length of the functional group (from 1 carbon atom (C1F0) to 10 carbon atoms (C10F0)) (Figure 1). The increasingly negative log Kp values (from −1.72 for the C1F0 to −3.40 for C10F0) reflect the increasing preference for the hydrocarbon phase.
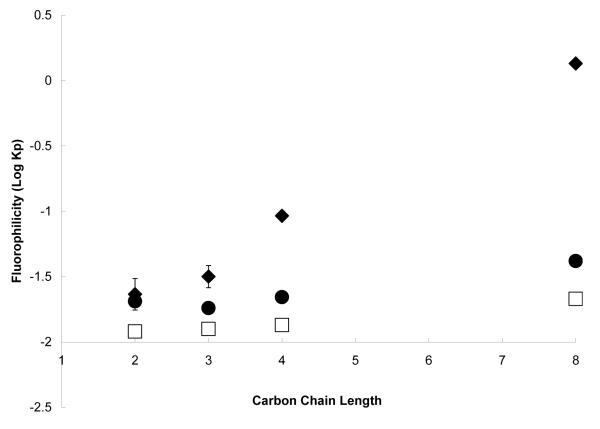
Figure 1.
Partition coefficients for perhydrocarbon nicotinates in PFMCH-Toluene as determined experimentally (◆) or from the application of regular solution theory based on solubility parameters estimated by group contribution (□) or molecular dynamics simulation (•). (The structures are provided in Scheme 1A).
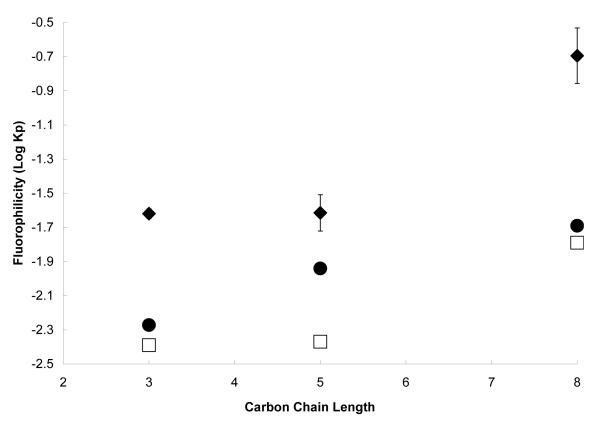
The partitioning behavior of the fluorinated nicotinates, categorized as fully fluorinated and partially fluorinated (Scheme 1), also spans three orders of magnitude (Figures 2 and 3), although the carbon chain length of the fluorinated nicotinates (two to eight carbon atoms) varies less than the perhydrocarbon nicotinates (one to twelve carbon atoms) investigated. This demonstrates the significant effect of substituting fluorine for hydrogen atoms on a compound’s chemical properties. For the fully fluorinated nicotinates, increasing the carbon chain length by four atoms (C4F7 to C8F15) results in a pronounced increase in fluorophilicity. The nicotinate with the longest fluorinated tail length is slightly fluorophilic, as indicated by its positive log Kp value (log Kp = 0.13 for C8F15). These trends follow the empirical rules suggested by Kiss et al. [13], where greater than 60% wt fluorine content is needed to impart “fluorophilic” behavior to a molecule. In our investigation, C8F15 is the only molecule which approaches this criterion (57 wt% fluorine).
Figure 2.
Partition coefficients for fully fluorinated functional chain length nicotinates in PFMCH-Toluene as determined experimentally (◆) or from the application of regular solution theory based on solubility parameters estimated by group contribution (□) or molecular dynamics simulation (•). (The structures are provided in Scheme 1B).
Figure 3.
Partition coefficients for partially fluorinated functional chain length nicotinates in PFMCH-Toluene as determined experimentally (◆) or from the application of regular solution theory based on solubility parameters estimated by group contribution (□) or molecular dynamics simulation (•). (The structures are provided in Scheme 1C).
