Abstract
This paper describes characterization of lyophilized lipid nanocapsules loaded with Alexa 488 by fluorescence correlation spectroscopy (FCS). Fluorimetry analysis of nanocapsules containing self-quenching concentrations of 5-(and-6)-carboxyfluorescein was performed to establish a point of reference for FCS. FCS results complemented the results obtained by fluorimetry for a bulk nanocapsule solution and provided additional information about the size and dye retention by individual nanocapsules. Using this method, we determined that nanocapsules composed of the thiol-functionalized lipids showed the best dye retention and the most consistent results. Dye retention, size, and photolysis efficiency of these thiol-functionalized nanocapsules doped with a far-red photosensitizer did not change substantially upon lyophilization and storage at −20 °C for up to two months, making lyophilization a suitable method for the long-term storage of nanocapsules with the appropriate lipid composition.
Keywords: nanocapsules, fluorescence correlation spectroscopy, lyophilization, dye retention, photolysis
Introduction
Lipid nanocapsules have received much attention as delivery vessels for drug and other biological stimuli to cells.1-3 Our group is interested in perturbing single cells with high spatiotemporal resolution, and thus has focused on the development of light-addressable nanocapsules as an alternative for use in the photolysis of caged compounds.4-8 Chemically caged molecules suffer from several drawbacks, including tediousness of design and synthesis as well as the difficulty in chemically caging large biological molecules, such as peptides and proteins. Nanocapsules, in contrast, offer a versatile platform for “caging” a wide range of biologically relevant molecules. Recently, we have also demonstrated the light-triggered release of biological stimuli from photosensitized nanocapsules using a single nanosecond laser pulse in the far red wavelength region,9,10 a feat that has yet to be achieved with chemically caged molecules.
Despite the versatility and potential usefulness of light-addressable lipid nanocapsules, there is one important practical limitation. Upon their storage in solution, lipid nanocapsules tend to degrade fairly fast (over a few days or less), and thus usually have to be freshly prepared for each experiment. To facilitate and disseminate the use of light-addressable nanocapsules, it is of high practical value to have nanocapsules that could be stored over long periods of time (weeks to months) without a substantial loss of encapsulated material and photolysis efficiency.
One of the most widely used techniques for storage of biologically-relevant materials is lyophilization, or freeze-drying. Indeed, liposomes have been successfully lyophilized previously and were shown to preserve their encapsulated content best when freeze-dried in solutions containing saccharides.11-16 The short and long-term storage of lyophilized liposomes was also investigated.17-19 For these studies, only the bulk behavior of the liposomes was monitored. Leakage from the liposomes was characterized using a fluorimetry method based on the analysis of liposomes loaded with self-quenching concentrations of 5-(and 6)-carboxyfluorescein (CF).11,12,17,18 Although the fluorimetry method is easy to use and is a well established technique, it measures parameters of the liposome solution in bulk, relies on self-quenching concentrations of encapsulated dyes, and does not offer any insight on what is happening at a single-nanocapsule level. For example, it would be impossible to tell from the results obtained by fluorimetry whether the decrease in retention of CF is caused by rupture of a few nanocapsules or whether all of the nanocapsules had leaked to some degree. It is important for our applications, however, to know the change in dye concentration and size of individual nanocapsules upon lyophilization and rehydration. To overcome the drawbacks of the standard fluorimetry method, this paper describes the use of fluorescence correlation spectroscopy (FCS) for monitoring the retention of dye molecules in nanocapsules, as well as the aggregation of nanocapsules, information that is inaccessible to the fluorimetry method.
FCS has been previously used by our group and others to study the parameters associated with single particles.20-24 In addition to the information about brightness, and therefore leakage, of individual nanocapsules, FCS can simultaneously collect data that can be used to determine nanocapsule size/aggregation. This paper provides the first report of the characterization of lyophilized lipid nanocapsules by FCS. Dye retention and change in size/aggregation of individual nanocapsules upon lyophilization and rehydration are reported. Using this method, we found thiol-functionalized lipid nanocapsules offer the best solution for long-term storage using lyophilization.