Similarly, the partitioning of the partially fluorinated molecules (C3F4 to C8F13) into the fluorinated phase increases with length of the fluorinated chain. However, all the partially fluorinated nicotinates have negative partition coefficients. The one order magnitude difference in partition coefficients between the fully fluorinated C8F15 and the partially fluorinated C8F13 (which differ by a single CH2 linkage replacing a CF2 group) highlights the drastic effect of fluorine substitution on the physicochemical properties of the nicotinates. The distribution and position of fluorine atoms in the molecule, as well as the amount of fluorination, are known to affect the fluorophilic behavior of perfluorinated compounds [13]. The demonstrated effect of a terminal H-atom in the fluorinated chain on the physicochemical properties of fluorinated compounds [30, 31] is apparent in the different partition values for the fully fluorinated nicotinate, C3F5, and its partially fluorinated analogue, C3F4, which have log Kp values of −1.50 and −1.62, respectively. The presence of the terminal H-atom in perfluorinated compounds introduces a large permanent dipole in the carbon chain, significantly increasing the hydrophilicity of the solute. In surfactant systems, this effect is observed as an increased critical micelle concentration (CMC). The CMC of both ionic [30] and non-ionic [31] perfluorinated surfactants with terminal hydrogen atoms have been reported to be 300% greater than the fully fluorinated analogues.
4.2 Prediction of Nicotinate Fluorophilicity from Molecular Structure
Predictive models of relative solubilities in biphasic solvents utilize solubility parameters, molar volumes, molecular surface area, van der Waals volume, molar refraction, solvent extended surface and solvatochromic parameters (β, α, π*) [12, 16, 18, 32, 33]. Although recent studies have demonstrated successful correlation of molecular surface area with partitioning behavior of fluorinated molecules [16, 17], the solubility parameter remains one of the most widely accepted parameters for predicting or interpreting physicochemical properties such as partitioning [12, 13]. We calculated the solubility parameter of the functionalized nicotinates from their molecular structure using Fedors structural group contribution method [21] and Accelrys computational molecular dynamics (Table 1). The estimated solubility parameters decrease with chain length in each group of nicotinates (perhydrocarbon, fluorinated, and partially fluorinated). Solubility parameter estimates by group contribution methods and molecular dynamics simulations (Table 1) differ by less than 10%. The solubility parameters calculated by simulation are consistently lower than those estimated by the group contribution method. For equal carbon chain length, the solubility parameter of the perhydrocarbon compounds is greater than the corresponding fluorinated compounds, an expected trend. Substituting a hydrogen atom on the terminal carbon of a fluorinated chain results in an increase in the solubility parameter (C3F5 vs. C3F4).
The partition values (log Kp) predicted using the estimated solubility parameters (Eq 3) are compared with the experimental values in Figures 1-3. Published values of solubility parameter were used for the organic solvents: toluene (δ = 18.2 (J/cm3)1/2) and perfluoromethylcyclohexane (δ = 12.5 (J/cm3)1/2) [21].
The group contribution estimates of partition coefficients for the perhydrocarbon nicotinates are closer to experimental values than the predictive estimates from simulation (Figure 1). Both predictive methods for the solubility parameter of the longer chain perhydrocarbon nicotinates (C6 and greater) result in partition coefficient estimates which overestimate the preference of the nicotinate for the fluorocarbon phase (i.e., higher log Kp). Excluding the simulated estimate for C10F0 (38% difference), all the predicted values in this group of nicotinates are in reasonable agreement with experimentally determined log Kp (i.e.< 20%).
Conversely, the predicted values for the fully fluorinated nicotinate prodrugs are lower than the experimental values (Figure 2), suggesting that the predictive tools underestimated the fluorophilicity of the compounds. The molecular dynamics estimates of partitioning behavior for the fluorinated nicotinates are closer to the experimental values than the estimates determined by group contribution methods. The predictive tools capture the experimental partition coefficients less accurately for fully fluorinated nicotinates than for perhydrocarbon nicotinates. For the fully fluorinated nicotinates, only the shortest chain functional groups (C2F3 and C3F5), have predicted values that are in reasonable agreement with experiment (< 30% difference).
Partially fluorinated nicotinates demonstrate similar partitioning trend to the fully fluorinated nicotinates. The predicted partition coefficients of partially fluorinated nicotinates (Figure 3) deviate significantly from experiment (> 20%), although the partition values predicted by the simulation method are slightly more accurate than the group contribution method. Also, the predictive methods do not accurately capture the effect of a terminal hydrogen atom (C3F4 and C5F8), underestimating the fluorophilicity of the molecules. Partitioning is primarily governed by the balance between the energy required to create a cavity for the solute in the solvent and the intermolecular interactions [33]. However other factors such as the change in shape, symmetry and moment of the molecule due to the exact position of the hydrogen in the molecule and the effects on partitioning [13] are excluded from partition estimates based on the group contribution estimation method.
The greatest differences between experimental and predicted partition coefficients are observed with the highest chained nicotinates (C8F15 and C8F13), where the fluorophilicity is underestimated. Both predictive methods show several orders magnitude deviation (group contribution and model simulation values are log Kp’s = −1.67, −1.38 for C8F15 and −1.79 and −1.69 for C8F13) from the experimental values for C8F15 (log Kp = 0.13) and C8F13 (log Kp = −0.7). While the predictive techniques provide a reasonable estimate of partitioning behavior for the long chain perhydrocarbon nicotinates, they fail to capture the fluorophilic contribution of long fluorinated carbon chains. Applying regular solution theory to large chain nicotinates requires a significant extrapolation of RST assumptions, namely the dispersion of solute molecules similarly sized solvents with no change in entropy and random mixing [23]. This combined with the errors in estimation of the solubility parameters would result in large additive errors in the final predicted values. The sensitivity of the partition coefficients to uncertainties in the Hildebrand solubility parameter (Fedors group contribution values in Table 1) was investigated at δ ± 0.1 and 1.0. The small change in Fedors values (δ ± 0.1), resulted in minimal variation in the predicted partition coefficients (< ±4%). However, δ ± 1 significantly impacted the predicted partition coefficients with a ±16 – 35% and ±14 – 32% variation from the calculated Fedors values for the fully and partially fluorinated series respectively. In effect, decreasing the calculated solubility parameter by 1 (δ – 1) results in a more “fluorophilic” solubility parameter and skews the predicted partition coefficients for the perfluorinated nicotinates toward the experimental partition coefficients.
Fluorophilicity was predicted to within 30% of the experimental log Kp for structurally similar compounds (i.e. eight fluorinated chains attached to pyridine headgroups) within Huque et al.’s [34] correlation of 90 organic compounds (both fluorinated and non-fluorinated) based on linear free energy relationships. Similarly, Kiss et al. [13] demonstrated the applicability of a neural network model to correlate and predict fluorophilicities using 59 fluorinated compounds. However, these techniques also employed the physicochemical properties of the compounds of interest in the statistical parameterization of their models.
An alternative description of fluorophilicity, specific fluorophilicity (Fspec) as defined by Kiss et al. [13] in equation (5), adjusts the experimental partition coefficient for the differences in molecular structure of the fluorocarbon solvent (PFMC) and the solute. It provides insight into how the trend in fluorophilicity is influenced by the difference in molecular size of the solute and the accommodating perfluorocarbon solvent. Specific fluorophilicity of the solutes closely follows the fluorophilicity trends observed for functionalized nicotinates as a function of fluorination and chain length of the alkyl group (Table 2 of supplementary information), For example, increasing the fluorocarbon chain length of the perhydrocarbon nicotinates results in an increase in both the specific fluorophilicity (−2.07 (C2F3) to 0.1 (C8F15) ) and the fluorophilicity (−1.64 (C2F3) to 0.13 (C8F15) ) as measured directly from their partition coefficients in fluorocarbon solvent (PFMC)/toluene. This suggests that as the chain length increases for the perfluorinated nicotinates, the size compensation for accommodating the solute is diminished.