Materials and Methods
Materials
All lipids and polycarbonate membranes were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Alexa 488 carboxylic acid succinimidyl ester mixed isomers (Alexa 488), 1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD), and 5-(and 6)-carboxyfluorescein mixed isomers (CF) were purchased from Invitrogen (Carlsbad, CA). Alexa 488 was hydrolyzed by addition of water to get a free dye. All other reagents were used as received. The miniextruder was purchased from Avestin (Ottawa, Ontario). Spin columns were purchased from Epoch Biolabs (Missouri City, TX). All other reagents and materials were purchased from Sigma-Aldrich (St. Luis, MO).
Nanocapsule Preparation
Nanocapsules were prepared by rehydration of dry lipid cakes in the buffer containing dye for the encapsulation. In a typical procedure, 0.2 mg of lipid or lipid mixture was dried from its solution in chloroform for one hour under nitrogen and then for a minimum of 2 hours under vacuum. To the dry lipid cake, we added 200 μL of 100 mM solution of CF in our lyophilization buffer (which was composed of 10 wt % sucrose and 20 mM HEPES of pH 7.4) or 200 μL of lyophilization buffer and 5 μL of 10 mg/mL Alexa 488 in water. Liposome mixture was then heated to 70 °C in a water bath, vortexed, and freeze-thawed 3 times by submersion in liquid nitrogen for 45 seconds and then thawing in room-temperature water bath. Liposome solutions were then heated to 70 °C and passed 21 times through 100 nm polycarbonate membrane using a miniextruder. Unencapsulated dye was then removed by running nanocapsule solutions through size-exclusion micro-spin columns containing Sephacryl-100HR equilibrated with the lyophilization buffer using a centrifuge with RCF 2,700 times g. Eluent was diluted 1:10 with lyophilization buffer to get the final lipid nanocapsule solution.
Lyophilization
200 μL of each of the lipid nanocapsule solutions were placed in 1.5 drum glass vials, frozen in liquid nitrogen for 2 minutes, and immediately placed under vacuum on a Labconco Freezone 6 freeze-dryer. The rest of the nanocapsule solutions were stored at 4 °C. After 18-20 hours, lyophilized samples were removed from the freeze-dryer and 180 μL of de-ionized water (Milli-Q) was added to each.
Fluorimetry
Solutions for fluorimetry experiments were typically prepared by adding 50 μL of nanocapsule solution to 3 mL of lyophilization buffer. A nanocapsule solution was analyzed right after its preparation, and after the analysis, part of the solution was lyophilized and the rest was stored at 4 °C. Rehydrated lyophilized solution and non-lyophilized solution stored at 4 °C were analyzed again on the following day. Fluorimetry spectra for each sample were obtained before and after the addition of 20 μL of 5% aqueous solution of Triton X100 detergent. Fluorimetry spectra were collected on Fluorolog Tau 3 fluorimetry with excitation at 488 nm by Xenon lamp. Fluorescence intensity was determined by integrating the emission spectra in the 500-700 nm area. Dye retention was determined as described in the literature:12
where Fb and Fa is the fluorescence intensity before and after the addition of detergent for nanocapsule solution right after preparation, and Fb’ and Fa’ is the fluorescence intensity before and after addition of detergent to lyophilized or non-lyophilized sample (part of the solution that was stored at 4°C while the lyophilized part was drying).
Fluorescence Correlation Spectroscopy (FCS)
Solutions for FCS experiments were used as prepared. In the same manner with fluorimetry experiments, a nanocapsule solution was analyzed by FCS right after its preparation. After the analysis, part of the solution was lyophilized and the rest was stored at 4 °C. Both lyophilized and non-lyophilized solutions were analyzed again on the following day. The setup and experimental conditions were previously described.20-23 In short, 40-50 μL droplets of solutions were placed on a glass coverslip above the objective and excited by 488 nm solid state diode pumped laser (Coherent, Santa Clara, CA). The laser probe volume was focused 25 μm above the surface of the coverslip. Fluorescence signal was collected via an avalanche photodiode (APD SPCM-AQR-16; Perkin-Elmer, Fremont, CA) and autocorrelated using Flex-02 digital correlator (Correlator.com, Bridgewater, NJ). At the start and end of each experiment, calibration data was collected using 190 nm polystyrene beads doped with Suncoast Yellow dye (Bangs Laboratories, Fishers, IN). Radius of the nanocapsules was determined from the diffusion coefficient obtained from fitting the autocorrelation data. To produce an autocorrelation curve, sample fluorescence was collected for 2 min, with 3 autocorrelation curves collected for each droplet. Three droplets for each sample were analyzed.