| (Eq. 5) |
Similar to our results, de Wolf and coworkers [12] report experimental and predicted differences in partitioning that range from 0% to >50% for over 50 fluorinated organic molecules based on Mobile Order and Disorder theory, which utilizes modified solubility parameters calculated by group contribution methods. Indeed de Wolf’s [12] expression for partitioning values is similar to equation (3) in this work. However, de Wolf’s specific cohesion parameters (δ’), which were determined from a database rich in fluorous compounds proved inadequate in prediction of our fluorinated nicotinates, which contain a substantial contribution from the hydrocarbon aromatic pyridine ring. Application of de Wolf’s approach to the prediction of partition values of the perfluorinated nicotinates (and utilizing their δ’ value for PFMC of 10.66 Mpa1/2)) results in increased discrepancies with the experimentally determined values (Table 2 of supplementary information). de Wolf’s method resulted in partition coefficients that differed from the experimental values by 31 - > 1000%, while direct application of Hildebrand solubility parameters results in lower discrepancies (17 - > 1000% (Fedors) and 3 - > 1000%, molecular simulation).
However, the utility of de Wolf’s method is more evident from a comparison of the contribution of only the hydrocarbon or fluorinated side chains of the nicotinates to the solubility parameters calculated by group contribution (Δδ (ΣFC) or Δδ (ΣHC)). The incremental contribution to the solubility parameter of the side chains of the perfluorinated nicotinates with increasing fluorinated alkyl groups is greater for Fedors method than for de Wolf’s approach [12] (i.e., Δδ = 0.38 (C3F5) – 1.52 (C8F15) and 1.32 (C3F4) – 1.64 (C5F8)), suggesting a smaller solubility parameter by the de Wolf approach. The difference between the perfluorocarbon tail contributions predicted by these methods increases with increasing chain length. An over-prediction of the contribution of the perfluorinated tail to the solubility parameter of the perfluorinated nicotinates using Fedors method may contribute to the differences in the experimental and predicted partition coefficients for the perfluorinated nicotinates, which increase with chain length. A full analysis of the nicotinates with de Wolf’s group contribution method is limited by the lack of values for the pyridine group or the fluorinated compounds with terminal hydrogen atoms such as our partially fluorinated nicotinates, C3F4 and C5F8.
5.0 Conclusion
Regular solution theory using the Hildebrand solubility parameter, determined by Fedors group contribution method and molecular dynamics simulations was applied to estimate the fluorous/organic partition coefficient of a series of functionalized nicotinic acid esters/nicotinates. This approach worked reasonably well with the series of perhydrocarbon nicotinates and the shorter chained perfluorinated nicotinates (C2F3 and C3F5). Significant deviation from experimental values with the higher chained perfluorinated nicotinates, for which the fluorophilicity is underestimated.
Supplementary Material
Acknowledgements
Special thanks to John May of the Environmental Research Training Laboratory (ERTL, University of Kentucky) for assistance with the GC analysis of the nicotinate partitioning. This work is funded by National Institute of Environmental Health Sciences, NIH, grants ES07380 and ES012475.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kani I, Sisman F. J Mol. Catal. A: Chem. 2006;259:142–149. [Google Scholar]
- [2].Yi W-B, Cai C. Catal. Commun. 2006;9:1291–1296. [Google Scholar]