Intensity data was collected concurrently with the autocorrelation data in 5 ms bins. 60,000 bins were collected for each droplet. Intensity data was then exported into MS Excel and analyzed by RobStat.xla Version 1.0 Robust Statistics Tool Kit, based on the Royal Chemical Society Analytical Methods Committee (RSC AMC) Technical Brief No.6, to get Median and Median Absolute Difference (MAD) values. The baseline (background) was set at a value equal to 3 × (Median + 3 × MAD) for a particular sample. The average intensity for each sample was then calculated as the sum of intensities in bins above the background divided by the number of bins above the background and minus the background. Average intensity of every sample was normalized to the average bead intensities measured before and after sample data collection.
Photolysis Efficiency
Lipid nanocapsules were photolyzed and photolysis efficiencies were calculated as previously described.10 Briefly, the photolysis efficiency was calculated as the percent of 50 nanocapsules that photolyzed upon a single laser pulse delivered to each nanocapsule. Three samples were analyzed for each data point.
Results and Discussion
Lipids for Nanocapsules
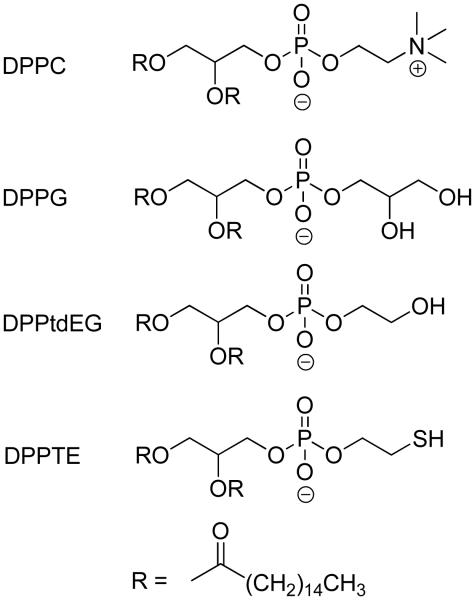
To select a lipid that produced the most robust nanocapsules, the following set of lipid nanocapsules was prepared: (1) DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine), (2) 9:1 DPPC:DPPG (1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-rac-glycerol) [sodium salt]), (3) DPPtdEG (1,2-dipalmitoyl-sn-glycero-3-phospho(ethylene glycol) [sodium salt]), and (4) DPPTE (1,2-Dipalmitoyl-sn-Glycero-3-Phosphothioethanol [Sodium Salt]) (Figure 1). All of the lipids have the same hydrophobic tail but vary in the hydrophilic head group. A neutral DPPC lipid is often used for liposome preparation. It is, however, frequently combined with negatively-charged DPPG to improve the robustness of the liposomes. Nanocapsules composed solely of DPPG, however, do not form well. We also attempted to form nanocapsules composed of the positively charged lipid DPTAP (1,2-dipalmitoyl-3-trimethylammonium-propane [chloride salt]), but failed to produce quality nanocapsules.
Figure 1.
Lipids used for nanocapsule preparation. All lipids have the same hydrophobic tail and differ only in the nature of their hydrophilic heads.
Besides increased robustness, negatively charged nanocapsules would also minimize non-specific cell adsorption. Thus, we selected two other negatively charged lipids not commonly used for liposome preparation, DPPtdEG and DPPTE, and tested them for liposome formation. We found both to form high quality lipid nanocapsules. DPPtdEG and DPPTE structures differ only in the end group of the hydrophilic head. DPPtdEG has a hydroxyl functionality and DPPTE contains a thiol functional group that has a potential to be oxidized to form disulfide bonds and render lipid nanocapsules even more robust.
Fluorimetry
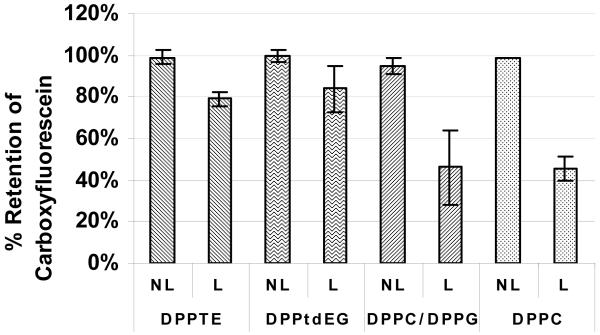
To establish a reference for FCS, fluorimetry experiments were carried out in a similar manner as described in the literature.12 Buffer containing 100 mM of CF was used as a loading solution, which produced a self-quenching CF concentration inside the liposomes. Figure 2 shows the amount of dye retained by the nanocapsules that were lyophilized then rehydrated as well as the non-lyophilized nanocapsules that were stored at 4 °C for 19 hours. It can be seen that lyophilized nanocapsules composed of purely negatively charged lipids, DPPTE and DPPtdEG, retained about 80% of the encapsulated CF, while liposomes made of DPPC and DPPC/DPPG retained only about 50%. Since all of the non-lyophilized nanocapsules stored at 4 °C retained close to 100% of the encapsulated dye, the leakage of encapsulated dye from lyophilized samples was triggered by freeze-drying and rehydration.
Figure 2.
Fluorimetry analysis of the retention of encapsulated 5-(and-6)-carboxyfluorescein (CF) by non-lyophilized (NL; part of the solution that was stored at 4 °C for 19 hours, while the lyophilized part was drying) and lyophilized (L; rehydrated right after lyophilization) lipid nanocapsules. Retention of CF was calculated as % Retention = [(Fa’ – Fb’)/Fa’]/[(Fa – Fb)/Fa]x100%, where Fb and Fa is the fluorescence intensity before and after the addition of detergent into a nanocapsule solution right after nanocapsules were made; and Fb’ and Fa’ is the fluorescence intensity before and after the addition of detergent to a lyophilized (L) or a non-lyophilized (NL) sample.
Although the fluorimetry technique is easy to use, is well established, and provides a good estimate for the dye retention by the nanocapsules, it suffers from several drawbacks. First, fluorimetry technique relies on self-quenching dye concentrations inside the liposomes. Upon dye leakage, there is a possibility that the concentration inside the nanocapsules will drop below the self-quenching level. In this case, the increase in fluorescence intensity will no longer be caused solely by the leaked dyes; dye retained within the liposomes may also contribute to an increase in fluorescence intensity, which would over estimate the amount of dye that has leaked from the liposomes. Second, it is impossible to tell whether the increase in leaked dye was caused by rupture of a small number of nanocapsules or was due to homogeneous leakage from all nanocapsules. To overcome these drawbacks, we performed FCS analysis of lipid nanocapsules.
Fluorescence Correlation Spectroscopy (FCS)
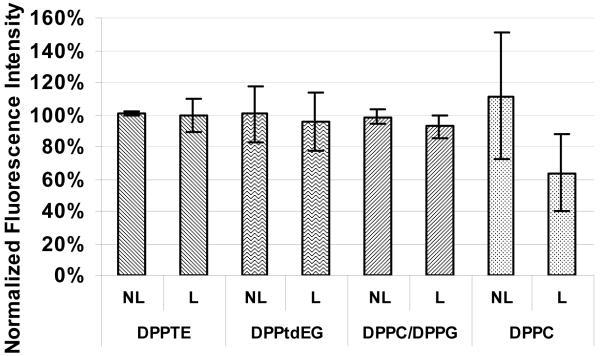
For FCS experiments, lipid nanocapsules were loaded with Alexa 488 dye, chosen for its better photostability than CF. It can be seen from Figure 3 that the average dye retention for the lyophilized lipid nanocapsules was at or above the levels obtained by the fluorimetry method. The average brightness of non-lyophilized nanocapsules measured after storage for 19 hours at 4°C was the same as it was right after the nanocapsules were prepared. In general, it was found that lipid nanocapsules containing the encapsulated dye retained their dye content at most for a few days upon storage at 4 °C. There is wide variability, however, in the duration that nanocapsules can be stored at 4 °C, mostly dependent on the type of lipids used to form the nanocapsules. For example, nanocapsules made from neutral DPPC only last for hours (less than one day) while those made with negatively charged lipids can last several days. It is notable that non-lyophilized DPPC nanocapsules displayed an increase in the average brightness, which can be attributed to aggregate formation, evident from the pronounced average radius increase of DPPC liposomes (Figures 4 and 5).