- [3].Krafft MP, Riess JG. Biochimie. 1998;80:489–514. doi: 10.1016/s0300-9084(00)80016-4. [DOI] [PubMed] [Google Scholar]
- [4].Reiss JG, Krafft MP. Biomaterials. 1998;19:1529–1539. doi: 10.1016/s0142-9612(98)00071-4. [DOI] [PubMed] [Google Scholar]
- [5].LoNostro P, Choi S-M, Ku C-Y, Chen S-H. J. Phys. Chem. B. 1999;103:5347–5352. [Google Scholar]
- [6].Hsu C-H, Jay M, Bummer PM, Lehmler H-J. Pharm. Res. 2003;20:918–925. doi: 10.1023/a:1023899505837. [DOI] [PubMed] [Google Scholar]
- [7].Courrier HM, Vandamme TF, Krafft MP. Colloids Surf., A. 2004;244:141–148. [Google Scholar]
- [8].Weers JG, Arlauskas RA, Tarara TE, Pelura TJ. Langmuir. 2004;20:7430–7435. doi: 10.1021/la049375e. [DOI] [PubMed] [Google Scholar]
- [9].Lehmler H-J, Bummer PM, Jay M. Chemtech. 1999;29:7–12. [Google Scholar]
- [10].Lehmler H-J. Expert Opin. Drug Delivery. 2007;4:247–262. doi: 10.1517/17425247.4.3.247. [DOI] [PubMed] [Google Scholar]
- [11].Lehmler H-J, Xu L, Vyas SM, Ojogun VA, Knutson BL, Ludewig G. Int. J. Pharm. 2008;353:35–44. doi: 10.1016/j.ijpharm.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].de Wolf E, Ruelle P, Broeke J, Deelman B-J, Koten G. J. Phys. Chem. B. 2004;108:1458–1466. [Google Scholar]
- [13].Kiss LE, Kovesdi I, Rabai J. J. Fluorine Chem. 2000;108:95–109. [Google Scholar]
- [14].Jacobson EL, Shieh WM, Huang AC. Mol. Cell. BioChem. 1999;193:69–74. [PubMed] [Google Scholar]
- [15].Rocaboy C, Rutherford D, Bennett BL, Gladysz JA. J. Phys. Org. Chem. 2000;13:596–603. [Google Scholar]
- [16].Daniels SD, Robert A, Saunders RA, Platts JA. J. Fluorine Chem. 2004;125:1291–1298. [Google Scholar]
- [17].Duchowicz PR, Fernandez FM, Castro EA. J. Fluorine Chem. 2005;125:43–48. [Google Scholar]
- [18].Ruelle P. J. Chem. Inf. Comput. Sci. 2000;40:681–700. doi: 10.1021/ci9902752. [DOI] [PubMed] [Google Scholar]
- [19].Danielsson L-G, Zhang Y-H. Trends in analytical chemistry. 1996;15:188–196. [Google Scholar]
- [20].Fedors RF. J. Polymer. Eng and Sci. 1974;14:147–154. [Google Scholar]
- [21].Fedors RF, Van Krevelen DW, Hoftyzer PJ. In: CRC Handbook of solubility parameters and other cohesion parameters. Barton AFM, editor. CRC Press; Boca Raton, FL: 1983. pp. 64–66. [Google Scholar]
- [22].Morishita S, Saito T, Hirai Y, Shoji M, Mishima Y, Kawakami M. J. Med. Chem. 1988;31:1205–1209. doi: 10.1021/jm00401a022. [DOI] [PubMed] [Google Scholar]
- [23].Prausnitz JM, Lichtenthaler RN, de Azevedo EG. Molecular Thermodynamics of Fluid-Phase Equilibria. third ed Prentice-Hall Inc.; Englewood Cliffs, New Jersey: 1986. [Google Scholar]
- [24].Allen MP, Tildesley DJ. Computer simulation of liquids. Oxford University Press; Oxford: 1987. [Google Scholar]
- [25].Spyriouni T, Vergelati C. Macromolecules. 2001;34:5306–5316. [Google Scholar]
- [26].Zhang M, Choi P, Sundararaj U. Polymer. 2003;44:1979–1986. [Google Scholar]
- [27].Sun H. J. Phys. Chem. B. 1998;102:7338–7364. [Google Scholar]
- [28].Rigby D, Sun H, Eichinger BE. Polym. Int. 1998;44:311–330. [Google Scholar]
- [29].Eichinger BE, Rigby D, Muir MH. Comput. Polym. Sci. 1995;5:147. [Google Scholar]
- [30].Downer A, Eastoe J, Pitt AR, Simister EA. J. Penfold, Langmuir. 1999;15:7591–7599. [Google Scholar]
- [31].Eastoe J, Paul A, Rankin A, Wat R, Penfold J, Webster JRP. Langmuir. 2001;17:7873–7878. [Google Scholar]
- [32].Sarraf EG. J. Chem. Soc. Faraday Trans. 1997;93:2519–2525. [Google Scholar]
- [33].Bodor N, Buchwald P. J. Chem. Phys. B. 1997;101:3404–3412. [Google Scholar]
- [34].Huque FTT, Jones K, Saunders RA, Platts JA. J. Fluorine Chem. 2002;115:119–128. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.