Figure 3.
FCS analysis of brightness of non-lyophilized (NL; part of the solution that was stored at 4 °C for 19 hours, while the lyophilized part was drying) and lyophilized (L; rehydrated right after lyophilization) lipid nanocapsules containing Alexa 488. Data normalized to intensity of nanocapsules right after their preparation.
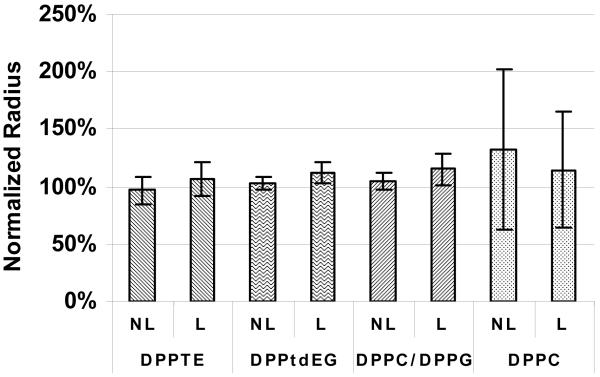
Figure 4.
Radius of non-lyophilized (NL; part of the solution that was stored at 4 °C for 19 hours, while the lyophilized part was drying) and lyophilized (L; rehydrated right after lyophilization) lipid nanocapsules containing Alexa 488 determined by FCS. Radius was calculated from the diffusion coefficient determined by autocorrelation curves. Data normalized to radius of nanocapsules right after their preparation.
Figure 5.
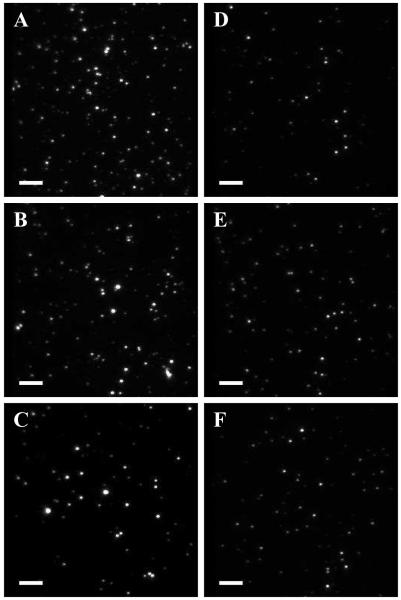
Images of lipid nanocapsules loaded with Alexa 488 obtained with Total Internal Reflection Fluorescence (TIRF) microscopy: A. DPPC right after preparation; B. DPPC after storage at 4 °C for 19 hours; C. DPPC after lyophilization and rehydration; D. DPPTE right after preparation; E. DPPTE after storage at 4 °C for 19 hours; F. DPPTE after lyophilization and rehydration. Scale bar is 5 μm.
The average dye retention of lyophilized nanocapsules appeared to be greater than the dye retention measured by fluorimetry for all nanocapsules. There are two possible reasons for such behavior: (1) dye retention measured by fluorimetry does not account for the release of the dye due to the nanocapsule rupture and (2) non-linear increase in fluorescence due to de-quenching of CF. For most applications, the presence of non-encapsulated material in the external matrix due to rupture of nanocapsules will not be an issue, because a quick filtration through the size exclusion chromatography (SEC) column should remove the non-encapsulated material. A significant difference in size between the lipid nanocapsules and the encapsulated molecules that makes purification via SEC possible is another advantage of using lipid nanocapsules instead of chemically caged compounds, which often are hard to separate from their dark hydrolysis products.
Figure 4 shows the radii of the lipid nanocapsules determined by FCS. DPPTE nanocapsules showed the smallest increase in size after lyophilization, with the normalized average radius after the lyophilization lying within the standard deviation of the average radius measured right after the preparation. The most pronounced increase in radius was observed for DPPC nanocapsules. The increase in size for DPPC nanocapsules can also be seen in Figure 5. Images of DPPC nanocapsules after storage at 4 °C for 19 hours (Figure 5B) and after lyophilization and rehydration (Figure 5C) contain spots that are much larger than spots in images taken right after nanocapsule preparation (Figure 5A), indicating the formation of aggregates and/or fused nanocapsules. Spots in images of DPPTE nanocapsules (Figures 5D-F) are of the same size before and after storage or lyophilization. We note that the number of observed spots in each image does not serve as an accurate indicator of the concentration of the nanocapsules, because the density of nanocapsules on the surface depends on many variables, including the settling time and the “stickiness” of the surface.
The slight increase in the radii of the DPPC/DPPG and DPPtdEG nanocapsules seen in Figure 4 was most probably caused by their slight aggregation and/or fusion. The images of these nanocapsules (data not provided) did not reflect any qualitative change in size, meaning that the size of these aggregated and/or fused nanocapsules would have to be smaller than the microscope resolution.
The increase in the viscosity of the nanocapsule solution after the lyophilized solutions were rehydrated could also have resulted in the increased measured radius, although we believe this to be unlikely. Lyophilized samples were rehydrated by addition of 180 μL of de-ionized water to the dry cake produced by freeze-drying of 200 μL of nanocapsule solution in 10 wt % sucrose buffer. Fluorimetry measurements of the samples after the addition of Triton X100 detergent showed that the total concentration of CF in the rehydrated samples on average was 102 ± 2% of the CF concentration in the non-lyophilized samples. Therefore, it can be assumed that the concentration of all the other solutes, including sucrose, in the lyophilized samples stayed within 4% of their original concentrations as well. A 4% increase in the sucrose concentration would have resulted in less than 2% increase in the calculated radius of the nanocapsules.
Effect of Lipid Head Group on Dye Leakage
Data in Figures 2 and 3 shows that there was virtually no leakage of the encapsulated dye from the non-lyophilized nanocapsules after their storage at 4 °C for 19 hours. Thus, the nature of the lipid head group did not affect the leakage of the encapsulated dyes from the nanocapsules stored in a solution. Upon lyophilization, however, nanocapsules composed of the negatively charged lipids retained more dye than the neutral DPPC nanocapsules. Because lyophilized DPPC nanocapsules also formed the most aggregated and/or fused species, this aggregation and/or fusion could be the leading cause of their increase in the dye leakage.
This hypothesis is also supported by the fact that the electrostatic repulsion between the negatively charged lipid nanocapsules would decrease their aggregation and/or fusion and result in a better retention of the encapsulated dye. Nanocapsules composed of purely negatively charged lipids, DPPTE and DPPtdEG, showed a better dye retention than DPPC/DPPG nanocapsules that contained only 10 mol % of negatively charged lipid. In addition, Figure 4 shows that DPPTE nanocapsules exhibited the smallest increase in their radius after the lyophilization. Since thiol groups in DPPTE are more acidic than their alcohol counterparts in DPPtdEG, the potential presence of an additional negative charge on the DPPTE head group could contribute to an even greater repulsion between the nanocapsules and even lower dye leakage due to the aggregation and/or fusion of nanocapsules.
Incorporation of Photosensitizer Dye
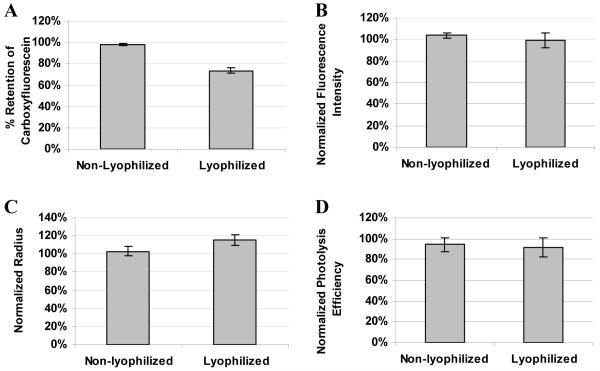
Based on the results from Figures 2-5, DPPTE was chosen as the best-performing lipid for the nanocapsule formation due to the lower dye leakage and a better reproducibility. To make nanocapsules photolyzable, a far-red absorbing lipophilic dye, DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate), was incorporated into the DPPTE nanocapsules at 6 mol %, an amount shown to have an optimal photolysis efficiency previously.10 The effect of DiD incorporation on the dye retention, size, and photolysis efficiency of lyophilized nanocapsules was investigated (Figure 6). A slight increase in the dye leakage in DPPTE/DiD nanocapsules compared to pure DPPTE nanocapsules was observed by fluorimetry, but FCS results showed 99% retention of Alexa 488 after lyophilization and rehydration. The average radius of DPPTE/DiD nanocapsules was measured to be 42 ± 2 nm and had increased slightly upon lyophilization (Figure 6C). The photolysis efficiency of lyophilized DPPTE/DiD nanocapsules was the same as with the non-lyophilized nanocapsules (Figure 6D).
Figure 6.
Analysis of non-lyophilized (part of the solution that was stored at 4 °C for 19 hours, while the lyophilized part was drying) and lyophilized (rehydrated right after lyophilization) DPPTE/DiD lipid nanocapsules. A. Retention of encapsulated 5-(and-6)-carboxyfluorescein determined by fluorimetry. B. Retention of Alexa 488 determined by FCS. C. Radius of nanocapsules loaded with Alexa 488, determined by FCS. D. Photolysis efficiency of nanocapsules containing Alexa 488. Data normalized to measurements of nanocapsules right after their preparation.
Long-Term Storage
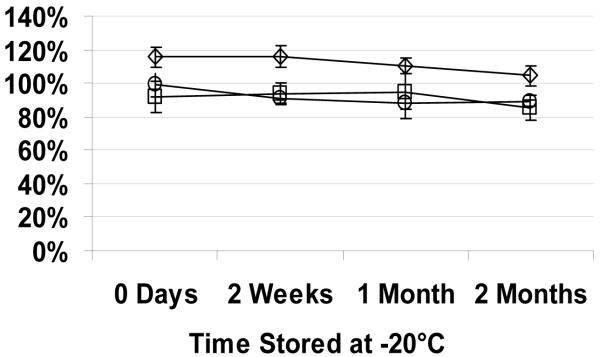
The effects of the long-term storage on the dye retention, size, and photolysis efficiency of lyophilized DPPTE/DiD nanocapsules stored at −20 °C can be seen in Figure 7. No significant change in photolysis efficiency or size was observed even after two months of storage. A slight decrease in the brightness after two weeks of storage was, in all probability, induced by freezing of the lyophilized samples at −20 °C, since no further decrease in brightness was observed after one and two months of storage. The lyophilized nanocapsules were stored at −20 °C to simulate the storage conditions of nanocapsules containing biologically active molecules. Overall, DPPTE/DiD nanocapsules appeared to be suitable for long-term storage with the encapsulated material.
Figure 7.
Fluorescence intensity (○), radius (◇), and photolysis efficiency (□) of lyophilized DPPTE/DiD nanocapsules containing Alexa 488 and stored at −20 °C over time. Data normalized to nanocapsule parameters measured right after their preparation.
Conclusions
This paper describes the first FCS characterization of lyophilized lipid nanocapsules. There were three major sets of findings: (1) FCS was more appropriate for the analysis of the dye retention by individual lyophilized lipid nanocapsules, since fluorimetry did not account for the dye leaked due to the rupture of a small population of the liposomes, (2) Out of all the lipids tested, DPPTE gave the most robust nanocapsules and the most consistent results, and (3) Lyophilization could be used for long-term storage of the photolyzable nanocapsules without substantial loss of the encapsulated material or of the photolysis efficiency. In addition, the conditions we used to lyophilize and store nanocapsules are similar to the conditions used to store sensitive biological materials, such as antibodies. The ability to store photolyzable lipid nanocapsules has an important practical advantage and opens new avenue for their use in a growing number of applications.
Acknowledgement
K.A. Dendramis gratefully acknowledge support by the NIH Training Program No. 1T32CA138312-01. This work is supported by the NSF (CHE-0924320) and the NIH (AG 029574).
References
- 1.Chrai SS, Murari R, Ahmad I. Biopharm-Appl. T. of Bio. 2002;15:40–43. 49. [Google Scholar]
- 2.Immordino ML, Dosio F, Cattel L. Int. J. Nanomed. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 3.Ruysschaert T, Germain M, Gomes J, Fournier D, Sukhorukov GB, Meier W, Winterhalter M. IEEE T Nanobiosci. 2004;3:49–55. doi: 10.1109/tnb.2004.824273. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Chiu DT. Spatially and temporally resolved delivery of stimuli to single cells. J. Am. Chem. Soc. 2003;125(13):3702–3703. doi: 10.1021/ja029942p. [DOI] [PubMed] [Google Scholar]
- 5.Sun B, Mutch SA, Lorenz RM, Chiu DT. Langmuir. 2005;21:10763–10769. doi: 10.1021/la0513297. [DOI] [PubMed] [Google Scholar]
- 6.Sun BY, Chiu DT. Langmuir. 2004;20:4614–4620. doi: 10.1021/la0364340. [DOI] [PubMed] [Google Scholar]
- 7.Sun BY, Chiu DT. Anal. Chem. 2005;77:2770–2776. doi: 10.1021/ac048439n. [DOI] [PubMed] [Google Scholar]
- 8.Sun BY, Lim DSW, Kuo JS, Kuyper CL, Chiu DT. Langmuir. 2004;20:9437–9440. doi: 10.1021/la048444m. [DOI] [PubMed] [Google Scholar]
- 9.Dendramis KA, Allen PB, Reid PJ, Chiu DT. Chem. Commun. 2008:4795–4797. doi: 10.1039/b806685j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dendramis KA, Chiu DT. J. Am. Chem. Soc. 2009;131:16771–16778. doi: 10.1021/ja904976r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ausborn M, Schreier H, Brezesinski G, Fabian H, Meyer HW, Nuhn P. J. Control. Release. 1994;30:105–116. [Google Scholar]
- 12.Crowe JH, Crowe LM. Biochim. Biophy. Acta. 1988;939:327–334. doi: 10.1016/0005-2736(88)90077-6. [DOI] [PubMed] [Google Scholar]
- 13.Hauser H, Strauss G. Adv. Exp. Med. Biol. 1988;238:71–80. doi: 10.1007/978-1-4684-7908-9_7. [DOI] [PubMed] [Google Scholar]
- 14.Hua ZZ, Li BG, Liu ZJ, Sun DW. Dry. Technol. 2003;21:1491–1505. [Google Scholar]
- 15.Miyajima K. Adv. Drug Deliver. Rev. 1997;24:151–159. [Google Scholar]
- 16.Vemuri S, Yu CD, Degroot JS, Wangsatornthnakun V, Venkataram S. Drug Dev. Ind. Pharm. 1991;17:327–348. [Google Scholar]
- 17.Sun WQ, Leopold AC, Crowe LM, Crowe JH. Biophys.l J. 1996;70:1769–1776. doi: 10.1016/S0006-3495(96)79740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Winden ECA, Crommelin DJA. J. Control. Release. 1999;58:69–86. doi: 10.1016/s0168-3659(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 19.vanWinden ECA, Crommelin DJA. Eur. J. Pharm. and Biopharm. 1997;43:295–307. [Google Scholar]
- 20.Budzinski KL, Allen RW, Fujimoto BS, Kensel-Hammes P, Belnap DM, Bajjalieh SM, Chiu DT. Biophys. J. 2009;97:2577–2584. doi: 10.1016/j.bpj.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadd JC, Kuyper CL, Fujimoto BS, Allen RW, Chiu DT. Anal. Chem. 2008;80:3450–3457. doi: 10.1021/ac8000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuyper CL, Budzinski KL, Lorenz RM, Chiu DT. J. Am. Chem. Soc. 2006;128:730–731. doi: 10.1021/ja0569252. [DOI] [PubMed] [Google Scholar]
- 23.Kuyper CL, Fujimoto BS, Zhao Y, Schiro PG, Chiu DT. J. Phys. Chem. B. 2006;110:24433–24441. doi: 10.1021/jp064865w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigler P, Meier W. J. Am. Chem. Soc. 2006;128(1):367–373. doi: 10.1021/ja056719u. [DOI] [PubMed] [Google Scholar]